Increased adiposity and direct effects of glucocorticoid excess on muscle, liver, and β-cells are responsible for the high prevalence of impaired glucose tolerance (IGT) and type 2 diabetes in patients with Cushing syndrome (CS) (1,2). In the SEISMIC study, the glucocorticoid receptor antagonist mifepristone improved glucose tolerance and produced weight loss over 24 weeks in CS patients (3). Using oral glucose tolerance test data from SEISMIC, our goal was to assess whole-body insulin sensitivity (Matsuda index), β-cell function (insulinogenic index, homeostasis model assessment-β [HOMA-β]), disposition index (4,5), weight (WT), and waist circumference (WC) over time. Complete data in patients not receiving insulin were available in 19 patients, 8 with diabetes and/or IGT (C-DM) and 11 with hypertension only (C-HT).
Within-group comparisons for change over time were analyzed with a mixed-effects repeated measures two-way ANOVA with cohort (C-DM and C-HT), time, and cohort by time interaction as fixed effects; unpaired Student t tests were used to assess differences between groups (Table 1). Matsuda index improved in the total population, with the greatest improvement occurring between baseline and week 6 and lesser changes occurring from week 6 to 24. Further analysis (piecewise linear mixed-model regression) showed that a two-phase model (0–6 weeks and 6–24 weeks) for Matsuda index change over time was better than the linear model (P = 0.007; Akaike information criteria). In contrast, WT and WC declined linearly over the 24 weeks, with the largest declines occurring during the final 18 weeks of treatment (baseline to week 6: WT, −1.19 ± 3.17%, P = 0.1; WC, −1.31 ± 3.38%, P = 0.07; week 6 to 24: WT, −6.66 ± 6.52%, P = 0.0003; WC, −6.36 ± 5.83%, P = 0.0002, ANOVA). At baseline, C-DM patients had compromised insulin secretory responses, as evidenced by lower insulinogenic index0–30, insulinogenic index0–120, and HOMA-β. C-HT patients experienced declines in insulinogenic index0–30, insulinogenic index0–120, and HOMA-β by week 24, whereas these parameters trended nonsignificantly up in C-DM patients. The disposition index was lower at baseline in C-DM patients than C-HT patients. Individual C-DM patients tended more often to have increases in disposition index than C-HT patients (3.079 ± 4.387 vs. −1.739 ± 5.444, P = 0.063, respectively). Adiponectin levels increased from baseline to week 24 in C-HT subjects only in a temporal pattern that closely followed changes in WT and WC.
Table 1.
Insulin sensitivity and secretory parameters in CS patients (C-DM and C-HT) treated with mifepristone
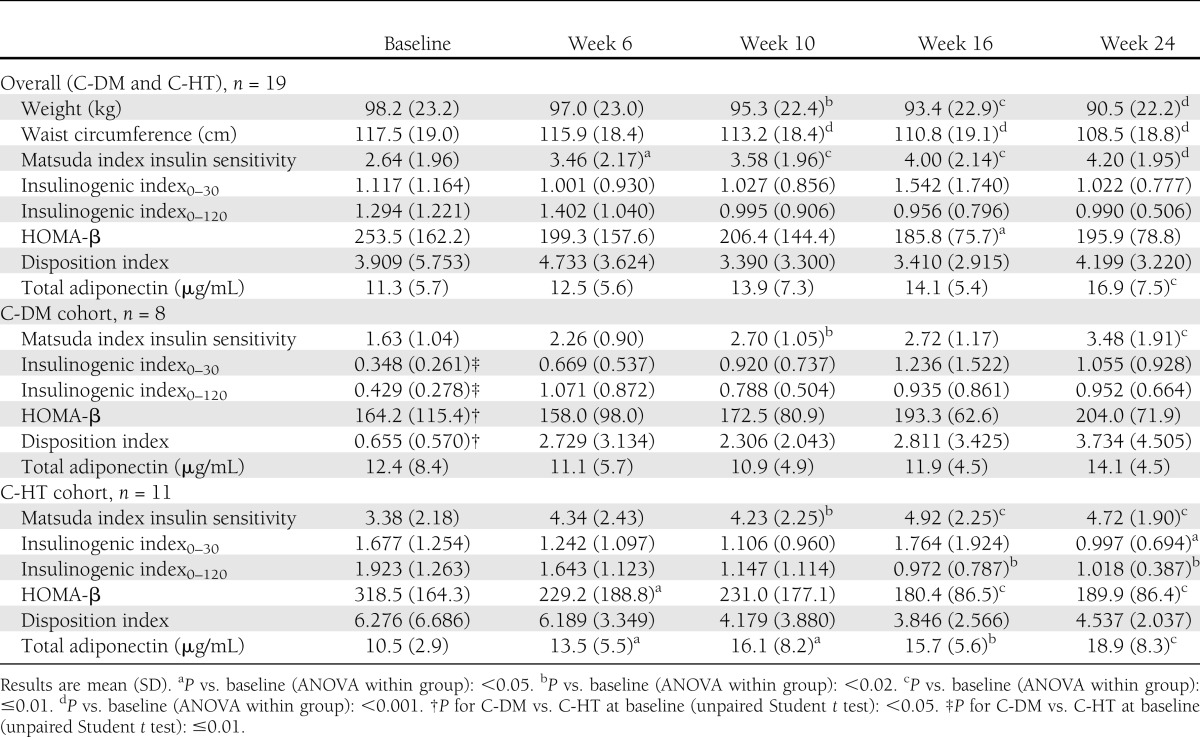
These findings suggest that rapid improvements in insulin sensitivity occurred due to direct effects of glucocorticoid blockade and longer-term improvements resulted from weight loss. Our data suggest that CS patients without underlying IGT or diabetes experience appropriate reductions in β-cell secretory response in proportion to their improved insulin sensitivity with insulin secretion decreasing in parallel (minimal change in disposition index). However, CS patients with IGT or diabetes manifest a baseline defect in β-cell secretory responsiveness that is partially retrievable along with improvement in insulin sensitivity (increase trend in disposition index) with mifepristone treatment. Adiponectin levels significantly increased with mifepristone throughout the course of treatment, particularly in patients without diabetes/IGT.
