Abstract
OBJECTIVE
Improvements in diabetes after Roux-en-Y gastric bypass (RYGB) often occur days after surgery. Surgically induced hormonal changes and the restrictive postoperative diet are proposed mechanisms. We evaluated the contribution of caloric restriction versus surgically induced changes to glucose homeostasis in the immediate postoperative period.
RESEARCH DESIGN AND METHODS
Patients with type 2 diabetes planning to undergo RYGB participated in a prospective two-period study (each period involved a 10-day inpatient stay, and periods were separated by a minimum of 6 weeks of wash-out) in which patients served as their own controls. The presurgery period consisted of diet alone. The postsurgery period was matched in all aspects (daily matched diet) and included RYGB surgery. Glucose measurements were performed every 4 h throughout the study. A mixed-meal challenge test was performed before and after each period.
RESULTS
Ten patients completed the study and had the following characteristics: age, 53.2 years (95% CI, 48.0–58.4); BMI, 51.2 kg/m2 (46.1–56.4); diabetes duration, 7.4 years (4.8–10.0); and HbA1c, 8.52% (7.08–9.96). Patients lost 7.3 kg (8.1–6.5) during the presurgery period versus 4.0 kg (6.2–1.7) during the postsurgery period (P = 0.01 between periods). Daily glycemia in the presurgery period was significantly lower (1,293.58 mg/dL·day [1,096.83–1,490.33) vs. 1,478.80 mg/dL·day [1,277.47–1,680.13]) compared with the postsurgery period (P = 0.02 between periods). The improvements in the fasting and maximum poststimulation glucose and 6-h glucose area under the curve (primary outcome) were similar during both periods.
CONCLUSIONS
Glucose homeostasis improved in response to a reduced caloric diet, with a greater effect observed in the absence of surgery as compared with after RYGB. These findings suggest that reduced calorie ingestion can explain the marked improvement in diabetes control observed after RYGB.
Roux-en-Y gastric bypass surgery (RYGB) is one of the most successful treatment strategies for diabetes accompanying morbid obesity. Long-term diabetes remission rates of 83% have been reported (1,2). Remarkably, diabetes can improve markedly within a few days of surgery. In-hospital diabetes remission rates have been reported to be as high as 89% (3,4). In one study, 30% of patients with diabetes were discharged from the hospital with normal blood glucose levels and not using any diabetes medication (4). Improvement often occurs before any significant weight loss. These findings have led to the suggestion that surgical shunting of food past the duodenum results in altered hormonal signaling that ameliorates diabetes within a few days (5–7).
The interpretation of the acute improvement in glycemia is confounded by the fact that postsurgical patients are placed on a severe calorie-restricted diet for at least 7–14 days after surgery. Severe calorie restriction alone can significantly improve diabetes within days (8,9). A series of 40 obese patients with type 2 diabetes underwent 40 days of a very-low-calorie diet. Fasting glucose levels improved significantly, and 87% of the improvement occurred within the first 10 days (10). A similar calorie-restricted diet reduced hepatic glucose production and insulin resistance within 7 days (11). In another study of patients with type 2 diabetes, a 600-calorie/day diet normalized plasma glucose levels and hepatic glucose output within 1 week (12).
The findings from the diet studies raise the question regarding whether the rapid improvement in diabetes after RYGB is caused by the low-calorie diet or by the surgery. Previous studies (13–15) have sought to answer this question, but the results are confounded because different patients (with different baseline characteristics) were subjected to the diet or surgery regimens and the dietary intake was different between groups. In the current study, we compared diet-only and diet plus surgery treatments in 10 patients, each of whom was subjected to both regimens. Both interventions were performed under strict inpatient supervision, and dietary intake was closely matched.
RESEARCH DESIGN AND METHODS
Patients with type 2 diabetes served as their own controls in a single-group, two-period study. Caloric ingestion, physical activity, and intravenous fluid administration were equivalent during the two study periods that occurred several months before (presurgery period) and immediately after the RYGB procedure (postsurgery period). The research protocol was reviewed and approved by the University of Texas Southwestern Medical School Institutional Review Board, and all participants signed informed consent forms before enrollment in the study.
