Abstract
OBJECTIVE
To examine the independent and combined association of isometric muscle strength of the abdomen and back and cardiorespiratory fitness (CRF) in youth with indices of glucose metabolism in young adulthood among boys and girls from the European Youth Heart Study.
RESEARCH DESIGN AND METHODS
We used data from a population-based prospective cohort study among youth followed up for up to 12 years (n = 317). In youth, maximal voluntary contractions during isometric back extension and abdominal flexion were determined using a strain-gauge dynamometer and CRF was obtained from a maximal cycle ergometer test. Insulin resistance (homeostasis model assessment of insulin resistance [HOMA-IR]) and β-cell function (homeostasis model assessment of β-cell function [HOMA-B]) were estimated from fasting serum insulin and glucose that were obtained in youth and at follow-up in young adulthood.
RESULTS
For each 1-SD difference in isometric muscle strength (0.16 N/kg) in youth, fasting insulin, HOMA-IR, and HOMA-B in young adulthood changed by −11.3% (95% CI −17.0 to −5.2), −12.2% (−18.2 to −5.7), and −8.9% (−14.4 to −3.0), respectively, in young adulthood after adjustment for CRF and personal lifestyle and demographic factors. Results for CRF were very similar in magnitude, and the magnitude of associations for both exposures was unchanged with additional adjustment for general or abdominal adiposity in youth. Combined associations of muscle strength and CRF with fasting insulin, HOMA-IR, and HOMA-B were additive, and adolescents in the highest sex-specific tertile for both isometric muscle strength and CRF had the lowest levels of these glucose metabolism outcomes.
CONCLUSIONS
Increasing muscle strength and CRF should be targets in youth primordial prevention strategies of insulin resistance and β-cell dysfunction.
Previously, type 2 diabetes was very rare in young people. Today, it is more common not only in young adults but also in youth, and a similar trend has been observed for impaired fasting glucose and impaired glucose tolerance (1–3), which are considered precursors of type 2 diabetes. Youth and young adults with type 2 diabetes or prediabetes are at risk for premature mortality and early complications (4,5), making prevention critical. Numerous prospective epidemiological studies among adults suggest that regular participation in aerobic moderate-to-vigorous physical activity (MVPA) and high cardiorespiratory fitness (CRF) reduce the risk of type 2 diabetes and are associated with healthier glucose metabolism (6,7). However, less is known from prospective studies about the importance of fitness in childhood and adolescence (8). In addition, it is unknown whether muscle strength in youth is associated with impaired glucose metabolism in adulthood independent of CRF. In this study, we aimed to examine the independent and combined association of isometric muscle strength of the abdomen and back and CRF in youth with fasting glucose, insulin, insulin resistance, and β-cell function in young adulthood among men and women from the European Youth Heart Study (EYHS) followed up for a period of up to 12 years. We also assessed the extent to which these associations were mediated or confounded by general and abdominal adiposity.
RESEARCH DESIGN AND METHODS
Design
We used data from the Danish cohort of the EYHS, an international, population-based, multicenter study that addresses cardiovascular disease risk factors in children and adolescents. A detailed description of the EYHS has been published elsewhere (9). In this study, a random sample of 658 15-year-olds was invited to participate in 1997–1998, of whom 429 (65%) agreed to take part in the study. Isometric muscle strength was assessed in a subgroup of 243 participants in 1997–1998. In 2003–2004, another random sample of 771 15-year-olds was invited, of whom 444 (58%) agreed to take part, and 441 of these participants had isometric muscle strength evaluated. In 2009–2010, a 6- or 12-year follow-up was conducted in which all originally invited participants from the 1997–1998 and 2003–2004 studies were reinvited. In this study, 317 participants had complete data for all outcomes, exposures, and covariates. Ninety-four percent of the participants were postpubertal based on Tanner stage evaluation. The local scientific Ethics Committee approved the study, and all participants gave informed consent to participate.
Muscle strength
We obtained isometric muscle strength during maximal voluntary contraction of abdominal and back muscles using a strain-gauge dynamometer (10). The participants were standing upright and positioned with a strap around the shoulders connected to the dynamometer. Abdominal maximal voluntary contraction was performed with the back against the dynamometer performing maximal forward flexion. For maximal voluntary contraction of the low back muscles, the participants were positioned with the front against the dynamometer, performing maximal backward extension. Isometric muscle strength was expressed as the mean of abdominal and back strength relative to body weight. High reliability of these particular isometric strength measures (intraclass correlation coefficient >0.9) has been reported in a previous study among Danish adults (11).
