Abstract
OBJECTIVE
To study whether N-terminal probrain natriuretic peptide (NT-proBNP) is a short-term independent predictor of both all-cause and cardiovascular (CV) mortality in type 2 diabetic patients and to establish whether albuminuria and C-reactive protein (CRP) affect this relationship.
RESEARCH DESIGN AND METHODS
The prospective study included 1,825 type 2 diabetic patients from the population-based cohort of the Casale Monferrato study. CV risk factors, preexisting CVD, and NT-proBNP levels were evaluated at baseline. All-cause and CV mortality were assessed 5.5 years after baseline examination. Multivariate Cox proportional hazards modeling was used to estimate mortality hazard ratios (HRs).
RESULTS
During the follow-up period, 390 people died (175 for CVD) out of 9,101 person-years of observations. A significantly increased mortality risk by quartiles of NT-proBNP was observed (test for trend, P < 0.001). NT-proBN P values >91 pg/mL conferred HRs of 2.05 (95% CI 1.47–2.86) for all-cause and 4.47 (2.38–8.39) for CV mortality, independently of CV risk factors, including CRP and albumin excretion rate (AER). The association was also significant for modest rises in NT-proBNP levels and in patients without microalbuminuria and CVD at baseline (upper quartiles HRs 3.82 [95% CI 1.24–13.75]) and 3.14 [1.00–9.94]). Albuminuria and NT-proBNP had an additive effect on mortality, though the association was stronger for NT-proBNP.
CONCLUSIONS
NT-proBNP is a strong independent predictor of short-term CV mortality risk in elderly people with type 2 diabetes, including those without preexisting CVD. This association is evident even in people with slightly increased values, is not modified by CRP, and is additive to that provided by AER.
Cardiac natriuretic peptides, atrial (ANP) and B-type (BNP), are secreted in response to myocardial stretching induced by volume overload. They play an important role in the control of blood pressure and both sodium and water balance by regulating both the renin-angiotensin and the sympathetic nervous systems (1). N-terminal probrain natriuretic peptide (NT-proBNP) is the inactive molecule resulting from cleavage of BNP prohormone (1). In the general population, circulating levels of NT-proBNP are used in the diagnosis of acute heart failure (2) and correlate with left ventricular dilatation, remodeling, and dysfunction (3).
NT-proBNP is strongly associated with the risk of cardiovascular (CV) disease (CVD) in both high-risk patients with established CVD and the general population (4–6). Patients with type 2 diabetes have an increased risk of vascular complications, which cannot be completely accounted for by conventional risk factors. Clinic-based studies have shown a role of NT-proBNP as a biomarker of CVD in diabetic patients; however, lack of prospective population-based studies does not allow data extrapolation to the entire diabetic population (7–10).
In type 2 diabetic patients, albuminuria is a well-established predictor of CV morbidity and mortality. In addition, C-reactive protein (CRP) is independently associated with short-term mortality risk even in type 2 diabetic individuals without preexisting CVD and albuminuria (11,12). However, there are no prospective data on the relationship between CRP, albuminuria, and NT-proBNP on CV mortality in people with type 2 diabetes. Our aim was to investigate in the population-based cohort of type 2 diabetic patients from the Casale Monferrato study whether NT-proBNP is a short-term predictor of all-cause and CV mortality, independently of CV risk factors, and whether albuminuria and CRP affect the relationship between NT-proBNP and short-term mortality.
RESEARCH DESIGN AND METHODS
Baseline study
The study base was comprised of 3,249 type 2 diabetic patients: residents in the year 2000 in the town of Casale Monferrato in the northwest of Italy (93,477 inhabitants) (13). Patients were identified based on independent data sources (diabetes clinics; administrative data sources), using the capture-recapture method, with a high degree of estimated ascertainment (95%). The date of diabetes diagnosis was retrieved and recorded for all recruited subjects. As previously described in detail, patients were interviewed and examined and blood samples collected after overnight fasting (13). Ethics committee approval was obtained, and all subjects provided written informed consent.
Smoking status was classified as current smoker, never smoker, or ex-smoker (smoking cessation at least a month prior to the visit). Hypertension was defined as systolic blood pressure >140 mmHg and/or diastolic blood pressure >90 mmHg or treatment with antihypertensive drugs. CVD was defined as a positive medical history of a CV event, including myocardial infarction, angina pectoris, coronary artery bypass graft and stroke, or ischemic changes on a resting 12-lead electrocardiogram, classified according to the Minnesota Code. The World Health Organization Rose questionnaire was also administered, and people with symptoms suggestive of CVD underwent further investigations to confirm the diagnosis. Congestive heart failure was clinically diagnosed based on medical history, physical examination, and drug treatment.
