Abstract
OBJECTIVE
To examine the efficacy and safety of adding the once-daily glucagon-like peptide-1 receptor agonist (GLP-1RA) lixisenatide to established basal insulin therapy alone or together with metformin, in people with type 2 diabetes and elevated glycated hemoglobin (HbA1c).
RESEARCH DESIGN AND METHODS
We conducted a double-blind, parallel-group, placebo-controlled trial. Patients (n = 495) with established basal insulin therapy but inadequate glycemic control were randomized to add lixisenatide 20 μg or placebo for 24 weeks. Basal insulin dosage was unchanged except to limit hypoglycemia. HbA1c reduction from baseline was the primary end point.
RESULTS
Mean duration of diabetes was 12.5 years, duration of insulin use was 3.1 years, insulin dosage was 55 units/day, and baseline HbA1c was 8.4%. With lixisenatide, the placebo-corrected change of HbA1c from baseline was –0.4% (95% CI –0.6 to –0.2; P = 0.0002), and mean HbA1c at end point was 7.8%. HbA1c <7.0% (53 mmol/mol) was attained by more lixisenatide (28%) than placebo (12%; P < 0.0001) participants. Lixisenatide reduced plasma glucose levels after a standardized breakfast (placebo-corrected reduction, –3.8 mmol/L; P < 0.0001); seven-point glucose profiles showed a reduction persisting through the day. Reductions in body weight (placebo corrected, –1.3 kg; P < 0.0001) and insulin dosage (–3.7 units/day; P = 0.012) were greater with lixisenatide. Main adverse events (AEs) with lixisenatide were gastrointestinal. Symptomatic hypoglycemia was 28% for lixisenatide and 22% for placebo; 4 of 328 subjects (1.2%) had severe hypoglycemia with lixisenatide vs. 0 of 167 with placebo.
CONCLUSIONS
By improving HbA1c and postprandial hyperglycemia without weight gain in type 2 diabetes with inadequate glycemic control despite stable basal insulin, lixisenatide may provide an alternative to rapid-acting insulin or other treatment options.
In type 2 diabetes, additional therapies are needed over time to maintain acceptable glycemic control (1–3). When lifestyle measures and oral antihyperglycemic agents are no longer sufficient, the addition of basal insulin optimized by systematic titration of dosage can restore glycated hemoglobin (HbA1c) to 7.0% for 50–60% of people with type 2 diabetes (2,4,5). However, some people do not initially achieve this glycemic target with basal insulin plus oral therapy, and others experience later deterioration of control (6–9). Further therapy, especially for postprandial hyperglycemia, is then needed. A traditional option has been to add one or more injections of prandial insulin (10), but adding a glucagon-like peptide-1 receptor agonist (GLP-1RA) is a recently proposed alternative that may improve glycemic control without additional weight gain and, perhaps, with less hypoglycemia. Drugs of this class have effects that complement those of basal insulin; they potentiate endogenous insulin responses to hyperglycemia, suppress inappropriately elevated glucagon secretion, and favor weight loss by promoting satiety (11,12). In addition, GLP-1RAs can slow gastric emptying, further blunting postprandial hyperglycemia. However, slowing of gastric emptying appears to be greater with short-acting than with long-acting GLP-1RAs (13), possibly related to the observation that, with time, continuous exposure of GLP-1 leads to a reduction in its effect on gastric emptying (14).
Lixisenatide is a novel GLP-1RA that, like other drugs of its class, has demonstrated significant improvements in glycemic control, low rates of hypoglycemia, and a beneficial effect on weight (15–17). Lixisenatide taken once daily (15) improves HbA1c levels by reducing fasting plasma glucose (FPG) and has robust postprandial glucose (PPG) effects (18,19). Lixisenatide was granted marketing authorization by the European Medicines Agency in February 2013 (20). The objective of this study was to examine the efficacy and safety of adding once-daily lixisenatide to established basal insulin therapy (dosage maintained except for the avoidance of hypoglycemia), alone or together with metformin, in people with long-duration type 2 diabetes and inadequate glycemic control.
RESEARCH DESIGN AND METHODS
Study design
This was a randomized, placebo-controlled, two-arm, parallel-group, double-blind phase III study with a 24-week treatment period. It was conducted in 111 centers in 15 countries (Brazil, Canada, Chile, Egypt, France, Germany, India, Italy, Republic of Korea, Mexico, Puerto Rico, Russian Federation, Turkey, U.K., and U.S.) from July 2008 to February 2011 (clinicaltrials.gov, NCT00715624). It was approved by the institutional review boards or ethics committees of the participating centers and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All participants gave written informed consent. An independent Data Monitoring Committee provided an ongoing review of unmasked efficacy and safety data, and an Allergic Reaction Assessment Committee (ARAC) and a Cardiovascular Event Adjudication Committee reviewed masked events.
