Abstract
OBJECTIVE
To evaluate ITCA 650, a continuous subcutaneous miniature osmotic pump delivery system of exenatide versus twice-daily exenatide injections (Ex-BID) in subjects with type 2 diabetes.
RESEARCH DESIGN AND METHODS
We conducted a randomized, two-stage, 24-week, open-label, phase 2 study in type 2 diabetes inadequately controlled with metformin. Stage I: 155 subjects were randomized to 20 or 40 μg/day of ITCA 650 or Ex-BID 5→10 μg. Stage II: 131 subjects were rerandomized to 20, 40, 60, or 80 μg/day of ITCA 650. Change from baseline for HbA1c, weight, and fasting plasma glucose were evaluated at weeks 12 and 24.
RESULTS
HbA1c was significantly lower in all groups after 12 and 24 weeks. Stage I: mean change in HbA1c from a mean baseline of 7.9–8.0% was −0.98, −0.95, and −0.72% for the 20 and 40 μg/day ITCA 650 and Ex-BID groups, respectively, with 63, 65, and 50% of subjects achieving HbA1c levels ≤7% (P < 0.05). Stage II: significant (P < 0.05) reductions in HbA1c (∼1.4% from baseline) were achieved with 60 and 80 μg/day ITCA 650, and 86 and 78% of subjects achieved HbA1c ≤7% at 24 weeks; respectively. Weight was reduced by 2.8–3.7 kg (P < 0.05) at 24 weeks in all except the 20→20 μg/day group. ITCA 650 was well tolerated; nausea was lower and transient with 20 μg/day relative to Ex-BID; and 60 μg/day had the best profile of tolerability and HbA1c lowering.
CONCLUSIONS
ITCA 650 significantly reduced HbA1c and weight and was well tolerated. The 20→60 μg/day regimen was considered the best dose for further examination in phase 3.
Glucagon-like peptide-1 (GLP-1) receptor agonists (RA) are widely recognized as effective in achieving glycemic control and producing modest weight reduction in patients with type 2 diabetes (1–3). Current guidelines for type 2 diabetes recommend GLP-1 RA as effective therapeutic options to add to metformin and lifestyle management in patients not achieving glycemic targets (4,5). Currently available GLP-1 RA require subcutaneous (SC) injection either once (liraglutide) or twice (exenatide BID) daily or once weekly (exenatide LAR). The need for repeated self-injections as well as the inconvenience of having to reconstitute and refrigerate the once-weekly formulation may create a barrier to initial use as well as long-term patient adherence and compliance with therapy (6–8). Associated gastrointestinal (GI) adverse events (AEs), especially nausea, and injection site reactions/discomfort often lead to discontinuation or further impair adherence to therapy (1–3). In addition, the weekly formulation of exenatide LAR requires 6 to 7 weeks to reach steady state and cannot be retrieved quickly in the event of side effects, as circulating therapeutic drug concentrations persist ∼10 weeks after the drug is discontinued.
ITCA 650 is a miniature osmotic pump system that is designed to deliver zero-order, continuous SC release of exenatide at a precise predetermined rate for up to 12 months with a single placement (9,10). The sterile product with dimensions similar to a small match stick is inserted SC in the abdominal region with a placement tool using aseptic technique during a short office procedure that can be performed by a physician, physician's assistant, or other licensed practitioner (9). Removal requires skin preparation and a small (∼5 mm) incision. The procedures to place, remove, and replace other nonbiodegradable drug delivery systems is reimbursed in the U.S. by insurance companies and other payers, and so it is expected to be the same with ITCA 650 in the future.
This novel delivery system for exenatide has several potential advantages for the treatment of type 2 diabetes such as more rapid attainment and maintenance of consistent therapeutic drug concentrations, 100% adherence with therapy, and improved glycemic control with improved tolerability, perhaps related to more constant and predictable exenatide levels (11). Once ITCA 650 is removed, the pharmacological effect of exenatide abates within 24 h, allowing quick retrieval of drug if needed due to AEs or other clinical considerations. For a chronic condition in which medication adherence is linked to clinical results, the potential to mitigate poor adherence with once or twice yearly chronic dosing with ITCA 650 may improve long-term outcomes as well as patient satisfaction.
A phase 1b study evaluated the safety and tolerability of 10, 20, 40, or 80 μg/day of ITCA 650 for 28 days in subjects with inadequately controlled type 2 diabetes (11). Fasting plasma glucose (FPG) levels decreased in all dose groups within 1 to 2 days, and reductions in HbA1c and body weight were observed in all groups. ITCA 650 was well tolerated, with mild local changes at the insertion site, largely due to the healing process, and transient nausea and vomiting that was generally mild and seen most often with the highest dose. Based on these results, a dose ranging study was undertaken to further investigate the efficacy, safety, and tolerability of ITCA 650 in subjects with type 2 diabetes inadequately controlled on metformin monotherapy.
RESEARCH DESIGN AND METHODS
This was a randomized, two-stage, 24-week, open-label, phase 2 study with an initial 12-week active-controlled period and 12-week dose-ranging period conducted at 50 centers in the U.S. The study was approved by an appropriately constituted institutional review board and conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines for Good Clinical Practice. All subjects gave written informed consent. This study was registered at ClinicalTrials.gov as NCT00943917.