Study participants
Patients were recruited from the medical weight loss/bariatric clinic at University of Texas Southwestern Medical Center. We enrolled adults (age older than 18 years) of any ethnicity and both sexes who met all criteria for and planned to undergo RYGB and who had a diagnosis of type 2 diabetes. Exclusion criteria were abnormal renal function (serum creatinine above the upper limit of normal for age and sex), significant anemia (hemoglobin <10 mg/dL), difficult venous access, and treatment with incretin mimetics or dipeptidyl peptidase IV inhibitors during the previous 3 months.
Study design
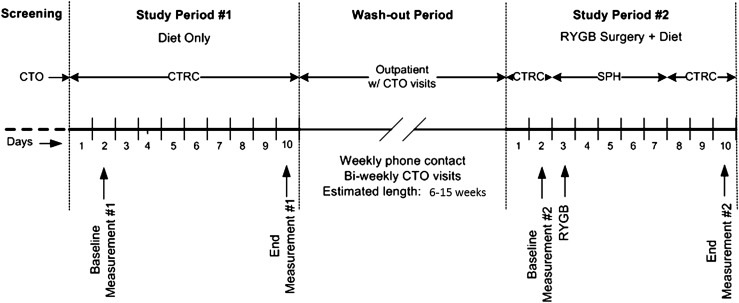
The protocol consisted of two inpatient study periods (10 days each) separated by a wash-out period of at least 6 weeks (Fig. 1). During the first study period (presurgery period), participants adopted the diet and activity protocol typical for patients after RYGB. Participants were admitted to the Clinical and Translational Research Center for this entire study period. On the first day, they underwent clinical evaluation (medical history and physical examination) and were fed a standard liquid preoperative diet that consisted of Glucerna (Abbott Laboratories, Columbus, OH; 26 g carbohydrates, 10 g protein, 200 calories/240 mL) 240 mL per meal and water ad libitum. On the second day at 8:00 a.m., they underwent a 6-h mixed meal challenge test (MMCT) using 240 mL chocolate Boost Plus (Nestle Healthcare Nutrition, Florham Park, NJ; 45 g carbohydrates, 14 g protein, 360 calories/240 mL), followed by 240 mL vegetable broth for lunch and 240 mL sugar-free gelatin for dinner. On the third day, they were NPO. On the fourth day, feeding was restarted after 12:00 p.m., and between 12:00 p.m. and 8:00 p.m. participants were allowed to have a maximum of 30 mL clear liquids (ice chips, water, Crystal Light, broth, or sugar-free gelatin) every hour. Normal saline 125 mL/h was infused from 8:00 a.m. on the third day through 8:00 a.m. on day 6 to prevent dehydration. On day 5, the volume of the clear liquids was advanced to a maximum of 30 mL every 30 min in the morning and every 15 min in the afternoon. On days 6–9, the maximum oral intake was 30 mL every 15 min between 8:00 a.m. and 8:00 p.m., and participants were encouraged to alternate intake between clear liquids and Glucerna. On day 10, the MMCT was repeated, followed by home discharge.
Figure 1.
Study design. CTO, Clinical Trials Office; CTRC, Clinical and Translational Research Center; SPH, St. Paul Hospital.
The second study period (postsurgery period) started 2 days before RYGB surgery and continued until postoperative day 7. The only difference between the two study periods was the performance of RYGB surgery on day 3 and a gastrografin study performed during the morning of day 4. The dietary protocol was identical to the first study period. Dietary intake (volume and content) was matched on a daily basis to the intake during the presurgery period. Participants spent the first 2 days and 2 nights at the Clinical and Translational Research Center, spent days 3 through 5 or 6 (at the surgeon’s discretion) at St. Paul University Hospital, and returned to the Clinical and Translational Research Center for the remaining of the study days (day 5 or 6 through 10). RYGB surgery was performed laparoscopically in all patients and using the same technique (16). A 25-mm EEA Stapler to create a gastro-jejunal anastomosis and a linear 60-mm stapler to create a jejuno-jejunal anastomosis were used. The length of the roux limb was 100 cm in all the patients.
Patients were instructed to stop using all oral anti-diabetic agents 3 days before each study period. Subcutaneous insulin treatment was withheld starting the day before admission and replaced with regular insulin intravenous boluses administered only when capillary glucose measurements exceeded 200 mg/dL. No insulin correction was administered within 10 h of MMCT. All routine nondiabetes medications were continued throughout the entire study at the same dose.
During the wash-out period, participants were instructed to return to their usual diets and activity levels, with the stated expectation that participants would return to a comparable metabolic baseline for the surgery period. The length of the wash-out period was a minimum of 6 weeks and was determined solely by the surgery schedule.
