Abstract
OBJECTIVE
When oral therapy for type 2 diabetes is ineffective, adding basal insulin improves glycemic control. However, when glycated hemoglobin (HbA1c) remains elevated because of postprandial hyperglycemia, the next therapeutic step is controversial. We examined the efficacy and safety of lixisenatide in patients with HbA1c still elevated after initiation of insulin glargine.
RESEARCH DESIGN AND METHODS
This double-blind, parallel-group trial enrolled patients with HbA1c 7–10% despite oral therapy. Insulin glargine was added and systematically titrated during a 12-week run-in, after which candidates with fasting glucose ≤7.8 mmol/L and HbA1c 7–9% were randomized to lixisenatide 20 µg or placebo for 24 weeks while insulin titration continued. The primary end point was HbA1c change after randomization.
RESULTS
The randomized population (n = 446) had mean diabetes duration of 9.2 years, BMI 31.8 kg/m2, and daily glargine dosage of 44 units. HbA1c had decreased during run-in from 8.6 to 7.6%; adding lixisenatide further reduced HbA1c by 0.71 vs. 0.40% with placebo (least squares mean difference, –0.32%; 95% CI, –0.46 to –0.17; P < 0.0001). More participants attained HbA1c <7% with lixisenatide (56 vs. 39%; P < 0.0001). Lixisenatide reduced plasma glucose 2 h after a standardized breakfast (difference vs. placebo –3.2 mmol/L; P < 0.0001) and had a favorable effect on body weight (difference vs. placebo –0.89 kg; P = 0.0012). Nausea, vomiting, and symptomatic hypoglycemia <3.3 mmol/L were more common with lixisenatide.
CONCLUSIONS
Adding lixisenatide to insulin glargine improved overall and postprandial hyperglycemia and deserves consideration as an alternative to prandial insulin for patients not reaching HbA1c goals with recently initiated basal insulin.
When oral therapy does not maintain acceptable glycemic control in type 2 diabetes, adding and titrating basal insulin improves control and frequently restores glycated hemoglobin (HbA1c) to <7.0% (<53 mmol/mol) (1–3). For those patients not fully successful with this regimen, the approach to treatment intensification is of current interest, especially with regard to improving control of postprandial hyperglycemia (4–8). Adding one or more injections of rapid-acting insulin with meals is effective for many patients but has drawbacks, including increased risk of hypoglycemia and weight gain (4,9). Another recently available option is addition of a glucagon-like peptide 1 receptor agonist (GLP-1RA) to previous oral and basal insulin therapy (10,11). Currently available GLP-1RAs are twice-daily exenatide (Byetta), once-daily liraglutide (Victoza), and once-weekly exenatide (Bydureon). Each has a glucose-dependent insulinotropic and glucagon-reducing effect, promotes satiety, and seldom causes hypoglycemia when used alone (12,13). However, short-acting exenatide appears more effective in controlling postprandial glucose (PPG) (14), whereas long-acting preparations, such as liraglutide and once-weekly exenatide, have greater effects on fasting hyperglycemia (13).
Lixisenatide is a novel GLP-1RA that shares the main features of these agents but has a profile of action that appears intermediate between short-acting exenatide and longer-acting agents (13,15). Lixisenatide is effective administered once-daily yet retains the ability to reduce PPG, an effect associated with slowing of gastric emptying (15–18). The objective of this study was to assess the efficacy and safety of adding lixisenatide in the problematic subgroup of people with type 2 diabetes who have relatively acceptable control of fasting plasma glucose (FPG) after initiating and titrating basal insulin but have HbA1c levels remaining persistently elevated (≥7.0%).
RESEARCH DESIGN AND METHODS
Study design
This phase III study (NCT00975286) was a randomized, double-blind, placebo-controlled, parallel-group protocol conducted from October 2009 to August 2011 in 140 centers in 25 countries. It included a 12-week run-in phase, during which insulin glargine was started and titrated, and a 24-week randomized treatment period for participants who were eligible after the run-in (Supplementary Fig. 1). The study was approved by the Institutional Review Boards or Ethics Committees of the participating centers, and it was conducted in accordance with the principles of the Declaration of Helsinki and International Conference on Harmonization–Good Clinical Practice guidelines. All participants provided written informed consent. An independent Data Monitoring Committee supervised conduct of the study. The Allergic Reaction and Cardiovascular Event Adjudication Committees performed masked adjudication of possible events in these categories.
Participants
Adults with type 2 diabetes for at least 1 year at the time of screening were eligible. Entry criteria included the following: use of metformin at a stable dose of at least 1.5 g/day for at least 3 months alone or in combination with a sulfonylurea or glinide or a thiazolidinedione (TZD), or a combination of these; HbA1c ≥7.0 and ≤10% (≥53 to ≤86 mmol/mol); and BMI >20 kg/m2. Exclusion criteria included the following: use of oral or injectable antihyperglycemic agents other than metformin, sulfonylureas, glinides, and TZDs within 3 months; use of weight-loss drugs if not at a stable dose for ≥3 months; history of hypoglycemia unawareness, gastrointestinal disease associated with prolonged nausea, and vomiting; and hypersensitivity to insulin glargine or allergic reaction to any GLP-1RAs. After enrollment, participants continued metformin and a TZD if previously used but stopped any secretagogue. Morning administration of insulin glargine was started at 10 units daily and was titrated weekly, targeting a fasting range of 4.4–5.6 mmol/L (80–100 mg/dL). At completion of the 12-week run-in, participants were eligible for randomization if they had HbA1c ≥7% and ≤9% (≥53 and ≤75 mmol/mol) and fasting self-measurement of plasma-referenced glucose (SMPG) for the past 7 days averaging ≤7.0 mmol/L (126 mg/dL) early in the trial or ≤7.8 mmol/L (140 mg/dL) after a protocol amendment in July 2010.
