Abstract
OBJECTIVE
To investigate the potential relationship between overweight, obesity, and severe obesity and the risk of hospitalization for heart failure (HF) in patients with type 1 diabetes.
RESEARCH DESIGN AND METHODS
We studied patients with type 1 diabetes included in the Swedish National Diabetes Registry during 1998–2003, and they were followed up until hospitalization for HF, death, or 31 December 2009. Cox regression was used to estimate relative risks.
RESULTS
In a sample of 20,985 type 1 diabetic patients (mean age, 38.6 years; mean BMI, 25.0 kg/m2), 635 patients were hospitalized with HF as a primary or secondary diagnosis during a median follow-up of 9.1 years. Cox regression adjusting for age, sex, diabetes duration, smoking, HbA1c, systolic and diastolic blood pressures, and baseline and intercurrent comorbidities (including myocardial infarction) showed a significant relationship between BMI and hospitalization for HF (P < 0.0001). In reference to patients in the BMI 20–25 kg/m2 category, hazard ratios (HRs) were as follows: HR 1.22 (95% CI, 0.83–1.78) for BMI <20 kg/m2; HR 0.94 (95% CI, 0.78–1.12) for BMI 25–30 kg/m2; HR 1.55 (95% CI, 1.20–1.99) for BMI 30–35 kg/m2; and HR 2.90 (95% CI, 1.92–4.37) for BMI ≥35 kg/m2.
CONCLUSIONS
Obesity, particularly severe obesity, is strongly associated with hospitalization for HF in patients with type 1 diabetes, whereas no similar relation was present in overweight and low body weight.
Heart failure (HF) is a growing public health issue, and it was recently estimated that nearly 6 million Americans older than age 20 had a clinical diagnosis of HF (1). By 2030, an additional 3 million people in the United States are predicted to have HF, a 25% increase in prevalence from 2010 (1). HF also is associated with poor prognosis, with an age-adjusted 5-year mortality rate of 50% after onset (2,3) even in the setting of modern treatment (4). Patients with symptomatic HF have a significant impact on health care spending in industrialized countries (5,6). Consequently, identifying risk factors and striving to prevent clinical HF through treatment should be a high priority, as should early identification of preclinical HF in high-risk populations, such as patients with diabetes.
Patients with type 1 diabetes have been shown to be at particularly high risk for HF. Recent work from our group demonstrated that not only poor diabetes control but also BMI was independently associated with increased risk of HF, but the nature of the association between BMI and hospitalization for HF was not explored (7).
Obesity also has been shown to be an important risk factor for HF in the general population (8), and in several studies obesity has shown a graded increase in risk across BMI categories (9,10). In this study, we examined whether an association exists between overweight or modest obesity and major increases in risk of HF, or if these risks primarily are limited to more severe stages of obesity.
RESEARCH DESIGN AND METHODS
Data were obtained from the Swedish National Diabetes Registry (NDR), a nationwide quality-assurance instrument in diabetes care that is linked with outcomes data from the Swedish Hospital Discharge Registries (7). We identified a cohort of all patients included within the NDR during 1998–2003 aged 18 or older who had type 1 diabetes and no known HF according to the hospital discharge register (registrations since 1987). Patients were followed-up until hospitalization for HF, death, or until 31 December 2009. Risk factors tracked in the NDR include blood pressure, HbA1c, blood lipids, weight, height, diabetes complications (e.g., microalbuminuria and retinopathy), and treatment regimens.
Type 1 diabetes is defined in the NDR as receiving treatment with insulin only and onset at age 30 years or younger. These characteristics previously have been validated as accurate in 97% of cases (11). The study was approved by the regional Ethics Review Board at the University of Gothenburg (Gothenburg, Sweden), and all participants provided verbal informed consent for inclusion in the NDR.
