Abstract
OBJECTIVE
To examine the interactive relationship between diabetic retinopathy (DR) and diabetic nephropathy (DN) in type 2 diabetic patients and to elucidate the role of DR and microalbuminuria on the onset of macroalbuminuria and renal function decline.
RESEARCH DESIGN AND METHODS
We explored the effects of DR and microalbuminuria on the progression of DN from normoalbuminuria and low microalbuminuria (<150 mg/gCr) to macroalbuminuria or renal function decline in the Japan Diabetes Complications Study (JDCS), which is a nationwide randomized controlled study of type 2 diabetic patients focusing on lifestyle modification. Patients were divided into four groups according to presence or absence of DR and MA: normoalbuminuria without DR [NA(DR−)] (n = 773), normoalbuminuria with DR [NA(DR+)] (n = 279), microalbuminuria without DR [MA(DR−)] (n = 277), and microalbuminuria with DR [MA(DR+)] (n = 146). Basal urinary albumin-to-creatinine ratio and DR status were determined at baseline and followed for a median of 8.0 years.
RESULTS
Annual incidence rates of macroalbuminuria were 1.6/1,000 person-years (9 incidences), 3.9/1,000 person-years (8 incidences), 18.4/1,000 person-years (34 incidences), and 22.1/1,000 person-years (22 incidences) in the four groups, respectively. Multivariate-adjusted hazard ratios of the progression to macroalbuminuria were 2.48 (95% CI 0.94–6.50; P = 0.07), 10.40 (4.91–22.03; P < 0.01), and 11.55 (5.24–25.45; P < 0.01) in NA(DR+), MA(DR−), and MA(DR+), respectively, in comparison with NA(DR−). Decline in estimated glomerular filtration rate (GFR) per year was two to three times faster in MA(DR+) (−1.92 mL/min/1.73 m2/year) than in the other groups.
CONCLUSIONS
In normo- and low microalbuminuric Japanese type 2 diabetic patients, presence of microalbuminuria at baseline was associated with higher risk of macroalbuminuria in 8 years. Patients with microalbuminuria and DR showed the fastest GFR decline. Albuminuria and DR should be considered as risk factors of renal prognosis in type 2 diabetic patients. An open sharing of information will benefit both ophthalmologists and diabetologists.
Diabetic retinopathy (DR) and nephropathy (DN) are two major chronic microvascular complications in long-standing type 1 and type 2 diabetic patients. However, it is still unclear whether these 2 complications are related to or affect each other or whether both of them progress simultaneously after their onset, although many epidemiological studies have shown the coexistence of DR and DN (1,2). In fact, we sometimes see proteinuric diabetic patients without DR or normoalbuminuric patients with proliferative DR, which is the most advanced stage of DR. For example, it was shown that only 36% had no DR, while 53% had nonproliferative, 9% moderate to severe, and 2% severe DR in 285 normoalbuminuric Caucasian type 1 diabetic patients (3). In addition, there was marked discordance between DR and DN, especially in normoalbuminuria or low-level microalbuminuria, while advanced renal histological severity has been related to advanced DR severity in Caucasian type 1 diabetic patients (4). On the other hand, diabetic patients treated by diabetologists sometime miss their visits to ophthalmologists; therefore, the relationships or detailed clinical courses of DR and DN can hardly be analyzed in most clinical sites.
All over the world, DN is a major cause of end-stage renal disease, which requires renal replacement therapy such as hemodialysis or renal transplantation (5,6). In Japan, the number of patients requiring renal replacement therapy has increased threefold in the last 15 years. Therefore, it is absolutely necessary to stop the progression of DN and to find biomarkers or easily available factors that represent the exact clinical course or prognosis of DN. However, it is not exactly known what factors affect an increase of urinary albumin excretion (UAE) or glomerular filtration rate (GFR) decline, which are typical clinical changes in DN.
Microalbuminuria is well known as a risk factor resulting in macroalbuminuria in type 1 and type 2 diabetic patients (7–9). In addition, some Caucasian type 2 diabetic patients with microalbuminuria showed rapid decline of GFR, although it was unclear whether these patients had more frequent DR compared with the patients without rapid GFR decline (10). On the other hand, DR was shown to be a risk factor of microalbuminuria and macroalbuminuria (2,11). In addition, proliferative DR was shown to be a predictor of macroalbuminuria in Caucasian type 1 diabetic patients (13), but this association has not been investigated in Asian populations. Although DR and glomerulosclerosis seemed to be parallel to progress using the investigation of serial renal biopsy specimens (14) when the blood glucose control was fair to poor, detailed interaction between two complications are still obscure in a large number of patients. Whether DR can predict renal functional decline in type 1 and type 2 diabetic patients remains to be clarified.
The Japan Diabetes Complications Study (JDCS) is a nationwide randomized controlled study of type 2 diabetic patients focusing on lifestyle modification (15,16). We have reported the extremely low transition rate from normoalbuminuria and low microalbuminuria in this Japanese cohort (9), as well as incidence and progression rates of DR that were also lower than in Caucasian populations (15). In addition, we have also shown that the incidence and progression rate of DR were lower than those in Caucasian populations and that glycemic control, duration of diabetes, and systolic blood pressure (SBP) were related to DR in the JDCS cohort (17). Here, we elucidated the relationships between DN and DR, and the risk factors of the UAE increase and GFR decline according to the presence or absence of microalbuminuria or DR in the JDCS cohort.