Subject selection
Men and women aged 18–70 years with a diagnosis of type 2 diabetes mellitus ≥6 months on a stable dose of metformin for ≥3 months were eligible if they had an HbA1c ≥7% and ≤10%, an FPG <240 mg/dL, BMI ≤40 kg/m2, and a stable body weight for 3 months prior to study entry. Women could not be pregnant or breastfeeding; had to be surgically sterile or ≥1 year postmenopausal; or, if of childbearing potential, were required to have a negative pregnancy test and practice an acceptable form of contraception during the study.
Subjects were excluded if they previously received treatment with exenatide, thiazolidinediones, sulfonylureas, dipeptidyl peptidase-IV inhibitors, or acarbose; insulin within 3 months of screening; or treatment with an investigational drug within 30 days of screening. Subjects with type 1 diabetes, overt diabetes complications, fasting triglyceride level >500 mg/dL, or medical conditions that could interfere with the conduct of the study were excluded.
Study design
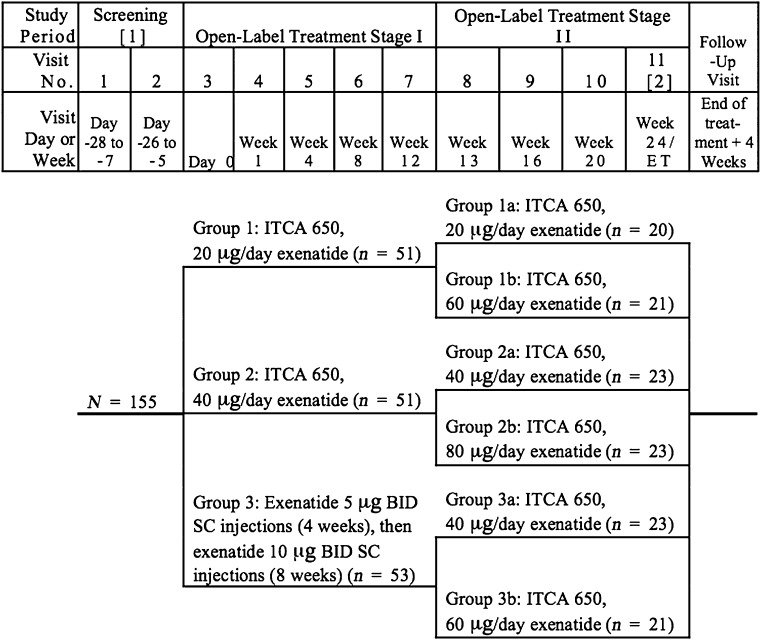
This study consisted of a 1–4-week screening period, two sequential 12-week open-label treatment stages, and a 4-week follow-up visit (Fig. 1). During stage I, eligible subjects were randomized in a 1:1:1 ratio to 20 or 40 μg/day of ITCA 650 or twice-daily exenatide injections (Ex-BID), 5 μg twice daily titrated after 4 weeks to 10 μg twice daily for 8 weeks. In stage II, subjects who received ITCA 650 in stage I were rerandomized to their previous dose of ITCA 650 or an increase in dose to either 60 or 80 μg/day. Therefore, subjects initially randomized to 20 μg/day of ITCA 650 received either 20 μg/day (20→20) or 60 μg/day (20→60). Subjects initially receiving ITCA 650 40 μg/day were randomized to receive either 40 μg/day (40→40) or 80 μg/day (40→80). Subjects who received Ex-BID in stage I were randomized to receive either ITCA 650 40 μg/day (Ex-BID→40) or 60 μg/day (Ex-BID→60) of ITCA 650 (Fig. 1). Treatment group assignments were determined using a dynamic randomization algorithm within the interactive voice response system. Medpace generated the randomization sequence. ITCA 650 study devices that delivered exenatide for 3 months were placed SC by site personnel using aseptic technique (Supplementary Fig. 1). Ex-BID was supplied as SC injection pens, and subjects were instructed in their proper use according to labeling for the marketed version of the product.
Figure 1.
Study flow chart.
Study assessments
HbA1c was measured at screening, baseline, and weeks 4, 8, 12, 16, 20, and 24. FPG was measured at baseline, 4, 12, 16, and 24 weeks. Weight was measured at screening, baseline, and weeks 12 and 24. Safety assessments included AEs, clinical laboratory measurements (chemistry, hematology, and urinalysis) measured at screening, baseline, weeks 4, 12, 16, 24, and at follow up, 12-lead electrocardiograms, vital signs, and physical examinations. Anti-exenatide antibodies were measured at baseline and at weeks 12 and 24. Assessment of anti-exenatide antibodies in human serum samples was conducted at Midwest BioResearch, LLC (Skokie, IL) using a validated ELISA.
Statistical analysis
Approximately 400 subjects were screened to ensure that between 150 and 160 subjects were randomized in stage I. The sample size of 50 patients per stage I group afforded 93% power to detect a significant decrease in HbA1c from baseline (α = 0.05, two-sided paired t test) in any of the groups as long as the true decrease was at least 0.5 at 12 weeks and assuming the SD of the between time point differences was 1.
The primary end point was change from baseline for HbA1c to end point for each stage (12 and 24 weeks) and for the overall 24-week trial (0–24 weeks). Percent changes from baseline were analyzed with an ANCOVA model with treatment as a factor and baseline as the covariate. Pairwise comparisons were reported using least square (LS) means and SEs for each treatment and LS means, SE, 95% CIs, and P values. Statistical analyses were performed using SAS version 9.1 (SAS Institute). The proportion of subjects who achieved HbA1c levels <7% was reported. For other data, descriptive statistics were reported.