Randomization and masking
Eligible participants were centrally randomized in a 1:1 ratio to add either lixisenatide or injectable placebo to their current regimens. Lixisenatide or placebo was packaged in accordance with the centrally generated randomized treatment kit number list. Corresponding treatment numbers for each randomized participant were allocated using a centralized interactive voice response system. Investigators did not have access to the randomization code, the bioanalyst was blinded, the Allergic Reaction Assessment Committee reviewed and adjudicated allergic reactions, and the Cardiovascular Event Adjudication Committee reviewed and adjudicated major cardiovascular events in a blinded manner. Randomization was stratified by HbA1c values after the run-in (<8%, ≥8% [64 mmol/mol]) and TZD use (yes or no). A two-step dosage increase was used with both placebo and lixisenatide (10 μg for 1 week, 15 μg for 1 week, and then 20-μg maintenance dosage if tolerated), with injections self-administered by participants ≤1 h before breakfast. Adjustment of dosage of insulin glargine was permitted throughout randomized treatment targeting fasting SMPG 4.4–5.6 mmol/L (80–100 mg/dL). Rescue therapy with short-acting insulin was permitted through week 8 if FPG was repeatedly >11.1 mmol/L (200 mg/dL) or if HbA1c was >9.0% (75 mmol/mol), and after week 8 if FPG was >10.0 mmol/L (180 mg/dL) or if HbA1c was >8.5% (69 mmol/mol).
Efficacy and safety measurements
The primary efficacy measure was the absolute change of HbA1c from baseline to week 24. Continuous secondary efficacy variables included the following: the change from baseline to week 24 in 2-h PPG and blood glucose excursions during a standardized breakfast meal test; seven-point plasma-calibrated SMPG; FPG; body weight; and average daily insulin glargine dosage. Measurements of body weight, insulin dosage, FPG, and HbA1c were recorded at baseline, at end point, and at intervals throughout the trial. The standardized breakfast meal (Ensure Plus drink; Abbott) contained 600 kcal and was consumed within a 15-min period 30 min after study drug administration. The PPG measurements were assessed 2 h after the meal, and the blood glucose excursion was calculated as the 2-h PPG–plasma glucose 30 min before the meal test before administration of lixisenatide or placebo. Additional categorical secondary efficacy variables included the percentage of participants achieving an HbA1c target of <7% or ≤6.5% (<53 or ≤48 mmol/mol) at week 24 and the percentage of participants requiring rescue therapy during the double-blind 24-week period.
Safety was assessed by report of treatment-emergent adverse events (TEAEs), symptomatic and severe symptomatic hypoglycemia, injection site reactions, allergic events, and laboratory tests. In addition to standard laboratory tests, amylase, lipase, calcitonin, plasma lipoproteins, and albumin-to-creatinine ratio were measured.
Statistical analysis
Sample sizes of 450 (225 per group) provided a power of 90% to detect a difference of 0.4% in the change of HbA1c from baseline to week 24 between lixisenatide and placebo, assuming the common SD was 1.3% with a two-sided test at the 5% significance level. Efficacy variables were assessed in the modified intention-to-treat population, defined as all randomized participants who received at least one dose of double-blind study drug, and had both a baseline assessment and at least one postbaseline assessment of any primary or secondary efficacy variables using the last observation carried forward procedure. Safety variables were assessed in the safety population, defined as all randomized participants exposed to at least one dose of the double-blind study drug, regardless of the amount of treatment administered. Safety was analyzed descriptively according to treatment groups during the on-treatment period.
The primary efficacy assessment used an ANCOVA model with treatment groups (lixisenatide or placebo), randomization strata (HbA1c values after the run-in [<8.0, ≥8.0%, 64 mmol/mol] and TZD use [yes or no]), and country as fixed effects, and baseline HbA1c value was used as a covariate. The difference between lixisenatide and placebo and two-sided 95% CIs, as well as P values, were estimated within the framework of ANCOVA. Continuous secondary efficacy variables used a similar ANCOVA model, and categorical secondary efficacy variables were analyzed using a Cochran–Mantel–Haenszel method with stratification by HbA1c values after run-in (<8.0, ≥8.0%) and TZD use (yes or no).