Measurements
Weight was measured every morning (except during the hospitalization) at 7:00 a.m. on the same scale, with participants wearing only a hospital gown. Waist (umbilical level at end expiration) and hip (largest part) circumference were measured on each admission and discharge with the same tape measure. Oral intake (amount, type, time) was monitored strictly and recorded every hour. The daily and per-study period caloric intake was calculated based on the nutritional content of each ingested product. The total caloric intake per study period is reported as all calories ingested from (and including) the baseline MMCT to the (and excluding) end-of-study MMCT.
Capillary blood glucose level was measured every 4 h using an AccuCheck Advantage glucose meter (Roche Diagnostics). The average of two readings obtained with two separate meters was reported for each time point. Total glycemic exposure during each study period was reported as “glycemia” and represents the area under the curve (AUC) for the daily average capillary blood glucose measurement over the entire study period. Regular human insulin was administered only if capillary glucose level was >200 mg/dL and at a dose estimated based on the participant’s preadmission total daily insulin dose. The total insulin dose administered from day 2 through day 9 (inclusive) is reported.
A 6-h MMCT was administered at 8:00 a.m. on days 2 and 10 of each study period. After obtaining two baseline samples (−10 and −5 min), 240 mL chocolate Boost Plus was ingested in 5 min. Blood samples for glucose measurement were collected at 5, 10, 15, 20, 25, 30, 40, 50, 60, 80, 100, 120, 150, 180, 210, 240, 300, and 360 min after ingestion. We report the average of the two fasting samples, the maximum postingestion glucose level, and the AUC for glucose obtained from all measurements. Whole-blood glucose was measured using YSI 2300 STAT Plus Glucose analyzer (Yellow Spring Instruments). HbA1c was measured by high-performance liquid chromatography in the Clinical Diabetes laboratory at University of Texas Southwestern.
Statistical analysis
The primary outcome of the study was glycemic control as measured by the glucose AUC during the 6-h MMCT. We hypothesized a greater reduction in glucose AUC in the postsurgery period compared with the presurgery period. A sample size of 10 patients was needed to achieve a power of 0.8 with α = 0.05 to detect a 10% (7,230 mg/dL/min) difference in the glucose AUC with an estimated SD of 7,300 mg/dL/min. Estimated baseline average glucose AUC was 72,300 ± 20,500 mg/dL/min based on a study that enrolled a similar study population (17). All other reported measurements are prespecified secondary outcomes. We conducted pair-wise comparisons of the changes that occurred during each study period using a paired t test. AUC for glucose was calculated using the trapezoidal rule. Data are reported as mean and 95% CI. Significance was established at P < 0.05.
RESULTS
Ten participants completed both study periods. One participant dropped out after the fourth day of the presurgery period because she decided to no longer pursue RYGB (Table 1, patient 5). A second participant completed the presurgery period but, because of acute medical problems unrelated to the study, the surgery was postponed indefinitely (Table 1, patient 11). The baseline characteristics of each participant are described in Table 1. The average age of the participants who completed the study was 53.2 years (95% CI, 48.0–58.4), with a diabetes duration of 7.4 years (4.8–10.0). All patients had fasting C-peptide levels >1 ng/dL. The average length of the wash-out period was 101.6 days (range, 38–218 days). The goal was for each participant to return to a similar metabolic baseline for the surgery period. During the wash-out period, participants regained only half of the weight lost during the presurgery period (P < 0.001 for the weight and BMI comparison between the two baseline values), but glycemic status was similar at the start of both study periods (Table 2, nonsignificant differences between baseline HbA1c, fasting glucose, maximal poststimulation glucose, and glucose AUC during MMCT).
Table 1.