Participants
Adults with type 2 diabetes diagnosed ≥1 year at the time of screening were eligible if they had used a basal insulin regimen for ≥3 months with a stable dose (±20%) ≥30 units/day for ≥2 months before screening and had HbA1c = 7–10%. Candidates using metformin must have taken a stable dose of at least 1.5 g/day (South Korea, at least 1.0 g/day) for at least 3 months before screening. Exclusion criteria included FPG >13.9 mmol/L (250 mg/dL); BMI ≤20.0 kg/m2; weight change >5.0 kg over the 3 months before screening; history of unexplained pancreatitis, end-stage renal disease, or allergic reaction to any GLP-1RA in the past; or pregnancy.
Randomization, masking, and dosing
Eligible participants were centrally randomized and entered a 24-week, double-blind treatment period (Supplementary Fig. 1). Lixisenatide or placebo was packaged into treatment kits and labeled with a number. Treatment numbers were allocated on day 1, using an interactive voice-response system, after completion of the baseline assessment. Investigators did not have access to the randomization code. In addition, reviews by the ARAC members were all blinded. Lixisenatide and placebo treatments were indistinguishable, but the injected volume differed according to the titration step (or maintenance period) and the injected volume was unblinded. Randomization was stratified by HbA1c (<8.0 and ≥8.0% [<64 and ≥64 mmol/mol]) and metformin use at screening (yes or no). Randomization was in a 2:1 ratio to once-daily lixisenatide or placebo in a two-step dose-increase regimen (10 μg for 1 week, 15 μg for 1 week, and then 20 μg if tolerated). Lixisenatide or placebo was given subcutaneously within 1 h before the morning meal. If used at enrollment, metformin was continued at a stable dose throughout the study. In general, basal insulin dosage was to remain relatively stable (±20%) throughout the study. However, if HbA1c was ≤7.5% (≤58 mmol/mol) at screening, the daily dosage of basal insulin was initially reduced by 20% at randomization to limit the risk of hypoglycemia and thereafter progressively increased between weeks 4 and 12 to the dosage used at the screening visit, unless prevented by the occurrence of hypoglycemia. After week 12, no further dose adjustments of basal insulin were to be made except for reductions in response to hypoglycemia. Rescue therapy, preferably with rapid-acting insulin, was permitted if FPG was >15.0 mmol/L (270 mg/dL) any time between randomization and week 8, FPG was >13.3 mmol/L (240 mg/dL) from week 8 through 12, and FPG was >11.1 mmol/L (200 mg/dL) or HbA1c >8.5% from week 12 through 24.
Efficacy and safety measurements
The primary end point was the change in HbA1c from randomization to week 24. Secondary measures included the percentage of patients attaining HbA1c <7.0 or ≤6.5% (<53 or ≤48 mmol/mol); change from baseline in FPG, body weight, seven-point self-measurement of plasma-referenced glucose (SMPG), and 2-h PPG after a standardized meal and glucose excursion (2-h PPG levels minus plasma glucose levels 30 min prior to the standardized meal before study drug administration); daily basal insulin dosage; and percentage of participants requiring rescue therapy.
Samples for FPG and HbA1c were measured at a certified (National Glycohemoglobin Standardization Program Level 1; Covance) central laboratory by high-performance liquid chromatography. Participants performed SMPG at least once a day, and, in addition, seven times over a 24-h period (before and 2 h after each meal and at bedtime) before randomization, and during the week before each subsequent visit. Additional tests were performed for symptoms consistent with hypoglycemia. Insulin dose, SMPG, and symptoms of hypoglycemia were recorded in diaries for review by study personnel. All participants ingested a standardized liquid breakfast meal (Ensure Plus drink; Abbott; 600 kcal with 54% carbohydrate, 17% protein, and 29% fat, consumed within a 10-min period, 30 min after study drug administration) before randomized treatment and at week 24 or last visit. Body weight was recorded at screening, randomization, and thereafter at weeks 4, 6, 8, 12, and 24 (or last visit); clinical histories were recorded at randomization and at each subsequent visit.
Safety was assessed by review of adverse events (AEs), symptomatic hypoglycemia, and clinical laboratory data. Laboratory tests were performed for hematology, creatinine, microalbuminuria, pregnancy (in females of childbearing potential), and serum chemistry (including amylase, lipase, lipoproteins, and calcitonin). The status and concentration of antilixisenatide antibodies were determined using highly sensitive surface plasmon resonance technology (Biacore, Uppsala, Sweden) at baseline and at weeks 2, 4, and 24.
