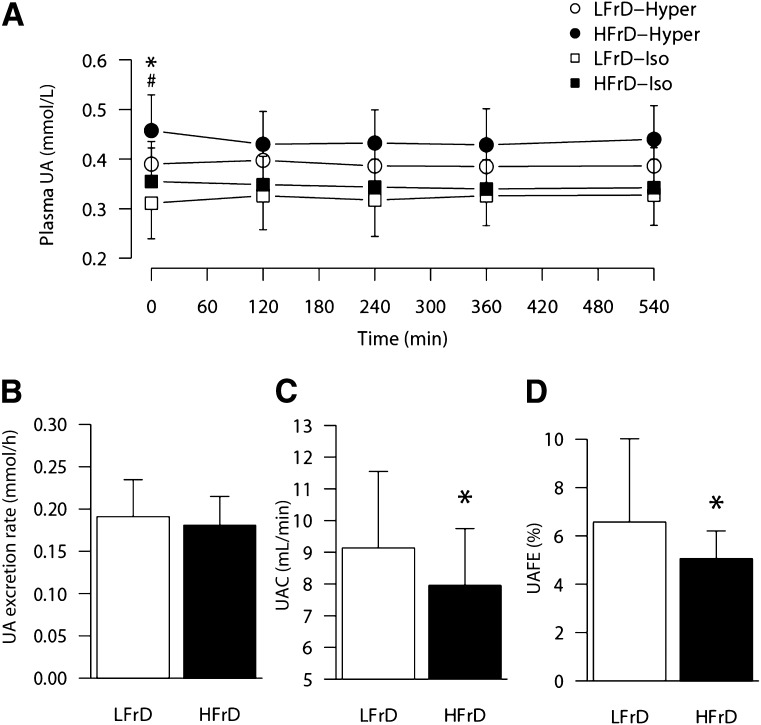
Ingestion of a high-fructose meal increases blood uric acid (UA) concentration in healthy subjects (1). Furthermore, high-fructose intake over several days is associated with increased fasting UA concentration (1). These effects are generally attributed to an increased UA production, as observed after intravenous fructose administration (2). It has not been assessed, however, whether UA also increases when fructose is administered as several small drinks instead of one single large load or whether a high-fructose diet (HFrD) impairs renal UA clearance (UAC) or fractional excretion (UAFE) as observed in rats (3). We therefore measured blood and urine UA and creatinine concentrations (RX Monza; Randox Laboratories, London, U.K.) and calculated UAC and UAFE in 16 healthy male subjects (mean ± SD age 22.6 ± 2.9, weight 69.7 ± 6.9 kg, BMI 22.1 ± 1.8 kg/m2, mean fat-free mass [FFM] 57.8 ± 5.4 kg) over a 9-h period during which they drank lemon-flavored beverages providing 0.2 g fructose/kg FFM every hour. These samples were collected while subjects were participating in two clinical trials (clinical trial reg. nos. NCT01121003 and NCT01119989; clinicaltrials.gov) that assessed different metabolic effects of HFrD but with very similar experimental designs (4,5). Each subject was studied twice after 1) 4–6 days on an isoenergetic, low-fructose diet (LFrD) (n = 16) and either 2) 6 days on a hyperenergetic HFrD with 34% excess energy as fructose (n = 8) or 3) 4 days on an weight maintenance HFrD in which 30% starch was substituted with fructose (n = 8). Because an initial repeated-measures ANOVA did not reveal any significant interaction between diet and energy content, all HFrD data were pooled to specifically test the hypothesis that HFrD decreased UAC and UAFE.
Although the cumulative fructose load was large (1.8 g/kg FFM), blood UA concentration did not increase above fasting values, irrespective of the diet consumed in the days before (Fig. 1A), suggesting that UA production increases mainly when the rate of hepatic fructose metabolism is abnormally high. Urinary UA excretion was not different after HFrD and LFrD (Fig. 1B), but fasting blood UA concentration was 18% higher (P = 0.004) (Fig. 1A), urinary UAC was 9.8 ± 21% lower (P = 0.015) (Fig. 1C), and UAFE was 11.5 ± 29.2% lower (P = 0.041) (Fig. 1D) after HFrD. Creatinine clearance was not altered by HFrD (LFrD vs. HFrD 153 ± 39 vs. 158 ± 19 mL/min, P = 0.62) (data not shown). These observations suggest that a decreased urinary UA excretion may contribute to fructose-induced hyperuricemia. This mechanism may substantially enhance the risk of gout in people who consume high amounts of sugar. Because the metabolic effects of fructose show significant sex differences, it remains to be assessed whether the same effects are observed in female subjects.
Figure 1.
A: Effect of 4–6 days of an HFrD on blood UA concentrations during a 9-h test of 0.2 g/kg FFM fructose ingested every hour. ○ and ●, subjects receiving a hyperenergetic diet in the HFrD condition (HFrD-Hyper trial); □ and ■, subjects receiving an isoenergetic diet in the HFrD condition (HFrD-Iso trial). Open symbols indicate data collected after 4–6 days of an isocaloric LFrD in either trial (LFrD-Hyper, LFrD-Iso). Closed symbols represent data collected after 4–6 days of HFrD. Data are mean ± SD. *Fasting values significantly (P < 0.05) different in subjects receiving an LFrD after the HFrD-Hyper trial. #Fasting values significantly (P < 0.05) different in subjects receiving an LFrD after the HFrD-Iso trial. B: Urinary UA excretion rate. C: UAC rate. D: UAFE. Data are mean ± SD. *Significant difference between HFrD and LFrD (pooled data P < 0.05).
Acknowledgments
This study was supported by grant 320030-138428 from the Swiss National Foundation for Science and by a grant from Ajinomoto Co. Inc., Japan.
L.T. received research support from Ajinomoto Co. Inc. for the clinical trial NCT01119989, and from Nestec S.A. for clinical trials unrelated to this work. No other potential conflicts of interest relevant to this article were reported.
V.L. and L.E. performed the statistical analysis. V.L., L.E., F.T., C.D., P.S., and L.T. revised the manuscript. V.L. and L.T. designed the study and drafted the manuscript. L.E., F.T., and P.S. recruited subjects and performed the tests and laboratory analyses. C.D. analyzed dietary intakes. L.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at Experimental Biology 2013, Boston, Massachusetts, 20–24 April 2013.
Footnotes
V.L., L.E., and F.T. contributed equally to this work.
References
- 1.Cox CL, Stanhope KL, Schwarz JM, et al. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr Metab (Lond) 2012;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelmalek MF, Lazo M, Horska A, et al. ; Fatty Liver Subgroup of Look AHEAD Research Group Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology 2012;56:952–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu QH, Wang C, Li JM, Zhang DM, Kong LD. Allopurinol, rutin, and quercetin attenuate hyperuricemia and renal dysfunction in rats induced by fructose intake: renal organic ion transporter involvement. Am J Physiol Renal Physiol 2009;297:F1080–F1091 [DOI] [PubMed] [Google Scholar]
- 4.Egli L, Lecoultre V, Theytaz F, et al. Exercise prevents fructose-induced hypertriglyceridemia in healthy young subjects. Diabetes 2013;62:2259–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theytaz F, Noguchi Y, Egli L, et al. Effects of supplementation with essential amino acids on intrahepatic lipid concentrations during fructose overfeeding in humans. Am J Clin Nutr 2012;96:1008–1016 [DOI] [PubMed] [Google Scholar]