Abstract
OBJECTIVE
Nonnutritive sweeteners (NNS), such as sucralose, have been reported to have metabolic effects in animal models. However, the relevance of these findings to human subjects is not clear. We evaluated the acute effects of sucralose ingestion on the metabolic response to an oral glucose load in obese subjects.
RESEARCH DESIGN AND METHODS
Seventeen obese subjects (BMI 42.3 ± 1.6 kg/m2) who did not use NNS and were insulin sensitive (based on a homeostasis model assessment of insulin resistance score ≤2.6) underwent a 5-h modified oral glucose tolerance test on two separate occasions preceded by consuming either sucralose (experimental condition) or water (control condition) 10 min before the glucose load in a randomized crossover design. Indices of β-cell function, insulin sensitivity (SI), and insulin clearance rates were estimated by using minimal models of glucose, insulin, and C-peptide kinetics.
RESULTS
Compared with the control condition, sucralose ingestion caused 1) a greater incremental increase in peak plasma glucose concentrations (4.2 ± 0.2 vs. 4.8 ± 0.3 mmol/L; P = 0.03), 2) a 20 ± 8% greater incremental increase in insulin area under the curve (AUC) (P < 0.03), 3) a 22 ± 7% greater peak insulin secretion rate (P < 0.02), 4) a 7 ± 4% decrease in insulin clearance (P = 0.04), and 5) a 23 ± 20% decrease in SI (P = 0.01). There were no significant differences between conditions in active glucagon-like peptide 1, glucose-dependent insulinotropic polypeptide, glucagon incremental AUC, or indices of the sensitivity of the β-cell response to glucose.
CONCLUSIONS
These data demonstrate that sucralose affects the glycemic and insulin responses to an oral glucose load in obese people who do not normally consume NNS.
Most people, like other mammals, are innately attracted to consuming sweet-tasting foods. Nonnutritive sweeteners (NNS) are food additives that provide a sweet taste to food but have few, if any, calories. Therefore, the use of NNS has become a popular approach to help reduce energy intake and glycemic load (1,2). Currently, seven NNS (sucralose, saccharin, aspartame, acesulfame potassium, neotame, stevia, and Luo han guo extract) are approved by the U.S. Food and Drug Administration and are widely used in thousands of food products.
Although it has been proposed that NNS do not affect glycemia (3), data from several recent studies suggest that NNS are not physiologically inert. First, it has been demonstrated that the gastrointestinal tract (4,5) and the pancreas (6,7) can detect sugars through taste receptors and transduction mechanisms that are similar to those indentified in taste cells in the mouth. Second, NNS-induced activation of gut sweet taste receptors in isolated duodenal L cells and pancreatic β-cells triggers the secretion of glucagon-like peptide 1 (GLP-1) (4,5) and insulin (6–9), respectively. Third, data from studies conducted in animal models demonstrate that NNS interact with sweet taste receptors expressed in enteroendocrine cells to increase both active and passive intestinal glucose absorption by upregulating the expression of sodium-dependent glucose transporter isoform 1 (5,10,11) and increasing the translocation of GLUT2 to the apical membrane of intestinal epithelia (12).
The relevance of the findings from studies conducted in cell systems and rodent models to human physiology is not clear because the NNS data obtained from studies conducted in people often fail to replicate the metabolic outcomes observed in vitro and in animal models (rev. in 13). The results from most (14–18), but not all (19,20), studies conducted in people have found that NNS do not affect plasma glucose, insulin, or GLP-1. However, these studies did not exclude people who regularly consumed NNS, which could have chronic effects on glucose metabolism (5,10,11) that would blunt any acute effects of sucralose intake.
The primary purpose of this study was to test the hypothesis that sucralose ingestion alters the glycemic and hormonal responses to glucose ingestion in obese subjects who are not regular users of NNS. We specifically studied obese people because NNS are often promoted to help decrease calorie intake and facilitate weight management in this population.