Acknowledgments
This study was supported by Corcept Therapeutics. M.E.M. and J.Q.P. have been consultants to Corcept Therapeutics. A.W., K.C., and M.E.M. have served as investigators on research grants to their institutions from Corcept Therapeutics. C.G. is an employee of Corcept Therapeutics. No other potential conflicts of interest relevant to this article were reported.
A.W. was the primary author of the manuscript and a study investigator, collected study data, interpreted data, provided input on statistical analysis, and contributed to the design of the post hoc study analysis. K.C. was a study investigator, reviewed and edited the manuscript, and collected and interpreted study data. J.Q.P. reviewed and edited the manuscript, interpreted data, provided statistical analysis, and contributed to the design of the post hoc study analysis. C.G. cowrote the manuscript, interpreted data, provided statistical analysis, and contributed to the design of the SEISMIC study and the post hoc study analysis. M.E.M. was a study investigator, reviewed and edited the manuscript, collected and interpreted study data, provided input on statistical analysis, and contributed to the design of the SEISMIC study and the post hoc study analysis. A.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
A portion of this work was presented in abstract form at the 94th Annual Meeting of The Endocrine Society, Houston, Texas, 23–26 June 2012.
The authors thank Dawn Marquez, Corcept Therapeutics, Menlo Park, California, for data management assistance.
Footnotes
Clinical trial reg. no. NCT00569582, clinicaltrials.gov.
References
- 1.Pivonello R, De Leo M, Vitale P, et al. Pathophysiology of diabetes mellitus in Cushing’s syndrome. Neuroendocrinology 2010;92(Suppl. 1):77–81 [DOI] [PubMed] [Google Scholar]
- 2.Clayton RN, Raskauskiene D, Reulen RC, Jones PW. Mortality and morbidity in Cushing’s disease over 50 years in Stoke-on-Trent, UK: audit and meta-analysis of literature. J Clin Endocrinol Metab 2011;96:632–642 [DOI] [PubMed] [Google Scholar]
- 3.Fleseriu M, Biller BM, Findling JW, Molitch ME, Schteingart DE, Gross C, SEISMIC Study Investigators Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J Clin Endocrinol Metab 2012;97:2039–2049 [DOI] [PubMed] [Google Scholar]
- 4.Albareda M, Rodríguez-Espinosa J, Murugo M, de Leiva A, Corcoy R. Assessment of insulin sensitivity and beta-cell function from measurements in the fasting state and during an oral glucose tolerance test. Diabetologia 2000;43:1507–1511 [DOI] [PubMed] [Google Scholar]
- 5.Kanat M, Winnier D, Norton L, et al. The relationship between β-cell function and glycated hemoglobin: results from the veterans administration genetic epidemiology study. Diabetes Care 2011;34:1006–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]


