Abstract
OBJECTIVE
Macrovascular disease may contribute to increased risk of accelerated cognitive decline in patients with type 2 diabetes. We aimed to determine associations of measures of macrovascular disease with cognitive change in a cognitively healthy older population with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Eight hundred thirty-one men and women (aged 60–75 years) attended two waves of the prospective Edinburgh Type 2 Diabetes Study (ET2DS). At baseline, clinical and subclinical macrovascular disease was measured, including cardiovascular event history, carotid intima-media thickness (cIMT), ankle brachial index (ABI), and serum N-terminal probrain natriuretic peptide (NT-proBNP). Seven neuropsychological tests were administered at baseline and after 4 years; scores were combined to a standardized general ability factor (g). Adjustment of follow-up g for baseline g assessed 4-year cognitive change. Adjustment for vocabulary (estimated premorbid ability) was used to estimate lifetime cognitive change.
RESULTS
Measures of cognitive decline were significantly associated with stroke, NT-proBNP, ABI, and cIMT, but not with nonstroke vascular events. The association of stroke with increased estimated lifetime cognitive decline (standardized β, −0.12) and of subclinical markers with actual 4-year decline (standardized β, −0.12, 0.12, and −0.15 for NT-proBNP, ABI, and cIMT, respectively) reached the Bonferroni-adjusted level of statistical significance (P < 0.006). Results altered only slightly on adjustment for vascular risk factors.
CONCLUSIONS
Stroke and subclinical markers of cardiac stress and generalized atherosclerosis are associated with cognitive decline in older patients with type 2 diabetes. Further investigation into the potential use of subclinical vascular disease markers in predicting cognitive decline is warranted.
Cognitive abilities are essential for independent living in later life, and some domains of cognitive functioning decline in mean level from relatively early adulthood (1). Age-related cognitive decline is accompanied by pathological changes in the brain, including cerebral microvascular changes, and although individual differences exist in the severity of age-related microvascular damage in the brain, this is difficult to investigate noninvasively. Systemic atherosclerotic changes in the body may serve as a marker of vascular-related changes in the brain (2) that, in turn, lead to cognitive deficits (3,4). However, the potential of large vessel changes distant from the brain itself to function as markers of cognitive decline remains unclear. We aimed to study a range of measures of clinical and subclinical macrovascular disease that focus on different areas of the vasculature or different underlying pathophysiological mechanisms to assess which of these might function as proxies of cognitive decline.
Understanding the role of macrovascular disease in age-related cognitive impairment is particularly important in diabetes, given the higher prevalence of atherosclerotic large vessel disease as well as the accelerated cognitive decline and increased risk of cognitive impairment (5,6) associated with this condition, and the potentially modifiable nature of macrovascular disease (7). The prevalence of stroke, of transient ischemic attack (TIA) (8), and of coronary heart disease (9) are higher in diabetic populations than in nondiabetic populations, and average natriuretic peptide levels, a marker of cardiac stress, are increased (10). Markers of subclinical atherosclerosis also are altered, with increased average carotid intima-media thickness (cIMT) (11) and reduced mean ankle brachial index (ABI) (12). Despite this, investigation into the role of macrovascular disease in age-related cognitive impairment in people with diabetes is limited compared with investigation into this issue in the general (predominantly nondiabetic) population. We set out to determine the association of a variety of measures of subclinical macrovascular disease and cardiovascular event categories with cognitive decline in a sample of older people, all of whom had diabetes (the Edinburgh Type 2 Diabetes Study [ET2DS]). We did so using two cognitive outcomes, actual late-life cognitive change over a 4-year period and estimated lifetime cognitive change. These analyses are timely given the increasing prevalence of diabetes at younger ages (13) that, together with greater survival (14) and greater lifetime exposure to diabetes in current generations, is likely to contribute to increasing prevalence of cognitive impairment.
RESEARCH DESIGN AND METHODS
Study population
In 2006, the ET2DS recruited a randomly selected sample of older adults with type 2 diabetes from the Lothian Diabetes Register, a population-based disease register that contains details of nearly all individuals with diagnosed diabetes living in the Lothian region of Scotland, U.K. The study had ethical approval from the Lothian Medical Research Ethics Committee. Of 5,454 individuals aged 60–74 years who were invited to participate, 1,066 were recruited. Details of recruitment have been described previously (15) and representativeness of the cohort has been demonstrated by comparing demographic and clinical characteristics of responders and nonresponders (16).