Cardiorespiratory fitness
CRF was assessed during a progressive maximal ergometer bicycle test (Ergomedic 839; Monark, Varberg, Sweden) as previously described (9). During the test, heart rate was recorded every 5 s using a heart rate monitor (Polar Vantage). Criteria for maximal effort were heart rate of ≥185 bpm and a subjective judgment by the observer that the participant could no longer continue, even after encouragement. Maximal power output (wattmax) was used to estimate maximal oxygen uptake using the following equation: VO2max (mL) = 0.465 + (0.0112*wattmax) + (0.172*sex), where sex represents boys = 1 and girls = 0 (12). This particular fitness test is highly reproducible (coefficient of variation 2.5–4.8%). Furthermore, a validation study among 15-year-olds has shown that this measure is highly correlated with VO2max assessed directly (r > 0.90; P < 0.001) (13).
Other covariates
Height and weight were measured while the participants were wearing light clothing, without shoes, using standard anthropometric procedures. Waist circumference was measured to the nearest 1 mm at the midpoint between the lower ribs and the iliac crest with a flexible tape. Smoking status (yes or no), television viewing (hours per day), monthly frequency of soft drink consumption, and monthly fruit and vegetable intake were obtained by self-report of adolescence using a computer-based questionnaire as described previously (9,14). Family history of diabetes (paternal or maternal, yes or no) and parental educational level were obtained by parental self-report. Parental educational status was defined according to the International Standard Classification of Education (United Nations Educational, Scientific, and Cultural Organization 1997). However, because the details obtained regarding the description of education were insufficient, the International Standard Classification of Education seven-point scale was combined into three new groups (I = level 1–2, II = level 3–4, and III = level 5–7). MVPA was assessed using accelerometry with data reduction as described previously (15). Specifically, an accelerometer output >2,000 counts/min (equivalent to walking ∼4 km/h) was defined as MVPA and expressed as percentage of total registered time. Weight-bearing activity such as resistance exercise is grossly underestimated when using accelerometry-measured activity.
Fasting insulin and glucose
A fasting blood sample (overnight) was taken in the morning from the antecubital vein. Samples were aliquoted and separated within 30 min and then stored at −80°C until they were transported to World Health Organization–certified laboratories in Bristol (U.K.) for analysis of baseline samples and Cambridge (U.K.) for analysis of follow-up samples. Samples were analyzed for serum glucose and insulin. Glucose was analyzed using the hexokinase method (Olympus AU600 autoanalyzer; Olympus Diagnostica, Hamburg, Germany) at baseline and on a Dade Behring Dimension RxL autonalyzer (Siemens Healthcare, Camberley, U.K.) at follow-up. Insulin was analyzed using enzyme immunoassay (microtiter plate format, Dako Diagnostics [at baseline]; 1235 AutoDELFIA automatic immunoassay [at follow-up]). Between-laboratory correlations for glucose and insulin for 30 randomly selected samples analyzed at both laboratories were 0.94–0.98 at baseline (16).
The homeostasis model assessment of insulin resistance (HOMA-IR; fasting glucose [mmol/L] × insulin [μU/mL] / 22.5) and homeostasis model assessment of β-cell function (HOMA-B; insulin [μU/mL] × 20 / glucose [mmol/L] − 3.5) were used to quantify the level of insulin resistance and secretion (17). Both these measures have been validated as indices of insulin resistance and pancreatic β-cell function in healthy adolescents (18).