Blood samples were analyzed at the Central Laboratory (13). Triglycerides, total cholesterol, HDL cholesterol, apolipoprotein (apo)A1, apoB, serum creatinine, and HbA1c (reference range 3.8–5.5%) were measured by standard laboratory techniques. LDL cholesterol was calculated from Friedewald formula when triglyceride values were <4.48 mmol/L. Glomerular filtration rate was estimated using the four-component abbreviated equation from the MDRD Study (14). Subjects with an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 were defined as having chronic kidney disease. High-sensitivity CRP was measured by immunoturbidimetry (Roche Diagnostics, Mannheim, Germany) (coefficient of variation 0.5%). Albumin excretion rate (AER) was measured by nephelometry (Behring Nephelometer Analyzer; Behring Institute, Marburg, Germany) (coefficient of variation 4%) on single overnight urine collections and categorized as either normoalbuminuria (<20 μg/min) or micro- or macroalbuminuria (≥20 μg/min). Serum NT-proBNP levels were measured by a two-site sandwich electrochemiluminescence immunoassay (Elecsys proBNP II; Roche Diagnostics), using a Modular Analytics Evo analyzer with an E170 module (Roche) as previously described (15). The intra-assay variation was <3.0%, and total coefficient of variation ranges were between 2.2 and 5.8% in low and high ranges of NT-proBNP.
Follow-up study
Six years after baseline examinations, mortality data up to 31 December 31 2006 were obtained from the demographical files of towns of residence and both hospital and autopsy records. Only one patient was lost to follow-up. Underlying causes of death were derived and coded by two authors according to ICD-9 with 100% agreement. CV mortality was defined by ICD-9 codes 390–459.
Statistical analysis
Variables distributed normally are presented as mean and SD, while variables with skewed distribution (triglycerides, AER, CRP, and NT-proBNP) were analyzed after logarithmic transformation and results presented as geometric means and 95% CIs. Comparisons were performed using the Student t test and the χ2 test as appropriate. Pearson correlation and multiple linear regression were used to assess relationships and covariance analysis to adjust log NT-proBNP values for potential confounders.
Mortality rates were calculated dividing the number of deaths, occurring during the study period, by the number of person-years of observation. Cox proportional hazards modeling was used to estimate the hazard ratios (HRs) and 95% CIs of CV and all-cause mortality by NT-proBNP values independently of conventional and new risk factors. Models for log NT-proBNP as a continuous measure were run to assess the multiplicative increase of each increment of NT-proBNP to mortality risk. Analyses were also performed using quartiles of NT-proBNP value distribution to examine the shape of the relationship between NT-proBNP values and mortality. In order to assess the incremental association of NT-proBNP with respect to CV risk factors, all models were adjusted for age, sex, diabetes duration (model 1), hypertension, HbA1c, smoking, apoA1/apoB, eGFR, AER, CVD, diabetes treatment (model 2), and CRP (model 3). Models were constructed with variables as continuous measures except AER (< 20 vs. ≥20 μg/min) and eGFR (<60 vs. ≥60 mL/min/1.73 m2) to provide maximum power for detecting an association between NT-proBNP and mortality. BMI and antihyperglycemic treatment (diet, oral drugs, and insulin) were also included in models 2 and 3, as they might provide residual confounding of the relationship between NT-proBNP and mortality. In addition, we allowed any of the following variables to enter the model if they added significantly or modified HRs of NT-proBNP: waist circumference; uric acid; total, LDL, and HDL cholesterol; triglycerides; fibrinogen; systolic and diastolic blood pressure; and antihypertensive treatment. Stratified mortality analysis by CVD and AER (<20 vs. ≥20 μg/min) at baseline was also performed. We tested for linear trends across categorical variables by entering a single ordinal term into the Cox regression model. The proportional hazard assumptions of explanatory variables were assessed on the basis of Schoenfeld residuals. The likelihood ratio test was used to assess the statistical significance of examined variables in nested models. We also carried out analyses that included terms for the interaction between NT-proBNP and AER. A P value of <0.05 was considered to indicate statistical significance. Analyses were performed with STATA software, version 10.0.