Statistical analysis
All efficacy end points were assessed for participants who received one or more doses of the allocated treatment and had a measurement at baseline (randomization) and at least one on-treatment measurement of any primary and secondary efficacy end point (modified intent-to-treat population). Data obtained after rescue therapy were excluded from the efficacy analyses. The safety population consisted of all randomized individuals who received at least one dose of the investigational product. The primary efficacy end point was analyzed using ANCOVA with treatment groups (lixisenatide and placebo), randomization strata and country as fixed effects, and baseline HbA1c as a covariate. Superiority of lixisenatide compared with placebo was assessed based on the predefined primary analysis of the least squares (LS) mean changes from baseline to week 24 in HbA1c. The sample size/power calculation based on this end point estimated that enrollment of 300 and 150 participants in the lixisenatide and placebo arms, respectively, would provide 86% power of detecting a 0.4% difference between treatments in change of HbA1c from baseline, with a two-sided test at the 5% significance level and a common SD of 1.3%. The 2:1 randomization ratio was chosen to provide sufficient participant exposure to lixisenatide for safety evaluation in the context of the whole phase 3 development program, as is required for regulatory review. For descriptive purposes, unadjusted mean values at distinct time points were calculated for the as-observed population, including all values available, even for individuals lacking data at baseline or week 24. Data for all continuous secondary efficacy end points were analyzed by a similar ANCOVA model, with the corresponding baseline value as a covariate. All categorical efficacy parameters were analyzed using a Cochran-Mantel-Haenszel method with adjustment for the stratification variables. In case of missing week-24 measurements, LS mean change was calculated with last observation carried forward (LOCF). A sample size of at least 450 participants (300 for lixisenatide and 150 for placebo) provided a power of 96% (or 86%) to detect a treatment difference of 0.5% (or 0.4%) in the absolute change in HbA1c from baseline to week 24. This assumed a common SD of 1.3% with a two-sided 5% significance level. Summaries and statistical analyses were generated using SAS version 9.1.3 (SAS, Carey, NC) or higher.
RESULTS
Participant flow and characteristics
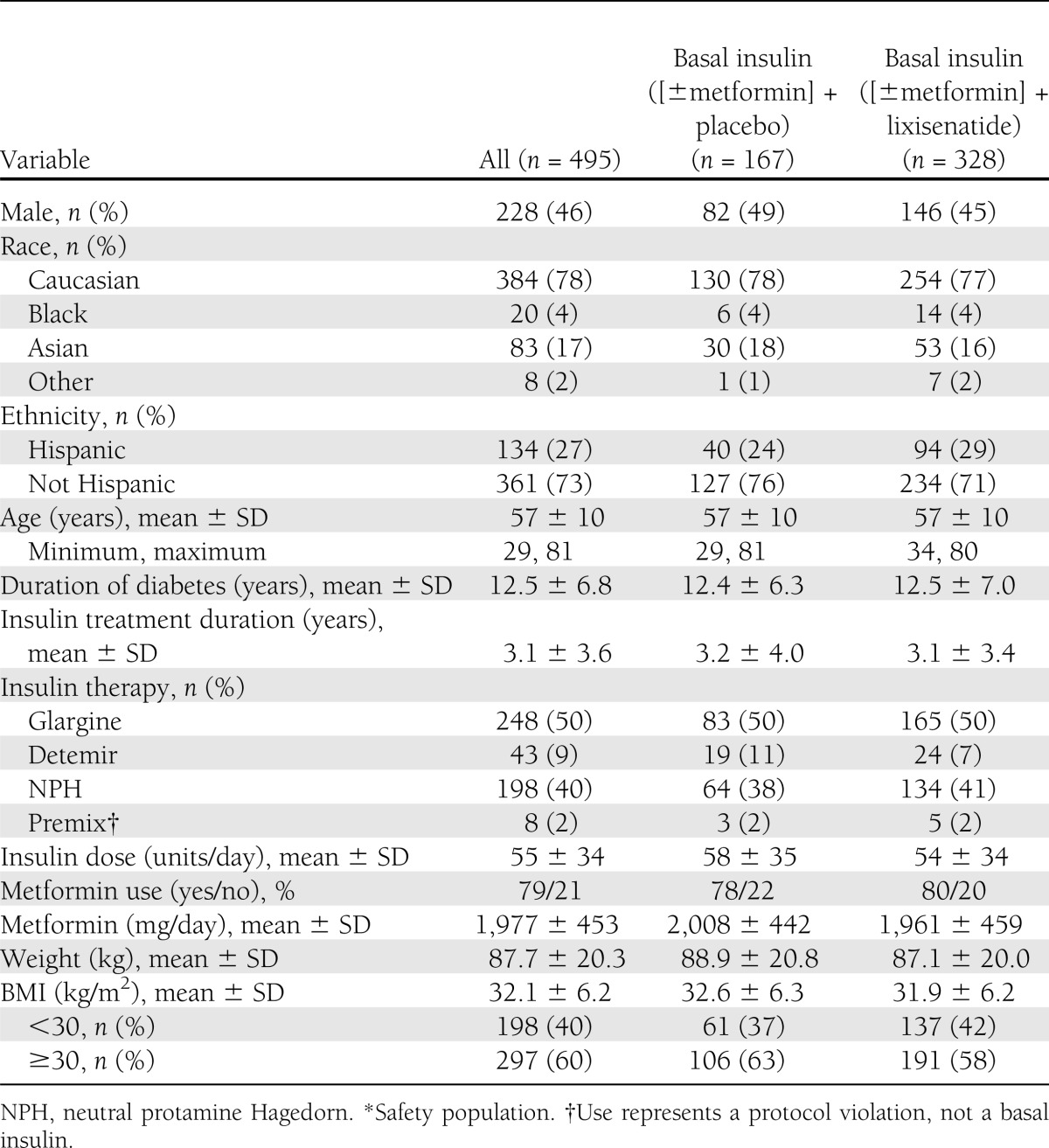
Of 879 candidates screened, 496 eligible participants were randomized and 495 received treatment (Supplementary Fig. 2). Baseline demographics and characteristics of participants were balanced between the two groups, although no formal statistical comparison between groups was conducted (Table 1). Overall, mean duration of diabetes was 12.5 years, mean duration of basal insulin use was 3.1 years, mean insulin dosage was 55 units daily, and mean HbA1c at baseline was 8.4% (Table 1). Basal insulin at entry was glargine (50%) or human neutral protamine Hagedorn (NPH; 40%) in most cases. Most participants (79%) were taking metformin.