RESULTS
Participant flow and characteristics
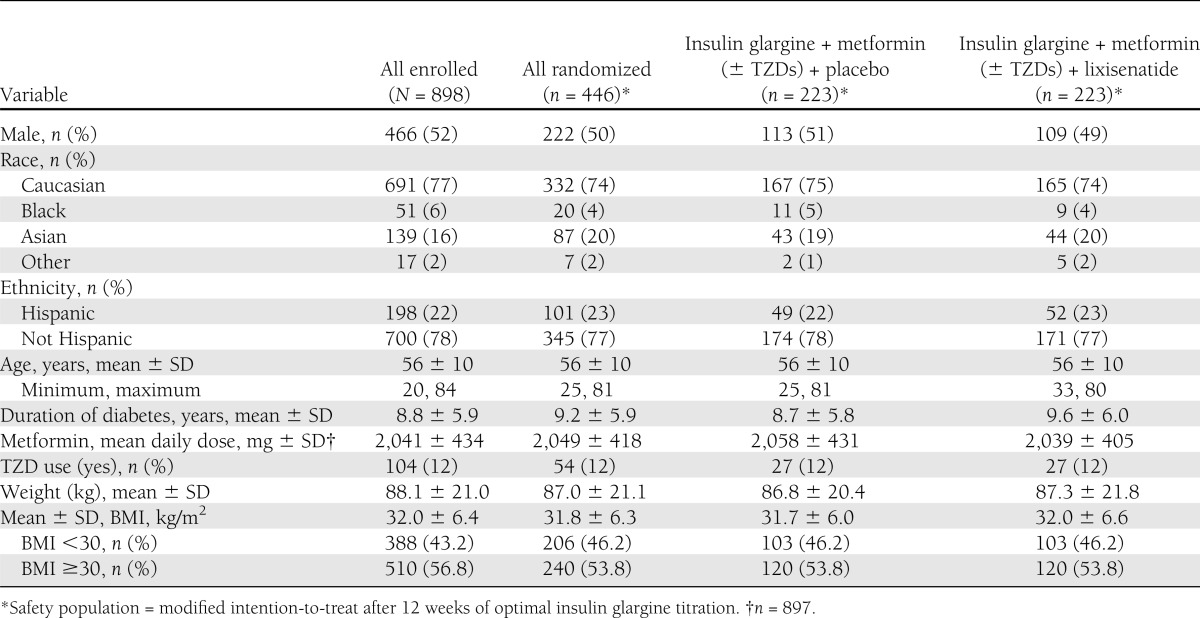
Of 1,470 candidates screened, 898 entered the 12-week run-in phase with insulin glargine (Supplementary Fig. 2). Of the 825 (92%) participants completing the run-in, 446 (54%) met the requirements for randomization. The reasons for disqualification (379 participants) were as follows: HbA1c <7.0 for 276; HbA1c >9.0% for 25; HbA1c between 7.0 and 9.0% but fasting SMPG >7.8 mmol/L for 56 participants; and other reasons for 22. The clinical characteristics of the participants are shown in Table 1. Randomized participants were similar to the originally enrolled population in their demographic and clinical features. Twenty-nine (13%) lixisenatide-treated participants and 12 (5%) placebo-treated participants discontinued randomized treatment, with the main reason being an adverse event (8.5% for lixisenatide and 4.0% for placebo; Supplementary Fig. 2). Gastrointestinal-related adverse effects were the major TEAEs leading to discontinuation for lixisenatide (10 participants [4.5%] and none with placebo), which were mainly related to nausea and vomiting (9 participants [4%]). One person in each group received rescue therapy.
Table 1.
Demographics and patient characteristics
Responses to therapy
HbA1c.