Variables assessed were age, sex, duration of diabetes, BMI, systolic and diastolic blood pressures, LDL cholesterol, HDL cholesterol, HbA1c, and smoking status. Blood pressure was registered using the standard method in Sweden as the mean value of two supine readings (Korotkoff 1–5) with an appropriately sized cuff and after at least 5 min of rest. Antihypertensive medications also were recorded in the NDR. BMI was calculated as body weight (kg) divided by height (m2).
In Sweden, it is mandatory to register principal and contributory discharge diagnoses for all patients in the hospital discharge register. Diagnosis at discharge is coded according to the ICD-10 after 1996 (ICD-10 1st and 2nd editions). For this study, data from the national hospital discharge and cause-specific death registers were linked through personal identification numbers, which are unique to each Swedish citizen. The diagnosis of HF in the Swedish Hospital Discharge Registry has been validated, showing 95% accuracy for primary diagnoses and 82% irrespective of diagnosis position (12).
Patients were followed-up from their first inclusion in the NDR in 1998–2003 until hospital admission with a primary or secondary discharge diagnosis for HF, death, or 31 December 2009. Analyses also were performed using only a primary diagnosis of HF as an end point. The ICD-10 code used for hospitalization for HF was I50. Besides HF, we retrieved diagnoses of atrial fibrillation (I48), valve disease (I05–I09 and I34–I36), and myocardial infarction (I21) from the hospital discharge register.
Hospitalization for HF was studied in relation to categories of BMI, adjusting for other risk factors as well as for cardiovascular comorbidities and antihypertensive medication.
Statistical analysis
Statistical modeling and analyses were performed using methods consistent with previous studies (7,13).
Incidence rates categorized by BMI
We calculated unadjusted incidence rates of HF by dividing the number of patients with HF by the number of patient-years of follow-up in a particular BMI category and reported these as events per 1,000 years of follow-up. Studied categories were estimated by updated mean BMI and classified as <20.0 kg/m2, 20–25 kg/m2, 25–30 kg/m2, 30–35 kg/m2, and ≥35 kg/m2. The updated mean BMI was defined as the mean value that was updated each time a new BMI measurement was made (e.g., when the third measurement from baseline was performed, the updated mean BMI was the mean of the first three values). The updated mean BMI variable takes into account all BMI values during the exposure period (14). Incidence rates were adjusted for age, sex, and diabetes duration, estimated using Poisson regression, and reported as 1,000 patient-years of follow-up.
Risk estimates
A Cox proportional hazards model was constructed to study potential relationships between patient characteristics and HF. Updated means of BMI and systolic and diastolic blood pressure were included as time-dependent covariates. Age, sex, and diabetes duration were used as baseline variables. Atrial fibrillation, valve disease, and myocardial infarction were included as 0/1 binary variables from year 1987 (myocardial infarction) or 1997 (atrial fibrillation, valve disease) in the following way: those who had experienced an event before study entry had an event indicator equal to 1 throughout their follow-up time, and those who experienced the event during follow up received an indicator value equal to 1 from that time and onward. Smoking was included as a time-dependent variable with three possible categories (smoker, nonsmoker, no information) at each registration during follow-up. Similarly, the information regarding blood pressure medications at each registration (yes, no, no information) was included as a time-dependent variable.
RESULTS
Patient characteristics
We identified 20,985 patients from the NDR with type 1 diabetes (45% women and 55% men). The patients were divided into five categories according to BMI (<20, 20–25, 25–30, 30–35, and ≥35 kg/m2). Baseline characteristics for the entire cohort and by category of BMI are shown in Table 1. There were no major differences in age between the groups at inclusion, but 71% of patients with BMI >35 kg/m2 were women compared with 45% of women in the overall cohort.
Table 1.