RESEARCH DESIGN AND METHODS
Seventeen obese subjects (BMI 41.0 ± 1.5 kg/m2, age 35.1 ± 1.0 years, 15 female and 2 male, 13 African American and 4 Caucasian), who were not markedly insulin resistant based on homeostasis model assessment of insulin resistance score ≤2.6 (21), participated in the study. Potential subjects were interviewed with a questionnaire used in previous studies (22) that inquired about 1) the type of sweetener used for coffee, tea, and other drinks; 2) current intake of diet beverages (including soda, juice, ice tea, and flavor water), 3) current intake of yogurt, pudding, gelatin, or other snacks foods sweetened with NNS; and 3) current use of gums containing NNS. For each type of product, potential participants were asked whether they had used it in the past month and, if so, on how many days per week and how many servings per day. Subjects who reported consuming more than one can of diet beverage or one spoonful of NNS a week (or its equivalent from foods) were excluded. In addition, those who smoked cigarettes in the last six months; were pregnant or breastfeeding; had a history of malabsorptive syndromes, bariatric surgery, or inflammatory intestinal disease; or were taking any medication that might affect glucose metabolism were excluded. This study was approved by the institutional review board at Washington University School of Medicine in St. Louis, and each subject gave informed written consent before participation.
Subjects were studied on two separate occasions, ∼7 days apart, in a crossover design. For each study, subjects were admitted in the morning to the Clinical Research Unit at Washington University School of Medicine at 0700 h after subjects fasted overnight (12 h) at home. A catheter was placed in a hand vein and heated in a warming box (55°C) to obtain arterialized venous samples (23). Blood samples were obtained to assess plasma glucose, insulin, C-peptide, glucagon, glucose-dependent insulinotropic polypeptide (GIP), and active GLP-1 concentrations at 20, 15, 10, 6, and 2 min before and at 10, 20, 30, 40, 60, 90, 120, 150, 180, 240, and 300 min after ingesting 75 g glucose. In randomized order, subjects drank 60 mL of 2 mmol/L sucralose (i.e., 48 mg sucralose) or an equivalent volume of distilled water 10 min before glucose ingestion. This concentration of sucralose was used because it is the effective concentration needed to stimulate GLP-1 secretion in human intestinal cells in vitro (4) and it matches the sweetness of a typical diet soda; i.e., it approximates the amount of sucralose in a standard 12-oz can of diet soda if it is all sweetened with sucralose (24).
Laboratory assessments
Biochemical measurements.
Plasma glucose was measured immediately after collection by using an automated glucose analyzer (YSI 2300 STAT plus; Yellow Springs Instruments, Yellow Spring, OH). Blood samples were also collected in chilled EDTA tubes containing a protease inhibitor cocktail (Millipore, Billerica, MA). These samples were placed on ice and centrifuged at 4°C, and the plasma was stored at −80°C for subsequent analyses. Plasma active GLP-1 and GIP were measured by using commercially available immunoassay kits from Meso Scale Discovery (Gaithersburg, MD) and Millipore, respectively. Plasma C-peptide was measured by using a solid-phase two-site chemiluminescent immunometric assay (Siemens Medical Solutions Diagnostics, Los Angeles, CA), plasma insulin concentrations were determined by using a two-site immunoenzymatic assay (DxI 800; Beckman Instruments, Chaska, MN), and plasma glucagon was measured by a direct, double-antibody radioimmunoassay (Millipore).
Calculations
Metabolic response to glucose ingestion.
The incremental areas under the curve (AUCs) above baseline concentrations for glucose, insulin, C-peptide, glucagon, GIP, and GLP-1 were calculated by using the trapezoid method (25).
Insulin sensitivity and clearance.
The insulin sensitivity index [SI: dL ⋅ kg−1 ⋅ min−1/(pmol/L)] was determined from a minimal model of the glucose concentration as a function of the insulin concentration (26). Insulin clearance rate from plasma was calculated by dividing the mean insulin secretion rate (ISR) by the mean plasma insulin concentration (27).
β-Cell function.
Plasma C-peptide and glucose concentrations were used to determine the ISR in response to the oral glucose load and the sensitivity of the β-cell response (ISR) to changes in plasma glucose by using a minimal model (25). This model provides an estimate of the total amount of insulin secreted in response to plasma glucose as a function of time (i.e., total ISR in pmol/min) and partitions this total response into a dynamic component (ISRdynamic), which represents the rapid release of a readily releasable pool of insulin secretory granules in response to the rate of increasing plasma glucose concentration, and a static component (ISRstatic), which represents the slower release of a reserve pool of insulin secretory granules in response to the ambient plasma glucose concentration (28). The β-cell response sensitivity parameters (Φtotal, Φdynamic, and Φstatic) corresponding to the total, dynamic, and static ISR in response to changes in plasma glucose were determined (29).