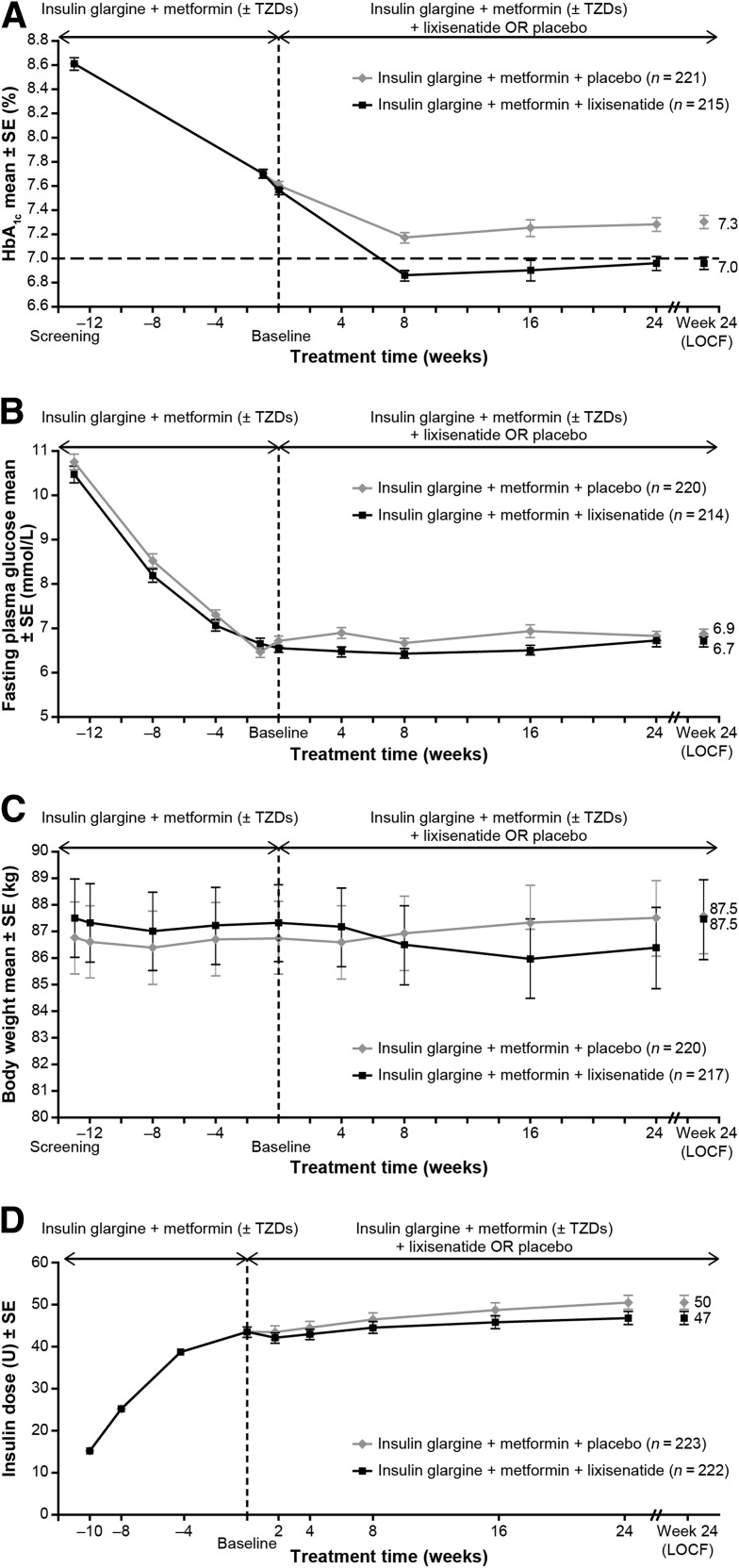
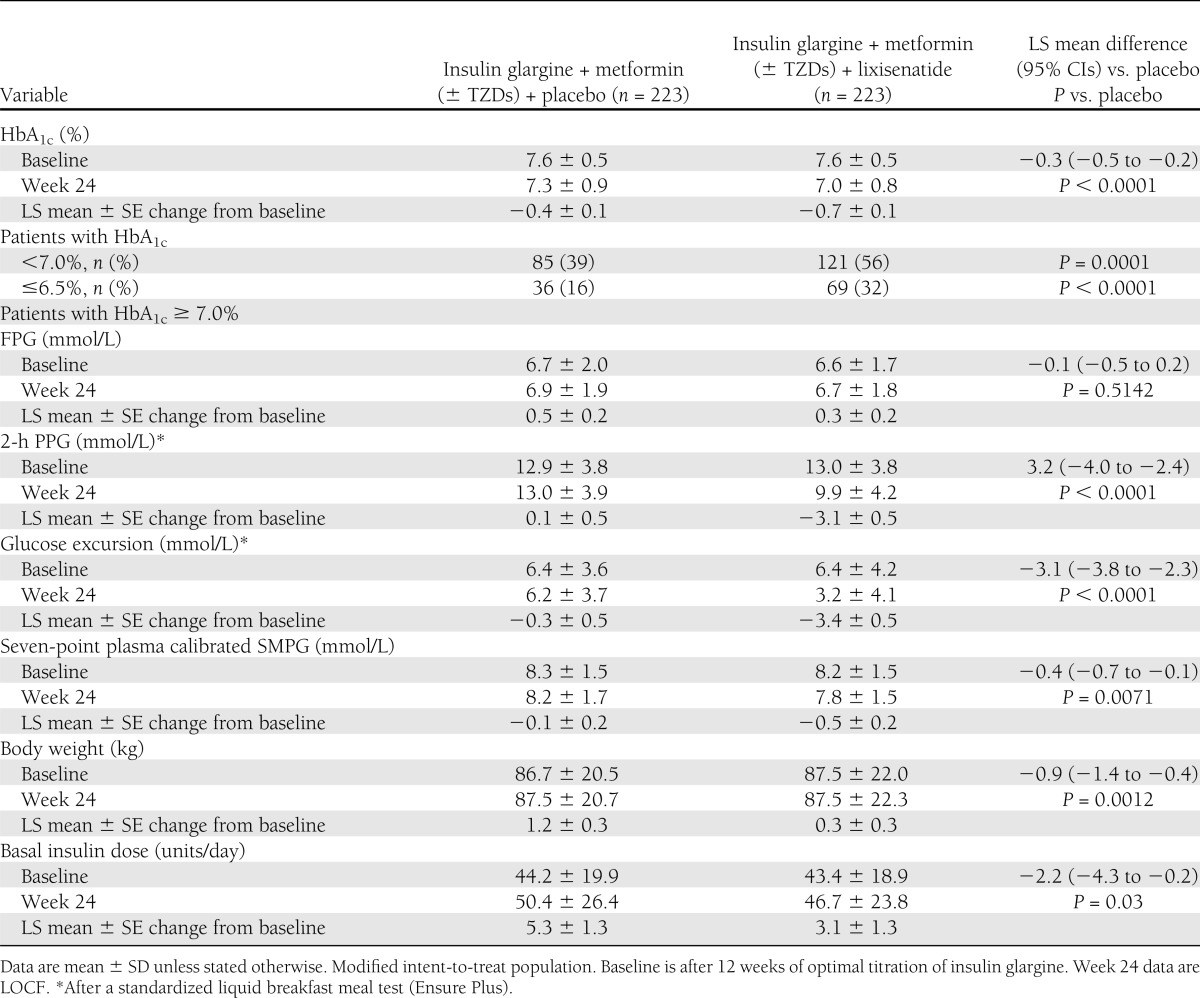
During the 12-week run-in phase, addition of insulin glargine to oral therapy decreased mean HbA1c levels from 8.6% (70 mmol/mol) at screening to 7.6% (60 mmol/mol) for both subsequent randomization groups (Fig. 1A, Table 2). After randomization, HbA1c decreased further to 7.0% (53 mmol/mol) at week 24 or LOCF in the lixisenatide group and 7.3% (56 mmol/mol) in the placebo group (Fig. 1A). The HbA1c decrease from baseline was significantly greater with lixisenatide compared with placebo; adjusted least squares (LS) mean changes were −0.7 and −0.4%, respectively, and LS mean difference for lixisenatide versus placebo was −0.3% (P < 0.0001). A significantly higher percentage of patients with lixisenatide attained target HbA1c <7.0 (53 mmol/mol; 56 vs. 39%; P = 0.0001) or ≤6.5% (48 mmol/mol; 32 vs. 16%; P < 0.0001; Table 2).
Figure 1.
Clinical responses to therapy from baseline to week 24 and end point with last observation carried forward (LOCF). A: Mean HbA1c (%) by visit. B: Mean fasting plasma glucose (mmol/L) by visit. C: Mean change in body weight (kg) from baseline by visit. D:Mean daily basal insulin dose (units/day) by visit. Values are mean ± SE. The end point with LOCF calculation used the modified intention-to-treat population. All analyses excluded measurements after the introduction of rescue medication or after treatment cessation plus 14 days, or both.