Table 1.
Demographics and patient characteristics*
Responses to therapy
HbA1c.
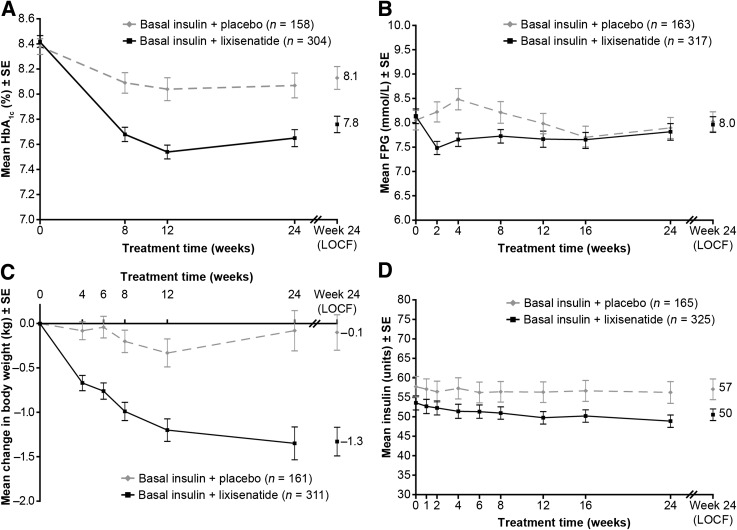
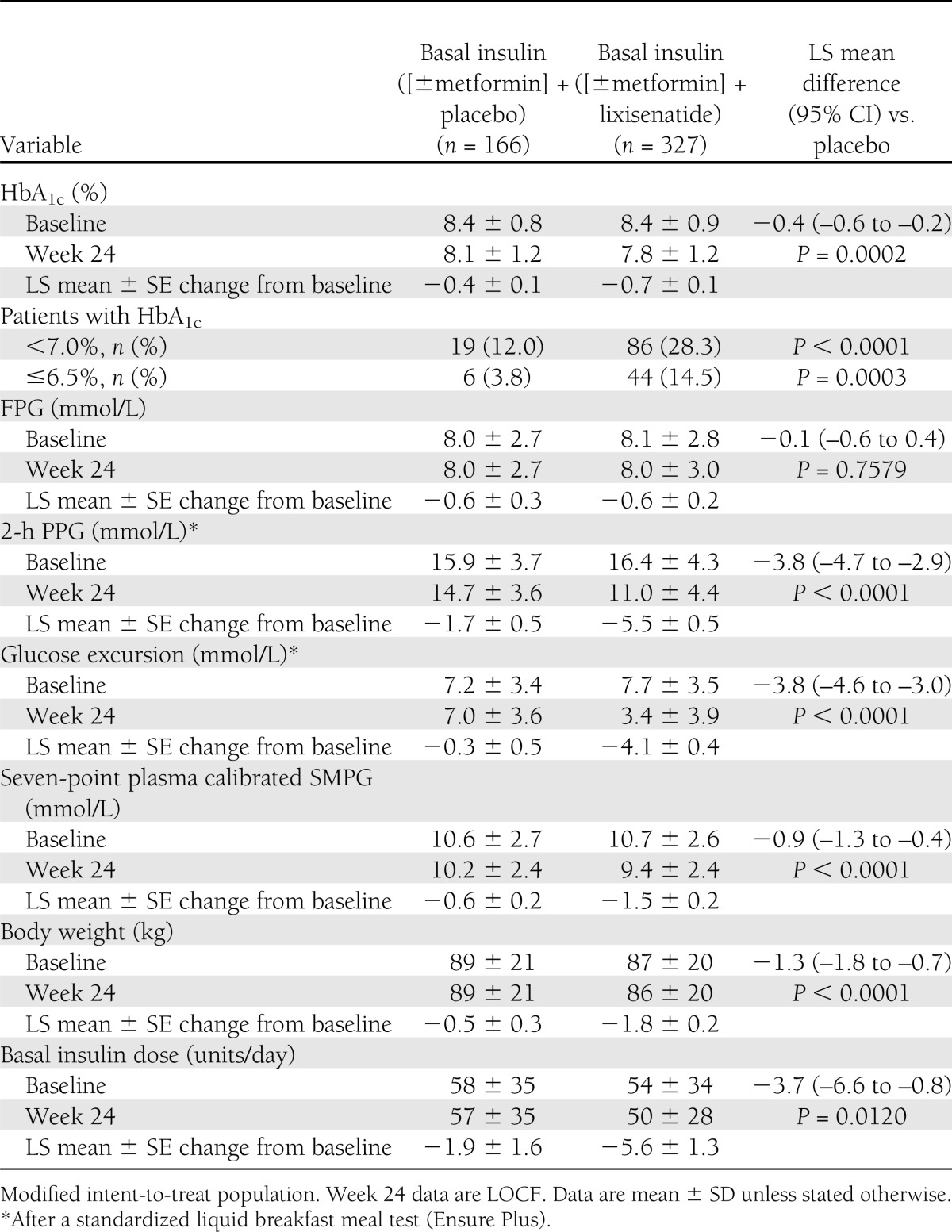
Mean HbA1c (±SD) declined from 8.4 ± 0.9% at randomization to 7.8 ± 1.2% at 24 weeks in the lixisenatide group and from 8.4 ± 0.8 to 8.1 ± 1.2% in the placebo group (LS mean change difference –0.4% [95% CI –0.6 to –0.2]; P = 0.0002) (Fig. 1A and Table 2). A higher percentage of participants treated with lixisenatide versus placebo had HbA1c <7% at week 24 (28.3 vs. 12.0%; P < 0.0001). Similarly, the percentage with HbA1c ≤6.5% at 24 weeks was higher with lixisenatide versus placebo (14.5 vs. 3.8%; P = 0.0003) (Table 2).