RESULTS
Of the 3,249 type 2 diabetic subjects enrolled in the Casale Monferrato Study, 1,825 (56.2%) patients showed no clinical evidence of heart failure and had samples available for NT-proBNP measurements. Compared with the entire cohort, selected patients were younger (67.6 ± 10.5 vs. 71.0 ± 12.2 years, respectively; P < 0.0001) and had slight lower diabetes duration (10.7 ± 8.0 vs. 11.5 ± 8.4 years; P = 0.02) and negligibly lower HbA1c values (7.0 ± 1.8 vs. 7.2 ± 1.8%, 53 ± 19.7 vs. 55 ± 19.7 mmol/mol; P = 0.01) but similar systolic blood pressure (146.0 ± 16.4 vs. 145.0 ± 17.2 mmHg; P = 0.16) and diastolic blood pressure (82.6 ± 8.2 vs. 83.2 ± 8.2 mmHg; P = 0.10) levels. At the baseline examination, most of the subjects (64.0%) were elderly, being ≥65 years old at recruitment.
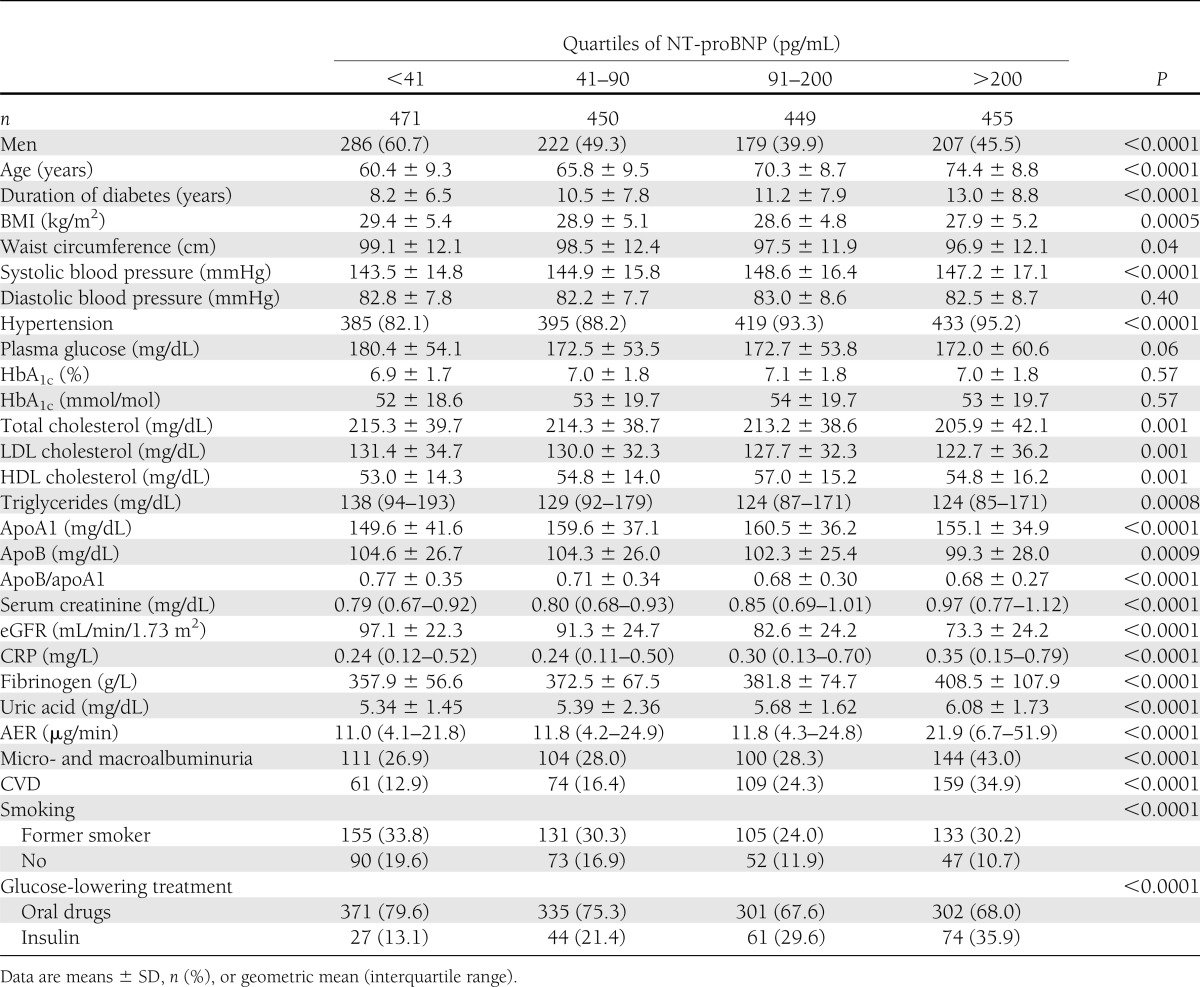
The distribution of NT-proBNP values was right skewed with a median value of 90 pg/mL (interquartile range 39–200). As expected, NT-proBNP levels were higher in women than in men (103.1 pg/mL [interquartile range 48–215]) vs. 76.2 pg/mL [30–185], P < 0.0001]), but differences disappeared after adjustment for age. In addition, overweight/obese patients had lower NT-proBNP values (77.3 vs. 112.7, P < 0.001) compared with normal-weight subjects, even after adjustment for age and sex (P = 0.003). Baseline characteristics of the 1,825 recruited patients by quartiles of NT-proBNP