Statistics
We analyzed the associations of isometric muscle strength and CRF in adolescence with fasting glucose, insulin, HOMA-IR, and HOMA-B in young adulthood using multiple linear regression analyses with baseline levels of respective variables included as a covariate. In basic models, age in adolescence, age in young adulthood, sex, and recruitment period were adjusted for. Values of insulin, HOMA-IR, and HOMA-B were natural log transformed. Thus, regression coefficients from these models were exponentiated to give ratios of geometric means (expressed in percent) per SD difference in isometric muscle strength and CRF. In multivariable analyses, we additionally adjusted for parental educational level, current smoking, family history of diabetes, frequency of intake of soft drinks, and intake of fruit and vegetables. Muscle strength and CRF in youth also were included in the same model to examine their independent influence on glucose, insulin, HOMA-IR, and HOMA-B in young adulthood. We then analyzed the association of muscle strength with the odds of insulin resistance, defined as HOMA-IR value >75th percentile in young adulthood (19), using multiple logistic regression adjusting for the same covariates as in the linear models including HOMA-IR at baseline. Finally, we assessed the joint association of muscle strength and CRF by constructing a joint variable of tertiles of muscle strength and CRF, respectively, and associated that with the outcomes in multivariable models. Because no sex-dependent or recruitment period–dependent associations for any outcomes were observed, we present all analyses for men, women, and recruitment period (follow-up time) combined, but with appropriate statistical adjustment. Standard linear regression diagnostics were performed, including examining linearity and normality of residuals.
In sensitivity analyses, we compared associations of the nonimputed sample with a sample with imputed data. We imputed missing information for covariates and outcomes (n = 12 to n = 556, depending on variable) among the total sampled population at baseline (n = 873) using chained equations (“mi impute chained” in STATA) (20). All covariates and respective outcomes were included in the imputation approach. We obtained β-coefficients and SEs based on 20 imputed datasets. We also performed an analysis additionally adjusting for accelerometry-measured MVPA to examine if any residual confounding by MVPA remained that CRF may not have captured. Because 35% of the participants with otherwise full data had missing information regarding accelerometer-measured MVPA, we imputed missing values for MVPA using a multiple linear regression imputation approach including all covariates and the outcome. All statistical analyses were performed in STATA 12.1 with α = 0.05 (two-sided).