The safety population was defined as all randomized subjects who received at least one dose of study medication. The intent-to-treat population was defined as all randomized subjects who received at least one dose of study medication and had at least one postbaseline assessment.
RESULTS
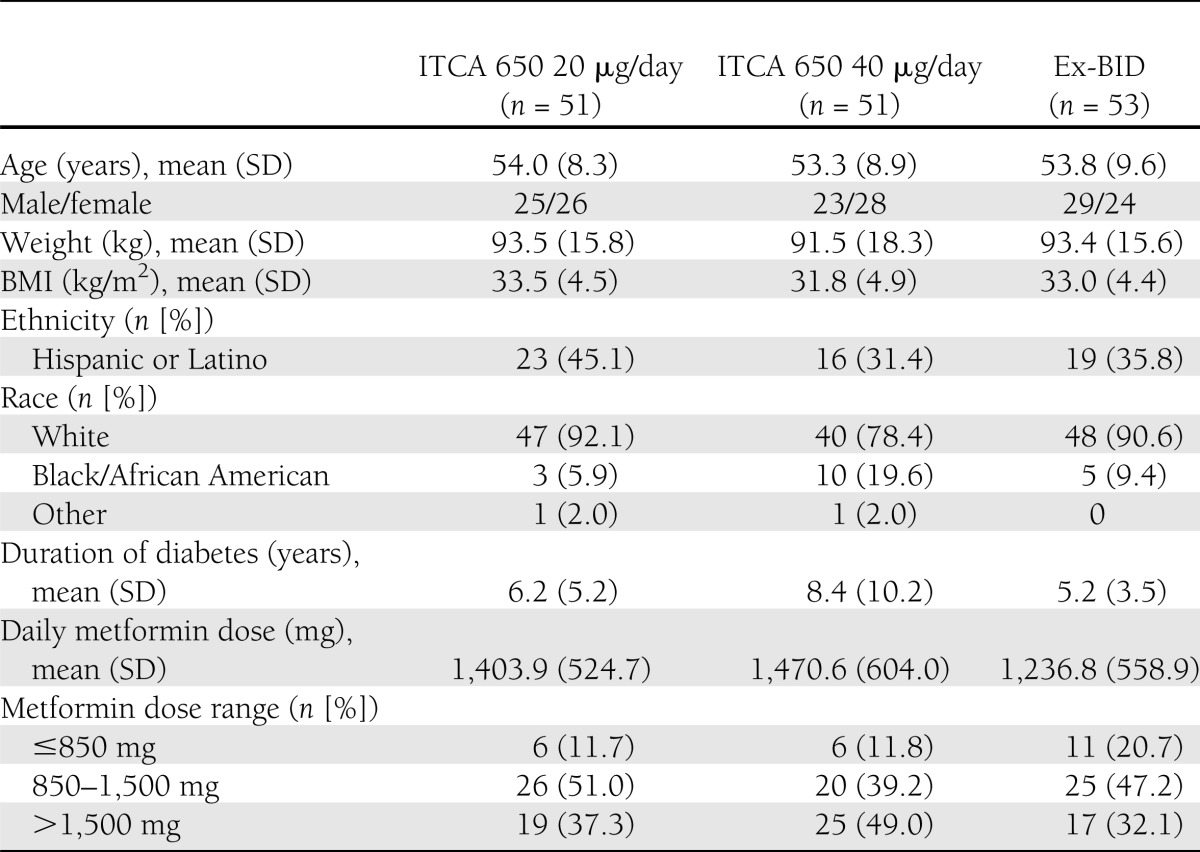
Subjects were recruited between 24 August 2009 and 10 August 2010. Treatment groups were generally comparable at baseline for demographic and clinical characteristics (Table 1). The 40 μg/day ITCA 650 group had a longer mean duration of diabetes (8.4 years) and a higher proportion of African American subjects (19.6%) compared with the other treatment groups. The mean daily dose of metformin was 1,385 mg/day.
Table 1.
Baseline demographics and clinical characteristics
The majority of subjects completed stage I (n = 155 [91.6%]). Thirteen subjects discontinued prematurely; 7.8, 5.9, and 11.3% from the 20 and 40 μg/day ITCA 650 and Ex-BID groups; respectively. Of the 131 subjects randomized into stage II, 94.7% completed the additional 12 weeks of treatment. A total of five discontinuations for an AE occurred across all doses (Supplementary Table 1).
Efficacy
Stage I.