Table 2.
Clinical responses to therapy
Fasting plasma glucose.
After randomization, FPG did not change significantly and there were no significant differences observed between the groups (Fig. 1B, Table 2).
Standardized meal study.
Glucose values after the standard breakfast were significantly reduced with lixisenatide treatment but not with placebo (Supplementary Fig. 3, Table 2). The LS mean difference of change of the 2-h postprandial value from baseline between lixisenatide and placebo treatment was −3.2 mmol/L (95% CI, −4.0 to −2.4; P < 0.0001). Most of this improvement was accounted for by reduction of the glucose excursion after the meal (i.e., the increment of glucose from the premeal value to the 2-h postprandial value; LS mean difference from baseline −3.1 mmol/L [95% CI, −3.8 to −2.3]).
SMPG profiles.
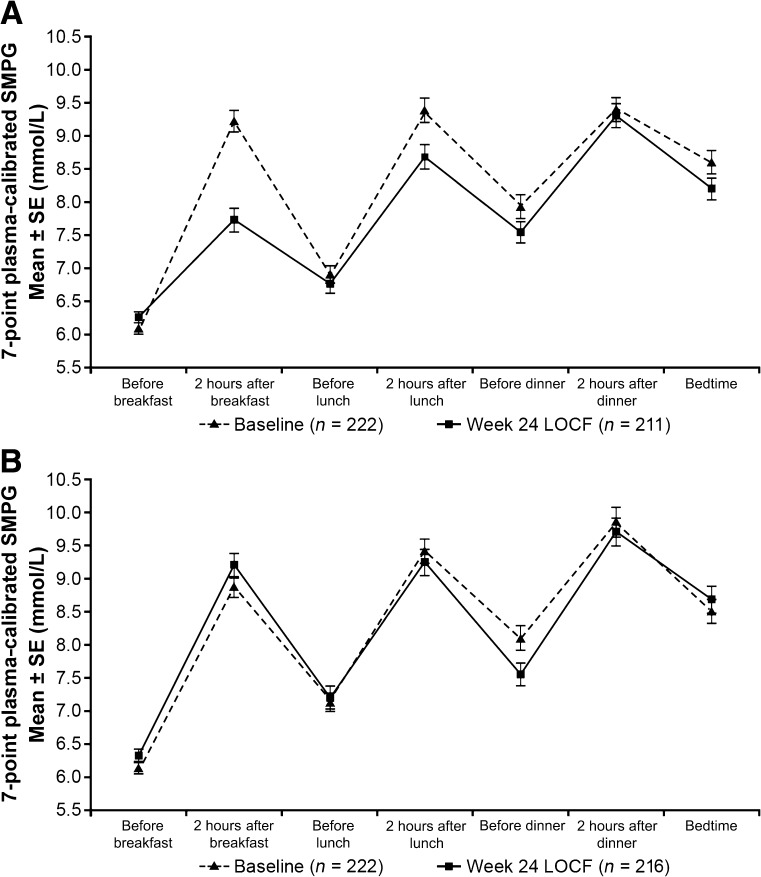
Average seven-point SMPG values (Fig. 2, Table 2) were reduced significantly during treatment with lixisenatide relative to placebo (LS mean difference −0.4 mmol/L; P = 0.0071). The effect of lixisenatide treatment was most evident after the morning meal.