Figure 1.
Clinical responses to therapy from baseline to week 24 and end point with LOCF. A: Mean HbA1c (%) by visit. B: Mean FPG (mmol/L) by visit. C: Mean change in body weight (kg) from baseline by visit. D: Mean daily basal insulin dose (units/day) by visit. Values are all mean ± SE, for the modified intent-to-treat population. All analyses excluded measurements after the introduction of rescue medication and/or after treatment cessation plus 3 days. For week 24 (LOCF), the analysis included measurements obtained up to 3 days after the last dose of study drug.
Table 2.
Clinical responses to therapy
FPG.
Mean FPG decreased slightly more initially with lixisenatide than placebo, but by week 24, there was no difference between treatments (Fig. 1B and Table 2).
Body weight.
Body weight decreased by 1.8 kg with lixisenatide and 0.5 kg with placebo between randomization and week 24 (LS mean change −1.3 kg; P < 0.0001) (Fig. 1C and Table 2).
Insulin dose.
Daily basal insulin dosage was similar in the treatment groups at randomization. Dosage change by week 24 was –5.6 units/day with lixisenatide versus –1.9 units/day with placebo (LS mean change –3.7 units/day; P = 0.012) (Fig. 1D and Table 2).
SMPG profiles.
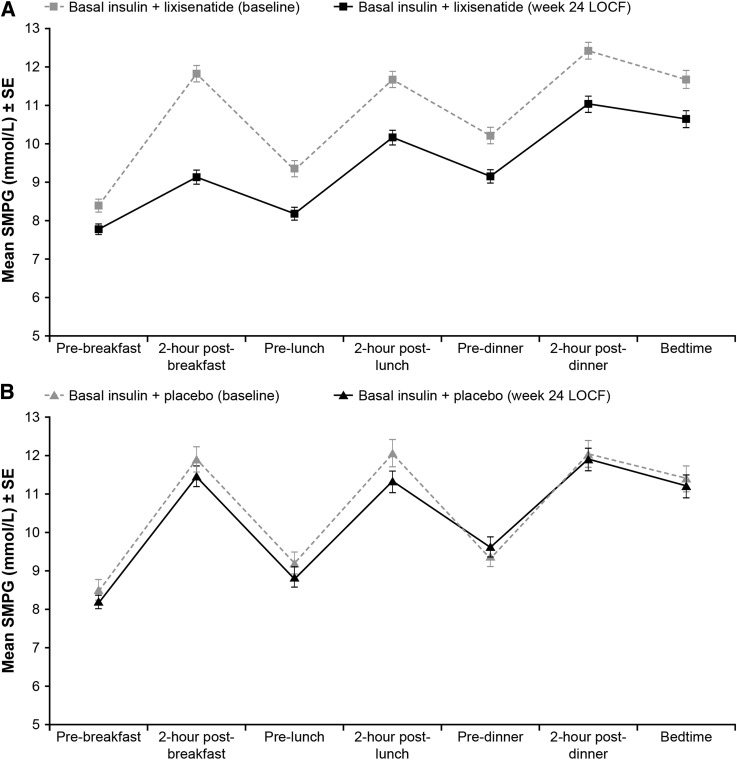
Mean seven-point values were 10.7 and 10.6 mmol/L at randomization and 9.4 and 10.2 mmol/L on treatment with lixisenatide and placebo, respectively (LS mean difference −0.9 mmol/L; P < 0.0001) (Table 2). Mean seven-point SMPG was lower throughout the day during treatment with lixisenatide compared with placebo (Fig. 2). The greatest LS mean difference was seen at 2 h postbreakfast (–2.4 mmol/L [–43 mg/dL]) and the smallest prebreakfast (–0.3 mmol/L [–5 mg/dL]).
Figure 2.
Mean seven-point SMPG (mmol/L). Mean ± SE seven-point SMPG (mmol/L) at baseline and week-24 LOCF in the modified intent-to-treat population for basal insulin + lixisenatide (A) and basal insulin + placebo (B).
Standardized meal study.