RESULTS
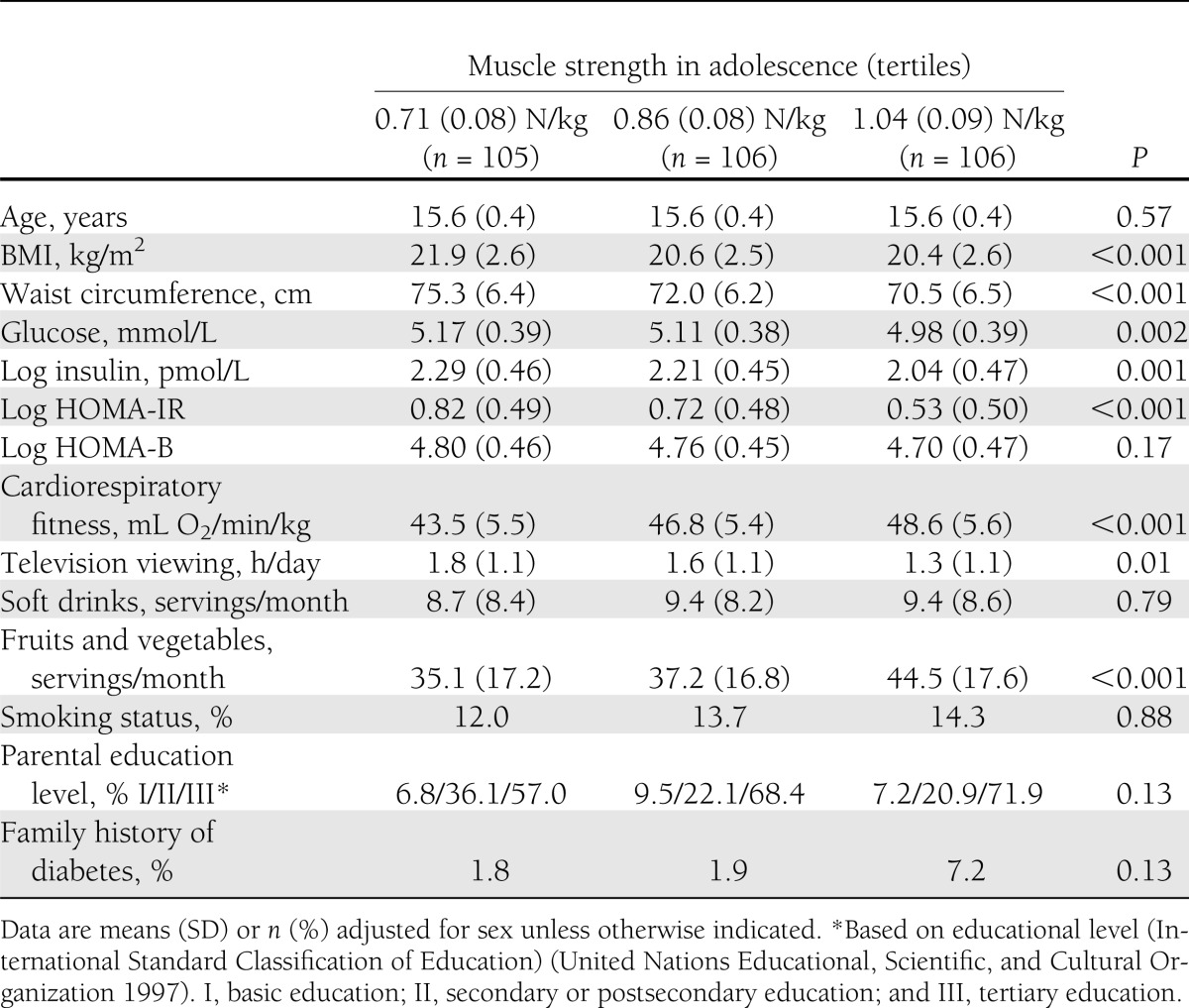
Baseline characteristics adjusted for sex by tertiles of isometric muscle strength in adolescence are shown in Table 1. Isometric muscle strength in adolescence was inversely associated with adolescence BMI, waist circumference, fasting glucose, fasting insulin, HOMA-IR, and television viewing and was positively associated with cardiovascular fitness and intake of fruits and vegetables at baseline.
Table 1.
Baseline characteristics adjusted for sex by tertiles of maximal voluntary isometric trunk muscle strength in adolescence
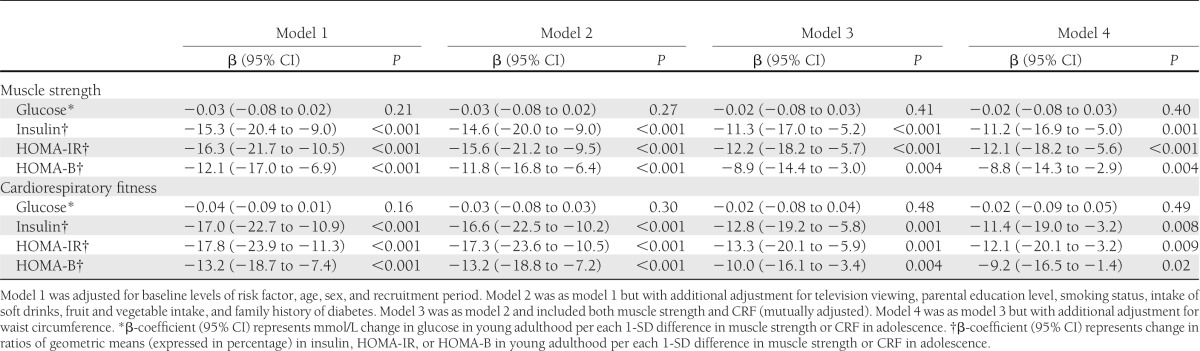
Isometric muscle strength and CRF in youth were both significantly inversely associated with fasting insulin, HOMA-IR, and HOMA-B in young adulthood in multivariable-adjusted analyses (Table 2). Although associations of adolescent muscle strength and CRF with fasting glucose in young adulthood were in the expected inverse direction, these did not reach statistical significance. When muscle strength and CRF were included in the same multivariable models, associations with insulin, HOMA-IR, and HOMA-B were only marginally attenuated for both variables. For each 1-SD difference in muscle strength (0.16 N/kg) in youth, fasting insulin, HOMA-IR, and HOMA-B in young adulthood changed by −11.3, −12.2, and −8.9%, respectively. The magnitudes of associations for CRF were fairly similar; for each SD difference in CRF in youth, fasting insulin, HOMA-IR, and HOMA-B in young adulthood changed −12.8, −13.3, and −10.0%, respectively. When we additionally adjusted our analyses for waist circumference measured at baseline, estimates of associations were only slightly attenuated for both exposures (Table 2, model 4). Using BMI instead of waist circumference as a confounder or mediator gave the same results (data not shown). Furthermore, additional adjustment for accelerometer-measured MVPA did not materially change the associations (data not shown). When we repeated the analyses based on imputed samples (n = 873), associations were essentially similar to the nonimputed analyses (Supplementary Table 1). Analyzing isometric abdominal and back strength separately also yielded fairly similar associations compared with using the mean of abdominal and back isometric strength (Supplementary Table 2).