Characteristics of 20,985 type 1 diabetic patients by categories of BMI at first inclusion in the register in 1998–2003
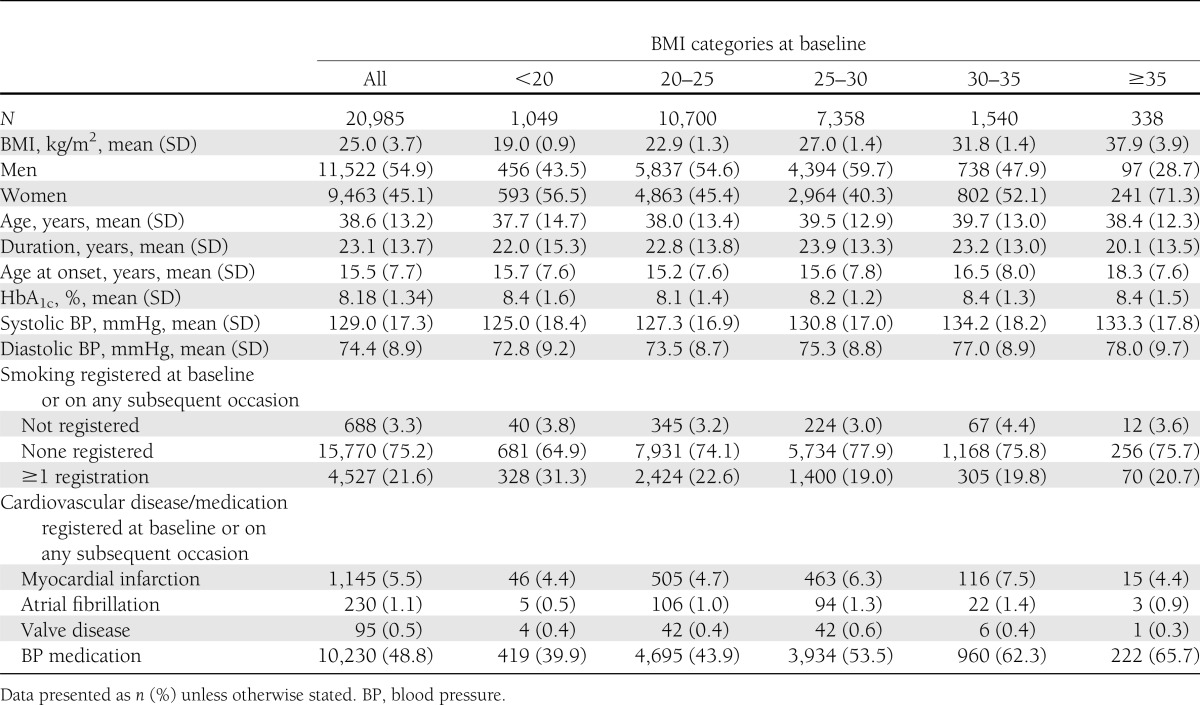
There were no major differences in glycemic control in the various BMI groups, whereas systolic and diastolic blood pressures showed an increasing trend with higher BMI. Smoking was most common in patients with low BMI (<20 kg/m2), with a prevalence of 31.3% compared with ∼20% for other BMI groups. A history of myocardial infarction was more common in patients with BMI 25–30 kg/m2 and 30–35 kg/m2 than in the other BMI categories.
Incidence of HF
Among all patients, 635 (3%) were admitted for a primary or secondary diagnosis of HF during a median follow-up of 9 years, with an incidence of 3.38 events per 1,000 patient-years (95% CI, 3.12–3.65). Unadjusted rates of HF by category of BMI are presented in Table 2. The relation between BMI and HF was slightly J-formed, with the unadjusted incidence of HF lowest for patients with BMI 20–25 kg/m2 being 2.83 (95% CI, 2.51–3.20) events per 1,000 patient-years. HF increased with higher BMI. The incidence of HF was 7.87 (95% CI, 5.36–11.6) events per 1,000 patient-years in patients with BMI >35 kg/m2.
Table 2.