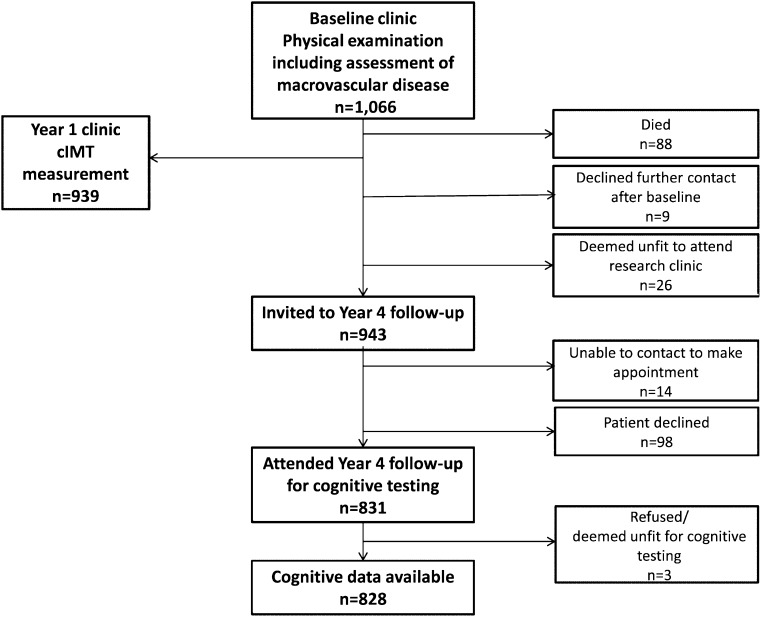
At baseline in 2006–2007, all study participants attended a dedicated research clinic for extensive physical and cognitive examination and 939 (88%) returned for further examination after ∼1 year (2007–2008). All participants were considered for repeat cognitive testing after 4 years (2010–2011). Between baseline and year 4, 88 participants had died, 9 declined further participation, and 26 were deemed unfit to continue participation. Invitations were sent out to 943 (88%) participants. Of these, 98 declined to attend and 14 could not be contacted; 831 (78%) ultimately attended the year 4 clinic (Fig. 1). Reasons for nonattendance at follow-up included poor health (including dementia), responsibilities as a carer, and loss of interest in participation. Nonattenders were followed-up using subject or general practitioner questionnaires, record linkage to hospital discharge and death certificate data, and review of hospital notes when required. Informed consent was obtained from all participants at each clinic attendance.
Figure 1.
Subject participation in the ET2DS.
Physical and cognitive examination
All assessments were performed by specially trained nurses following standard operating procedures and have been described in detail previously (15). At baseline, fasting blood samples were taken for measurement of total serum cholesterol, HbA1c, glucose, and plasma N-terminal probrain natriuretic peptide (NT-proBNP). Participants completed a questionnaire with standard questions about vascular disease and vascular risk factors, including smoking history, doctor diagnosis of myocardial infarction (MI), angina, and stroke, the World Health Organization chest pain questionnaire, and the Edinburgh Claudication Questionnaire (17).
Height and weight, brachial blood pressure, and a standard 12-lead electrocardiogram were recorded and measurement of right and left brachial, posterior tibial, and dorsalis pedis systolic pressures were used to calculate the ABI. Scottish Morbidity Record (SMR01) data on all medical and surgical discharges from Scottish hospitals between 1981 and 2011 were obtained. Any ICD-10 or ICD-9 codes for cardiovascular or cerebrovascular disease recorded between 1981 and 2007 were extracted and used together with questionnaire data on vascular disease and electrocardiogram findings (as well as review of clinical notes when required) to define MI, angina, stroke, and TIA, as detailed previously (16,18). Peripheral arterial disease was defined as presence of intermittent claudication on the Edinburgh Claudication Questionnaire.
At year 1, carotid IMT was measured bilaterally in three separate images of the common carotid artery, 1 to 2 cm below the bifurcation and in areas free of plaque using a Sonoline Elegra Ultrasound Imaging System (Siemens Medical Systems). Mean cIMT was calculated for the left and right carotid arteries; the higher of the two values was used for analyses.
At baseline and at 4-year follow-up, seven neuropsychological tests were used to measure several domains of cognitive ability. The Borkowski Verbal Fluency Test (BVFT) examined executive function. The Logical Memory (LM) subtest of the Wechsler Memory Scale, third edition, assessed immediate and delayed verbal declarative memory. Nonverbal memory was measured by the Faces subtest of the same scale. The Trail-Making Test B (TMT-B) examined mental flexibility and executive function. The Digit Symbol Coding (DSC) subtest of the Wechsler Adult Intelligence Scale, third edition, measured speed of information processing. The Letter-Number Sequencing and the Matrix Reasoning (MR) subtests from the Wechsler Adult Intelligence Scale, third edition, assessed working memory and nonverbal reasoning, respectively. Scores on the combined junior and senior Mill Hill Vocabulary Scale (MHVS) for synonyms, which correlate strongly with scores on the more traditionally used National Adult Reading Test (19), tested vocabulary abilities. Symptoms of depression and anxiety were self-reported on the Hospital Anxiety and Depression Scale (20). The Mini-Mental-State Examination (MMSE) (21) assessed global cognitive function. A cut-point of <24 out of 30 is traditionally used to identify individuals with possible dementia (22). However, because of the low validity and reliability of this method (23), we applied the following additional criteria to define dementia: using medication for dementia at baseline or year 4, or both; dementia coding (F00, F01, F02, F03, or G30) from SMR01 or on death certificate between 1981 and completion of year 4 follow-up in 2011; report of psychiatrist diagnosis of dementia in a questionnaire sent to general practitioners of all subjects with MMSE <24 at baseline or follow-up and subjects who did not attend the year 4 clinic; a psychiatrist’s diagnosis of dementia obtained from a review of psychiatry or hospital notes performed after completion of year 4 follow-up; and self-reported or relative-reported dementia. Dementia was recorded if two of these criteria were met or, in subjects with MMSE score <24 at baseline or follow-up, or missing at follow-up, if one of the first four criteria was met.
Statistical analysis
Preparation of data.
Because of skewed distributions, NT-proBNP and TMT-B were transformed to their natural logarithm values. When data for one, two, or three out of the seven cognitive tests were missing, multiple imputation accounting for age and sex was performed. In accordance with previous literature (24), mild cognitive impairment (MCI) was defined as scoring in the lowest fifth percentile of memory scores (LM), with MMSE ≥24 and failure to meet criteria for dementia.
Univariate analyses.