Table 2.
Isometric trunk muscle strength and cardiorespiratory fitness in youth and fasting glucose, insulin, HOMA-IR, and HOMA-B in young adulthood
For the association of muscle strength and CRF in youth (in the same multivariable-adjusted model) with the odds of insulin resistance in young adulthood, each 1-SD difference in muscle strength (0.16 N/kg) and CRF (6.8 mL O2/min/kg) in youth was significantly associated with 0.56 (95% CI 0.39–0.81) and 0.63 (0.43–0.94) lower odds of adverse levels of HOMA-IR in young adulthood, respectively. Participants in the third sex-specific tertile of isometric muscle strength had 0.31 (0.15–0.66) lower odds of insulin resistance in young adulthood. Furthermore, participants in the third sex-specific tertile of CRF had 0.48 (0.23–1.01) lower odds of insulin resistance in young adulthood. There were no indications of the associations of muscle strength or CRF with HOMA-IR being nonlinear in these models.
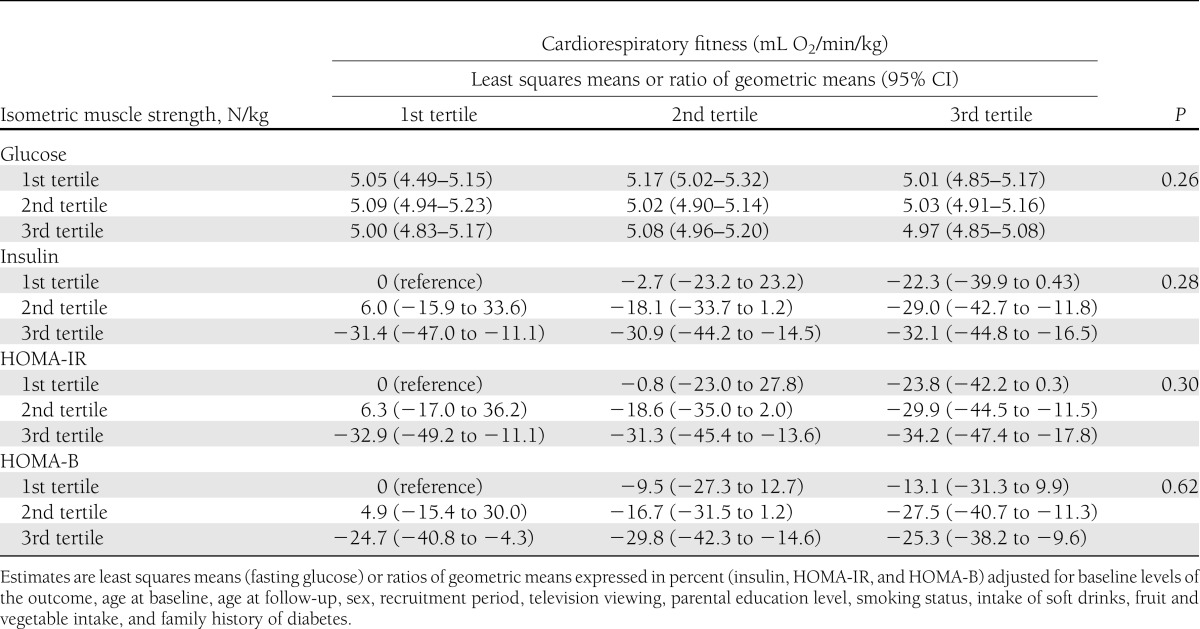
Finally, Table 3 shows the joint associations of isometric muscle strength and CRF in adolescence with fasting glucose, insulin, HOMA-IR, and HOMA-B in young adulthood. The inverse associations of isometric muscle strength with insulin, HOMA-IR, and HOMA-B in young adulthood were generally observed in each tertile of CRF. There was no statistical evidence of multiplicative interactions between muscle strength and CRF on these outcomes, and results suggested an additive effect of muscle strength and CRF on glucose metabolism outcomes.