RESEARCH DESIGN AND METHODS
This study is a part of the JDCS, a Japanese nationwide multicentered randomized trial (15). In 1996, 2,205 patients aged 40–70 years with previously diagnosed type 2 diabetes and HbA1C levels of >6.5% were recruited and registered from 59 hospitals specializing in diabetes care. The protocol for the study, which is in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical/Epidemiological Studies of the Japanese Ministry of Health, Labor and Welfare, received ethics approval from the institutional review boards of all the participating institutes. Written informed consent was obtained from all patients enrolled. The inclusion criteria for participating patients have previously been described (15). Those who had impaired glucose tolerance and major ocular disease including glaucoma, dense cataract, or history of cataract surgery were excluded. A final total of 2,033 patients aged 58.5 ± 6.9 (mean ± SD) years were included in the study, and their known diabetes duration was 10.9 ± 7.2 years.
The protocol originally specified that patients with nondiabetic nephropathy, macroalbuminuria, serum creatinine levels >120 μmol/L, and mean values of two spot urine examinations for an albumin excretion rate of >150 mg/g creatinine were excluded in the analysis of nephropathy, making up the analysis population of 1,558 patients (9). We excluded the patients with high microalbuminuria (150–300 mg/gCr) because the INNOVATION (Incipient to Overt: Angiotensin II Receptor Blocker, Telmisartan, Investigation on Type 2 Diabetic Nephropathy) trial showed a higher transition rate from high microalbuminuria to macroalbuminuria (18). After exclusion of 83 patients without DR assessment, the remaining 1,475 patients were divided into four groups according to the absence or presence of DR and microalbuminuria as follows: normoalbuminuria without DR [NA(DR−)] (n = 773), normoalbuminuria with DR [NA(DR+)] (n = 279), microalbuminuria without DR [MA(DR−)] (n = 277), and microalbuminuria with DR [MA(DR+)] (n = 146).
Assessment of DR
The presence and severity of DR were determined annually by qualified ophthalmologists at each institute by mydriatic indirect ophthalmoscopic examination and slit lamp biomicroscopic fundus examination using precorneal lens with the international DR and diabetic macular edema disease scales including minor modifications (17,19). To validate the consistency of staging between study sites, we cross-examined fundus images and evaluated the agreement in