Exploratory analyses determined associations among baseline predictors, correlations between cognitive scores measured at baseline and at follow-up, and 4-year change in mean cognitive scores. Mean ABI, cIMT, and NT-proBNP and prevalence of macrovascular disease were compared in participants with dementia, in those who had conversion to MCI during follow-up, and in the remaining sample.
Multivariate analyses.
Multivariate regression analyses tested for associations between baseline macrovascular disease and follow-up cognitive scores, estimated lifetime, and 4-year cognitive change. To estimate lifetime cognitive change, follow-up cognitive scores were adjusted for baseline MHVS scores. Because vocabulary tests measure "crystallized" intelligence and show little age-related declines, they may be used to estimate peak previous cognitive ability (25). Details of this approach have been published previously (26); essentially, by controlling for a well-validated estimate of peak cognitive ability, it was possible to assess associations of macrovascular disease variables with estimated decline from best-ever level of cognitive function. To measure actual 4-year cognitive change, follow-up scores were adjusted for baseline scores on the respective cognitive test.
General ability factor g.
Principal components analysis was performed on cognitive test scores with extraction of components with Eigen values >1 to function as additional cognitive outcomes. A single component of general cognitive ability (usually referred to as g) was determined for follow-up (factor loading: Faces, 0.51; LM, 0.61; DSC, 0.62; BVFT, 0.62; MR, 0.69; Letter-Number Sequencing, 0.75; DSC and TMT-B, 0.81), which accounted for 47.4% of the total variance. Strictly speaking, principal components analysis does not extract "factors," but the usage is common and we adopted it here. It has been found that individuals who perform well on one cognitive test tend to perform well on another (27). Adjustment of follow-up g for baseline g to signify 4-year cognitive change requires that both are standardized in the same population. To achieve this, cognitive data were arranged in the statistical program so that each of seven columns included cognitive scores obtained at baseline and at follow-up, a single principal components analysis was performed, and saved regression scores of the first principal component were separated into two columns according to time point. The resulting baseline g and follow-up g, which therefore have identical factor loading (Faces, 0.43; LM, 0.55; BVFT, 0.65; MR, 0.67, Letter-Number Sequencing, 0.72; DSC, 0.75; TMT-B, 0.79), were then used for all analyses of 4-year change in g.
Covariates and correction for multiple comparisons.
Linear regression analyses were further adjusted for conventional cardiovascular risk factors (brachial blood pressure, serum total cholesterol, and history of cigarette smoking). Post hoc analyses controlling for baseline MHVS determined the independence of associations with 4-year cognitive change from premorbid ability. To counteract risk of type I error, Bonferroni corrections are commonly applied to the cut-point for statistical significance, but this increases the risk of accepting incorrect null hypotheses (type II error). To balance these risks, and because all three cognitive outcomes were derived from one variable, we applied a Bonferroni correction on the basis of nine individual analyses only (to reflect nine predictor variables; cut-point P < 0.006). Analyses were performed using SPSS for Windows version 19.0 (IBM).
RESULTS
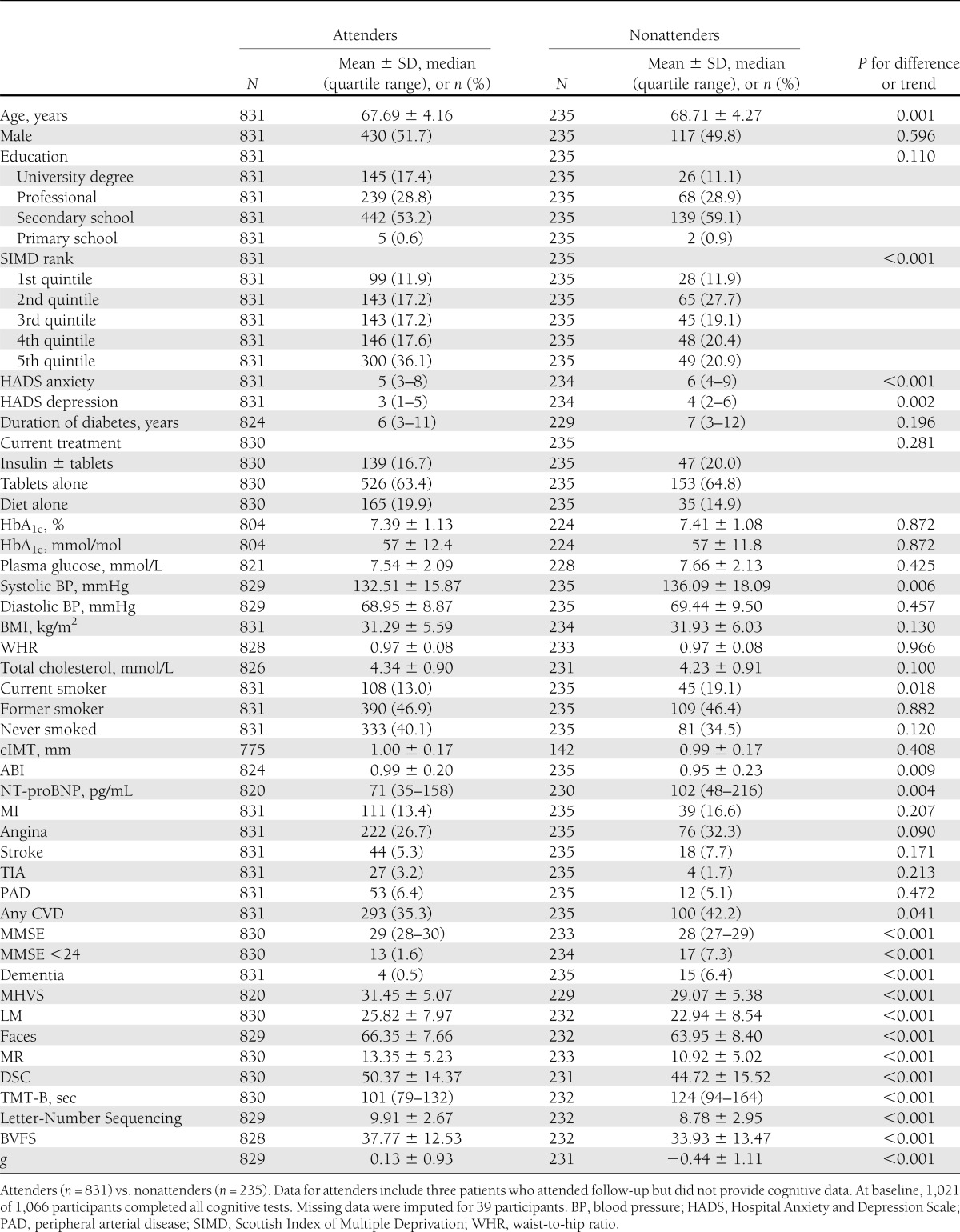
A comparison of baseline sociodemographic and physical characteristics revealed modest differences between participants who returned for year 4 follow-up ("attenders") and those who did not ("nonattenders") (Table 1). Attenders had significantly better cognitive ability at baseline and were less likely to have dementia by year 4 (n = 4, 0.5%) compared with nonattenders (n = 15, 6.4%). Further analyses were performed on data from attenders; in this group, mean age at baseline was 67.7 years and 293 participants (35.3%) had had at least one cardiovascular event (stroke, TIA, MI, angina, or intermittent claudication).