First hospitalizations for HF per 1,000 patient-years with 95% CIs by updated BMI categories estimated using Poisson regression
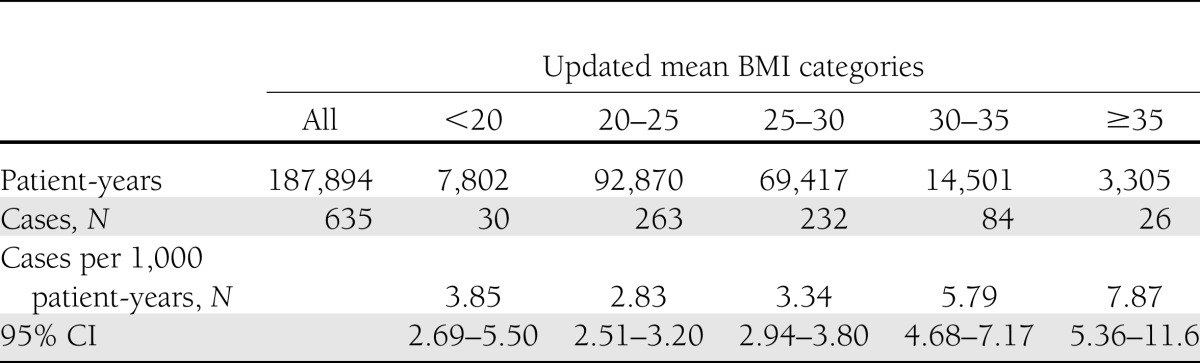
Fig. 1 displays the incidence of HF for patients 41–50 years old and 51–60 years old by category of updated mean BMI and according to diabetes onset at ages 11–20 years and older than 20 years. Men 61 years or older with BMI ≥35 kg/m2 who had diabetes onset at ages 0–10 years experienced the highest rates of HF events, 108 (95% CI, 70–168) per 1,000 patient-years. The rate in women corresponding to the same categories was 88 (95% CI, 57–134) events per 1,000 patient-years.
Figure 1.
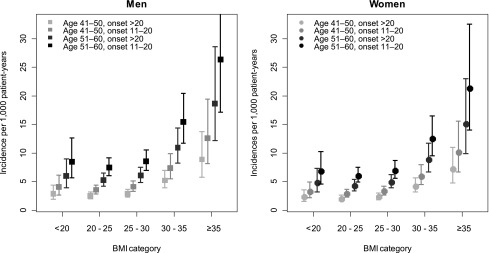
Incidence rates with 95% CIs for HF by updated mean BMI category in patients with type 1 diabetes diagnosed at 11–20 years of age or after 20 years of age and belonging to two different age groups. Incidence estimations were made using a Poisson regression model including age, age at onset, sex, and updated mean BMI as explanatory variables.
The estimated mean incidence rates for those with age at onset of 10 years or younger are consistently higher, but with wider CIs because of fewer numbers and events, as compared with those with older age at onset in the age groups shown in Fig. 1.
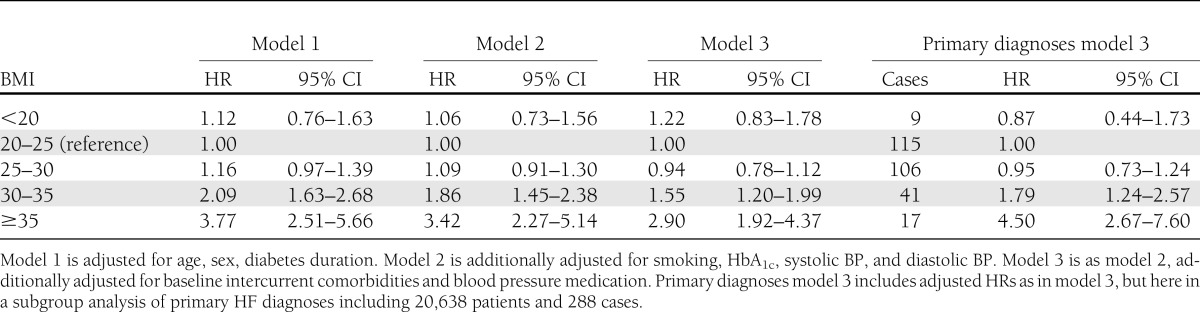
Relative risks of HF
Table 3 shows that patients with BMI <20 kg/m2 or BMI 25–30 kg/m2 did not have significantly increased risks of HF compared with patients with a BMI of 25–30 kg/m2. Significantly increased risks for HF were noted among patients with BMI 30–35 and BMI ≥35 kg/m2, with hazard ratio (HR) of 2.09 (95% CI, 1.63–2.68) and HR of 3.77 (95% CI, 2.51–5.66), respectively, after adjustment for age, sex, and duration of diabetes. Further adjustment for smoking, HbA1c, and systolic and diastolic blood pressures slightly reduced these estimates (Table 3). In a final model, additionally adjusting for cardiovascular comorbidities and antihypertensive medications, BMI 30–35 was associated with HR of 1.55 (95% CI, 1.20–1.99) and BMI ≥35 kg/m2 was associated with HR of 2.90 (95% CI, 1.92–4.37; Table 3). Restricting the analysis in the fully adjusted model to the 288 patients with a principal diagnosis of HF yielded similar results; no increased risks for hospitalization for HF were found at BMI <20 kg/m2 or BMI 25–30 kg/m2 (Table 3). The relative risk increase of hospitalization for HF for the two upper categories of BMI were numerically higher than when analyzing primary and secondary diagnoses as a composite end point but CIs were wide (Table 3).
Table 3.
Adjusted HRs for the development of HF and 95% CI for updated BMI categories examined using Cox regression
CONCLUSIONS