Mean 2-h PPG declined from 16.4 mmol/L at randomization to 11.0 mmol/L at week 24 in the lixisenatide group and from 15.9 to 14.7 mmol/L in the placebo group (LS mean difference −3.8 mmol/L; P < 0.0001) (Table 2 and Supplementary Fig. 3A). The PPG excursion was similarly reduced for lixisenatide versus placebo (LS mean difference −3.8; P < 0.0001) (Table 2 and Supplementary Fig. 3B).
Rescue therapy.
Rescue therapy with rapid-acting insulin or increase of basal insulin of >20% was required by 19 (6%) participants assigned to lixisenatide and 12 (7%) participants receiving placebo (P = 0.540).
Hypoglycemia
Symptomatic documented hypoglycemia of <3.3 mmol/L (60 mg/dL) was reported by 26.5% of participants using lixisenatide versus 21.0% with placebo (P = 0.174) (Supplementary Table 1). Most events were diurnal and occurred during the first weeks of treatment. Four individuals in the lixisenatide group each experienced a single severe hypoglycemic event; a missed or delayed meal was reported for two of these participants.
Other AEs
A total of 73.5% of participants experienced at least one treatment-emergent AE with lixisenatide versus 68.3% with placebo (Supplementary Table 1). AEs led to discontinuation for 7.6% in the lixisenatide group and 4.8% from the placebo group. The most common symptoms reported in lixisenatide-treated patients were gastrointestinal, mainly nausea or vomiting. Most reports of nausea occurred in the first 2 months of treatment in both groups. Injection site reactions were reported by the investigator for four participants (1.2%) in the lixisenatide group and one (0.6%) in the placebo group; none of these were considered severe by the investigator, and no participant discontinued as a result. The frequency of events adjudicated as allergic reactions by the ARAC was 1.5% in the lixisenatide group and 1.8% in the placebo group. Three events were considered by the ARAC to be possibly related to the treatment: two events in the lixisenatide group, reported by investigators as “allergic reaction,” which rapidly improved after treatment with oral diphenhydramine (both events were adjudicated as low grade [1°] anaphylaxis), and one in the placebo group (severe tongue edema, treated with antihistamines and steroids, adjudicated by the ARAC as angioedema grade 4). Approximately 70% of participants were antilixisenatide antibody positive at week 24; of the antibody-positive participants, 70% had an antibody concentration below the limit of quantification. At week 24, there were no substantial differences in the reduction from baseline in HbA1c levels or on the treatment-emergent AE profile between the antibody-positive and antibody-negative populations. No significant changes in heart rate or blood pressure occurred in either group, and no confirmed cases of acute pancreatitis were reported. No participants in the lixisenatide group had a calcitonin value ≥50 ng/L. One participant in the lixisenatide group died suddenly at home on day 25. This event was assessed at autopsy as sudden cardiac death and deemed not to be treatment related by the investigator.
CONCLUSIONS
This study demonstrated statistically and clinically significant improvements of glycemic control after adding once-daily lixisenatide to prior treatment with basal insulin with or without metformin in type 2 diabetes. The dosage of basal insulin was meant to be relatively constant to test the effect of lixisenatide itself, which resulted in reductions in HbA1c by 0.7% from a baseline of 8.4%, and 0.4% more than placebo, despite lower insulin doses to minimize the risk of hypoglycemia. A corresponding improvement of SMPG profile values accompanied treatment with lixisenatide; the mean placebo-adjusted reduction was 0.9 mmol/L. Both the SMPG profiles and a standardized breakfast test showed a prominent glucose reduction after the first meal of the day. Moreover, body weight decreased significantly after lixisenatide treatment relative to placebo.
Several features of the study design require comment. First, by enrolling participants with relatively poor glycemic control despite using basal insulin and oral therapy, the study addressed a population for which improving glycemic control may be expected to be difficult. For comparison, in the 4T study, a year of treatment with titrated basal insulin therapy (insulin detemir once or twice daily; median dosage = 42 units daily) led to a mean HbA1c of 7.6% in a previously insulin-naive population (21). Adding three prandial injections of rapid-acting insulin to basal insulin for an additional 2 years allowed most (63%) of these patients to attain HbA1c ≤7.0%, but with significant weight gain and hypoglycemia as unwanted effects (22). The population in the current study entered with a mean HbA1c of 8.4% after taking basal insulin for an average of >3 years at a mean dosage of 55 units daily, and thus potentially presented an even more significant challenge for restoring glycemic control. Second, the study was designed to minimize changes of the type, timing, and dosage of insulin used to assess the effects of lixisenatide itself. Hence, the full potential of the combination of basal insulin with lixisenatide was not tested. Although more than twice as many participants attained HbA1c <7.0% with lixisenatide as with placebo (28 vs. 12%), over two-thirds of them did not reach this goal. Further titration of basal insulin dosage in combination with lixisenatide might have allowed a greater number of participants to reach HbA1c <7.0%.