Table 1.
Baseline characteristics of ET2DS participants attending and not attending 4-year follow-up for cognitive testing
Many measures of macrovascular disease were associated with each other; participants with low ABI tended to have higher cIMT (r = −0.10; P = 0.004) and increased plasma NT-proBNP levels (r = −0.16; P < 0.001). NT-proBNP correlated positively with cIMT (r = 0.13; P < 0.001). Participants with history of any cardiovascular disease (CVD), stroke, or angina all had significantly higher mean NT-proBNP and cIMT, and lower mean ABI compared with the remaining population (all associations P < 0.001 for any CVD; P < 0.05 for stroke and angina). History of TIA was associated with lower mean ABI (P = 0.009). Mean NT-proBNP (P < 0.001) and cIMT (P = 0.012) were increased in participants with MI.
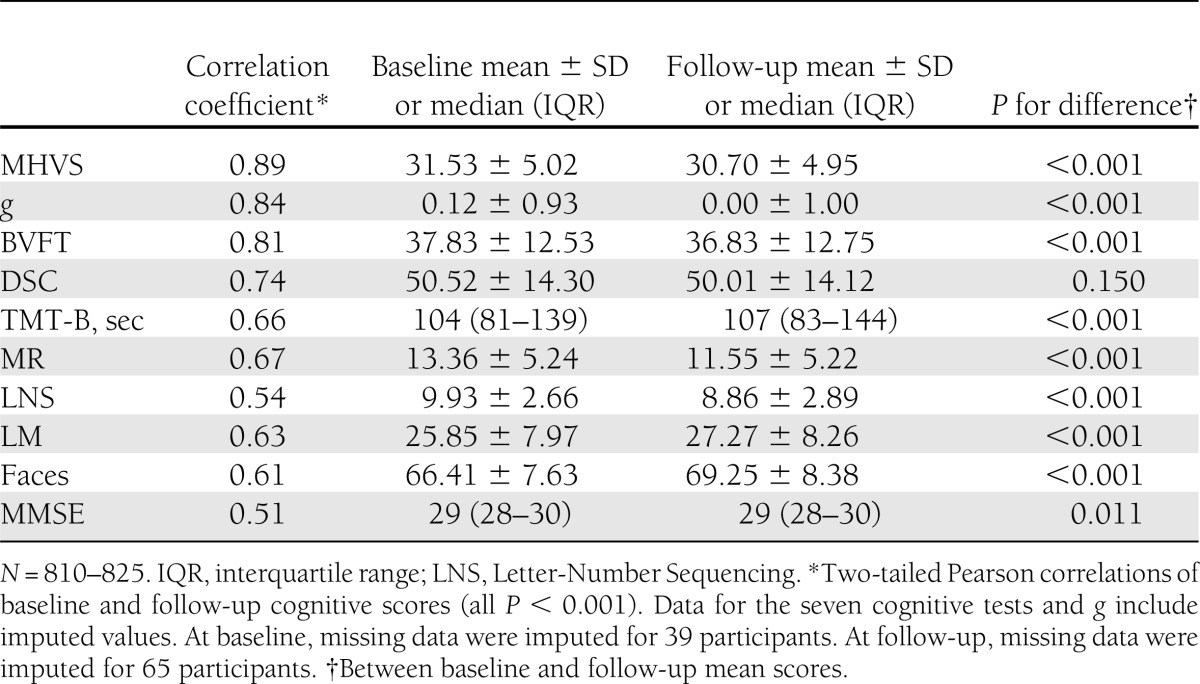
Of all subjects attending follow-up (n = 831), three were not cognitively tested because of refusal or physical disability. Performance on each cognitive test correlated significantly with performance on all other cognitive tests at baseline (r = 0.19–0.63; all P < 0.001) and at follow-up (r = 0.26–0.65; all P < 0.001). Individual cognitive test scores and g correlated strongly between baseline and follow-up (Table 2). Mean scores declined slightly but significantly (P < 0.001) for all tests except Faces and LM, in which scores improved (P < 0.001), and for DSC (P = 0.150) (Table 2). Effect sizes and significance levels were similar when analyses were repeated using original rather than imputed data (data not shown).