Statistical analyses
The statistical significance of the effect of sucralose on glucose, insulin, C-peptide, glucagon, GIP, and active GLP-1 concentrations and ISR after a glucose load was determined by conducting separate repeated ANOVAs for each outcome variable with condition (sucralose and water) and time point as within-subject factors. When differences in values were statistically significant, a post hoc Bonferroni adjustment to Fisher least significant differences analyses was conducted. Active GLP-1, SI, and Φstatic data were positively skewed and required logarithmic transformation to approximate a normal distribution. The significance of differences in incremental peaks and AUCs, SI, and insulin clearance was evaluated by using a paired t test or Wilcoxon matched pairs test, as appropriate. Data in the tables and figures are presented as means ± SEM or median (semi-interquartile range: [75th–25th percentile]/2) for skewed datasets. All analyses were performed with STATISTICA 8.0 (StatSoft, Tulsa, OK), and criterion for statistical significance was P < 0.05.
RESULTS
Plasma glucose and hormonal responses to a glucose load were altered by sucralose
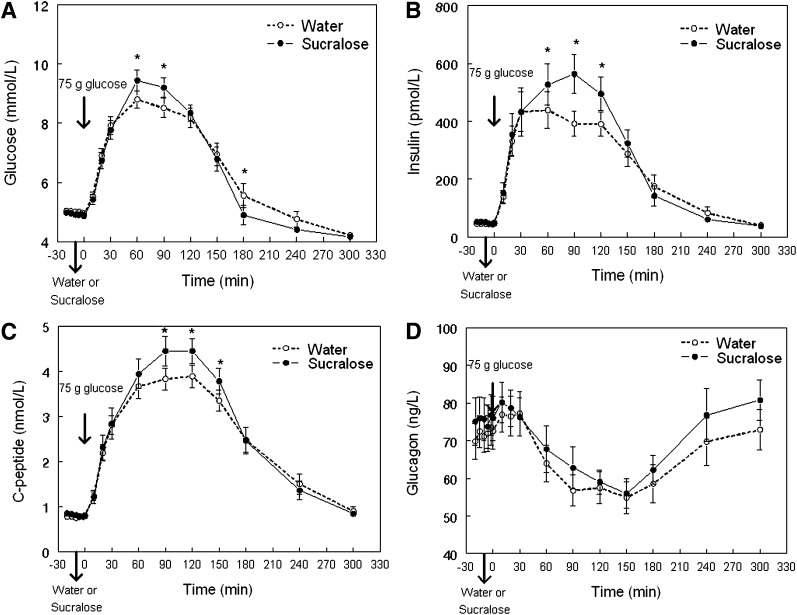
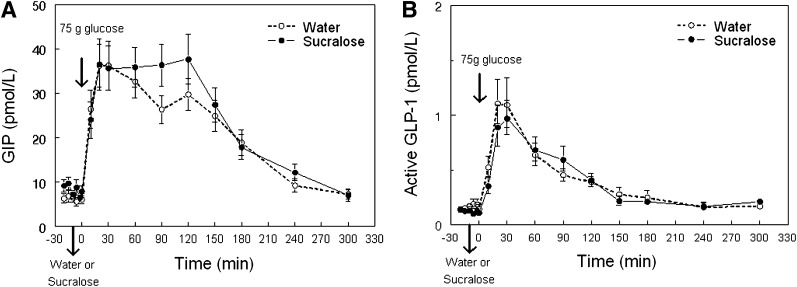
Mean peak plasma glucose concentration was higher and the subsequent nadir was lower after sucralose than after water ingestion (Fig. 1A and Table 1). Peak plasma insulin and C-peptide concentrations were also higher after sucralose than after water ingestion (Fig. 1B and C and Table 1). No significant differences in the incremental AUC of glucose or C-peptide were detected in response to the glucose load after sucralose and water ingestion. However, the incremental AUC of insulin was 20 ± 8% greater after sucralose than after water ingestion (P < 0.03) (Table 1). Plasma glucagon concentration and the decremental glucagon AUC after the glucose load were similar after sucralose and water ingestion (Fig. 1D). Although average plasma GIP concentrations tended to be higher after sucralose than after water ingestion (20 ± 8 vs. 18 ± 7 pmol/L), the difference was not statistically significant (P = 0.08) (Fig. 2A). Plasma active GLP-1 concentration, incremental GLP-1 AUC, and incremental GIP AUC after the glucose load were not different after sucralose or water ingestion (Fig. 2B and Table 1).