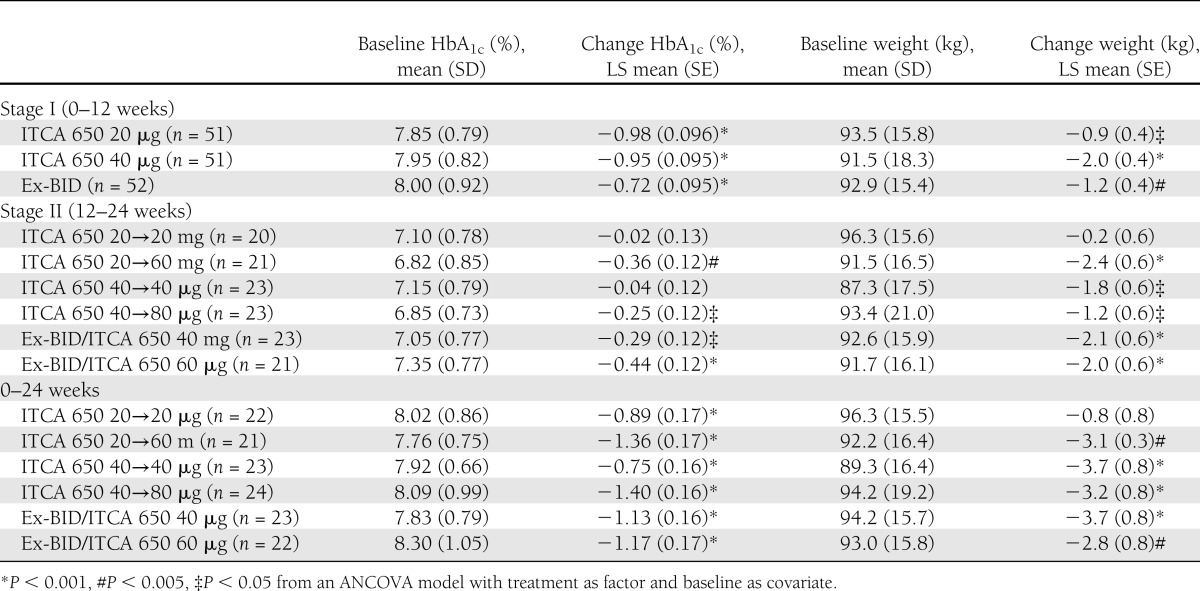
At week 12, significant (P < 0.001) reductions from baseline in mean HbA1c were observed in all three treatment groups (Table 2). The LS mean change in HbA1c from a mean baseline of 7.9–8.0% was −0.98, −0.95, and −0.72% for the 20 and 40 μg/day ITCA 650 groups and the Ex-BID group; respectively. HbA1c ≤7% was achieved in 63 and 65% of subjects treated with 20 and 40 μg/day ITCA 650 and in 50.0% with Ex-BID. Significant (P < 0.05) reductions from baseline in mean FPG were observed for all groups.
Table 2.
Mean changes in HbA1c and body weight at 0–12 weeks (stage I), 12–24 weeks (stage II), and overall (0–24 weeks)
Stage II.
During weeks 12–24, further significant (P < 0.05) incremental reductions in mean HbA1c were noted in subjects who were rerandomized to higher doses of ITCA 650 (20→60 and 40→80) or from exenatide injections to ITCA 650 (Ex-BID→40 and Ex-BID→60) compared with those subjects rerandomized to remain on the same dose of ITCA 650 (20 →20 or 40→40). Mean FPG decreased significantly (P < 0.05) only in the (20→60) ITCA 650 group. Mean body weight significantly (P < 0.05) decreased in all treatment groups except the ITCA 650 20→20 μg/day group (Table 2).
Day 0 to week 24.
Statistically significant (P < 0.001) reductions from baseline (day 0) to week 24 end point in mean HbA1c were observed in all treatment groups with the greatest reductions observed in the 20→60 μg/day ITCA 650 group (LS mean change, −1.36%) and the 40→80 μg/day ITCA 650 group (LS mean change, −1.4%) (Table 2). Treatment with 20→60 μg/day ITCA 650 resulted in a significantly greater reduction in HbA1c than 20→20 or 40→40 μg/day ITCA 650 (P < 0.001). Similarly, treatment with 40→80 μg/day ITCA 650 resulted in a significantly greater reduction in HbA1c than 40→40 μg/day ITCA 650 (P < 0.001). In addition, significant incremental reductions in HbA1c were noted from weeks 12 to 24 in groups initially treated with Ex-BID and then switched to 40 or 60 μg /day ITCA 650 at week 12 (P < 0.05). The proportion of subjects at HbA1c ≤7% at 24 weeks was 60% with ITCA 20→20 μg/day, 86% with ITCA 650 20→60 μg/day, 65% with ITCA 650 40→40 μg/day, and 78% with ITCA 650 40→80 μg/day.
During the day 0 to week 24 period, significant (P < 0.05) reductions from baseline in mean FPG were observed for all groups except the ITCA 650 40→40 μg/day group. Significant (P < 0.005) reductions from baseline for mean body weight were observed for all groups except the ITCA 650 20→20 μg/day group.
Tolerability
During stage I, the incidence of treatment-emergent AEs (TEAEs) was slightly higher in the 40 μg/day than the 20 μg/day ITCA 650 and Ex-BID groups. One subject had a serious AE of acute cholecystitis associated with cholelithiasis and mild gallstone-induced pancreatitis during stage I that was considered unrelated to study drug. Discontinuation for drug-related AEs occurred in 3.9% of subjects on ITCA 650 compared with 5.7% of subjects on Ex-BID (Table 3). ITCA 650 was generally well tolerated. The most common drug-related AEs were nausea, vomiting, and administration site events. These events were most commonly observed early in the course of treatment and improved over time.
Table 3.