Baseline characteristics of each study participant
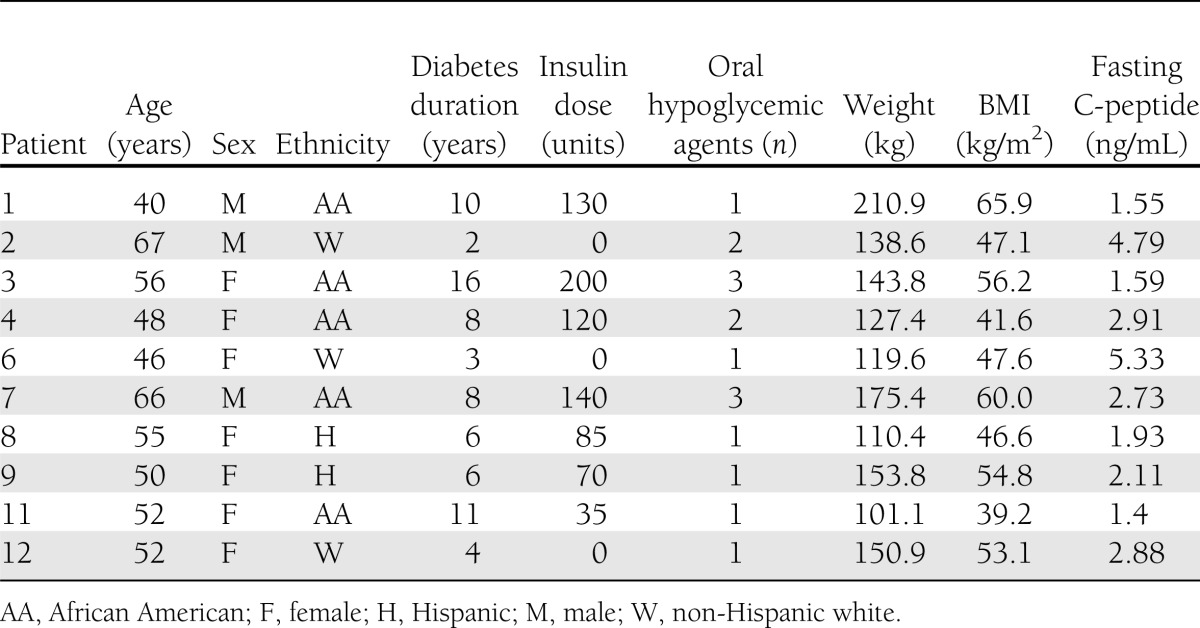
Table 2.
Metabolic characteristics and their changes during each study period
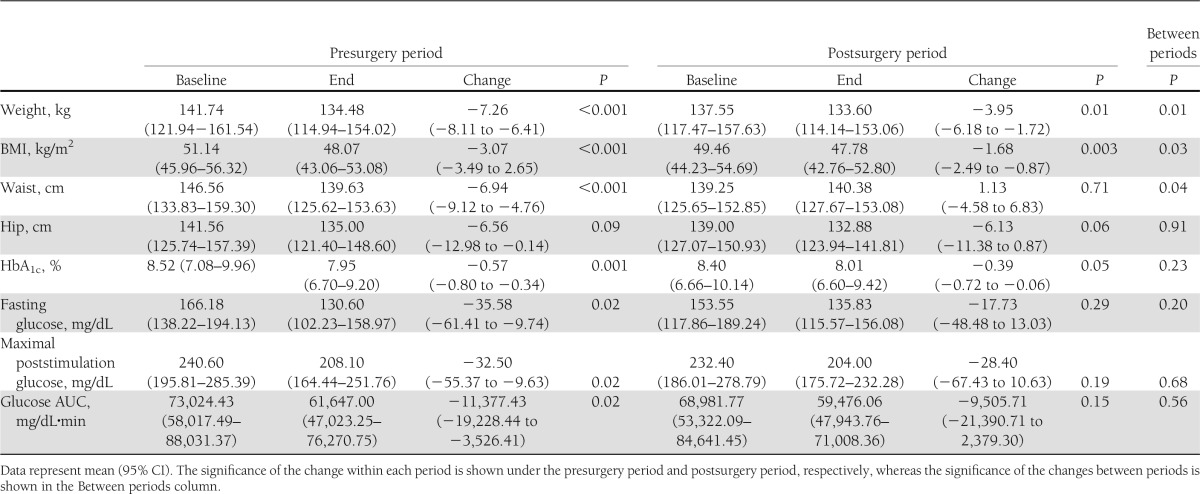
The total caloric intakes for the study periods (total of 7 days) were 1,736 calories (95% CI, 1,364.6–2,106.9) and 1,597 calories (1,312.6–1,881.9), respectively (P = 0.43 between periods). Oral intake was well-matched on a day-to-day basis (Fig. 2A). A few patients delayed their progression to a protein-based liquid diet on day 6 of the surgery period, but the difference in caloric intake on this day between study periods was not significant. There were no surgery-related adverse events during the study.