Table 3.
Joint association of sex-specific tertiles of isometric trunk muscle strength and cardiorespiratory fitness in adolescence with fasting glucose, insulin, HOMA-IR, and HOMA-B in young adulthood
CONCLUSIONS
In this prospective study of a population sample of Danish men and women, isometric muscle strength and CRF in youth were inversely associated with fasting insulin and inversely associated with markers of insulin resistance and β-cell function in young adulthood. These associations were independent of adiposity and demographic, personal, and lifestyle factors, and they suggest that muscle strength in youth is equally important as CRF for maintaining healthy insulin sensitivity and β-cell function later in life.
The current guidelines for physical activity among children and adults recommend participation in activities that maintain or increase muscular strength and endurance for ≥2 days (adults) or ≥3 days (children and adolescents) each week in addition to participation in aerobic MVPA (≥30 min/day for adults and ≥60 min/day for youth) (21,22). Our results generally support these guidelines; however, they also suggest that an even greater emphasis could be placed on maintaining or increasing muscle strength among youth. Because associations between CRF and strength with insulin resistance and β-cell function were independent of each other, this supports the view that aerobic activities and muscle strengthening activities should be targeted separately. Furthermore, the analyses of continuous trait and binary outcomes suggested that muscle strength and CRF were linearly associated with fasting insulin, insulin resistance, and β-cell function, indicating that there is no clear threshold effect of an increase in insulin secretion or action at a particular low level of fitness or muscle strength. Efforts to shift the population distribution of muscle strength and CRF upwards are therefore likely to be valuable for primordial prevention of type 2 diabetes.
We are aware of three randomized controlled trials conducted among youth comparing the effect of resistance training on insulin resistance or glycemic control with a pure control group. A small-scale trial among 22 overweight Latino adolescent males found that 16 weeks of resistance training performed twice per week markedly increased insulin sensitivity (23). Another randomized trial among 78 overweight or obese children and adolescents from New Zealand reported that the effect of 8 weeks of resistance training performed twice per week had no significant effect on insulin resistance; however, results were in the expected direction and the training improved abdominal and general adiposity (24). A recent efficacy trial among 45 obese adolescent boys reported that both aerobic exercise and resistance training were effective for reducing adiposity, but only the resistance exercise group improved insulin sensitivity (25). Although we have no data to support that participants with high isometric muscle strength of the abdomen and back engage more often in muscle-strengthening activities compared with participants with low muscle strength, findings from these and other exercise training studies clearly indicate that resistance training increases muscular strength (26). Our results are also largely in agreement with three previous cross-sectional studies among children and adolescents. A population-based study among Norwegian children and adolescents found that muscle fitness indicated by handgrip strength, standing broad jump, abdominal muscle endurance, and back muscle endurance were inversely associated with insulin resistance independent of CRF (27). A study among European children and adolescents have reported inverse associations of handgrip strength and standing long jump with insulin resistance; however, it was not reported whether these associations were independent of cardiovascular fitness (28). Finally, in a cross-sectional study among children and adolescents from New Zealand, maximal upper body muscle strength (bench press) was inversely associated with insulin resistance independent of CRF (29). Our results extend these previous observations by the prospective nature of our study and the adjustments for putative lifestyle behaviors and sociodemographic confounders. The finding that CRF in childhood or youth is important for the prevention of insulin resistance in adulthood is supported by a previous study among Australian children and adolescents followed up for a period of 20 years (8).
The similar magnitude of association of muscle strength and cardiovascular fitness with insulin resistance that we observed in the current study is in agreement with findings from experimental and observational studies among adults. The two largest trials among individuals with type 2 diabetes have not provided clear evidence that aerobic exercise is superior to resistance exercise for glycemic control (30,31). However, these studies indicated that the combination of aerobic and resistance exercise results in greatest improvement in glycemic control compared with either type of activity alone. The comparable effects of these two exercise regimes are also supported by a recent experimental study reporting that a single session of either aerobic or resistance exercise provided similar effects on 24-h postexercise glycemic control in insulin-resistant individuals with and without type 2 diabetes (32). Finally, in a prospective study of men from the Health Professionals Follow-up study, engagement in weight training and aerobic MVPA were both independently associated with reduced risk of incident type 2 diabetes with fairly comparable risk reduction sizes (33).