staging between local ophthalmologists and retinal specialists (17). Severity of DR was categorized following the international clinical diabetic retinopathy severity scales into five categories as “no retinopathy” (equivalent to the Early Treatment of Diabetic Retinopathy Study [ETDRS] scale level 10), “mild nonproliferative DR” (stage 1; equivalent to ETDRS level 20), “moderate nonproliferative DR” (stage 2; equivalent to ETDRS levels 35, 43, and 47), “severe nonproliferative DR” (stage 3; equivalent to ETDRS levels 53A–53E), and “proliferative DR” (stage 4; equivalent to ETDRS levels ≥61) (19). History of ocular surgery (e.g., cataract, glaucoma, and vitreoretinal surgery) was also surveyed.
Measures of kidney function
We followed up these groups for 8 years and measured their body weight and blood pressure at least twice a year. HbA1c, fasting plasma glucose, serum lipids, and serum creatinine levels were also determined twice a year. Spot urinary albumin-to-creatinine ratio (UACR) was also determined at least twice a year using the turbidmetric immunoassay to measure the urinary albumin concentration. We defined normoalbuminuria as a UACR <30 mg/gCr and low microalbuminuria as a UACR of 30–150 mg/gCr. Estimated GFR (eGFR) was calculated using serum creatinine levels and ages according to the Modification of Diet in Renal Disease formula modified for the Japanese population (20).
Statistical analysis
The annual increase rate of UACR and decline rate of eGFR in each group was determined by linear mixed models. The transition from normo- or low microalbuminuria to macroalbuminuria (≥300 mg/gCr) was determined in two consecutive urine samples. Transition to macroalbuminuria was summarized by the annual transition rate to macroalbuminuria, and the remission proportion was defined by patients whose mean value of UACR at the final two visits was <30 mg/gCr. Hazard ratios of the NA(DR+), MA(DR−), and MA(DR+) groups compared with the NA(DR−) group as a reference adjusted for age, sex, HbA1c, known duration of diabetes, SBP, and current smoking were estimated by Cox regression. All P values are two sided, and the significance level is 0.05. All statistical analyses and data management were conducted at a central data center using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
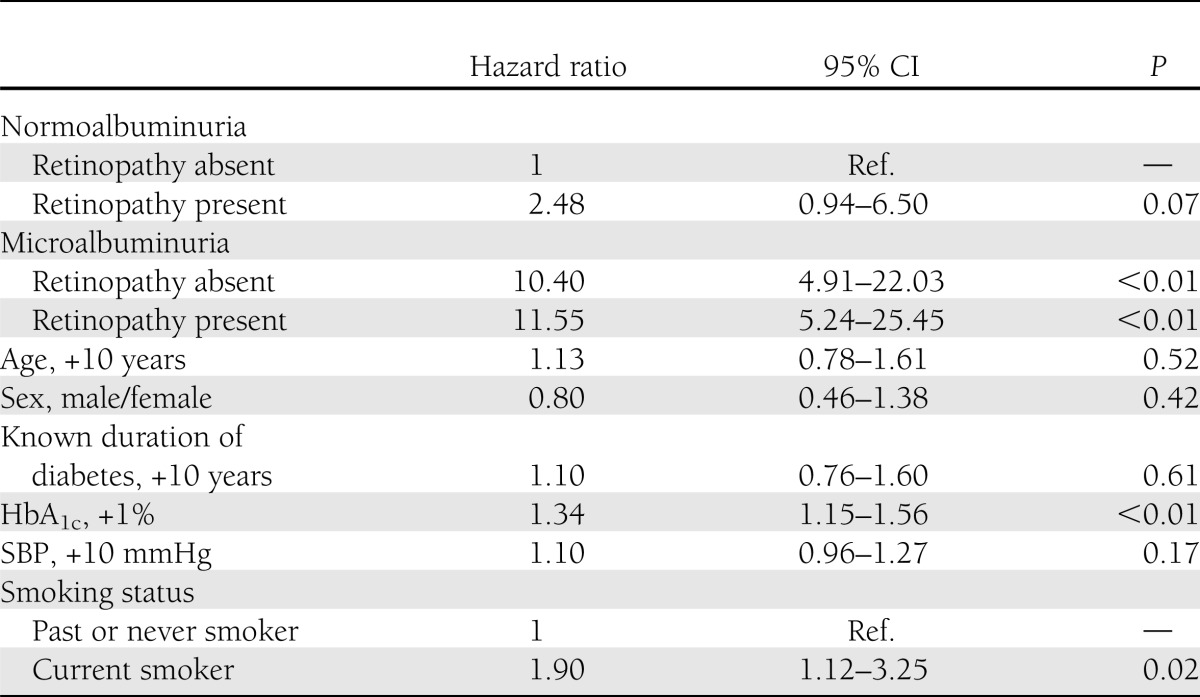
Baseline clinical characteristics between four diabetic groups