Figure 1.
Mean plasma glucose (A), insulin (B), C-peptide (C), and glucagon (D) concentrations in obese subjects after drinking either sucralose or water 10 min before ingestion of a 75-g glucose load (given at time = 0 min). *Value significantly different from corresponding water condition value, P < 0.004.
Table 1.
Metabolic response to an oral 75-g glucose load preceded by either sucralose or water ingestion
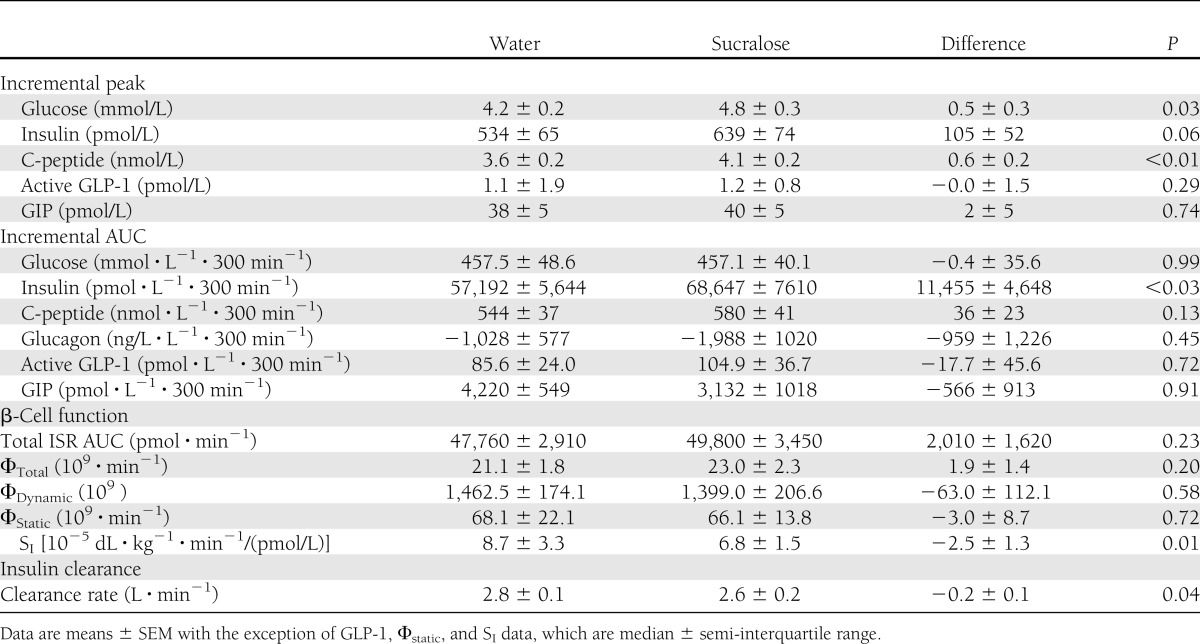
Figure 2.
Mean plasma GIP (A) and active GLP-1 (B) concentrations in obese subjects after drinking either sucralose or water 10 min before ingestion of a 75-g glucose load (given at time = 0 min).
Insulin clearance and insulin sensitivity were altered by sucralose
Sucralose ingestion decreased the insulin clearance rate after ingesting the glucose load by 7 ± 4% (P < 0.05). The median SI value was 23 ± 20% lower after sucralose than after water ingestion (P < 0.01) (Table 1).
β-Cell response to glucose ingestion is altered by sucralose
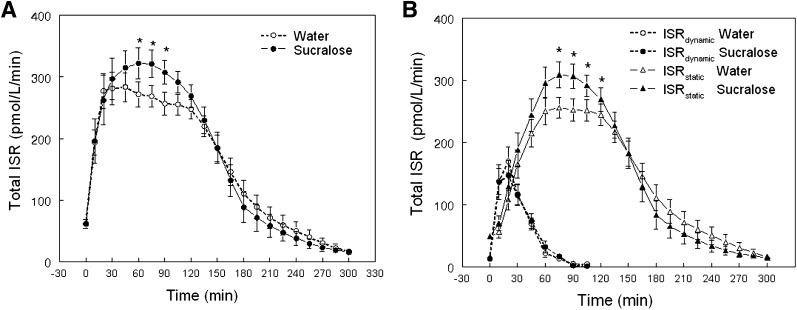
Total ISR AUCs in response to the oral glucose load and the sensitivity of insulin secretion to plasma glucose (Φtotal, Φdynamic, and Φstatic) were not different between sucralose and water conditions (Table 1). However, differences in plasma glucose concentration between conditions caused a higher peak in the ISR after sucralose than after water (Fig. 3A), which was exclusively due to an average increase of ∼22 ± 4% in the static ISR between 60 and 105 min (P < 0.005); the dynamic ISR curves were the same after sucralose and water ingestion (Fig. 3B).