An important strength of the current study was that we were able to examine the independent associations for strength and CRF, and we were able to control for important confounding factors. Furthermore, all participants were young and healthy at baseline and, therefore, very likely to be free from subclinical conditions that may have affected muscle strength at baseline and progression of insulin resistance and β-cell dysfunction during follow-up. There are also a number of limitations to the study. First, the attrition analyses indicated a possibility of selective nonresponse; however, associations were very similar in imputed and nonimputed samples, which suggests that associations are unaffected by selection bias, and our results may have wider external validity. Second, the moderate study size precluded us from adequately powered subgroup analysis. Third, although we used a standardized test for the assessment of isometric muscle strength of the abdomen and back, additional components of strength such as dynamic strength also may be important and their assessment would have provided more extensive information on overall muscle strength. Fourth, the observational nature of our study precludes us from excluding the possibility that unknown confounders or residual confounding explain our results. One such likely factor is diet, because the assessment of dietary intake was relatively crude in this study. Finally, a caveat of the study was that we assessed insulin resistance and β-cell function via HOMA-IR and HOMA-B, which mainly describe hepatic insulin resistance and steady-state insulin secretion, and generalizability to peripheral insulin resistance and insulin secretion in the stimulated state is uncertain (34).
In conclusion, our results show that lower isometric muscle strength and CRF in youth were independently associated with adverse levels of fasting insulin, insulin sensitivity, and β-cell function in young adulthood. The magnitude of associations for isometric muscle strength and for CRF were very similar, suggesting that participation in muscle-strengthening activities may be equally important as participating in aerobic activities in youth for maintaining healthy insulin sensitivity and β-cell function later in life. Furthermore, because associations for isometric muscle strength and CRF with these outcomes appeared additive, it may be beneficial to increase muscle strength at any level of CRF. Further studies are warranted to examine which specific physical activities explain the associations of isometric muscle strength with insulin sensitivity and β-cell function, and to what extent these associations are explained by skeletal muscle mass relative to body size. In addition, further studies should investigate whether the effects of strength and fitness in adolescence persist in adulthood despite changes in these physical fitness characteristics in adulthood.
Acknowledgments
This work was supported by the Danish Council for Strategic Research (grant number 2101-08-0058), The Danish Heart Foundation, the Danish Health Fund (Sygekassernes Helsefond), and the Trygfoundation (Trygfonden).
No potential conflicts of interest relevant to this article were reported.
A.G. researched data and wrote the manuscript. M.R.-L. researched data and reviewed and edited the manuscript. U.E. contributed to discussion and reviewed and edited the manuscript. K.F. researched data and contributed to discussion. S.B. contributed to discussion and reviewed and edited the manuscript. L.B.A. researched data, contributed to discussion, and reviewed and edited the manuscript. A.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2252/-/DC1.
References
- 1.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol 2012;8:228–236 [DOI] [PubMed] [Google Scholar]
- 2.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 2011;34:1249–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li C, Ford ES, Zhao G, Mokdad AH. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005-2006. Diabetes Care 2009;32:342–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med 2010;362:485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA 2006;296:421–426 [DOI] [PubMed] [Google Scholar]
- 6.Carnethon MR, Sternfeld B, Schreiner PJ, et al. Association of 20-year changes in cardiorespiratory fitness with incident type 2 diabetes: the coronary artery risk development in young adults (CARDIA) fitness study. Diabetes Care 2009;32:1284–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sui X, Jackson AS, Church TS, et al. Effects of cardiorespiratory fitness on aging: glucose trajectory in a cohort of healthy men. Ann Epidemiol 2012;22:617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dwyer T, Magnussen CG, Schmidt MD, et al. Decline in physical fitness from childhood to adulthood associated with increased obesity and insulin resistance in adults. Diabetes Care 2009;32:683–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riddoch C, Edwards D, Page A, et al. The European Youth Heart Study-cardiovascular disease risk factors in children: rationale, aims, design and validation of methods. J Phys Act Health 2005;2:115–129 [Google Scholar]