Drug-related AEs occurring in >5% overall of subjects during stages I and II
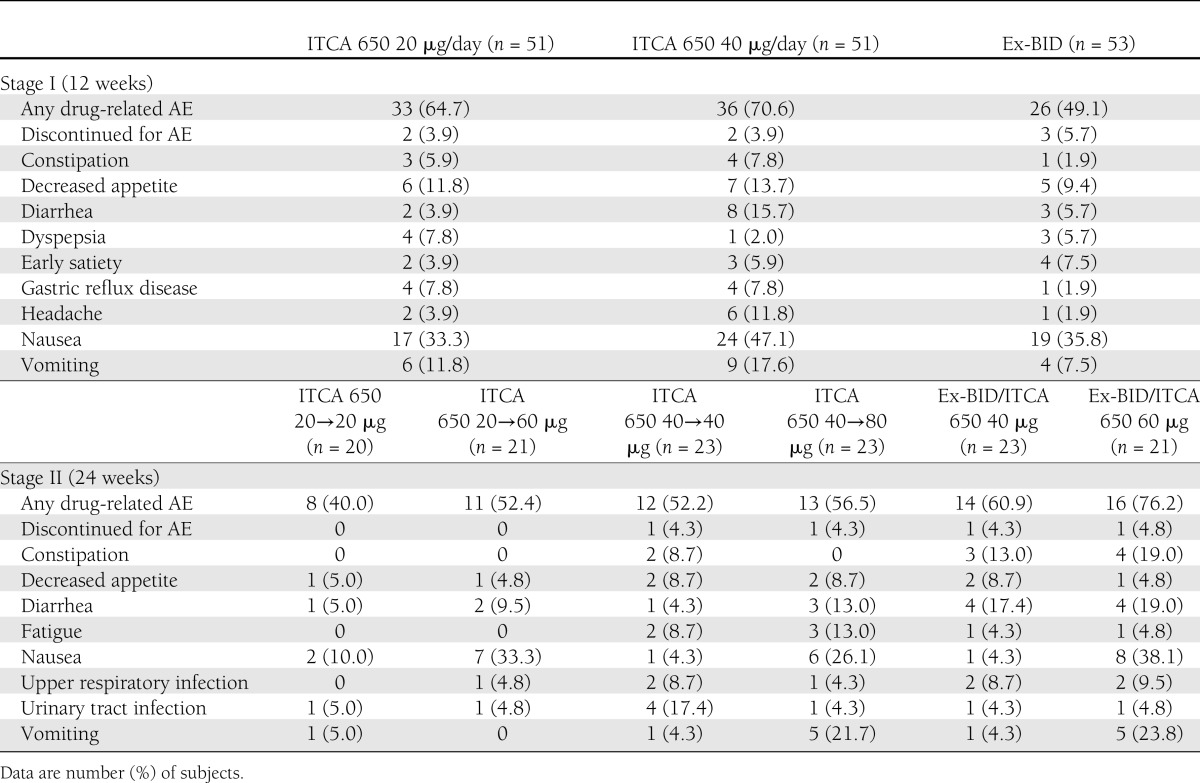
As expected with exenatide treatment, GI side effects were among the most common reported AEs. During week 1, the incidence of nausea was similar with 20 μg/day ITCA 650 and Ex-BID 5 μg (25.5 vs. 24.5%, respectively) but declined rapidly to <10% from week 3 to 2.2% by the end of stage I with 20 μg/day ITCA 650 compared with 20.8% at week 12 with Ex-BID 10 μg. Most episodes were mild or moderate and resolved without treatment. One subject in each treatment group reported severe nausea. The incidence of vomiting was highest during week 1 (7.8, 13.7, and 3.8% with ITCA 650 20 μg, 40 μg, and Ex-BID, respectively) and declined rapidly during stage I. No episodes of vomiting were reported with the 20-μg dose of ITCA 650 after week 4. Most events were also mild or moderate, with only one subject in the 40 μg/day ITCA 650 group reporting severe vomiting.
During stage II, the lowest incidence of TEAEs occurred in the 20→20 μg/day group and was similar in the other five treatment groups. The most common were nausea, diarrhea, vomiting, constipation, and decreased appetite (Table 3). Across all dose groups, five (3.8%) subjects had a TEAE that led to discontinuation from the study, and six (4.6%) subjects had a serious AE, none of which was considered related to study drug by the investigator. One subject died due to cryptococcal meningitis that was considered unrelated to study drug.
Subjects who remained on the same dose of ITCA 650 had a lower incidence of nausea than subjects whose dose was increased to 60 or 80 μg /day; no subjects on 20→60 μg/day experienced vomiting or discontinued therapy during stage II. The highest incidence was seen in the ITCA 650 groups rerandomized from Ex-BID (Table 3). Most episodes of nausea and vomiting were of mild or moderate intensity. Severe nausea occurred in two subjects in the Ex-BID→60 μg/day and one subject in the 20→20 μg/day groups. One subject in the Ex-BID→60 μg/day and 20→20 μg/day groups experienced severe vomiting.
Several different observations were reported in about half of subjects related to the site of administration of the ITCA 650 that mostly reflect the result of the normal healing process. Events such as bruising, pruritis, or pain local to the site of administration were usually reported within days of the placement and were typically mild and transient, resolving without any need for intervention. No subjects had a severe AE related to the site of administration of ITCA 650 during the study.
Subjects taking Ex-BID injections reported hematoma (5.9%), hemorrhage (1.9%), and erythema (1.9%) at the injection site. No clinically meaningful safety findings or trends in safety laboratory parameters or vital signs were noted. No clinically relevant effects on serum calcitonin, thyroid-stimulating hormone, serum amylase, or serum lipase were reported during 24 weeks of study (Supplementary Table 2).