values are reported in Table 1. At baseline, there was a direct significant correlation between NT-proBNP levels and age, systolic blood pressure, HDL cholesterol, uric acid, log-AER, fibrinogen, and log CRP. A significant negative correlation was found with plasma glucose, BMI, waist circumference, total and LDL cholesterol, apoB/apoA1, triglycerides, and eGFR (data not shown). In multiple linear regression, variables independently associated with log NT-proBNP were age, apoB/apoA1, eGFR, log AER, fibrinogen, and log CRP, and they accounted for 35% of NT-proBNP variability (P < 0.0001).
Table 1.
Baseline characteristics of persons with type 2 diabetes of the Casale Monferrato study by NT-proBNP quartiles
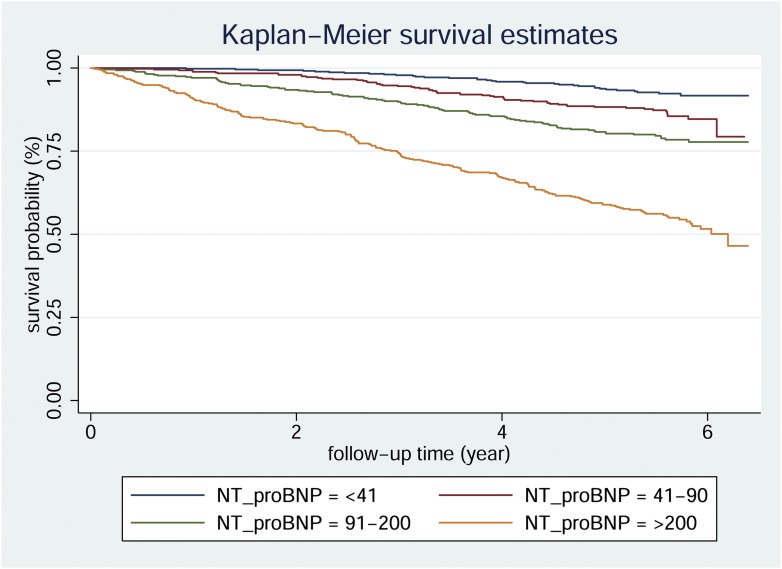
During the 5-year follow-up period (median 5.5 years [range 0.1–7.3]), 390 people died out of 9,101.6 person-years. CV mortality accounted for 175 (44.9%) deaths and out of them 101 deaths occurred in subjects without preexisting CVD. All-cause and CV mortality rates per 1,000 person-years were 42.8 (95% CI 38.8–47.3) and 19.2 (16.6–22.3), respectively. Kaplan-Meier curves for overall mortality according to quartiles of NT-proBNP are shown in Fig. 1 (log-rank test P < 0.0001)
Figure 1.
Kaplan-Meier survival curves for type 2 diabetic patients from the Casale Monferrato study categorized by NT-proBNP quartiles at baseline. Log-rank test P < 0.0001.