Table 2.
Baseline and follow-up cognitive test performance of attenders
At Bonferroni-corrected P = 0.006, follow-up g was found to be significantly lower in participants with a preexisting cardiovascular event at baseline (β = −0.18; P < 0.001); for individual event categories, findings were statistically significant for angina (β = −0.11; P = 0.002) and stroke (β = −0.16; P < 0.001). Lower follow-up g also appeared to be associated with measures of subclinical macrovascular disease, although only the association with higher NT-proBNP reached statistical significance at the Bonferroni-corrected level (β = −0.10; P = 0.005) (Table 3).
Table 3.
Macrovascular disease and generalized cognitive ability (g)
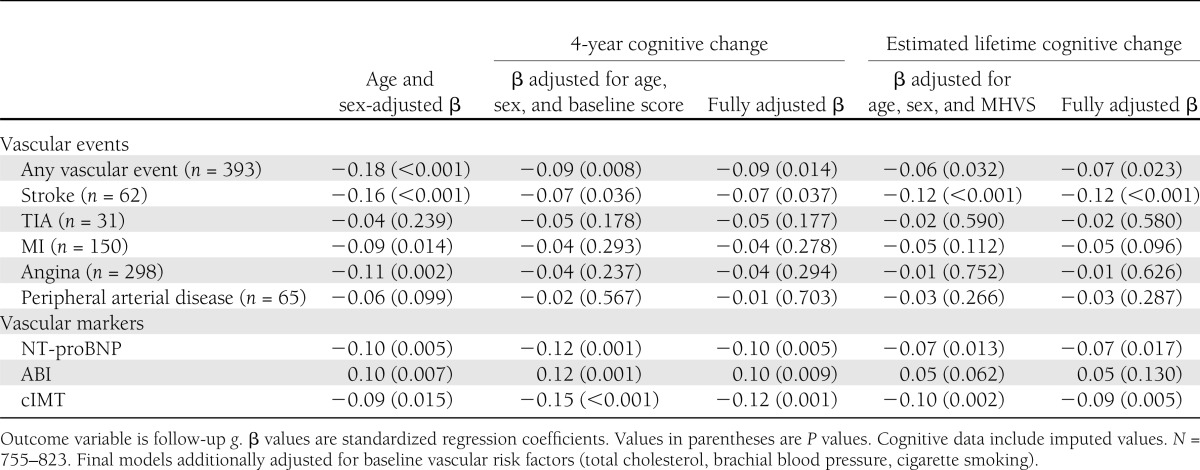
In multivariate analyses adjusting for baseline cognitive test scores or for MHVS (and for vascular risk factors), the only cardiovascular event category that remained significantly associated with lower g at follow-up was stroke (Table 3), suggesting that people with stroke experienced steeper cognitive decline compared with stroke-free individuals. For estimated lifetime cognitive change, the association was statistically significant at the Bonferroni-adjusted level of significance (β = −0.12; P < 0.001) and persisted after adjustment for cIMT (β = −0.10; P = 0.001). In terms of individual test scores, stroke appeared to affect predominantly processing speed (Supplementary Tables).
All markers of subclinical macrovascular disease were significantly associated with 4-year decline in g, although the association with ABI, which appeared to be driven by processing speed (TMT-B: β = −0.08; P = 0.001; Supplementary Tables), only lost statistical significance at the Bonferroni-adjusted level when vascular risk factors were controlled for (P = 0.009) (Table 3).
Further analyses of the individual cognitive tests revealed statistically significant associations of NT-proBNP with 4-year decline in verbal fluency at Bonferroni-corrected level (BVFT: β = −0.07; P = 0.001) (Supplementary Tables). Associations between increased cIMT and 4-year decline in processing speed (DSC: β = −0.08; P = 0.001) and reasoning (MR: β = −0.08; P = 0.004) appeared to be the main contributors to the association with decline in g. All associations of cIMT and NT-proBNP with g and individual cognitive tests (except between cIMT and MR; P = 0.007) remained statistically significant (P < 0.006) after adjustment for vascular risk factors (Table 3 and Supplementary Tables). In post hoc analyses, the association of cIMT with 4-year decline in g survived the additional adjustment for MHVS (β = −0.13; P = 0.001) as well as addition of stroke, ABI, and NT-proBNP into the model (β = −0.12; P = 0.002). The association of NT-proBNP with decline in g lost statistical significance when MHVS (P = 0.008) or when stroke, ABI, and cIMT were controlled for (P = 0.055).
A similar pattern of associations was found for the subclinical vascular markers and MHVS-adjusted cognitive test performance to signify estimated lifetime cognitive change, although overall the associations were weaker, such that only the association with cIMT reached the Bonferroni-adjusted level of statistical significance (β = −0.09; P = 0.005). The finding also became less significant when stroke was additionally controlled for (P = 0.011).