Figure 2.
Mean seven-point SMPG (mmol/L) at baseline and week 24 or end point with last observation carried forward (LOCF). A: Before and after treatment profiles for the insulin glargine plus metformin (with or without TZDs) plus lixisenatide group. B: Profiles for insulin glargine plus metformin (with or without TZDs) plus placebo. The dashed lines show the profiles at baseline and the solid lines show those after treatment. Values are mean ± SE. Calculations used the modified intention-to-treat population.
Body weight.
After randomization, body weight increased by 1.2 kg in the placebo group and 0.3 kg in the lixisenatide group (Fig. 1C, Table 2). The between-treatment difference favoring lixisenatide was statistically significant (LS mean difference −0.9 kg; P = 0.0012) at the end of treatment.
Insulin dose.
After 12 weeks of titration during the run-in, mean daily insulin dosage at baseline was 43.4 units (SD, 18.9) for the lixisenatide group and 44.2 units (SD, 19.9) for placebo (Fig. 1D, Table 2). After randomization, the insulin dose increased in both treatment groups. The LS mean changes were +3.1 and +5.3 units for lixisenatide and placebo groups, respectively. The mean between-group difference was –2.2 units (P = 0.03).
Hypoglycemia
Symptomatic glucose-confirmed hypoglycemia (<3.3 mmol/L [60 mg/dL]) during randomized treatment occurred in 20.2% of participants using lixisenatide and 11.7% of those using placebo (Supplementary Table 1). The annualized event rate for confirmed hypoglycemia was 0.80 per participant-year for lixisenatide and 0.44 for placebo. The higher rate of hypoglycemic events in the lixisenatide group was observed primarily during the first 6 weeks of treatment, which included the stepwise increase of dosage. One participant in the lixisenatide group (0.4%) had a severe hypoglycemic event, which the investigator determined possibly related to lixisenatide or to a delayed meal.
Other adverse events
The proportion of patients with TEAEs was higher with lixisenatide than placebo (79.8 vs. 68.2%; Supplementary Table 1), and this disproportion was driven by gastrointestinal events. A serious TEAE was reported in 7.6% of participants using lixisenatide and in 4.5% using placebo. Two participants receiving placebo had TEAEs leading to death (one myocardial infarction and one multiple myeloma), but no fatal TEAEs occurred with lixisenatide. Two participants using lixisenatide (0.9%, both urticaria) and one using placebo (0.4%, dermatitis) had an allergic reaction adjudicated as possibly related to study treatment. Fifteen participants (6.7%) in the lixisenatide group and five (2.2%) in the placebo group had injection site reactions; two lixisenatide-treated participants discontinued for this reason. One participant in the placebo group had a TEAE of pancreatitis compared with none in the lixisenatide group. Two participants receiving placebo had a TEAE of calcitonin increase (≥20 ng/L) compared with none in the lixisenatide group. No significant changes in blood pressure or heart rate occurred in either group.
CONCLUSIONS
These results demonstrate a clinically relevant improvement in glycemic control by addition of once-daily lixisenatide to basal insulin glargine together with oral therapy in a subpopulation of people for whom treatment options are of current interest. Initiation of basal insulin in patients not responding to oral therapy is a successful strategy for many people, yet 40–50% do not attain HbA1c <7.0, presumably because PPG levels remain elevated (4). The study participants evaluated had a meaningful initial HbA1c reduction after addition and titration of insulin for 12 weeks but had not attained HbA1c ≤7.0%. The possibility of poor adherence to titration of insulin was minimized by requiring participants to have, in addition to HbA1c >7.0%, at least moderate control of FPG (≤7.8 mmol/L). Approximately one-third of participants completing the run-in were not eligible because of having attained HbA1c ≤7.0%, and 7% were not eligible despite HbA1c values between 7 and 9% because FPG was found to be >7.8 mmol/L. At randomization, the mean FPG was 6.6 mmol/L and mean HbA1c was 7.6%. Thus, although further improvement in FPG was needed, residual postprandial hyperglycemia had to be an important contributor to the continued elevation of HbA1c.