LDL cholesterol, eGFR, and treatment of dyslipidemia did not differ between the four groups. Age, sex, HbA1c, known duration of diabetes, BMI, blood pressure, HDL cholesterol, triglyceride, percent of current smokers, or treatment of diabetes and hypertension were different among the four groups at the baseline. UACR did not show any difference between NA(DR−) and NA(DR+) at normoalbuminuric levels or between MA(DR−) and MA(DR+) at microalbuminuric levels. The majority of the DR-positive patients had mild nonproliferative DR (stage 1) (Table 1).
Table 1.
Baseline characteristics of the 1,475 type 2 diabetic patients
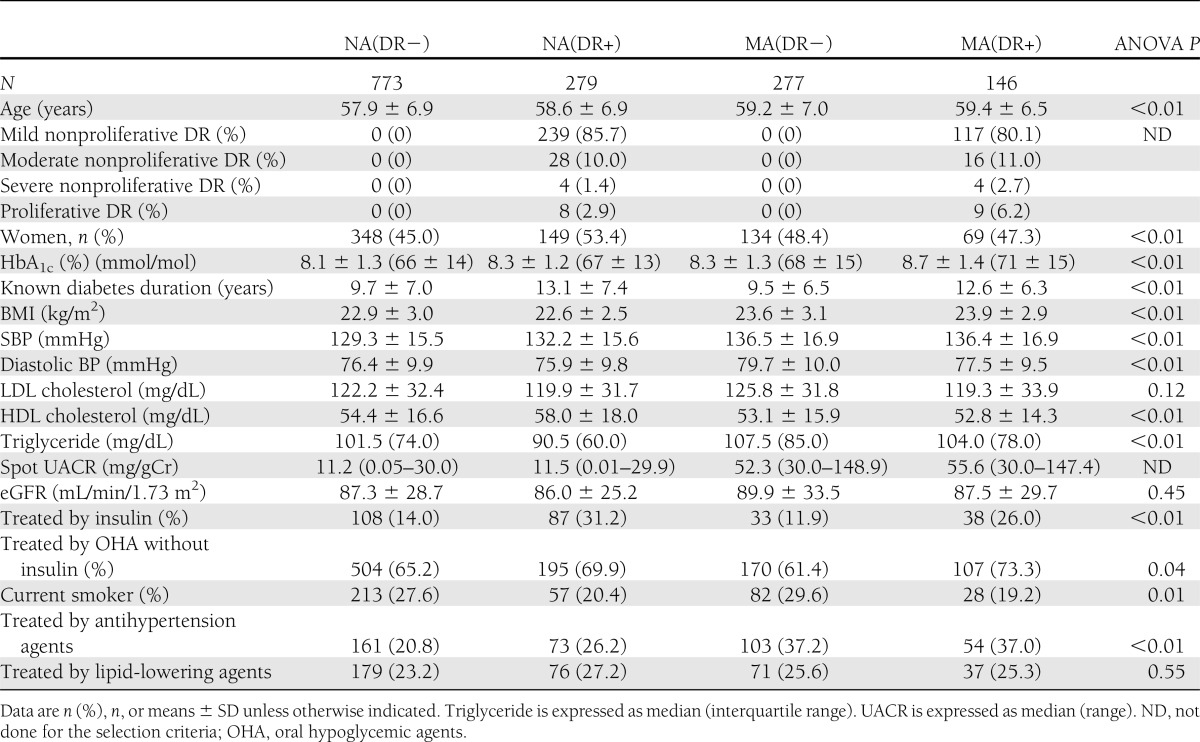
Cox regression analysis for baseline albuminuria/retinopathy and incidence of macroalbuminuria
During the follow-up of a median of 8.0 years, a total of 73 progressions to macroalbuminuria were observed. The follow-up rate at 8 years was 78%. The numbers of death were 58 (3.9%) during the observation. Annual incidence rates of macroalbuminuria were 1.6/1,000 person-years (9 incidences), 3.9/1,000 person-years (8 incidences), 18.4/1,000 person-years (34 incidences), and 22.1/1,000 person-years (22 incidences) in the NA(DR−), NA(DR+), MA(DR−), and MA(DR+) groups, respectively. Table 2 shows multivariate-adjusted hazard ratios for the progression to macroalbuminuria. As shown, the hazard ratio of NA(DR+) compared with NA(DR−) was 2.48 (95% CI 0.94–6.50, P = 0.07). However, hazard ratios in MA(DR−) and MA(DR+) were 10.40 (4.91–22.03, P < 0.01), 11.55 (5.24–25.45, P < 0.01), and significantly higher than NA(DR−). These results also indicate that the hazard ratio for the direct comparisons between MA(DR+) and MA(DR−) is 0.90 (0.51–1.56, P = 0.72). Further, quantitatively similar trends are observed across severity of DR and microalbuminuria; hazard ratios of normoalbuminuria with stage 1, normoalbuminuria with stage 2–4, MA(DR−), microalbuminuria with stage 1 and microalbuminuria with stage 2–4 compared with NA(DR−) were 2.56 (0.95–6.90, P = 0.06), 2.38 (0.30–18.85, P = 0.41), 10.41 (4.92–22.01, P < 0.01), 10.06 (4.36–23.21, P < 0.01), and 21.30 (7.84–57.87, P < 0.01), respectively, using the same adjustment variables.
Table 2.
Cox regression analysis for the incidence of macroalbuminuria by presence or absence of baseline albuminuria/retinopathy and other risk characteristics
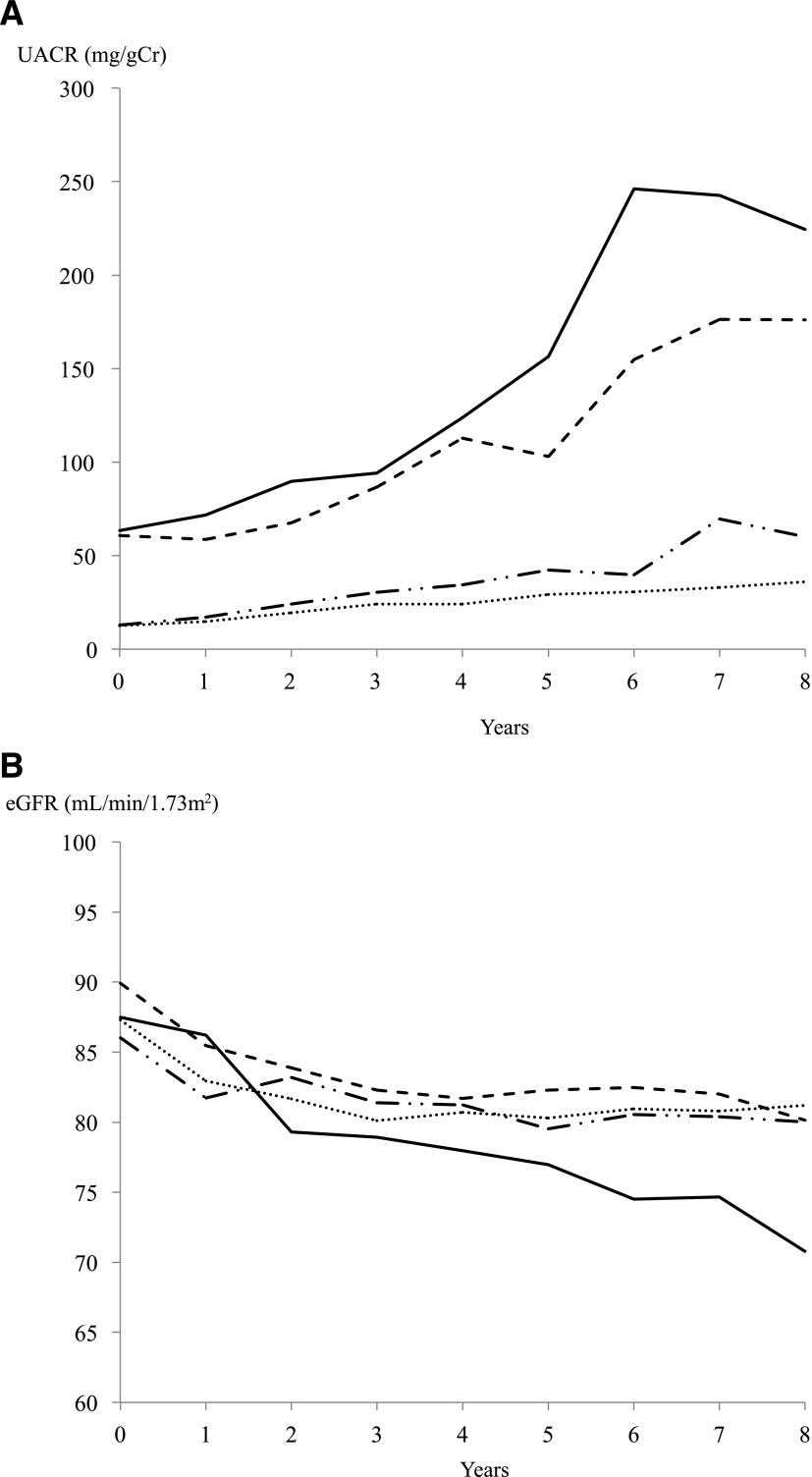
Clinical course of urinary albumin and eGFR among four groups >8 years old