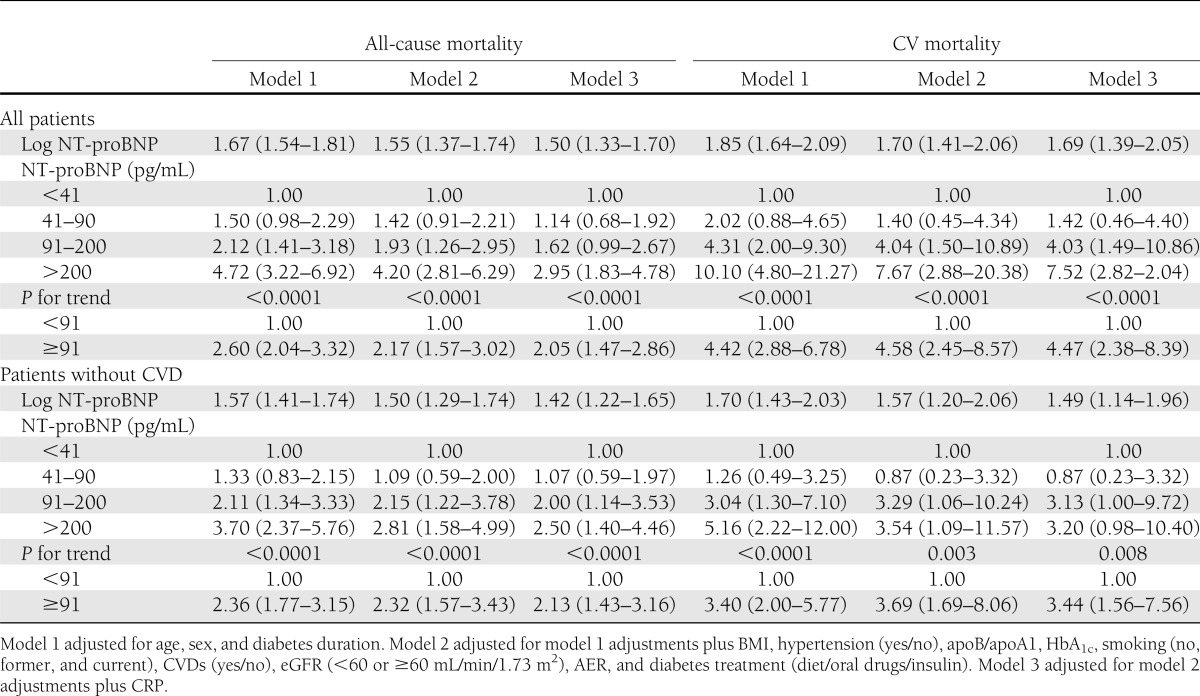
In multivariable Cox proportional hazard models for all-cause and CV mortality, HRs were significantly higher in persons with NT-proBNP values in the third and fourth quartiles compared with persons with values in the lowest quartile. This was only slightly reduced by multiple adjustment for CV risk factors, potential confounders, and preexisting CVD (Table 2, models 1 and 2). After further adjustment for CRP (Table 2, model 3), HRs for all-cause mortality were slightly reduced, while HRs for CV mortality were left unchanged. Trends of HRs across NT-proBNP quartiles were statistically significant in all examined models. NT-proBNP values greater than the median value (91 pg/mL) conferred fully adjusted HRs of 2.05 (95% CI 1.47–2.86) for all-cause mortality and 4.47 (2.38–8.39) for CV mortality (Table 2, model 3). NT-proBNP conferred higher HRs than both AER and CRP. Indeed, in the final model 3, micro- and macroalbuminuria conferred HRs of 1.37 (1.03–1.84) and 1.29 (0.82–2.05) for all-cause and CV mortality, respectively, and log CRP of 1.17 (1.04–1.32) and 1.06 (0.88–1.29). Similarly, analyses performed using log NT-proBNP as a continuous variable showed a 50% increase risk of all-cause mortality and a 69% increase risk of CV mortality for each increment of NT-proBNP (1 natural logarithm = 0.35 pg/mL) in the fully adjusted models (Table 2, model 3). In the subgroup of patients without CVD at baseline, results were comparable with those obtained in the whole cohort (Table 2). Of relevance, in patients without micro/macroalbuminuria and CVD at baseline, HRs for CV mortality in the second and in the third quartiles of NT-proBNP were 3.82 (1.24–13.75) and 3.14 (1.00–9.94), respectively.
Table 2.