Traditionally, the next therapeutic option in this setting has been addition of rapid-acting insulin at mealtime. This can involve initiation of full basal bolus therapy, but a stepwise process beginning with a single injection at the main meal has recently been proposed (19–22). Early experience with this approach suggests that a further reduction of 0.3–0.5% HbA1c can be obtained with addition of a single mealtime insulin injection, but with a requirement for additional SMPG tests to guide dosing, and with the potential for further weight gain. The present findings suggest that adding a single injection of lixisenatide can provide a similar improvement of glycemic control as adding a single injection of short-acting insulin, but without the need for additional glucose testing and with a modest beneficial effect on weight rather than weight gain. The mean reduction in HbA1c attained by participants treated with lixisenatide was −0.7% compared with −0.4% with placebo, and 56% of the lixisenatide group achieved HbA1c <7.0%. As expected in a study in which lixisenatide was added to basal insulin glargine, no additional improvement of FPG was evident, although other studies have demonstrated that lixisenatide—as monotherapy or add-on to oral antidiabetic agents—significantly reduces both FPG and PPG, as well as HbA1c levels (23,24). The seven-point SMPG profiles and the standardized breakfast meal test show that improvement of HbA1c in this study was mainly attributable to reduction of increments of glucose above the fasting level. Approximately 50% reduction of the increase of glucose after breakfast was evident in the profiles, and there was a smaller reduction after the midday meal as well. Results of the standardized breakfast test confirmed a marked reduction in PPG.
As expected, there was a modest increase in confirmed hypoglycemia accompanying the improvement of glycemic control with lixisenatide, occurring primarily in the first 6 weeks of treatment. One severe hypoglycemic event with lixisenatide was reported. The other adverse effects of lixisenatide in this population were similar to those demonstrated in other studies; nausea and vomiting were the most common and led to discontinuation in 4% of participants. A three-fold increase of injection-site skin reactions occurred with lixisenatide, although only two (0.9%) patients discontinued treatment for this reason. Addition of lixisenatide was not associated with increased heart rate or blood pressure changes compared with placebo. The two TEAEs leading to death during the treatment period and the TEAE of pancreatitis were in the placebo group.
This study has limitations and did not address some clinically relevant questions. Approximately 40% of participants randomized to lixisenatide did not attain the 7.0% HbA1c goal, and what further measures might improve their success remains unknown. The run-in period was relatively short (only 12 weeks for initiation and optimization of basal insulin glargine), and further dosage increases of glargine before randomization might have been possible if the run-in period had been longer. Also, although it has been shown that a 20-g dose of lixisenatide administered once daily in the morning had generally comparable effects as other lixisenatide regimens when added to metformin, whether the effectiveness of adding lixisenatide to basal insulin may be improved by alterations in the timing of administration of basal insulin glargine by administering it in the evening and administering lixisenatide at breakfast, or by administering basal insulin in the morning and lixisenatide at the main meal, often dinner, is unknown. In addition, lixisenatide was evaluated versus placebo rather than an active comparator, such as mealtime insulin. Determination of which individual patients might fare better with lixisenatide and which would fare better with mealtime insulin would be clinically helpful. Finally, further studies to examine directly the tolerability and effectiveness of lixisenatide relative to other therapies, especially other GLP-1RA agents, DPP-4 inhibitors, and α-glucosidase inhibitors when added to basal insulin therapy, would be of great interest.
In summary, for people whose HbA1c remained 7.0% or higher after initiation and titration of insulin glargine and continuation of oral therapy, adding lixisenatide to their treatment regimen significantly improved HbA1c and reduced PPG, allowing a majority to attain HbA1c <7.0%. Adding lixisenatide prevented weight gain and led to an expected increase in the frequency of gastrointestinal side effects and modestly increased rates of hypoglycemia. This treatment approach deserves consideration as an alternative method of intensifying treatment for people not reaching HbA1c goals with recently initiated basal insulin.
Acknowledgments
The study was funded by Sanofi, the manufacturer of lixisenatide. Representatives from Sanofi were responsible for the study design, protocol, statistical analysis plans, and analysis of the results. Editorial support was provided to the authors in preparing the manuscript by Frances Gambling, BA (Medicus International), and funded by Sanofi.
M.C.R. has received research grant support from Amylin, Eli Lilly, and Sanofi, and has received honoraria for consulting and/or speaking from Amylin, Eli Lilly, Hoffmann-La Roche, Sanofi, and Valeritas. This potential conflict of interest has been reviewed and managed by Oregon Health and Science University. R.A. has received research support and/or consulting honoraria from Eli Lilly, Novo Nordisk, Sanofi, and Takeda. T.F. has received speaker fees and advisory fees from Sanofi. L.S.-R. has attended medical advisory boards for Hoffmann-La Roche and has received honoraria fees for lectures for Boehringer Ingelheim and Eli Lilly. E.S., L.S., and L.P. are employees of the sponsor Sanofi. J.R. has served on scientific advisory boards and received honorarium or consulting fees or grants/research support from insulin and GLP-1RA manufacturers, Amylin, Eli Lilly, GlaxoSmithKline, Hoffmann-La Roche, Novo Nordisk, and Sanofi. No other potential conflict of interest relevant to this article was reported.
M.C.R. was involved in the development of the protocol, clinical conduct of the study, analysis of the data, and led the writing of the manuscript. T.F., R.A., and L.S.-R. were involved in the clinical conduct of the study and preparation of the manuscript. E.S. and L.S. designed and wrote the protocol, and were responsible for the set-up and medical supervision of the study, data review, and preparation of the manuscript. L.P. was responsible for medical supervision of the study as the Clinical Study Director, data review, and preparation of the manuscript. J.R. was involved in the development of the protocol, clinical conduct of the study, analysis of the data, and preparation of the manuscript. All authors had full access to all of the data in the study, contributed to the writing of the manuscript, and had final responsibility for the decision to publish. M.C.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012 (Rosenstock M et al. Diabetes 2012;61:A18 [62-OR]), and at the 48th Annual Meeting of the European Association for the Study of Diabetes, Berlin, Germany, 1–5 October 2012 (Rosenstock J et al. Diabetologia 2012;55 Supplement).