In stage I (week 12), high anti-exenatide antibody titers were observed in 0, 2.1, and 4.5% of subjects treated with 20 and 40 mg /day of ITCA 650 and Ex-BID, respectively. In stage II (week 24), high titers were observed in 5.3, 7.0, 7.3, and 10% of subjects receiving 20, 40, 60, and 80 mg /day of ITCA 650, respectively. Data from the three antibody-positive subjects with high titers (>7,500) did not reveal any apparent relationship between antibody titer and loss of activity. Two subjects with high titers had reductions in both HbA1c and body weight at the end of stages I and II, and the third subject had increases in both HbA1c and body weight, even as titer levels dropped in stage II. At the end of stage II, nine antibody-positive subjects had high titers. Of these, six subjects had reductions in both HbA1c and body weight. The other three subjects showed a reduction in HbA1c or a reduction in body weight. The magnitude of activity observed in the high antibody titer subjects, which was similar to that observed in antibody-negative subjects, suggests that the presence of anti-exenatide antibodies did not reduce the activity of ITCA 650 in this study.
CONCLUSIONS
In this study, treatment for up to 24 weeks with ITCA 650 resulted in significant improvements in HbA1c, FPG, and body weight in subjects with type 2 diabetes inadequately controlled on metformin monotherapy. Stage I of the study established that both initial doses of ITCA 650 (20 and 40 µg/day) resulted in significant HbA1c reductions. However, the ITCA 650 20 µg/day dose resulted in less overall nausea from weeks 1–12 compared with 40 µg/day and Ex-BID. In stage II, doses ranging up to 80 µg/day of ITCA 650 demonstrated incremental reductions in HbA1c levels at the higher doses. The 60 µg/day dose of ITCA 650 produced similar HbA1c reductions and appeared to be better tolerated than the 80 µg/day dose. Switching directly from Ex-BID to higher doses of ITCA 650 was explored and also resulted in incremental reduction in HbA1c levels with somewhat more nausea.
The most troublesome tolerability issue with GLP-1 RA is the incidence and duration of GI side effects, in particular nausea and vomiting, that may account in clinical practice for the low treatment adherence and higher withdrawal rates, which are a greater problem than what has been reported in clinical trials (1,2,12). The reported incidence of nausea with Ex-BID and once-weekly injection was 34.5 and 26.4%, respectively (13). Vomiting has been reported in 18.6 and 10.8% with twice-daily and once-weekly dosing, respectively. A similar incidence has been reported in other comparative trials of once-weekly and Ex-BID (14,15). However, during a 74-week open-label extension phase, nausea persisted in 12% of patients taking once-weekly exenatide (15). The incidence of nausea with liraglutide in phase 3 clinical trials ranged from 10–40% (16). Nausea was dose-related and occurred at higher rates within the first weeks of treatment. In this study, the incidence of nausea with ITCA 650 and Ex-BID was generally comparable during the first week after treatment was initiated; however, the incidence declined rapidly over 12 weeks with the 20-µg dose of ITCA 650 compared with the other groups.
In this study, local placement site AEs were mild and transient, and no subjects had a severe local AE related to ITCA 650. Injection site reactions including pain, induration, erythema, and bleeding have been reported with exenatide once-weekly injections (14,15). Injection site nodules occurred in 77% of subjects in one study (8). Pruritus of mild intensity was observed in 18% of patients on exenatide once weekly versus 1.4% with Ex-BID, which improved and resolved with continued treatment (13). Another study reported injection site pruritus and erythema in 18 and 7% of patients, respectively, taking exenatide once-weekly injections during 30 weeks of treatment (14). These effects persisted at an incidence of 4% during a 74-week open-label extension phase.
An important barrier to optimizing glycemic control in patients with type 2 diabetes is poor adherence and persistence with chronic long-term treatment. In general, poor adherence to medications is known to result in poor disease control and increased health care costs (17). Major factors contributing to poor adherence to diabetes medications are concerns about the risks of therapy, inconvenience, fear of injections, and complex dosing regimens (18–22). Rates of adherence to insulin injections in patients with type 2 diabetes were reported to range from <60–80%, and poor adherence impacts glycemic control and patient satisfaction (23–28). Examination of persistence with injectable antidiabetes drugs over 12 months from a pharmacy claims database found a mean persistence of 7.8 months among patients on Ex-BID therapy (6), and only two-thirds of subjects prescribed Ex-BID persisted on treatment 1 year after initiation (26). The need for repeated injections of GLP-1 RA creates a barrier to long-term patient adherence and compliance with therapy (6–8). ITCA 650 can provide continuous and controlled SC delivery of exenatide for up to 12 months (9,10). The potential to ensure 100% adherence with ITCA 650 may improve long-term therapeutic outcomes.
The limitations of this study include an open-label study design, absence of a control arm during stage II, and small sample size. These limitations will be addressed in phase 3 clinical studies of ITCA 650.
ITCA 650 is an innovative delivery system for the GLP-1 RA exenatide, being investigated for once- or twice-yearly administration in subjects with type 2 diabetes. Results from this phase 2 dose ranging trial showed significant dose-related effects on HbA1c, FPG, and body weight that were maintained for up to 24 weeks. ITCA 650 demonstrated a tolerability profile that may offer important advantages over exenatide BID injections and other long-acting injectable GLP-1 RA compounds. ITCA 650 was generally well tolerated, and patient acceptance and placement/removal of the delivery system were well accepted. The most common drug-related AEs were GI and administration site events that were generally observed early in the course of treatment and transient. Uniquely, ITCA 650 offers the potential of ensuring rapid attainment and maintenance of stable therapeutic exenatide levels, 100% compliance of drug delivery, and adherence to the prescribed regimen. In addition, pharmacological effects can be terminated within 24 h of removal if necessitated by the presence of side effects or other clinical considerations. The results of this study support additional clinical development of ITCA 650 in subjects with type 2 diabetes.