Figure 1A and B show the UACR and eGFR over 8 years. The UACR had a trend toward an increase over time, and eGFR were decreased over time in all four groups. Annual increase rates of UACR in NA(DR+), MA(DR−) and MA(DR+) were 6.76 mg/gCr/year (95% CI 4.53–8.99, P < 0.01), 16.35 mg/gCr/year (13.97–18.74, P < 0.01), and 25.27 mg/gCr/year (22.13–28.41, P < 0.01), respectively, and they were significantly higher than in NA(DR−), which was 3.05 mg/gCr/year (1.72–4.39, P < 0.01). GFR decline per year in MA(DR+) was −1.92 mL/min/1.73 m2/year (−2.28 to −1.55, P < 0.01) and was significantly faster than in NA(DR−), NA(DR+), and MA(DR−), which were −0.54 mL/min/1.73 m2/year (−0.70 to −0.39, P < 0.01), −0.69 mL/min/1.73 m2/year (−0.96 to −0.42, P < 0.01), and −0.69 mL/min/1.73 m2/year (−0.96 to −0.42, P < 0.01), respectively.
Figure 1.
The annual increase rate of UACR and decline rate of eGFR in each group. Dotted line, both normal; dash-dot line, retinopathy only; dashed line, albuminuria only; solid line, both abnormal. A: Two microalbuminuric groups showed a striking increase in UACR during the 8-year observation, although UACR in the two normoalbuminuric groups gradually increased more or less. B: The eGFR decline rate in the MA(DR+) group was significantly faster than that in the other three groups.
Course of UAE according to baseline diabetic retinopathy
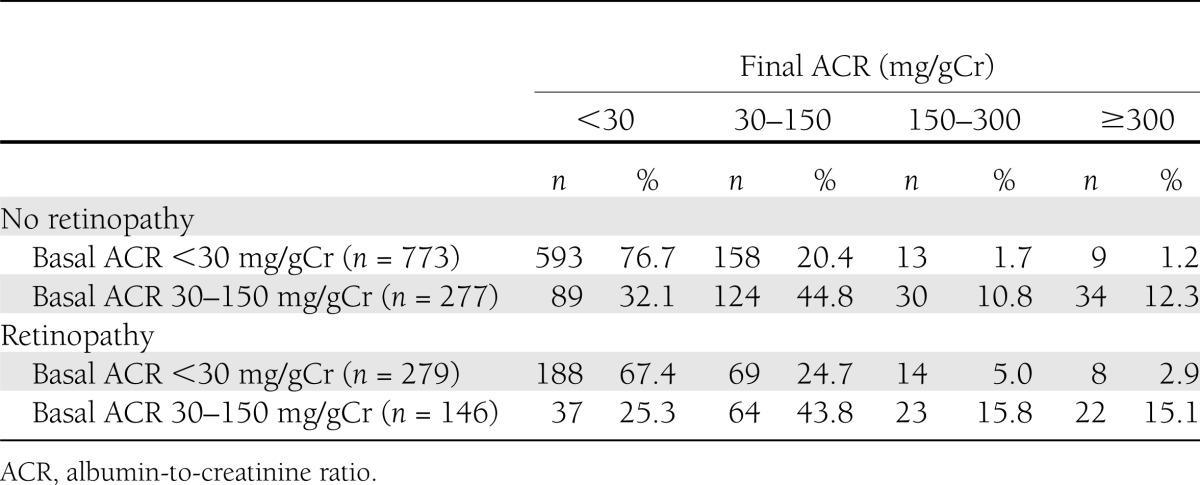
As we found in the multivariate analysis in Table 2, patients with DR progressed from normoalbuminuria to high microalbuminuria (UACR; 150–300 mg/gCr) or macroalbuminuria (UACR >300 mg/gCr) more frequently than those without DR. However, remission rates of MA(DR−) and MA(DR+) groups from low microalbuminuria (UACR; 30 to 150 mg/gCr) at baseline to normoalbuminuria at the 8-year follow-up were 32.1 and 25.3%, respectively, showing no significant difference (P = 0.18) (Table 3).
Table 3.
Course of UAE according to baseline diabetic retinopathy
CONCLUSIONS
In the previous report about DN in JDCS (9), we showed that the progression rate to macroalbuminuria from normoalbuminuria and low microalbuminuria was very low, and remission, i.e., normalization of low microalbuminuria to normoalbuminuria, was observed in 30.3% of patients. In the current study, we have shown that progression to macroalbuminuria was 2.48, 10.40, and 11.55 times faster than DR-free normoalbuminuria if patients had NA(DR+), MA(DR−), or MA(DR+), respectively. Of interest is the observation that the presence of both DR and microalbuminuria might be an important predictor of GFR decline in normoalbuminuric and low microalbuminuric type 2 diabetic patients during 8 years of follow-up.