The authors acknowledge editorial support provided by Frances Gambling, BA (Medicus International).
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2462/-/DC1.
Clinical trial reg. no. NCT00975286, clinicaltrials.gov.
References
- 1.Riddle MC. Timely initiation of basal insulin. Am J Med 2004;116(Suppl. 3A):3S–9S [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for the Study of Diabetes Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009;52:17–30 [DOI] [PubMed] [Google Scholar]
- 3.Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–3086 [DOI] [PubMed] [Google Scholar]
- 4.Raccah D, Bretzel RG, Owens D, Riddle M. When basal insulin therapy in type 2 diabetes mellitus is not enough—what next? Diabetes Metab Res Rev 2007;23:257–264 [DOI] [PubMed] [Google Scholar]
- 5.Woerle HJ, Neumann C, Zschau S, et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract 2007;77:280–285 [DOI] [PubMed] [Google Scholar]
- 6.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 2003;26:881–885 [DOI] [PubMed] [Google Scholar]
- 7.Peter R, Dunseath G, Luzio SD, Chudleigh R, Choudhury SR, Owens DR. Relative and absolute contributions of postprandial and fasting plasma glucose to daytime hyperglycaemia and HbA(1c) in subjects with Type 2 diabetes. Diabet Med 2009;26:974–980 [DOI] [PubMed] [Google Scholar]
- 8.Riddle M, Umpierrez G, DiGenio A, Zhou R, Rosenstock J. Contributions of basal and postprandial hyperglycemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care 2011;34:2508–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barag SH. Insulin therapy for management of type 2 diabetes mellitus: strategies for initiation and long-term patient adherence. J Am Osteopath Assoc 2011;111(Suppl. 5):S13–S19 [PubMed] [Google Scholar]
- 10.Garg SK. The role of basal insulin and glucagon-like peptide-1 agonists in the therapeutic management of type 2 diabetes—a comprehensive review. Diabetes Technol Ther 2010;12:11–24 [DOI] [PubMed] [Google Scholar]
- 11.Tzefos M, Olin JL. Glucagon-like peptide-1 analog and insulin combination therapy in the management of adults with type 2 diabetes mellitus. Ann Pharmacother 2010;44:1294–1300 [DOI] [PubMed] [Google Scholar]
- 12.Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in Basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2011;154:103–112 [DOI] [PubMed] [Google Scholar]
- 13.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006;368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 14.Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG, GWAA Study Group Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005;143:559–569 [DOI] [PubMed] [Google Scholar]
- 15.Barnett AH. Lixisenatide: evidence for its potential use in the treatment of type 2 diabetes. Core Evid 2011;6:67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratner RE, Rosenstock J, Boka G, DRI6012 Study Investigators Dose-dependent effects of the once-daily GLP-1 receptor agonist lixisenatide in patients with Type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled trial. Diabet Med 2010;27:1024–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werner U, Haschke G, Herling AW, Kramer W. Pharmacological profile of lixisenatide: A new GLP-1 receptor agonist for the treatment of type 2 diabetes. Regul Pept 2010;164:58–64 [DOI] [PubMed] [Google Scholar]
- 18.Lorenz M, Pfeiffer C, Steinsträßer A, Ruus P. Effects of lixisenatide once daily on gastric emptying and relationship to postprandial glycemia in type 2 diabetes mellitus. Diabetes Care 2012;61(Suppl. 1):A212–A344 (Abstract 1085-P) [DOI] [PubMed]
- 19.Owens DR, Luzio SD, Sert-Langeron C, Riddle MC. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab 2011;13:1020–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lankisch MR, Ferlinz KC, Leahy JL, Scherbaum WA, Orals Plus Apidra and LANTUS (OPAL) study group Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single-dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab 2008;10:1178–1185 [DOI] [PubMed] [Google Scholar]
- 21.Meneghini L, Mersebach H, Kumar S, Svendsen AL, Hermansen K. Comparison of 2 intensification regimens with rapid-acting insulin aspart in type 2 diabetes mellitus inadequately controlled by once-daily insulin detemir and oral antidiabetes drugs: the step-wise randomized study. Endocr Pract 2011;17:727–736 [DOI] [PubMed] [Google Scholar]
- 22.Davidson MB, Raskin P, Tanenberg RJ, Vlajnic A, Hollander P. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract 2011;17:395–403 [DOI] [PubMed] [Google Scholar]
- 23.Fonseca VA, Alvarado-Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE, EFC6018 GetGoal-Mono Study Investigators Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care 2012;35:1225–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seino S, Takahashi H, Takahashi T, Shibasaki T. Treating diabetes today: a matter of selectivity of sulphonylureas. Diabetes Obes Metab 2012;14(Suppl. 1):9–13 [DOI] [PubMed] [Google Scholar]