Only four subjects attending the year 4 follow-up had a diagnosis of dementia. Effect sizes and P values changed only marginally when analyses were repeated with exclusion of these cases (data not shown). Although there was a suggestion that the prevalence of vascular events was higher in all subjects with dementia, including TIA (P = 0.046) and MI (P = 0.027), this group of subjects (n = 19) was too small for further subgroup analysis. Twenty-three participants experienced conversion from normal functioning to MCI during follow-up. One (4.3%) had a history of TIA, three had stroke or peripheral arterial disease (13.0%, respectively), six (26.9%) had MI, 10 (43.5%) had angina, and 14 (60.9%) had any CVD. Age- and sex-adjusted analyses revealed increased prevalence of any CVD (P = 0.039) and significantly higher mean NT-proBNP levels (geometric mean: 131.1 pg/mL and 95% CI 81.1–211.7 vs. 74.07 pg/mL and 95% CI 68.4–80.3; P = 0.021) in the "conversion to MCI" group compared with the remaining population. None of the remaining macrovascular predictors was related to conversion to MCI (all P > 0.05).
CONCLUSIONS
In this cohort of initially cognitively healthy older people with type 2 diabetes, markers of subclinical macrovascular disease, including higher circulating levels of the natriuretic peptide NT-proBNP and increased cIMT as well as a history of stroke, were associated with cognitive decline.
Stroke is a well-established risk factor associated with cognitive dysfunction and impairment (28), and also may contribute to accelerated cognitive decline (29), although the relationship in exclusively diabetic populations has not been extensively investigated. In the ET2DS, stroke was associated with steeper cognitive decline between peak premorbid ability, which was estimated by vocabulary, and late-life ability. Contrary to previous investigations in older adults with type 2 diabetes (30,31), stroke also appeared to be associated with rate of actual late-life cognitive decline over the 4-year follow-up, although these associations were weaker and less statistically significant, suggesting that cognitive function deteriorates from prestroke to immediate poststroke levels, with preexisting stroke having relatively lower impact on subsequent cognitive decline.
The associations of cognitive decline with subclinical measures of macrovascular disease, including higher levels of NT-proBNP, increased thickness of the cIMT, and lower ankle brachial pressure (a further measure of systemic atherosclerotic disease as well as of lower limb peripheral arterial disease) (32), appeared slightly stronger for 4-year cognitive decline compared with associations of these vascular markers with estimated lifetime cognitive decline. This pattern of results is of interest because, in terms of identifying elderly subjects at risk for subsequent cognitive decline, information on a patient’s future decline may be more valuable compared with information that incorporates past decline. Higher natriuretic peptide levels as a marker of cardiac stress previously have been associated with low level of cognitive function (33) and risk of future dementia (34). However, to our knowledge, this was the first prospective investigation of NT-proBNP and cognitive decline in a cognitively healthy population with type 2 diabetes. In addition to associations with continuously measured decline in ability, higher baseline peptide levels also predicted increased risk of conversion from normal cognitive functioning to suspected MCI. Increased cIMT has been shown to be a marker of coexistent CVD, including cerebrovascular disease (35), and may indicate systemic atherosclerosis as well as more localized disease. Evidence for associations with cognitive decline is mixed (36,37), however, and although our finding of accelerated 4-year cognitive decline in individuals with higher cIMT is consistent with one previous study performed in a nondiabetic population (36), investigations of patients with type 2 diabetes are lacking. For cIMT, but not for NT-proBNP, associations in the current study were unrelated to potential effects of premorbid ability in late-life cognitive decline and risk of late-life subclinical vascular damage.
Contrary to previous investigations performed in predominantly nondiabetic populations (38,39), we found an association between low ABI and steeper late-life cognitive decline. Individuals exposed to lifestyle-associated vascular risk, such as cigarette smoking, hypertension, and hypercholesterolemia, may develop subclinical macrovascular disease, evident in lower ABI, and also may experience accelerated late-life cognitive decline. However, the association of ABI with cognitive decline was only partially dependent on conventional cardiovascular risk factors.
It is also possible that the ABI may function as a vascular risk marker in a slightly different manner in diabetic populations because of effects of stiffened arteries on the measurement of ankle pressures (12), and this could contribute to any disparity between findings for ABI and cognitive decline in diabetic compared with nondiabetic populations.
The assessment of links between vascular disease and cognition is notoriously difficult because of the interrelationships between different vascular measures and the problems associated with measuring or estimating change in cognitive abilities over appropriate time periods in relation to the development of vascular disease markers. This somewhat explains inconsistencies in the current literature on the role of large and small vessel vascular disease in the etiology of cognitive decline. Compared with some previous studies, the relatively large size, population-based approach, and prospective design are strengths of the current study. The use of an exclusively diabetic older population and the measurement of a large number of different but commonly used markers of subclinical and clinical macrovascular disease provides a unique contribution to the existing literature that has neglected investigations in diabetes patients despite increased prevalence of macrovascular disease and cognitive impairment (5,8,11). The general ability factor is a universal empirical finding, a robust measure of overall cognitive functioning, and also the locus of much of the age effects on cognition (1). A conservative Bonferroni correction was applied to the cut-point for statistical significance; results were discussed both in terms of the corrected and the traditional cut-point (P < 0.05) with the aim of offering an overall interpretation of findings with minimal risk of statistical error. Adjustment for a vocabulary-based cognitive test enabled estimation of long-term change in ability, although the MHVS only served as an estimate of peak premorbid ability and caution is warranted in the interpretation of this outcome. Analyses of actual longitudinal change in cognitive test scores were presumably subject to some error. Practice effects and selective attrition, which favor participants with slower rates of decline (40), lead to underestimation of actual cognitive decline in the population. Individuals with higher ability at baseline were more likely to attend follow-up and overall 4-year cognitive decline for attenders was small. Although we set out to recruit a cognitively healthy population, subsequent consideration of information from a wide variety of sources revealed that a very small number of subjects were likely to have been experiencing dementia at baseline. However, exclusion of dementia cases from the analyses only marginally changed the results reported here.