The favorable responses of glycemic control and weight just described are in keeping with previous studies of lixisenatide, in which a single daily dose taken in the morning improved postprandial and day-long glycemic control while limiting body weight gain or even reducing weight (18,19). In addition, the safety measures in this study are consistent with prior observations (18,19). No excess of serious AEs was observed. The nonsevere AEs included, as in other studies of lixisenatide and other GLP-1RAs, mainly gastrointestinal symptoms (which were usually transitory) and a 1–2% incidence of injection site reactions (18,19,23).
The findings reported here add to the limited information currently available on the effects of combining a GLP-1RA with basal insulin for treatment of type 2 diabetes. Other studies have shown improved glycemic control with little or no weight gain after initiating combined therapy with exenatide (24–27). In a 30-week, randomized study of twice-daily exenatide added to basal insulin (with vigorous titration) with or without metformin and/or pioglitazone (28), exenatide reduced HbA1c by –0.7% and body weight (by –2.7 kg relative to placebo), and insulin dose was increased in both groups. Recently, a 26-week randomized study of combining liraglutide with basal insulin has been reported (28), in which the inverse order of the introduction of drugs was tested. That is, patients who did not attain HbA1c <7.0% despite treatment with liraglutide and oral agents were randomized to add titrated basal insulin or to continue liraglutide without insulin. Adding basal insulin to liraglutide led to a greater additional reduction of HbA1c (–0.5%) than that seen with continued liraglutide without basal insulin. No weight gain was observed in either randomized group, and the reduction was 0.8 kg more with the continuation of liraglutide alone than when insulin was added. In an earlier, 24-week, placebo-controlled study in an Asian population, lixisenatide added to basal insulin and oral therapy (19) resulted in a reduction in HbA1c of −0.9% versus placebo. Weight increased by 0.1 kg with placebo and decreased by 0.4 kg (P = 0.086) with lixisenatide. The HbA1c and weight differences relative to placebo in the current study (–0.4% HbA1c and –1.3 kg) are consistent with findings in the other studies, and collectively all the studies support the efficacy and safety of combining a GLP-1RA with basal insulin.
Limitations of the present placebo-controlled study include the lack of comparison of lixisenatide with other possible ways of intensifying treatment with basal insulin and metformin. Further studies are needed to directly compare lixisenatide with exenatide or liraglutide in combination with basal insulin, and also to determine the relative efficacy and safety of lixisenatide versus prandial insulin added to basal insulin in similar populations. A direct comparison of glycemia, body weight, and other effects of adding lixisenatide with those of adding a DPP-4 inhibitor or another newer oral therapy would also be of interest. Finally, the long-term effects of this treatment approach may be clarified by the controlled extension of the current study and by other long-term studies.
In summary, addition of once-daily lixisenatide to basal insulin, with or without metformin and with insulin dosage kept stable except to limit hypoglycemia, resulted in a significant improvement of HbA1c, PPG level after a breakfast meal, and seven-point glucose profiles. Additionally, body weight was reduced. Hypoglycemia and nausea were increased compared with placebo, although there was no excess of serious AEs. These favorable findings in a challenging clinical population suggest that addition of once-daily lixisenatide to prior treatment with basal insulin offers an alternative to rapid-acting insulin or other agents to improve glycemic control in this setting and deserves further study.
Acknowledgments
The study was funded by Sanofi, the manufacturer of lixisenatide. M.C.R. has received research grant support from Amylin, Eli Lilly and Company, and Sanofi and has received honoraria for consulting and/or speaking from Amylin, Eli Lilly and Company, Hoffmann-La Roche, Sanofi, and Valeritas; this potential conflict of interest has been reviewed and managed by Oregon Health & Science University. R.A. has received research support and/or consulting honoraria from Eli Lilly and Company, Sanofi, Takeda, and Novo Nordisk. P.H. has received honoraria for services as an advisor and/or speaker or grants for clinical research from AstraZeneca/BMS Alliance, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Merck Sharp & Dohme, Novartis Pharmaceuticals, Novo Nordisk, Pfizer, Hoffmann-La Roche, SkyePharma, and Sanofi. M.M. has received honoraria for consulting and/or speaking from Abbott, Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, Servier, and Sanofi. E.N., P.M., L.P., and J.Y. are employees of the sponsor Sanofi. J.R. has served on scientific advisory boards and received honoraria, consulting fees, or grants/research support from insulin and GLP-1RA manufacturers Amylin, Eli Lilly and Company, GlaxoSmithKline, Novo Nordisk, Hoffmann-La Roche, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
M.C.R. developed the protocol, analyzed the data, and led the writing of the manuscript. R.A., P.H., M.M., and J.R. were involved in the clinical conduct of the study, interpreted the data, and prepared the manuscript. E.N. and P.M. designed and wrote the protocol, setup, and medical supervision of the study, and reviewed the data. L.P. was responsible for medical supervision of the study as the Clinical Study Director and reviewed the data. J.Y. performed the statistical analyses. All authors had full access to all of the data in the study and had final responsibility for the decision to publish. Editorial support was provided to the authors in the following ways: manuscript preparation by Frances Gambling (Medicus International London, U.K.) and funding from Sanofi. M.C.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The data included in this report were published previously in abstract form for the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012, and the 48th Annual Meeting of the European Association for the Study of Diabetes, Berlin, Germany, 1–5 October 2012.