Microalbuminuria, a phenotype of early DN, is one of the risk factors of macroalbuminuria (7,8). In addition, macroalbuminuria itself is known to be a risk factor resulting in renal function decline. In fact, a subset of microalbuminuric patients showed a rapid deterioration of renal function, which was evaluated with cystatin C–based eGFR (10). Another report (21) showed that normoalbuminuric type 2 diabetic patients had a decline in GFR similar to that in normal control subjects, while microalbuminuric patients showed more GFR loss for a 10-year follow-up. However, these reports (10,21) showed little information regarding DR. Microalbuminuria was indicated as a risk factor of DR in type 1 diabetic patients but not in type 2 diabetic patients (1). Thus, whether microalbuminuria itself or DR itself results in GFR decline must be examined to elucidate the exact and detailed clinical course of DN including both UAE and GFR changes (1).
Recently, DR has become known as a risk factor for all-cause mortality (22), cardiovascular event, and subclinical atherosclerosis (23) or cardiovascular disease (24). However, it is still obscure whether DR had some effects on UAE increase or GFR decline, especially in normoalbuminuric and low microalbuminuric diabetic patients. Thirty-eight Caucasian type 2 diabetic patients with macroalbuminuria showed higher rates of GFR decline during 6 years of observation when the patients had DR compared with the patients without DR (25). On the other hand, 25 Danish type 1 diabetic patients without macroalbuminuria revealed higher transition rates to macroalbuminuria when the patients had proliferative DR (13). In the current study, microalbuminuric patients with DR obviously revealed GFR decline, while normoalbuminuric patients with DR had a trend toward increase of UAE. In addition, hazard ratio was increased according to DR severity grade, especially in microalbuminuric patients. Therefore, we need to perform ophthalmological examination to detect DR carefully, especially in the patients with normo- or microalbuminuria, to identify persons at higher risk of developing macroalbuminuria. It has not been shown that DR itself is related to renal function decline, although some reports have shown that urinary abnormalities, microalbuminuria, or macroalbuminuria predicts DR (26), and microalbuminuria has been indicated to have a greater impact on predicting DR than GFR decline in type 2 diabetic patients (27).
There are few reports that both microalbuminuria and DR predict renal function loss. In Chinese populations, the reduction of eGFR of >50% or progression to eGFR <15 mL/min/1.73 m2 or end-stage renal disease was predicted in the type 2 diabetic patients with microalbuminuria or DR compared with the patients with no complications (28). The risk of the renal outcome was obviously increased when both DR and microalbuminuria or macroalbuminuria were present (28). However, the report (28) did not show that the slope of GFR decline was related to the presence of microalbuminuria and DR. Therefore, the current study demonstrates for the first time that the rate of GFR decline was faster in patients with microalbuminuria and DR at the early stage of DN.
One of the reasons why UAE trended to be increased in normoalbuminuric patients with DR or GFR decreased in microalbuminuric patients with DR in the current study might be related to the severity of the renal histological changes including glomerular basement membrane (GBM) thickening and mesangial expansion. In fact, in normoalbuminuric type 1 diabetic patients, abnormal values of GBM thickness and mesangial expansion were more frequently seen in the patients with DR, and these histological changes aggravated according to DR grade (3). Another report (29) revealed that type 2 diabetic patients with macroalbuminuria showed more frequent Kimmelstiel-Wilson nodular lesions when the patients had proliferative DR. The patients with nodular glomerulosclerosis frequently had an increase of serum creatinine within 5 years of follow-up (30). In addition, it was shown that renal histological changes were heterogeneous in microalbuminuric type 2 diabetic patients, and three histological categories of renal injury patterns were previously indicated (31). In the report (31), DR was present in all the patients with typical diabetic glomerular sclerosis. No proliferative DR was seen in the patients with a normal/near-normal pattern or atypical renal histological injury, while background DR was observed in 50 and 57% of patients, respectively. More recently, a link between quantitative assessment of the retinal vessel caliber size and change in glomerulopathy index including mesangial expansion and GBM thickening during 5 years follow-up was reported in normotensive normoalbuminuric Caucasian type 1 diabetic patients (32). Change in the retinal vessel caliber size has been speculated to be reflecting inflammation, endothelial dysfunction, and prevalence and incidence of DR (33). Therefore, DR might reflect renal histological severity as diabetic glomerulosclerosis regardless of albuminuria. In microalbuminuric Caucasian type 1 diabetic patients, the rate of annual GFR decline was related to renal histological change (34). In addition, in normo- and microalbuminuric Japanese type 2 diabetic patients, it was shown that UAE increased 5.6 years after the renal biopsy when renal histological changes, including mesangial expansion, were more severe (35). Thus, the patients with DR have more severe diabetic glomerular changes, which are followed by UAE increase or GFR decline compared with the patients without DR. Further examinations will be warranted to confirm the difference of histological changes among the four groups shown in the current study.
In the JDCS cohort, both blood pressure and glycemic control were risk factors of the occurrence of DR and the transition from normo- and low microalbuminuria to macroalbuminuria, whereas the duration of diabetes was a predictor for DR and smoking was a predictor for DN (9,17). In the previous study (9), progression to macroalbuminuria was independently associated with higher baseline HbA1c and SBP levels in addition to an elevated baseline UACR; and smoking was a significant predictor of macroalbuminuria as well. These factors were also significant predictors of macroalbuminuria in the current study. Therefore, achievement of good glycemic and blood pressure control and smoking cessation education would be valuable to avoid GFR decline in type 2 diabetic patients, which will be examined in larger populations.