Our results provide additional evidence that the impact of vascular disease on cognition may not be restricted to localized cerebral small vessel disease or altered blood flow and ischemic damage as a consequence of stroke, and that cognitive decline could reflect systemic atherosclerotic changes. NT-proBNP and cIMT both appear to function as biomarkers of risk of late-life cognitive decline, despite their strong associations with subclinical macrovascular disease in different areas of the vasculature (left ventricle and carotid artery, respectively) and may offer valuable information beyond traditional vascular risk factors. Given that stroke was only weakly associated with 4-year cognitive decline, and that none of the remaining measures of symptomatic macrovascular disease were related to this outcome, determination of subclinical macrovascular disease using continuous measures may be preferable over symptomatic categorically assessed macrovascular disease for the early identification of individuals at risk for late-life cognitive decline. However, it must be recognized that the categorical nature of "events" data compared with the continuous distribution of the subclinical measures is likely to have influenced the relative levels of statistical significance for these variables. Ascertainment of subclinical macrovascular disease may aid the identification of not only individuals at risk for future symptomatic macrovascular disease (and its cognitive consequences) but also those at risk for late-life cognitive decline in absence of symptomatic disease. With the proximity of the carotid artery to the brain, cIMT could be hypothesized to represent the most accurate noninvasive marker of asymptomatic cerebral vascular damage. Certainly, its potential as a vascular risk marker seems to exceed that of simply a risk factor for stroke.
In conclusion, we have found confirmatory evidence of a link between stroke and cognitive decline in people with type 2 diabetes but, more importantly, we have identified associations between a number of different measures of subclinical macrovascular disease and late-life cognitive change. Further studies are warranted to determine whether these markers could be useful clinical measures as risk predictors for cognitive impairment and subsequent targeting of interventions aimed at reducing cognitive decline.
Acknowledgments
The sponsor for the ET2DS was the University of Edinburgh. The study was funded by the Medical Research Council (U.K.), the Chief Scientist Office of the Scottish Executive, and Pfizer. The funders had no role in the design, analysis, or writing of the manuscript.
J.F.P. and I.J.D. are members of The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross-council Lifelong Health and Wellbeing Initiative (which has funding from the Biotechnology and Biological Sciences Research Council [BBSRC], Engineering and Physical Sciences Research Council [EPSRC], Economic and Social Research Council [ESRC], and the Medical Research Council [MRC]; G0700704/84698). No other potential conflicts of interest relevant to this article were reported.
I.F. performed the statistical analysis and drafted the manuscript. M.K., C.M.R., J.R.M., R.M.W., L.D.N., S.M., N.S., P.W., R.M.R., T.C.R., M.W.J.S., and J.F.P. were involved in the interpretation of findings and preparation of the final manuscript, including commenting on the final draft. I.J.D. supervised statistical analysis and was involved in the interpretation of findings and preparation of the final manuscript, including commenting on the final draft. M.W.J.S. conceived and designed the ET2DS, oversaw the acquisition and analysis of data, and was involved in the interpretation of findings and preparation of the final manuscript, including commenting on the final draft. J.F.P. supervised statistical analysis, conceived and designed the ET2DS, and oversaw the acquisition and analysis of data. J.F.P. and I.F. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank all patients and research staff involved in the ET2DS.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2241/-/DC1.
References
- 1.Salthouse TA. When does age-related cognitive decline begin? Neurobiol Aging 2009;30:507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gállego J, Martínez-Vila E. Asymptomatic cerebrovascular disease and systemic diagnosis in stroke, atherothrombosis as a disease of the vascular tree. Cerebrovasc Dis 2005;20(Suppl. 2):1–10 [DOI] [PubMed] [Google Scholar]
- 3.Rafnsson SB, Deary IJ, Smith FB, Whiteman MC, Fowkes FGR. Cardiovascular diseases and decline in cognitive function in an elderly community population: the Edinburgh Artery Study. Psychosom Med 2007;69:425–434 [DOI] [PubMed] [Google Scholar]
- 4.Singh-Manoux A, Britton AR, Marmot M. Vascular disease and cognitive function: evidence from the Whitehall II Study. J Am Geriatr Soc 2003;51:1445–1450 [DOI] [PubMed] [Google Scholar]
- 5.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes—systematic overview of prospective observational studies. Diabetologia 2005;48:2460–2469 [DOI] [PubMed] [Google Scholar]
- 6.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64–74 [DOI] [PubMed] [Google Scholar]
- 7.Golden SH. Emerging therapeutic approaches for the management of diabetes mellitus and macrovascular complications. Am J Cardiol 2011;108(Suppl.):59B–67B [DOI] [PubMed] [Google Scholar]
- 8.Mankovsky BN, Ziegler D. Stroke in patients with diabetes mellitus. Diabetes Metab Res Rev 2004;20:268–287 [DOI] [PubMed] [Google Scholar]
- 9.Rydén L, Standl E, Bartnik M, et al. Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) European Association for the Study of Diabetes (EASD) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. Eur Heart J 2007;28:88–136 [DOI] [PubMed] [Google Scholar]