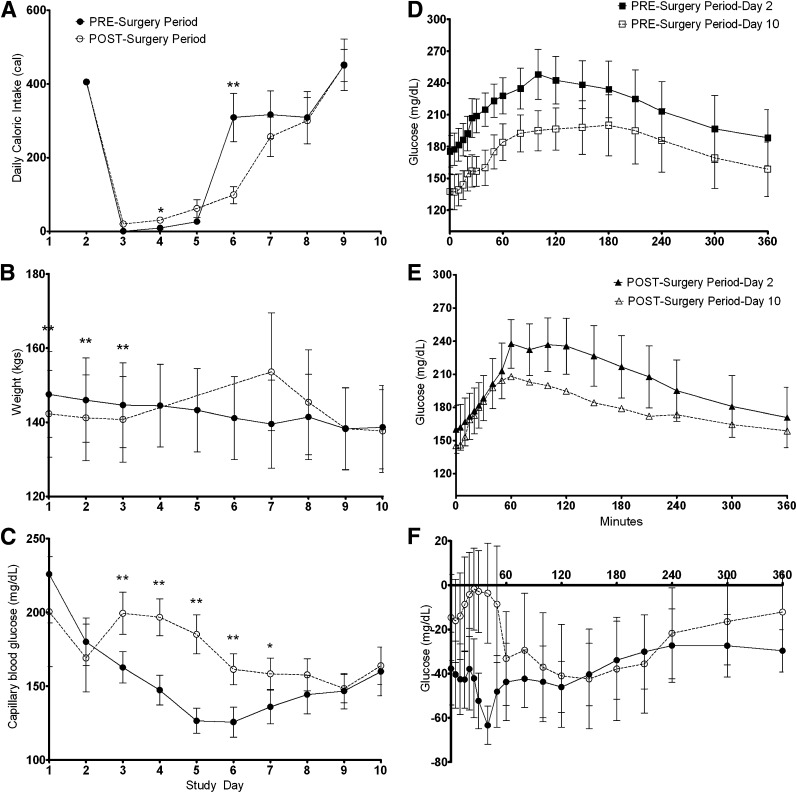
Figure 2.
Comparison of the changes observed during the presurgery period and postsurgery period. A: Daily caloric intake (calories). B: Daily weight (kg). C: Daily average capillary blood glucose (mg/dL). D: The improvement in the glucose profile over a 6-h MMCT in the presurgery period. E: The improvement in the glucose profile over a 6-h MMCT in the postsurgery period. F: The change in glucose (delta) for each time point during the MMCT within each study period. Data are mean and SE. Continuous line with filled circles (●) indicates presurgery period; dotted line with open circles (○) indicates postsurgery period; continuous line with filled squares (■) indicates baseline testing in presurgery period; dotted line with open squares () indicates end of period testing in presurgery period; continuous line with filled triangles (▲) indicates baseline testing in postsurgery period; dotted line with open triangles (▵) indicates end of period testing in postsurgery period. *P < 0.05; **P < 0.01.
Weight and anthropometric measurements
Total weight loss (and BMI change) was significantly greater during the presurgery period (Table 2). Participants lost 5.3% (95% CI, 5.96–4.57) of their total body weight during the presurgery period and lost 2.8% (4.46–1.16) during the postsurgery period (P = 0.02 between periods). The pattern of weight loss was different in the two study periods, with a steady daily loss during the presurgery period and postoperative gain, followed by rapid loss during the postsurgery period (Fig. 2B).
Hip circumference decreased by 6.6 cm and 6.1 cm, respectively, during each period (P = 0.91 between periods). Waist circumference decreased by 6.9 cm over the diet period, but increased by 1.1 cm over the surgery period (P = 0.04 between periods), which is probably explained by postsurgical swelling and residual intra-abdominal gas.
Measures of glycemia
The overall glycemia (reported as AUC for the daily average capillary glucose measurements) was 1,293.58 mg/dL·day (95% CI, 1,096.83–1,490.33) during the presurgery period versus 1,478.80 mg/dL·day (1,277.47–1,680.13) during the postsurgery period (P = 0.01 between periods). This difference in overall glycemia was observed despite a (nonsignificant) lower caloric intake and higher exogenous insulin requirement during the postsurgery period. The average total dose of insulin required during the entire presurgery period was 47.5 units/patient compared with 90.7 units/patient in the postsurgery period (P = 0.20 between periods). Of note, both of these insulin requirements were significantly lower than the patient’s home insulin dose (average, 78 units/day). On each of days 3–7, capillary glucose was significantly lower during the presurgery period compared with the postsurgery period (Fig. 2C). By day 8, the glucose profile became comparable between the two periods and continued to track on an identical course on days 9 and 10 (Fig. 2C).