Our study has certain limitations. The first is that death before the onset of macroalbuminuria is a competing risk that potentially influences the association between macroalbuminuria and its risk factors. We handled such patients as censored because the mortality in this study was low (only 58 deaths [3.9%]) and seemed not to have much effect on the results. The second is that the prognosis of DR has not been examined yet. It is very important to clarify whether the presence of baseline microalbuminuria affects the progression of DR. Therefore, further study is warranted to analyze the prognosis of DR using the same cohort in the future. The final one is that the current study was established when angiotensin II receptor blockers (ARBs) were not widely used in our country, unfortunately. Since ARBs became available in 1998 in Japan, the number of renin-angiotensin system (RAS) inhibitor usage was small—12.3% at baseline—and was increased gradually to 28.4% at 8 years’ follow-up based on each physician’s decision—not on the protocol (9). It is well known that RAS inhibitors including ACE inhibitors and ARBs remit microalbuminuria (36) or retard the onset of microalbuminuria (37). It is important to examine the detailed course of albuminuria or GFR prospectively, focusing on the effects of RAS inhibitors in a Japanese cohort in the future.
In conclusion, the presence of microalbuminuria and/or DR profoundly affects renal function in type 2 diabetic patients. Therefore, diabetologists and ophthalmologists should share their acquired information regarding DN and DR, even if it is at milder stages.
Acknowledgments
The Ministry of Health, Labor and Welfare of Japan funded the study. The sponsor had no role in the design or conduct of the study.
No potential conflicts of interest relevant to this article were reported.
T.M. researched data, contributed to the discussion, and wrote the manuscript. S.T. analyzed data. R.K. contributed to the discussion and reviewed and edited the manuscript. Y.O. analyzed data. Y.A. and N.Y. contributed to discussion. H.S. contributed to discussion and reviewed and edited the manuscript. H.Y. contributed to discussion. S.K. contributed to the discussion and reviewed and edited the manuscript. H.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank many diabetologists and patients at the 59 participating institutes throughout Japan.
Footnotes
Clinical trial reg. no. UMIN CTR C000000222, www.umin.ac.jp/ctr.
References
- 1.Pedro RA, Ramon SA, Marc BB, Juan FB, Isabel MM. Prevalence and relationship between diabetic retinopathy and nephropathy, and its risk factors in the North-East of Spain, a population-based study. Ophthalmic Epidemiol 2010;17:251–265 [DOI] [PubMed] [Google Scholar]
- 2.Parving HH, Gall MA, Skøtt P, et al. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int 1992;41:758–762 [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Zinman B, Gardiner R, et al. Renin-Angiotensin System Study The relationship of diabetic retinopathy to preclinical diabetic glomerulopathy lesions in type 1 diabetic patients: the Renin-Angiotensin System Study. Diabetes 2005;54:527–533 [DOI] [PubMed] [Google Scholar]
- 4.Chavers BM, Mauer SM, Ramsay RC, Steffes MW. Relationship between retinal and glomerular lesions in IDDM patients. Diabetes 1994;43:441–446 [DOI] [PubMed] [Google Scholar]
- 5.Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med 1999;341:1127–1133 [DOI] [PubMed] [Google Scholar]
- 6.Caramori ML, Fioretto P, Mauer M. The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes 2000;49:1399–1408 [DOI] [PubMed] [Google Scholar]
- 7.Mogensen CE. Microalbuminuria as a predictor of clinical diabetic nephropathy. Kidney Int 1987;31:673–689 [DOI] [PubMed] [Google Scholar]
- 8.Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med 1984;311:89–93 [DOI] [PubMed] [Google Scholar]
- 9.Katayama S, Moriya T, Tanaka S, et al. Japan Diabetes Complications Study Group Low transition rate from normo- and low microalbuminuria to proteinuria in Japanese type 2 diabetic individuals: the Japan Diabetes Complications Study (JDCS). Diabetologia 2011;54:1025–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 2007;18:1353–1361 [DOI] [PubMed] [Google Scholar]
- 11.Wirta O, Pasternack A, Mustonen J, Laippala P, Lähde Y. Retinopathy is independently related to microalbuminuria in type 2 diabetes mellitus. Clin Nephrol 1999;51:329–334 [PubMed] [Google Scholar]
- 12.Kim KS, Koh JM, Song KH, et al. Incidence of overt proteinuria and coronary artery disease in patients with type 2 diabetes mellitus: the role of microalbuminuria and retinopathy. Diabetes Res Clin Pract 2004;65:159–165 [DOI] [PubMed] [Google Scholar]
- 13.Karlberg C, Falk C, Green A, Sjølie AK, Grauslund J. Proliferative retinopathy predicts nephropathy: a 25-year follow-up study of type 1 diabetic patients. Acta Diabetol 2012;49:263–268 [DOI] [PubMed] [Google Scholar]
- 14.Takazakura E, Nakamoto Y, Hayakawa H, Kawai K, Muramoto S. Onset and progression of diabetic glomerulosclerosis; a prospective study based on serial renal biopsies. Diabetes 1975;24:1–9 [DOI] [PubMed] [Google Scholar]