Figure 3.
ISR in response to a glucose load after subjects drank either sucralose 10 min before ingestion of 75 g glucose (given at time = 0 min). Total ISR (A) and ISRdynamic and ISRstatic components (B). *Value significantly different from corresponding water condition value, P < 0.002.
CONCLUSIONS
The results from the current study demonstrate that the ingestion of sucralose alters the metabolic response to an oral glucose load in obese people who are not regular consumers of NNS. The peak increase plasma glucose, C-peptide, and insulin concentrations and total insulin AUC after an oral glucose load were greater when subjects consumed sucralose than when they consumed water before glucose ingestion. In addition, insulin clearance from plasma was slower after sucralose than after water ingestion. These data suggest that sucralose ingestion is not physiologically inert but affects the glycemic response to an oral glucose load and potentiates glucose-stimulated insulin secretion in obese people.
The finding that glucose-induced glucagon suppression was the same after both sucralose and water ingestion makes it unlikely that glucagon was responsible for the differences between conditions. The mechanisms responsible for the sucralose effect on plasma glucose after an oral glucose load are not clear but must involve an alteration in the rate of glucose absorption, disposal, or endogenous production. Data from previous studies conducted in animal models showed that sucralose augments glucose absorption by increasing intestinal glucose transport (11,12). Our results support this notion because sucralose increased the early peak in plasma glucose but did not affect the indices of β-cell sensitivity (Φtotal, Φdynamic, and Φstatic) to plasma glucose.
We found that SI decreased after sucralose ingestion, suggesting that sucralose caused insulin resistance. However, the minimal model used to calculate SI from a modified OGTT is unable to determine whether the decrease in SI was caused by insulin resistance in a specific organ (e.g., liver or skeletal muscle) or multiple organs. Additional studies that involve the use of glucose tracers to quantify the effect of sucralose on insulin-mediated suppression of endogenous glucose production and insulin-stimulated muscle glucose uptake are needed to further explore our findings.
Sucralose ingestion did not affect the GLP-1 response to a glucose load in our subjects, which is consistent with the data reported in previous studies conducted in human subjects (15,18). This finding suggests that the observed increase in the ISR observed in our subjects was mediated by a GLP-1–independent mechanism. There was a trend suggesting that GIP contributed to the potentiated glucose-stimulated insulin secretion, and a confirmatory study is needed. In contrast, the data from previous studies found that the oral ingestion of a NNS before a glucose load augmented GLP-1 (19,20) but did not affect GIP secretion. However, in those studies total GLP-1—not the biologically active form of GLP-1—was measured (19,20). Although it is possible that the discrepancy between GLP-1 findings of those and our study is the result of this methodological difference, we think it is unlikely because active and total GLP-1 are highly correlated with each other (30). A second methodological difference is that in those studies a diet cola sweetened with both sucralose and acesulfame potassium (19,20) was used, so it is unclear whether the enhanced glucose-stimulated GLP-1 response was mediated by acesulfame potassium, a synergistic effect of both sweeteners, or other ingredients contained in the carbonated drinks (20). Additional studies that examine the metabolic effects of different NNS and potential interactions with other dietary ingredients are needed.
We found a modest reduction in insulin clearance after sucralose was ingested. This finding suggests that intestinal sweet taste receptors (4), which are activated by NNS, are involved in regulating insulin metabolism. This observation also raises the possibility that sweet taste receptors contribute to the unexplained reduction in insulin clearance observed after an oral, but not intravenous, glucose load (31,32).