Fasting glucose, maximum poststimulation glucose, and glucose AUC during the MMCT (the primary outcome of the study) all improved significantly during the presurgery period (Fig. 2D), but not during the postsurgery period (Fig. 2E and Table 2). The shape of the glucose curve during the MMCT at the end of the postsurgery period was different compared with the same curve at the end of the presurgery period (earlier peak attributable to early and rapid glucose absorption), but the 6-h total AUC was similar (Fig. 2F and Table 2). HbA1c decreased significantly in both study periods by 0.57% (95% CI, 0.80–0.34) and 0.39% (0.72–0.06), respectively (P = 0.23 between periods).
The glucose AUC during the 6-h MMCT (primary outcome of the study) improved during both study periods (Table 2), with the difference of the improvement between study periods (difference of the deltas of each period) being −4,042.66 (95% CI, −12,898.23 to 4,812.92) in favor of the diet-only intervention. These results confirm that a clinically meaningful difference in favor of surgery is not present, and the two tested interventions are comparable or a diet-only intervention is superior.
CONCLUSIONS
We found that diabetes improves rapidly when a post-bariatric diet is provided to obese patients with diabetes before they undergo surgery. In fact, the improvement in overall glycemic control was more profound with the dietary intervention alone than after RYGB (with a perfectly matched diet). These findings support the hypothesis that rapid diabetes resolution after RYGB is mediated by caloric restriction and that there is no need to invoke a direct hormonally mediated mechanism related to bypassing the duodenum.
The strength of this study is in its design and implementation, which allow for the precise evaluation of the individual contribution of diet versus surgery to diabetes improvement in the immediate postsurgical period. First, we exclusively studied patients with type 2 diabetes to assure the highest relevance to the posted question. Second, we designed a one-group, two-period study to ensure identical baseline characteristics of the participants, and this also allowed us to perform a more powerful paired data analysis. Third, the entirely inpatient direct-observation implementation ensured perfect compliance with the dietary intervention (as well as activity level and intravenous fluid administration), precise matching of the oral intake between study periods, standardized frequent evaluations of the variables of interest, and standardization of any necessary treatment (i.e., insulin administration).
Two previous studies reached similar conclusions (14,15) but were not definitive because of study design issues (18). Both studies compared a diet intervention with surgery in two distinct groups of obese participants, but the patients’ baseline characteristics were not well-matched. Campos et al. (15) randomized some, but not all, of a cohort of obese patients without diabetes to undergo RYGB (n = 12) or diet (n = 10). Two weeks postoperatively or after being placed on the diet, patients lost similar amounts of weight (9.9 ± 2.4 kg for RYGB patients and 8.2 ± 2.3 kg for controls). Although the RYGB patients had large increases in glucagon-like peptide and gastric inhibitory polypeptide meal responses relative to the diet-only group, insulin sensitivity was the same between groups. This study showed that glucose dynamics improved in patients without diabetes as a function of weight loss, independent of an increased incretin response in RYGB patients. Isbell et al. (14) performed a similar study involving nine RYGB patients and nine controls, half of whom in each group had diabetes. Insulin sensitivity was assessed by homeostasis model assessment in a baseline examination and then again at any time from 2–7 days after RYGB or after a 4-day period of caloric restriction for control patients. Similar to what Campos et al. (15) observed, insulin resistance improved after RYGB or diet, incretin response was much larger in RYGB patients than in diet patients, and the improved glucose disposal appeared to be independent of incretin responses (14). Both these studies were further limited by examining glucose dynamics in patients without diabetes who might not have the same defect in incretin physiology that is hypothesized to occur in obese patients with diabetes (19,20) whose diabetes improves after RYGB (21). Our study evaluated only patients with type 2 diabetes in a completely controlled environment with strict matching of the dietary intake and timing of the evaluations among the two study periods (diet only and diet plus surgery). This design enabled us to isolate the surgery-specific effects from the dietary-induced effects. Under these strict experimental conditions, we found that glucose control improved significantly as a result of caloric restriction, and there were no additional glycemic effects observed that could be directly attributed to the RYGB surgery per se.