- 10.Andersen LB, Henckel P. Maximal voluntary isometric strength in Danish adolescents 16-19 years of age. Eur J Appl Physiol Occup Physiol 1987;56:83–89 [DOI] [PubMed] [Google Scholar]
- 11.Essendrop M, Schibye B, Hansen K. Reliability of isometric muscle strength tests for the trunk, hands and shoulders. Int J Ind Ergon 2001;28:379–387 [Google Scholar]
- 12.Kolle E, Steene-Johannessen J, Andersen LB, Anderssen SA. Objectively assessed physical activity and aerobic fitness in a population-based sample of Norwegian 9- and 15-year-olds. Scand J Med Sci Sports 2010;20:e41–e47 [DOI] [PubMed] [Google Scholar]
- 13.Anderssen SA, Cooper AR, Riddoch C, et al. Low cardiorespiratory fitness is a strong predictor for clustering of cardiovascular disease risk factors in children independent of country, age and sex. Eur J Cardiovasc Prev Rehabil 2007;14:526–531 [DOI] [PubMed] [Google Scholar]
- 14.Grøntved A, Ried-Larsen M, Møller NC, et al. Youth screen-time behaviour is associated with cardiovascular risk in young adulthood: the European Youth Heart Study. Eur J Prev Cardiol. 5 July 2012 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Kristensen PL, Moeller NC, Korsholm L, et al. The association between aerobic fitness and physical activity in children and adolescents: the European youth heart study. Eur J Appl Physiol 2010;110:267–275 [DOI] [PubMed] [Google Scholar]
- 16.Ekelund U, Brage S, Froberg K, et al. TV viewing and physical activity are independently associated with metabolic risk in children: the European Youth Heart Study. PLoS Med 2006;3:e488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 18.Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr 2004;144:47–55 [DOI] [PubMed] [Google Scholar]
- 19.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabetic Med 1999;16:442-443. [DOI] [PubMed]
- 20.Royston P. Multiple imputation of missing values. Stata J 2004;4:227–241 [Google Scholar]
- 21.Physical Activity Guidelines Advisory Committee Report. Washington, DC, US Department of Health and Human Services, 2008. Available from http://www.health.gov/paguidelines/guidelines/default.aspx Accessed 28 October 2012 [Google Scholar]
- 22.World Health Organization Global Recommendations on Physical Activity for Health. Geneva, Switzerland, WHO Press, 2010 [PubMed] [Google Scholar]
- 23.Shaibi GQ, Cruz ML, Ball GD, et al. Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Med Sci Sports Exerc 2006;38:1208–1215 [DOI] [PubMed] [Google Scholar]
- 24.Benson AC, Torode ME, Fiatarone Singh MA. The effect of high-intensity progressive resistance training on adiposity in children: a randomized controlled trial. Int J Obes (Lond) 2008;32:1016–1027 [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes 2012;61:2787–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behm DG, Faigenbaum AD, Falk B, Klentrou P. Canadian Society for Exercise Physiology position paper: resistance training in children and adolescents. Appl Physiol Nutr Metab 2008;33:547–561 [DOI] [PubMed] [Google Scholar]
- 27.Steene-Johannessen J, Anderssen SA, Kolle E, Andersen LB. Low muscle fitness is associated with metabolic risk in youth. Med Sci Sports Exerc 2009;41:1361–1367 [DOI] [PubMed] [Google Scholar]
- 28.Artero EG, Ruiz JR, Ortega FB, et al. HELENA Study Group Muscular and cardiorespiratory fitness are independently associated with metabolic risk in adolescents: the HELENA study. Pediatr Diabetes 2011;12:704–712 [DOI] [PubMed] [Google Scholar]
- 29.Benson AC, Torode ME, Singh MA. Muscular strength and cardiorespiratory fitness is associated with higher insulin sensitivity in children and adolescents. Int J Pediatr Obes 2006;1:222-231. [DOI] [PubMed]
- 30.Sigal RJ, Kenny GP, Boulé NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med 2007;147:357–369 [DOI] [PubMed] [Google Scholar]
- 31.Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA 2010;304:2253–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Dijk JW, Manders R, Tummers K, et al. Both resistance- and endurance-type exercise reduce the prevalence of hyperglycaemia in individuals with impaired glucose tolerance and in insulin-treated and non-insulin-treated type 2 diabetic patients. Diabetologia 2012;55:1273–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grøntved A, Rimm EB, Willett WC, Andersen LB, Hu FB. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Arch Intern Med 2012;172:1306–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]