Acknowledgments
Funding for this study was provided by Intarcia Therapeutics, Inc. (Hayward, CA).
R.R.H. is a government employee who received financial support to conduct the study, serves as a consultant and/or on the Advisory Board for Amgen, AstraZeneca, Intarcia Therapeutics, Inc., Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Eli Lilly and Company, Johnson & Johnson, Novo Nordisk Inc., Merck, Roche/Genentech, Sanofi, Medtronic, Daiichi Sankyo, and Elcelyx Therapeutics Inc., and has received grant/research support as the principle investigator from AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, Sanofi, and Medtronic. J.R. received financial support to conduct the study, has served on scientific advisory boards and received honoraria/consulting fees from Amylin Pharmaceuticals, Inc., Boehringer Ingelheim Pharmaceuticals, Daiichi Sankyo, Eli Lilly and Company, GlaxoSmithKline, Johnson & Johnson, MannKind Corporation, Novartis, Novo Nordisk, Pfizer, Inc., Roche, Sanofi, and Takeda Pharmaceuticals North America, Inc., and has also received grants/research support from Amylin Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly and Company, Forest Laboratories, GlaxoSmithKline, Johnson & Johnson, MannKind Corporation, Merck, Novartis, Novo Nordisk, Pfizer, Inc., Roche, Sanofi, and Takeda Pharmaceuticals North America, Inc. D.K.L. is an employee of Medpace, which was the contract research organization for this trial. T.R.A. and M.A.B. are employees of Intarcia Therapeutics, Inc. K.L. was an employee of Intarcia Therapeutics, Inc. at the time this study was conducted. No other potential conflicts of interest relevant to this article were reported.
R.R.H. and J.R. were involved in study design, data interpretation, review, and revision of the manuscript for critical content and review and approval of the manuscript. D.K.L. was involved in data interpretation, review, and revision of the manuscript for critical content and review and approval of the manuscript. T.R.A. was involved in study design, data analysis and interpretation, review, and revision of the manuscript for critical content and review and approval of the manuscript. K.L. was involved in study concept and design, data analysis and interpretation, and review and approval of the manuscript. M.A.B. was involved in data analysis and interpretation, review and revision of the manuscript for critical content, and review and approval of the manuscript. R.R.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was previously presented as an abstract at the 46th European Association for the Study of Diabetes Annual Meeting, 20–24 September 2010, Stockholm, Sweden and the 48th European Association for the Study of Diabetes Annual Meeting, 1–5 October 2012, Berlin, Germany.
The following investigators participated in this study: Atoya Adams, MD, Las Vegas, NV; Eddie Armas, MD, Miami, FL; Richard Beasley, MD, Rapid City, SD; Gary Bedel, MD, Franklin, OH; Richard Bergenstal, MD, Minneapolis, MN; Maria Bermudez, MD, Miramar, FL; Patricia Buchanan, MD, Eugene, OR; Rafael Canadas, MD, Dallas, TX; James Capo, Jr., Atlanta, GA; David Carter, MD, Austin, TX; Louis Chaykin, MD, Bradenton, FL; Scott Conard, MD, Irving, TX; Lisa Connery, MD, Norman, OK; John Earl, MD, Hickory, NC; Richard Egelhof, MD, Wichita, KS; Rochelle Elijah, MD, Pueblo, CO; Eli Engle, MD, PhD, Valley Village, CA; Mildred Farmer, MD, St. Petersburg, FL; Neil Fraser, MD, Troy, MI; Kenneth Hershon, MD, New Hyde Park, NY; Frederick Jenkin, DO, National City, CA; Dean Kereiakes, MD, Cincinnati, OH; Mark Kipnes, MD, San Antonio, TX; Eric Klein, MD, Olympia, WA; Douglas Logan, MD, Cincinnati, OH; Sunder Mudaliar, MD, San Diego, CA; Abel Murillo, MD, Miami, FL; Thomas Nussdorfer, MD, Traverse City, MI; E. David Pampe, MD, Austin, TX; Naynesh Patel, MD, Kettering, OH; Andres Patron, DO, Pembroke Pines, FL; Monica Perlman, MD, La Jolla, CA; David Podlecki, MD, Longmont, CO; Geri Poss, MD, San Antonio, TX; Luis Quintero, MD, Miami, FL; George Raad, MD, Charlotte, NC; Julio Rosenstock, MD, Dallas, TX; Michael Sanson, Ypsilanti, MI; Gladstone Sellers, MD, Sandy Springs, GA; Ronald Stegemoller, MD, Avon, IN; Danny Sugimoto, MD, Chicago, IL; Jeffrey Unger, MD, Chino, CA; Carol Wysham, MD, Spokane, WA; and Douglas Young, MD, Sacramento, CA.
The authors thank Richard S. Perry, PharmD, RP Consulting, for editorial assistance in the preparation of the manuscript, which was supported by Intarcia Therapeutics, Inc.
Footnotes
Clinical trial reg. no. NCT00943917, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2410/-/DC1.