HRs (95% CI) of 5-year all-cause and CV mortality in persons with type 2 diabetes of the Casale Monferrato study
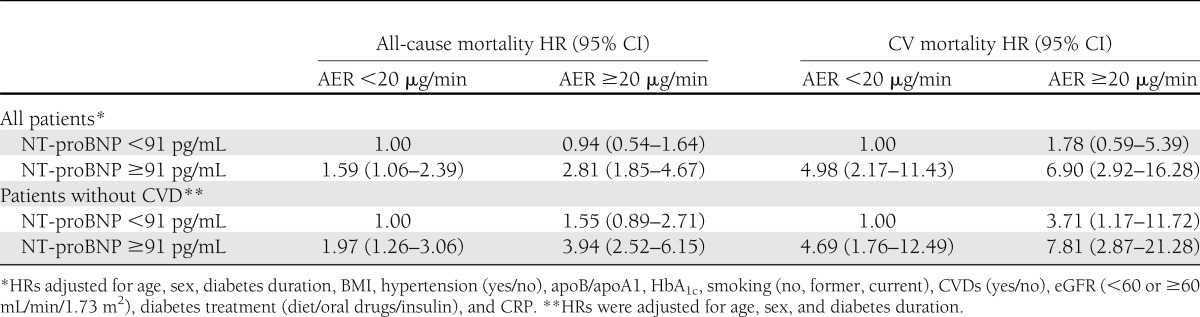
As shown in Table 3, when fully adjusted models were fitted replacing NT-proBNP and AER terms with an indicator variable with four categories defined by their joined effects, we found that with respect to the reference category (AER <20 μg/min and NT-proBNP <91 pg/mL), HRs of both CV and all-cause mortality were significantly increased in patients with NT-proBNP ≥91 pg/mL, irrespective of presence or absence of micro- and macroalbuminuria, but no evidence of an effect modification was found. Indeed, the expected HRs obtained by the multiplication of the HRs in each category of NT-proBNP and AER were higher than the observed HRs. No significant interactions were found between AER and NT-proBNP values (P = 0.30 for all-cause mortality and P = 0.58 for CV mortality). In patients without micro- or macroalbuminuria and CVD at baseline (Table 3), NT-proBNP values ≥91 pg/mL conferred a statistically significant twofold increased risk of all-cause mortality and a fivefold increased risk of CV mortality compared with patients with lower NT-proBNP values, and an additive effect of AER and NT-proBNP on both all-cause and CV mortality was also observed.
Table 3.
HRs of 5-year all-cause and CV mortality in persons with type 2 diabetes of the Casale Monferrato study by AER and NT-proBNP values
CONCLUSIONS
Our population-based study indicates that NT-proBNP is a strong independent predictor of short-term CV mortality in type 2 diabetic people and that this effect is evident even in normoalbuminuric patients without CVD at baseline. The association between NT-proBNP and mortality was additive to that provided by micro- and macroalbuminuria, was not modified by acute-phase inflammation markers, and was evident even in people with slightly increased NT-proBNP values. Both conventional and new CV risk factors (AER, eGFR, and uric acid) had a quite limited effect on the strength of the association between NT-proBNP and death, providing evidence that they do not act as mediators or as confounders. This finding suggests that they might be minimally involved in the pathogenetic cascade linking increased NT-proBNP values and CV mortality.
Our findings are original, and to our knowledge no previous prospective population-based study has examined in patients with type 2 diabetes the predictive role of NT-proBNP and its potential interrelationships with the main markers of CV mortality in these patients, AER and CRP. Our results are consistent and expand observations from the few clinic-based prospective studies examining this issue (7–9). In the Danish cohort, including 315 diabetic patients followed up to 15 years, NT-proBNP values in the upper tertile only increased significantly the HR for all-cause mortality after adjustment for risk factors (7). Another study including 1,071 diabetic patients followed for up to 3 years showed a linear multiplicative association between log NT-proBNP and mortality, but no dose response was analyzed (9). In our study, NT-proBNP was included in multivariate analyses both as continuous variable and as quartiles, allowing us to show that even minimally elevated values in the third quartile (91–200 pg/mL) conferred a statistically increased risk. With respect to the median NT-proBNP value of 91 pg/mL, higher values conferred a significant twofold increased risk of overall mortality and a threefold increased risk of CV mortality, which was only slightly affected by adjustment for traditional and new risk factors, including AER, CRP, fibrinogen, uric acid, and eGFR.