A series of studies conducted in human subjects have reported that sucralose does not affect the glycemic or hormonal responses to intraduodenal (15) or oral administration of glucose or other carbohydrates (16,18,33) (rev. in 13). The reason(s) for the discrepancy between findings may be related to study subject selection. To increase sample homogeneity, we only included subjects who were obese, were insulin sensitive based on a homeostasis model assessment score ≤2.6, and were not regular users of NNS. By decreasing variability, it is likely that our study had a greater statistical power than previous studies to detect a sucralose effect. In addition, most subjects in previous studies were Caucasian (15,16,18,20), whereas most subjects in our study were African American. We believe this study is the first to evaluate the acute effects of sucralose in subjects who are not regular users of NNS. Data from studies conducted in animal models have shown that chronic inclusion of NNS in the diet upregulates the expression of sodium-dependent glucose transporter isoform 1, which in turn increases the initial rate of Na+-dependent glucose uptake (5,10) and increases glycemic responses after an oral glucose tolerance test (34). Therefore, we speculate that regular users of NNS would have a higher glycemic response after an oral glucose tolerance test on the control day than irregular users and that the acute effects of sucralose intake would be blunted because differences between water and sucralose conditions would be smaller in regular than in irregular users of NNS.
In conclusion, the results from our study demonstrate that sucralose affects the glycemic and hormonal responses to an oral glucose load in obese people who do not normally consume NNS. These findings support the notion that sucralose is not metabolically inert but has physiologic effects. Additional studies of other NNS, conducted in distinct study populations, including children and chronic NNS users, and that evaluate the effect of NNS on the metabolic response to mixed-meal ingestion are needed.
Acknowledgments
This study was supported by National Institutes of Health (NIH) Clinical and Translational Sciences Award UL1 TR000448, subaward KL2 TR000450, and NIH grants DK088126, DK37948, and DK56341 (Nutrition Obesity Research Center).
No potential conflicts of interest relevant to this article were reported.
M.Y.P. helped with the study design and concepts, collated and analyzed data, wrote the manuscript, obtained funding, had overall responsibility for the project, and approved the final draft of the manuscript. C.D.T. helped with subject recruitment and collecting data, reviewed and edited the manuscript, and approved the final draft of the manuscript. B.W.P. analyzed and interpreted data, reviewed and edited the manuscript, and approved the final draft of the manuscript. B.M.W. performed active GLP-1 analyses, helped with data analysis and interpretation, reviewed and edited the manuscript, and approved the final draft of the manuscript. S.K. helped with the study design and concepts and data analysis and interpretation, reviewed and edited the manuscript, and approved the final draft of the manuscript. M.Y.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank C. Bourne for help with subject recruitment; J. Sonnenschein, I. Gruchevska, and S. Wang for technical assistance; the staff of the Clinical Research Unit for help in performing the studies; Dr. F. Magkos and J.P. Miller for statistical advice; the study subjects for participation; and Tate & Lyle (London, U.K.) for providing sucralose.
Footnotes
Clinical trial reg. no. NCT01128829, clinicaltrials.gov.
References
- 1.Sylvetsky AC, Welsh JA, Brown RJ, Vos MB. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr 2012;96:640–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardner C, Wylie-Rosett J, Gidding SS, et al. American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity and Metabolism, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Disease in the Young. American Diabetes Association Nonnutritive sweeteners: current use and health perspectives: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2012;35:1798–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raben A, Richelsen B. Artificial sweeteners: a place in the field of functional foods? Focus on obesity and related metabolic disorders. Curr Opin Clin Nutr Metab Care 2012;15:597–604 [DOI] [PubMed] [Google Scholar]
- 4.Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 2007;104:15069–15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 2007;104:15075–15080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakagawa Y, Nagasawa M, Yamada S, et al. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS ONE 2009;4:e5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci USA 2012;109:E524–E532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malaisse WJ, Vanonderbergen A, Louchami K, Jijakli H, Malaisse-Lagae F. Effects of artificial sweeteners on insulin release and cationic fluxes in rat pancreatic islets. Cell Signal 1998;10:727–733 [DOI] [PubMed] [Google Scholar]