References
- 1.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007;298:194–206 [DOI] [PubMed] [Google Scholar]
- 2.Fakhoury WK, Lereun C, Wright D. A meta-analysis of placebo-controlled clinical trials assessing the efficacy and safety of incretin-based medications in patients with type 2 diabetes. Pharmacology 2010;86:44–57 [DOI] [PubMed] [Google Scholar]
- 3.Monami M, Marchionni N, Mannucci E. Glucagon-like peptide-1 receptor agonists in type 2 diabetes: a meta-analysis of randomized clinical trials. Eur J Endocrinol 2009;160:909–917 [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009;15:540–559 [DOI] [PubMed] [Google Scholar]
- 6.Cooke CE, Lee HY, Tong YP, Haines ST. Persistence with injectable antidiabetic agents in members with type 2 diabetes in a commercial managed care organization. Curr Med Res Opin 2010;26:231–238 [DOI] [PubMed] [Google Scholar]
- 7.Jose B, Tahrani AA, Piya MK, Barnett AH. Exenatide once weekly: clinical outcomes and patient satisfaction. Patient Prefer Adherence 2010;4:313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bydureon [package insert]. San Diego, CA, Amylin Pharmaceuticals, 2012 [Google Scholar]
- 9.Rohloff CM, Alessi TR, Yang B, Dahms J, Carr JP, Lautenbach SD. DUROS technology delivers peptides and proteins at consistent rate continuously for 3 to 12 months. J Diabetes Sci Tech 2008;2:461–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang B, Negulescu C, D’vaz R, et al. Stability of ITCA 650 for continuous subcutaneous delivery of exenatide at body temperature for 12 months. Abstract presented at the Ninth Annual Diabetes Technology Meeting, 5–7 November 2009, San Francisco, California [Google Scholar]
- 11.Luskey K, McNally J, Dahms J, Alessi T. Continuous subcutaneous delivery of exenatide via ITCA 650 lowers plasma glucose, HbA1c and reduces weight in a 28-day phase 1b study in type 2 diabetes. Abstract presented at the Ninth Annual Diabetes Technology Meeting, 5–7 November 2009, San Francisco, California [Google Scholar]
- 12.Shyangdan DS, Royle PL, Clar C, Sharma P, Waugh NR. Glucagon-like peptide analogues for type 2 diabetes mellitus: systematic review and meta-analysis. BMC Endocr Disord 2010;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drucker DJ, Buse JB, Taylor K, et al. DURATION-1 Study Group Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008;372:1240–1250 [DOI] [PubMed] [Google Scholar]
- 14.Taylor K, Gurney K, Han J, Pencek R, Walsh B, Trautmann M. Exenatide once weekly treatment maintained improvements in glycemic control and weight loss over 2 years. BMC Endocr Disord 2011;11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blevins T, Pullman J, Malloy J, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab 2011;96:1301–1310 [DOI] [PubMed] [Google Scholar]
- 16.Edavalath M, Stephens JW. Liraglutide in the treatment of type 2 diabetes mellitus: clinical utility and patient perspectives. Patient Prefer Adherence 2010;4:61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–497 [DOI] [PubMed] [Google Scholar]
- 18.Bailey CJ, Kodack M. Patient adherence to medication requirements for therapy of type 2 diabetes. Int J Clin Pract 2011;65:314–322 [DOI] [PubMed] [Google Scholar]
- 19.Mann DM, Ponieman D, Leventhal H, Halm EA. Predictors of adherence to diabetes medications: the role of disease and medication beliefs. J Behav Med 2009;32:278–284 [DOI] [PubMed] [Google Scholar]
- 20.Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care 2010;33:240–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubin RR, Peyrot M, Kruger DF, Travis LB. Barriers to insulin injection therapy: patient and health care provider perspectives. Diabetes Educ 2009;35:1014–1022 [DOI] [PubMed] [Google Scholar]
- 22.Kuritzky L. Overcoming barriers to insulin replacement. J Fam Pract 2009;58(Suppl. 8):S25–S31 [PubMed] [Google Scholar]
- 23.Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med 2005;118(Suppl. 5A):27S–34S [DOI] [PubMed] [Google Scholar]
- 24.Cramer JA, Pugh MJ. The influence of insulin use on glycemic control: How well do adults follow prescriptions for insulin? Diabetes Care 2005;28:78–83 [DOI] [PubMed] [Google Scholar]
- 25.Donnelly LA, Morris AD, Evans JM, DARTS/MEMO collaboration Adherence to insulin and its association with glycaemic control in patients with type 2 diabetes. QJM 2007;100:345–350 [DOI] [PubMed] [Google Scholar]
- 26.Fabunmi R, Nielsen LL, Quimbo R, et al. Patient characteristics, drug adherence patterns, and hypoglycemia costs for patients with type 2 diabetes mellitus newly initiated on exenatide or insulin glargine. Curr Med Res Opin 2009;25:777–786 [DOI] [PubMed] [Google Scholar]
- 27.Alvarez Guisasola F, Tofé Povedano S, Krishnarajah G, Lyu R, Mavros P, Yin D. Hypoglycaemic symptoms, treatment satisfaction, adherence and their associations with glycaemic goal in patients with type 2 diabetes mellitus: findings from the Real-Life Effectiveness and Care Patterns of Diabetes Management (RECAP-DM) Study. Diabetes Obes Metab 2008;10(Suppl. 1):25–32 [DOI] [PubMed] [Google Scholar]
- 28.Ross SA, Tildesley HD, Ashkenas J. Barriers to effective insulin treatment: the persistence of poor glycemic control in type 2 diabetes. Curr Med Res Opin 2011;27(Suppl. 3):13–20 [DOI] [PubMed] [Google Scholar]