Laferrere et al. (13) studied the effect of RYGB on diabetes but matched for weight loss rather than caloric intake. In a completers-only analysis of a nonrandomized study in which patients underwent either RYGB (n = 9) or 10-kg diet-induced weight loss (n = 10), there was a substantial incretin response to oral glucose in postoperative RYGB not seen in diet patients. However, the study interpretation is limited by the differential rate of weight loss and study length between the two groups. RYGB patients lost the target weight of 10 kg in 32.3 ± 13.1 days compared with 55.0 ± 9.9 days in the diet group (P < 0.001), a difference determined by the caloric content of their diets: 600–800 calories/day in the RYGB group compared with 1,000 calories/day in the diet group.
Our study results are also in line with those of recent reports obtained after longer-term follow-up after RYGB. Bradley et al. (22) compared patients without diabetes who underwent either RYGB or laparoscopic adjustable gastric banding (a primarily restrictive procedure that does not involve intestinal bypass). Similar improvements in insulin sensitivity, β-cell function, hepatic glucose output, and intrahepatic fat content were noted after having lost 20% of their body weight at 4–6 months after surgery. Furthermore, a large retrospective review of patients with diabetes who underwent RYGB showed that 68.2% of patients attained diabetes resolution within 5 years of surgery, but 35.1% of these experienced a subsequent relapse (23). Weight trajectories after surgery were significantly different for never-remitters, relapsers, and durable remitters, for whom greater weight loss predicted a durable remission. These studies, along with a growing body of literature (22), suggest that caloric restriction and weight loss are significant contributors to diabetes remission both in the early phase and long-term after RYGB. Furthermore, weight regain is one of the main predictors of diabetes relapse after surgery.
We attribute the slight difference in overall glycemia between our two study periods to the surgery-related stress response. We noted a significant difference in the daily glycemia starting the day of surgery (day 3), a difference that gradually narrowed over the next 5 days, with the values converging over the last 2 days in the study (Fig. 2C). Our final evaluations (MMCT) occurred 7 days after the surgery, a time at which the stress response has receded as evidenced by the convergence of the glycemic curves and normalization of the acute stress response indicators of heart rate (79.4 beats per minute; 95% CI, 71.9–86.8), white blood cell count (8.79 106/mL;7.06–10.52), platelet count (286.9 109/L;247.77–326.03), and cortisol level (12.48 μg/dL; 10.12–14.84). Therefore, although the results of the overall glycemia during the entire study period were likely attributable to surgery-induced stress response, we conclude that the results of the MMCT performed 7 days after surgery were not affected by the postsurgical stress response, and the results show the true effect of the dietary intervention versus diet plus surgery.
We acknowledge a limitation of the study in that the baseline weight for the two study periods was not identical. Patients did not regain the entire weight lost during the diet period, which potentially could limit the effect size of the intervention in the second study period. We do not think this factor played a major role in the results we observed because all glycemic parameters were comparable at the start of both study periods and, furthermore, although glycemia improved substantially in both study periods, it did not completely normalize. Therefore, if the surgery would have had any additional effects beyond the diet intervention alone, then further lowering of the glucose levels toward normal levels should have been observed.
Our study led us to hypothesize that different mechanisms for improvement in diabetes prevail at different time points after RYGB. In the immediate postoperative period (at least 1 week, likely up to 2–4 weeks), the severely restricted oral caloric intake is primarily responsible for the changes in glycemia. Of note is that the majority of diabetes improvement occurs in this timeframe. As weight loss ensues and insulin sensitivity improves, consolidation of this improvement occurs over the following few months, an improvement that is commensurate with the amount of weight lost (24). In the long-term, it is possible that chronic glucagon-like protein 1 stimulation (and other incretin hormones) leads to β-cell regeneration or hypertrophy and increased insulin secretion, which can protect from diabetes recurrence as a small amount of weight is regained, although to date no studies have been able to prove this hypothesis. We believe the main effect of the surgically induced enhancement in the incretin hormones is centrally mediated, resulting in satiety and aiding the long-term adherence to such significant dietary restrictions. The dietary restrictions are ultimately responsible for the diabetes improvement and remission as well as the weight loss.
The clinical implication of our finding is that nonsurgical interventions that successfully achieve and maintain such strict caloric restriction have the potential to resolve diabetes to a comparable degree as RYGB. Long-term studies comparing the effect to RYGB surgery with identical nonsurgical caloric restriction are warranted to fully evaluate this concept.