- 10.Magnusson M, Melander O, Israelsson B, Grubb A, Groop L, Jovinge S. Elevated plasma levels of Nt-proBNP in patients with type 2 diabetes without overt cardiovascular disease. Diabetes Care 2004;27:1929–1935 [DOI] [PubMed] [Google Scholar]
- 11.Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med 2006;23:609–616 [DOI] [PubMed] [Google Scholar]
- 12.Potier L, Abi Khalil C, Mohammedi K, Roussel R. Use and utility of ankle brachial index in patients with diabetes. Eur J Vasc Endovasc Surg 2011;41:110–116 [DOI] [PubMed] [Google Scholar]
- 13.Harron KL, Feltbower RG, McKinney PA, Bodansky HJ, Campbell FM, Parslow RC. Rising rates of all types of diabetes in south Asian and non-south Asian children and young people aged 0-29 years in West Yorkshire, U.K., 1991-2006. Diabetes Care 2011;34:652–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregg EW, Cheng YJ, Saydah S, et al. Trends in death rates among U.S. adults with and without diabetes between 1997 and 2006: findings from the National Health Interview Survey. Diabetes Care 2012;35:1252–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price JF, Reynolds RM, Mitchell RJ, et al. The Edinburgh Type 2 Diabetes Study: study protocol. BMC Endocr Disord 2008;8:18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marioni RE, Strachan MWJ, Reynolds RM, et al. Association between raised inflammatory markers and cognitive decline in elderly people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes 2010;59:710–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol 1992;45:1101–1109 [DOI] [PubMed] [Google Scholar]
- 18.Ding J, Strachan MW, Reynolds RM, et al. Edinburgh Type 2 Diabetes Study Investigators Diabetic retinopathy and cognitive decline in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes 2010;59:2883–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Carroll RE, Gilleard CJ. Estimation of premorbid intelligence in dementia. Br J Clin Psychol 1986;25:157–158 [DOI] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–370 [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 22.Lancu I, Olmer A. The minimental state examination—an up-to-date review. Harefuah 2006;145:687–690, 701 [in Hebrew] [PubMed] [Google Scholar]
- 23.O’Connor DW, Pollitt PA, Hyde JB, et al. The reliability and validity of the Mini-Mental State in a British community survey. J Psychiatr Res 1989;23:87–96 [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC, Thomas RG, Grundman M, et al. Alzheimer’s Disease Cooperative Study Group Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005;352:2379–2388 [DOI] [PubMed] [Google Scholar]
- 25.Deary IJ, Whalley LJ, Crawford JR. An ‘instantaneous' estimate of a lifetime's cognitive change [article online], 2004. Available from http://www.sciencedirect.com/science/article/pii/S0160289603000679 Accessed 18 March 2013 [Google Scholar]
- 26.Rafnsson SB, Deary IJ, Smith FB, et al. Cognitive decline and markers of inflammation and hemostasis: the Edinburgh Artery Study. J Am Geriatr Soc 2007;55:700–707 [DOI] [PubMed] [Google Scholar]
- 27.Spearman C. ‘General intelligence', objectively determined and measured. Am J Psychol 1904;15:201–292 [Google Scholar]
- 28.Pendlebury ST, Rothwell PM. Risk of recurrent stroke, other vascular events and dementia after transient ischaemic attack and stroke. Cerebrovasc Dis 2009;27(Suppl. 3):1–11 [DOI] [PubMed] [Google Scholar]
- 29.Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA 1999;282:40–46 [DOI] [PubMed] [Google Scholar]
- 30.Reijmer YD, van den Berg E, de Bresser J, et al. Utrecht Diabetic Encephalopathy Study Group Accelerated cognitive decline in patients with type 2 diabetes: MRI correlates and risk factors. Diabetes Metab Res Rev 2011;27:195–202 [DOI] [PubMed] [Google Scholar]
- 31.Bruce DG, Davis WA, Casey GP, et al. Predictors of cognitive decline in older individuals with diabetes. Diabetes Care 2008;31:2103–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu D, Zou L, Xing Y, et al. Diagnostic value of ankle-brachial index in peripheral arterial disease: a meta-analysis. Can J Cardiol 2013;29:492–498 [DOI] [PubMed] [Google Scholar]
- 33.Gunstad J, Poppas A, Smeal S, et al. Relation of brain natriuretic peptide levels to cognitive dysfunction in adults > 55 years of age with cardiovascular disease. Am J Cardiol 2006;98:538–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerola T, Nieminen T, Hartikainen S, Sulkava R, Vuolteenaho O, Kettunen R. B-type natriuretic peptide as a predictor of declining cognitive function and dementia—a cohort study of an elderly general population with a 5-year follow-up. Ann Med 2010;42:207–215 [DOI] [PubMed] [Google Scholar]
- 35.Saleh C. Carotid artery intima media thickness: a predictor of cognitive impairment? Front Biosci (Elite Ed) 2010;2:980–990 [DOI] [PubMed] [Google Scholar]
- 36.Wendell CR, Zonderman AB, Metter EJ, Najjar SS, Waldstein SR. Carotid intimal medial thickness predicts cognitive decline among adults without clinical vascular disease. Stroke 2009;40:3180–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knopman D, Boland LL, Mosley TH, et al. Atherosclerosis Risk in Communities (ARIC) Study Investigators Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001;56:42–48 [DOI] [PubMed] [Google Scholar]
- 38.Johnson W, Price JF, Rafnsson SB, Deary IJ, Fowkes FGR. Ankle—brachial index predicts level of, but not change in, cognitive function: the Edinburgh Artery Study at the 15-year follow-up. Vasc Med 2010;15:91–97 [DOI] [PubMed] [Google Scholar]
- 39.Price JF, McDowell S, Whiteman MC, Deary IJ, Stewart MC, Fowkes FGR. Ankle brachial index as a predictor of cognitive impairment in the general population: ten-year follow-up of the Edinburgh Artery Study. J Am Geriatr Soc 2006;54:763–769 [DOI] [PubMed] [Google Scholar]
- 40.Stern Y. Cognitive reserve. Neuropsychologia 2009;47:2015–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]