- 15.Sone H, Tanaka S, Iimuro S, et al. Japan Diabetes Complications Study Group Long-term lifestyle intervention lowers the incidence of stroke in Japanese patients with type 2 diabetes: a nationwide multicentre randomised controlled trial (the Japan Diabetes Complications Study). Diabetologia 2010;53:419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sone H, Ito H, Saito Y, et al. The long-term effects of self-management education for patients with type 2 diabetes on glycemic control: response to Norris et al. Diabetes Care 2002;25:2115–2116 [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki R, Tanaka S, Tanaka S, et al. Japan Diabetes Complications Study Group Incidence and progression of diabetic retinopathy in Japanese adults with type 2 diabetes: 8 year follow-up study of the Japan Diabetes Complications Study (JDCS). Diabetologia 2011;54:2288–2294 [DOI] [PubMed] [Google Scholar]
- 18.Makino H, Haneda M, Babazono T, et al. INNOVATION Study Group Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care 2007;30:1577–1578 [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson CP, Ferris FL, 3rd, Klein RE, et al. Global Diabetic Retinopathy Project Group Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–1682 [DOI] [PubMed] [Google Scholar]
- 20.Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982–992 [DOI] [PubMed] [Google Scholar]
- 21.Murussi M, Gross JL, Silveiro SP. Glomerular filtration rate changes in normoalbuminuric and microalbuminuric Type 2 diabetic patients and normal individuals A 10-year follow-up. J Diabetes Complications 2006;20:210–215 [DOI] [PubMed] [Google Scholar]
- 22.Kramer CK, Rodrigues TC, Canani LH, Gross JL, Azevedo MJ. Diabetic retinopathy predicts all-cause mortality and cardiovascular events in both type 1 and 2 diabetes: meta-analysis of observational studies. Diabetes Care 2011;34:1238–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawasaki R, Cheung N, Islam FM, et al. Multi-Ethnic Study of Atherosclerosis Is diabetic retinopathy related to subclinical cardiovascular disease? Ophthalmology 2011;118:860–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Son JW, Jang EH, Kim MK, et al. Diabetic retinopathy is associated with subclinical atherosclerosis in newly diagnosed type 2 diabetes mellitus. Diabetes Res Clin Pract 2011;91:253–259 [DOI] [PubMed] [Google Scholar]
- 25.Trevisan R, Vedovato M, Mazzon C, et al. Concomitance of diabetic retinopathy and proteinuria accelerates the rate of decline of kidney function in type 2 diabetic patients. Diabetes Care 2002;25:2026–2031 [DOI] [PubMed] [Google Scholar]
- 26.Estacio RO, McFarling E, Biggerstaff S, Jeffers BW, Johnson D, Schrier RW. Overt albuminuria predicts diabetic retinopathy in Hispanics with NIDDM. Am J Kidney Dis 1998;31:947–953 [DOI] [PubMed] [Google Scholar]
- 27.Chen YH, Chen HS, Tarng DC. More impact of microalbuminuria on retinopathy than moderately reduced GFR among type 2 diabetic patients. Diabetes Care 2012;35:803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong PC, Kong AP, So WY, et al. Interactive effect of retinopathy and macroalbuminuria on all-cause mortality, cardiovascular and renal end points in Chinese patients with Type 2 diabetes mellitus. Diabet Med 2007;24:741–746 [DOI] [PubMed] [Google Scholar]
- 29.Schwartz MM, Lewis EJ, Leonard-Martin T, Lewis JB, Batlle D, Grp CS, The Collaborative Study Group Renal pathology patterns in type II diabetes mellitus: relationship with retinopathy. Nephrol Dial Transplant 1998;13:2547–2552 [DOI] [PubMed] [Google Scholar]
- 30.Suzuki Y, Ueno M, Hayashi H, et al. Morphologic study of the kidney in Japanese non-insulin-dependent patients. J Diabet Complications 1991;5:79–81 [DOI] [PubMed] [Google Scholar]
- 31.Fioretto P, Mauer M, Brocco E, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia 1996;39:1569–1576 [DOI] [PubMed] [Google Scholar]
- 32.Klein R, Knudtson MD, Klein BE, et al. The relationship of retinal vessel diameter to changes in diabetic nephropathy structural variables in patients with type 1 diabetes. Diabetologia 2010;53:1638–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen TT, Wong TY. Retinal vascular changes and diabetic retinopathy. Curr Diab Rep 2009;9:277–283 [DOI] [PubMed] [Google Scholar]
- 34.Rudberg S, Osterby R. Decreasing glomerular filtration rate—an indicator of more advanced diabetic glomerulopathy in the early course of microalbuminuria in IDDM adolescents? Nephrol Dial Transplant 1997;12:1149–1154 [DOI] [PubMed] [Google Scholar]
- 35.Moriya T, Tanaka K, Hosaka T, Hirasawa Y, Fujita Y. Renal structure as an indicator for development of albuminuria in normo- and microalbuminuric type 2 diabetic patients. Diabetes Res Clin Pract 2008;82:298–304 [DOI] [PubMed] [Google Scholar]
- 36.Araki S, Haneda M, Sugimoto T, et al. Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes 2005;54:2983–2987 [DOI] [PubMed] [Google Scholar]
- 37.Haller H, Ito S, Izzo JL, Jr, et al. ROADMAP Trial Investigators Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 2011;364:907–917 [DOI] [PubMed] [Google Scholar]