We conclude that the rapid improvement in diabetes after RYGB is mediated through caloric restriction without a need to invoke a primary direct hormonal mechanism related to duodenal bypass.
Acknowledgments
The study was supported by a Pilot Award to I.L. from NIH 3UL1 RR024982-05S1. I.L. was supported by NIH K23RR024470.
No potential conflicts of interest relevant to this article were reported.
I.L. designed and performed the study, analyzed the data, and wrote the manuscript. E.G. and A.I. helped with study recruitment and edited the manuscript. E.L. helped with study design and edited the manuscript. I.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors extend their deepest appreciation to Michael Brown, MD (Department of Molecular Genetics), for assistance in planning the study and editing the manuscript and to Laura Golici, BA, and Madhuri Poduri, MS (Endocrinology Division, Department of Internal Medicine) for their expert help in protocol implementation, patient care, and data collection. The authors would also like to thank all the study volunteers, the Clinical and Translational Research Center staff, Bariatric Clinic staff, University Hospital Staff, Adam Coster, Lori Evans (all at University of Texas Southwestern Medical Center), and Nic Burtea.
Footnotes
Clinical trial reg. no. NCT01153516, clinicaltrials.gov.
A.I. is currently affiliated with the Department of Surgery, Vascular Surgery Section, Wake Forest University, Winston-Salem, North Carolina.
E.L. is currently affiliated with JAMA, Chicago, Illinois.
References
- 1.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 2.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339–350; discussion 350–352 [DOI] [PMC free article] [PubMed]
- 3.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg 2005;15:474–481 [DOI] [PubMed] [Google Scholar]
- 4.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003;238:467–484; discussion 484–485 [DOI] [PMC free article] [PubMed]
- 5.Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 2006;244:741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubino F, Schauer PR, Kaplan LM, Cummings DE. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med 2010;61:393–411 [DOI] [PubMed] [Google Scholar]
- 7.Sandoval D. Bariatric surgeries: beyond restriction and malabsorption. Int J Obes (Lond) 2011;35(Suppl. 3):S45–S49 [DOI] [PubMed] [Google Scholar]
- 8.Schauer PR, Rubino F. International Diabetes Federation position statement on bariatric surgery for type 2 diabetes: implications for patients, physicians, and surgeons. Surg Obes Relat Dis 2011;7:448–451 [DOI] [PubMed] [Google Scholar]
- 9.Doar JW, Wilde CE, Thompson ME, Sewell PF. Influence of treatment with diet alone on oral glucose-tolerance test and plasma sugar and insulin levels in patients with maturity-onset diabetes mellitus. Lancet 1975;1:1263–1266 [DOI] [PubMed] [Google Scholar]
- 10.Henry RR, Scheaffer L, Olefsky JM. Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1985;61:917–925 [DOI] [PubMed] [Google Scholar]
- 11.Kelley DE, Wing R, Buonocore C, Sturis J, Polonsky K, Fitzsimmons M. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1993;77:1287–1293 [DOI] [PubMed] [Google Scholar]
- 12.Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011;54:2506–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008;93:2479–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isbell JM, Tamboli RA, Hansen EN, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care 2010;33:1438–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campos GM, Rabl C, Peeva S, et al. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg 2010;14:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton EC, Sims TL, Hamilton TT, Mullican MA, Jones DB, Provost DA. Clinical predictors of leak after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Endosc 2003;17:679–684 [DOI] [PubMed] [Google Scholar]
- 17.Cervera A, Wajcberg E, Sriwijitkamol A, et al. Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab 2008;294:E846–E852 [DOI] [PubMed] [Google Scholar]
- 18.Ozer K, Abdelnour S, Alva AS. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery: comment on Isbell et al. Diabetes Care 2010;33:e176; author reply e177 [DOI] [PubMed]
- 19.Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986;29:46–52 [DOI] [PubMed] [Google Scholar]
- 20.Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 2001;86:3717–3723 [DOI] [PubMed] [Google Scholar]
- 21.Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg 2004;240:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradley D, Magkos F, Klein S. Effects of bariatric surgery on glucose homeostasis and type 2 diabetes. Gastroenterology 2012;143:897–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg 2013;23:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadera BE, Lum K, Grant J, Pryor AD, Portenier DD, DeMaria EJ. Remission of type 2 diabetes after Roux-en-Y gastric bypass is associated with greater weight loss. Surg Obes Relat Dis 2009;5:305–309 [DOI] [PubMed] [Google Scholar]