NT-proBNP was a significant predictor of mortality also in the subgroup of patients without CVD at baseline. This finding is in agreement with a recent prospective population-based study performed in old men from the general population (16) and suggests that NT-proBNP may be useful in primary prevention for the identification of patients with a hitherto unknown high risk of death.
In type 2 diabetic patients, elevated urinary AER is considered the most consistent independent predictor of adverse outcomes (17) and CRP is independently associated with short-term mortality risk (11). Nonetheless, in the current study NT-proBNP was a stronger predictor of mortality than albuminuria and CRP, particularly for CV deaths. In keeping with this finding, a recent clinic-based prospective study in diabetic patients reported that NT-proBNP was superior to albuminuria for predicting cardiac events (9). On the other hand, our data provide evidence of an additive effect of NT-proBNP and micro- and macroalbuminuria in predicting the risk of CV mortality, and this suggests that the parallel assessment of both AER and NT-proBNP may provide additional information for risk stratification.
Identification of new biomarkers for CV risk assessment is of particular importance in diabetic patients without micro- or macroalbuminuria. Our data showing that normoalbuminuric patients without CVD had a fivefold higher risk of CV mortality when NT-proBNP values were >91 pg/mL indicate that NT-proBNP may be a promising prognostic marker in this relevant subgroup of diabetic patients. Consistent with this result, in the normoalbuminuric subgroup of the Danish study elevated NT-proBNP levels were significantly associated with increased CV mortality, thought people with and without baseline CVD were not examined separately (7).
Our data do not allow inferences about causality of the relationship between NT-proBNP and CV mortality. However, causality is more likely if the response is graded and multiple adjustments for potential confounders/mediators do not modify results as observed in our study. Left ventricular hypertrophy (LVH) and silent ischemia, leading to subclinical cardiac stress, may link NT-proBNP and CV mortality. Indeed, increased values of BNP have been found to be associated with increased LVH and deteriorated diastolic function in an 8-year follow-up, particularly in diabetic patients (18). In addition, we have recently reported that, in type 1 diabetic patients from the EURODIAB case-control study, the association between NT-proBNP and vascular complications was dependent on tumor necrosis factor (TNF)-α, suggesting a role for TNF-α in linking NT-proBNP to CVD (15). Recently, an independent association between NT-proBNP and TNF-α has also been reported in hypertensive patients with LVH (19). However, the underlying molecular mechanisms remains uncertain.
Strengths of this study are the prospective design; the recruitment of a large population-based cohort of type 2 diabetic people, increasing both precision and generalizability; the centralized assessment of CV risk factors and biomarkers, allowing analysis of the potential confounding effect of covariates; and the high degree of completeness of follow-up data. There are, however, certain limitations. Only 56% patients of the original population-based cohort were recruited in the study, and they were younger than unrecruited people. As age was positively correlated with NT-proBNP, the association between NT-proBNP and mortality might have been even greater in the entire cohort. However, we cannot exclude the possibility that risk factors negatively associated with NT-proBNP, such as BMI, might have acted in the opposite direction in unrecruited patients. Our results are based on an observational prospective cohort and, though multivariate methods of analyses were used to control for the effect of known confounders in the relationship between NT-proBNP and mortality, we cannot rule out the possibility of residual or undetected confounding effects on our results. A single NT-proBNP measurement was performed using stored samples collected at baseline. Although the stability of NT-proBNP after long-term storage is unknown, all samples were treated and stored under the same conditions. The study is based on a cohort of elderly subjects and, though this is a group of coniderable clinical interest because they constitute a high-risk group in whom traditional risk factors become less predictive (20), our results need further confirmation in similar middle-aged populations. Finally, no data on structural and functional cardiac abnormalities were available.
In conclusion, this study shows that NT-proBNP is a strong independent predictor of short-term mortality risk in elderly people with type 2 diabetes, even in those without CVD at baseline. The association between NT-proBNP and mortality is additive to that provided by AER, is not modified by CRP, and is evident even in people with slightly increased values, suggesting its role as an early marker of vascular abnormalities.
Acknowledgments
This work was supported by the Piedmont Region.
No potential conflicts of interest relevant to this article were reported.