Footnotes
Clinical trial reg. no. NCT007715624, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2454/-/DC1.
References
- 1.IDF Clinical Guidelines Task Force. Global Guideline for Type 2 Diabetes Brussels, International Diabetes Federation, 2005
- 2.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for the Study of Diabetes Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009;52:17–30 [DOI] [PubMed] [Google Scholar]
- 3.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–559 [DOI] [PubMed] [Google Scholar]
- 4.Riddle MC. Timely initiation of basal insulin. Am J Med 2004;116(Suppl. 3A):3S–9S [DOI] [PubMed] [Google Scholar]
- 5.Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–3086 [DOI] [PubMed] [Google Scholar]
- 6.Barag SH. Insulin therapy for management of type 2 diabetes mellitus: strategies for initiation and long-term patient adherence. J Am Osteopath Assoc 2011;111(Suppl. 5):S13–S19 [PubMed] [Google Scholar]
- 7.Davis SN, Renda SM. Psychological insulin resistance: overcoming barriers to starting insulin therapy. Diabetes Educ 2006;32(Suppl. 4):146S–152S [DOI] [PubMed] [Google Scholar]
- 8.Raccah D. Options for the intensification of insulin therapy when basal insulin is not enough in type 2 diabetes mellitus. Diabetes Obes Metab 2008;10(Suppl. 2):76–82 [DOI] [PubMed] [Google Scholar]
- 9.Raccah D, Bretzel RG, Owens D, Riddle M. When basal insulin therapy in type 2 diabetes mellitus is not enough—what next? Diabetes Metab Res Rev 2007;23:257–264 [DOI] [PubMed] [Google Scholar]
- 10.Owens DR, Luzio SD, Sert-Langeron C, Riddle MC. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab 2011;13:1020–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg SK. The role of basal insulin and glucagon-like peptide-1 agonists in the therapeutic management of type 2 diabetes—a comprehensive review. Diabetes Technol Ther 2010;12:11–24 [DOI] [PubMed] [Google Scholar]
- 12.Tzefos M, Olin JL. Glucagon-like peptide-1 analog and insulin combination therapy in the management of adults with type 2 diabetes mellitus. Ann Pharmacother 2010;44:1294–1300 [DOI] [PubMed] [Google Scholar]
- 13.Kapitza C, Coester H, Poitiers F, et al. Pharmacodynamic characteristics of lixisenatide once daily vs. liraglutide once daily in patients with T2DM inadequally controlled with metformin. Diabetes Obes Metab. February 2013 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 2011;60:1561–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratner RE, Rosenstock J, Boka G, DRI6012 Study Investigators Dose-dependent effects of the once-daily GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled trial. Diabet Med 2010;27:1024–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratner RE, Rosenstock J, Boka G, Silvestre L. Post-meal pharmacodynamic profile of ACE0010, a once-daily GLP-1 receptor agonst, in patients with type 2 diabetes inadequally controlled on metformin. Diabetologia 2009;52:S60 [Google Scholar]
- 17.Werner U, Haschke G, Herling AW, Kramer W. Pharmacological profile of lixisenatide: A new GLP-1 receptor agonist for the treatment of type 2 diabetes. Regul Pept 2010;164:58–64 [DOI] [PubMed] [Google Scholar]
- 18.Fonseca VA, Alvarado-Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE, EFC6018 GetGoal-Mono Study Investigators Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care 2012;35:1225–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seino Y, Min KW, Niemoeller E, Takami A, EFC10887 GETGOAL-L Asia Study Investigators Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab 2012;14:910–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Medicines Agency. Lixisenatide (Lyxumia) marketing approval in the EU. Available at http://ec.europa.eu/health/documents/community-register/html/alfregister.htm Accessed 18 March 2013
- 21.Holman RR, Thorne KI, Farmer AJ, et al. 4-T Study Group Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007;357:1716–1730 [DOI] [PubMed] [Google Scholar]
- 22.Holman RR, Farmer AJ, Davies MJ, et al. 4-T Study Group Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009;361:1736–1747 [DOI] [PubMed] [Google Scholar]
- 23.Buse JB, Rosenstock J, Sesti G, et al. LEAD-6 Study Group Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39–47 [DOI] [PubMed] [Google Scholar]
- 24.Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2011;154:103–112 [DOI] [PubMed] [Google Scholar]
- 25.Arnolds S, Dellweg S, Clair J, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof-of-concept study. Diabetes Care 2010;33:1509–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheffield CA, Kane MP, Busch RS, Bakst G, Abelseth JM, Hamilton RA. Safety and efficacy of exenatide in combination with insulin in patients with type 2 diabetes mellitus. Endocr Pract 2008;14:285–292 [DOI] [PubMed] [Google Scholar]
- 27.Viswanathan P, Chaudhuri A, Bhatia R, Al-Atrash F, Mohanty P, Dandona P. Exenatide therapy in obese patients with type 2 diabetes mellitus treated with insulin. Endocr Pract 2007;13:444–450 [DOI] [PubMed] [Google Scholar]
- 28.DeVries JH, Bain SC, Rodbard HW, et al. Liraglutide-Detemir Study Group Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1C targets. Diabetes Care 2012;35:1446–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]