- 9.Corkey BE. Banting lecture 2011: hyperinsulinemia: cause or consequence? Diabetes 2012;61:4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran AW, Al-Rammahi MA, Arora DK, et al. Expression of Na+/glucose co-transporter 1 (SGLT1) is enhanced by supplementation of the diet of weaning piglets with artificial sweeteners. Br J Nutr 2010;104:637–646 [DOI] [PubMed] [Google Scholar]
- 11.Stearns AT, Balakrishnan A, Rhoads DB, Tavakkolizadeh A. Rapid upregulation of sodium-glucose transporter SGLT1 in response to intestinal sweet taste stimulation. Ann Surg 2010;251:865–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 2007;582:379–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown RJ, Rother KI. Non-nutritive sweeteners and their role in the gastrointestinal tract. J Clin Endocrinol Metab 2012;97:2597–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma J, Bellon M, Wishart JM, et al. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol 2009;296:G735–G739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J, Chang J, Checklin HL, et al. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br J Nutr 2010;104:803–806 [DOI] [PubMed] [Google Scholar]
- 16.Ford HE, Peters V, Martin NM, et al. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur J Clin Nutr 2011;65:508–513 [DOI] [PubMed] [Google Scholar]
- 17.Steinert RE, Frey F, Töpfer A, Drewe J, Beglinger C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br J Nutr 2011;105:1320–1328 [DOI] [PubMed] [Google Scholar]
- 18.Wu T, Zhao BR, Bound MJ, et al. Effects of different sweet preloads on incretin hormone secretion, gastric emptying, and postprandial glycemia in healthy humans. Am J Clin Nutr 2012;95:78–83 [DOI] [PubMed] [Google Scholar]
- 19.Brown RJ, Walter M, Rother KI. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care 2009;32:2184–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown RJ, Walter M, Rother KI. Effects of diet soda on gut hormones in youths with diabetes. Diabetes Care 2012;35:959–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care 2003;26:3320–3325 [DOI] [PubMed] [Google Scholar]
- 22.Klein DA, Boudreau GS, Devlin MJ, Walsh BT. Artificial sweetener use among individuals with eating disorders. Int J Eat Disord 2006;39:341–345 [DOI] [PubMed] [Google Scholar]
- 23.McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol 1976;41:565–573 [DOI] [PubMed] [Google Scholar]
- 24.All about sucralose [article online], 2012. Available from http://sucralose.org/questions/default.asp Accessed 4 February 2013
- 25.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care 1995;18:245–250 [DOI] [PubMed] [Google Scholar]
- 26.Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C. Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am J Physiol Endocrinol Metab 2004;287:E637–E643 [DOI] [PubMed] [Google Scholar]
- 27.Tillil H, Shapiro ET, Miller MA, et al. Dose-dependent effects of oral and intravenous glucose on insulin secretion and clearance in normal humans. Am J Physiol 1988;254:E349–E357 [DOI] [PubMed] [Google Scholar]
- 28.Campioni M, Toffolo G, Shuster LT, Service FJ, Rizza RA, Cobelli C. Incretin effect potentiates beta-cell responsivity to glucose as well as to its rate of change: OGTT and matched intravenous study. Am J Physiol Endocrinol Metab 2007;292:E54–E60 [DOI] [PubMed] [Google Scholar]
- 29.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 2001;50:150–158 [DOI] [PubMed] [Google Scholar]
- 30.Heijboer AC, Frans A, Lomecky M, Blankenstein MA. Analysis of glucagon-like peptide 1; what to measure? Clin Chim Acta 2011;412:1191–1194 [DOI] [PubMed] [Google Scholar]
- 31.Madsbad S, Kehlet H, Hilsted J, Tronier B. Discrepancy between plasma C-peptide and insulin response to oral and intravenous glucose. Diabetes 1983;32:436–438 [DOI] [PubMed] [Google Scholar]
- 32.Meier JJ, Holst JJ, Schmidt WE, Nauck MA. Reduction of hepatic insulin clearance after oral glucose ingestion is not mediated by glucagon-like peptide 1 or gastric inhibitory polypeptide in humans. Am J Physiol Endocrinol Metab 2007;293:E849–E856 [DOI] [PubMed] [Google Scholar]
- 33.Brown AW, Bohan Brown MM, Onken KL, Beitz DC. Short-term consumption of sucralose, a nonnutritive sweetener, is similar to water with regard to select markers of hunger signaling and short-term glucose homeostasis in women. Nutr Res 2011;31:882–888 [DOI] [PubMed] [Google Scholar]
- 34.Swithers SE, Laboy AF, Clark K, Cooper S, Davidson TL. Experience with the high-intensity sweetener saccharin impairs glucose homeostasis and GLP-1 release in rats. Behav Brain Res 2012;233:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]