G.B. researched and analyzed data and wrote the manuscript. A.L., F.B., G.G., C.B., L.S., A.S., and T.P. researched data. P.C.P. reviewed the manuscript. G.G. wrote the manuscript. G.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Ventura HO, Silver MA. Natriuretic peptides as markers of cardiovascular risk: the story continues. Mayo Clin Proc 2011;86:1143–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunlay SM, Gerber Y, Weston SA, Killian JM, Redfield MM, Roger VL. Prognostic value of biomarkers in heart failure: application of novel methods in the community. Circ Heart Fail 2009;2:393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groenning BA, Nilsson JC, Sondergaard L, et al. Detection of left ventricular enlargement and impaired systolic function with plasma N-terminal pro brain natriuretic peptide concentrations. Am Heart J 2002;143:923–929 [DOI] [PubMed] [Google Scholar]
- 4.Blankenberg S, Zeller T, Saarela O, et al. MORGAM Project Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: the MONICA, risk, genetics, archiving, and monograph (MORGAM) biomarker project. Circulation 2010;121:2388–2397 [DOI] [PubMed] [Google Scholar]
- 5.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004;350:655–663 [DOI] [PubMed] [Google Scholar]
- 6.Di Angelantonio E, Chowdhury R, Sarwar N, et al. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation 2009;120:2177–2187 [DOI] [PubMed] [Google Scholar]
- 7.Tarnow L, Hildebrandt P, Hansen BV, Borch-Johnsen K, Parving HH. Plasma N-terminal pro-brain natriuretic peptide as an independent predictor of mortality in diabetic nephropathy. Diabetologia 2005;48:149–155 [DOI] [PubMed] [Google Scholar]
- 8.Tarnow L, Gall MA, Hansen BV, Hovind P, Parving HH. Plasma N-terminal pro-B-type natriuretic peptide and mortality in type 2 diabetes. Diabetologia 2006;49:2256–2262 [DOI] [PubMed] [Google Scholar]
- 9.Clodi M, Resl M, Neuhold S, et al. A comparison of NT-proBNP and albuminuria for predicting cardiac events in patients with diabetes mellitus. Eur J Prev Cardiol 2012;19:944–951 [DOI] [PubMed] [Google Scholar]
- 10.Huelsmann M, Neuhold S, Strunk G, et al. NT-proBNP has a high negative predictive value to rule-out short-term cardiovascular events in patients with diabetes mellitus. Eur Heart J 2008;29:2259–2264 [DOI] [PubMed] [Google Scholar]
- 11.Bruno G, Fornengo P, Novelli G, et al. C-reactive protein and 5-year survival in type 2 diabetes: the Casale Monferrato Study. Diabetes 2009;58:926–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sattar N, Hingorani AD. C-reactive protein and prognosis in diabetes: getting to the heart of the matter. Diabetes 2009;58:798–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruno G, Merletti F, Bargero G, et al. Changes over time in the prevalence and quality of care of type 2 diabetes in Italy: the Casale Monferrato surveys, 1988 and 2000. Nutr Metab Cardiovasc Dis 2008;18:39–45 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 15.Gruden G, Barutta F, Chaturvedi N, et al. NH2-terminal probrain natriuretic peptide is associated with diabetes complications in the EURODIAB Prospective Complications Study: the role of tumor necrosis factor-α. Diabetes Care 2012;35:1931–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wannamethee SG, Welsh P, Lowe GD, et al. N-terminal pro-brain natriuretic Peptide is a more useful predictor of cardiovascular disease risk than C-reactive protein in older men with and without pre-existing cardiovascular disease. J Am Coll Cardiol 2011;58:56–64 [DOI] [PubMed] [Google Scholar]
- 17.Gall M-A, Borch-Johnsen K, Hougaard P, Nielsen FS, Parving H-H. Albuminuria and poor glycemic control predict mortality in NIDDM. Diabetes 1995;44:1303–1309 [DOI] [PubMed] [Google Scholar]
- 18.Kroon MH, van den Hurk K, Alssema M, et al. Prospective associations of B-type natriuretic peptide with markers of left ventricular function in individuals with and without type 2 diabetes: an 8-year follow-up of the Hoorn Study. Diabetes Care 2012;35:2510–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roselló-Lletí E, Calabuig JR, Morillas P, et al. Variability of NT-proBNP and its relationship with inflammatory status in patients with stable essential hypertension: a 2-year follow-up study. PLoS ONE 2012;7:e31189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kannel WB. Coronary heart disease risk factors in the elderly. Am J Geriatr Cardiol 2002;11:101–107 [DOI] [PubMed] [Google Scholar]