In this study of 20,985 type 1 diabetic patients followed-up over 9 years, we found obesity (defined as BMI >30 kg/m2) to be associated with a markedly increased risk of hospitalization for HF. However, overweight, defined as BMI 25–30 kg/m2, was not associated with any increased risk of hospitalization for HF. The incidence of HF was high, especially in patients with obesity and severe obesity (≥35 kg/m2), demonstrating that BMI has a large clinical impact on the need for hospitalization for HF in this population. Similar results were found when analyzing HF as a primary diagnosis and as a composite end point of primary and secondary diagnoses of HF. Although not a predefined study objective, it is noteworthy that >70% of type 1 patients with diabetes with BMI >35 kg/m2 were women compared with 45% in the total cohort.
To our knowledge, there are no previous studies investigating the level of BMI associated with the lowest risk for HF in patients with type 1 diabetes or the risks that exist for HF at various BMI levels in this patient group. The increase in risk of hospitalization for HF in patients with BMI ≥35 kg/m2 in this study was almost three-times that for patients with normal weight. In patients with BMI 30–35 kg/m2, there was also a marked increase in risk of 55%, with the lower bound of the CI at 20%. For overweight patients, no significant increase in risk was found in contrast to studies based on general population samples in which graded associations in some studies have been present (9,10,15,16), although a higher BMI cut-off for defining overweight was used in one of these studies.
In contrast to type 2 diabetes, obesity is not implicated as a causal factor in type 1 diabetes and maintaining normal weight is accordingly less of a focus in clinical practice of patients with type 1 diabetes. Because most patients with type 2 diabetes are overweight or obese and glucose levels can normalize in some patients after weight reduction, this is usually an important part of integrated diabetes care. Our findings indicate that given the substantial risk of cardiovascular disease in type 1 diabetic patients, it is crucial for clinicians to also address weight issues in type 1 diabetes. Because many patients are normal weight when diabetes is diagnosed, careful monitoring of weight with a view to maintaining normal weight is probably more essential than previously thought. Although overweight was not associated with an increased risk of HF, higher BMI levels probably increase the risk of future obesity. Our finding that 71% of patients with BMI >35 kg/m2 were women is potentially important, although this should be tested in other populations given that it could be a random finding. If not random, especially because the proportion was much higher than in the entire cohort (45%), then it may indicate that severe obesity is a greater problem in women than in men with type 1 diabetes.
There are some limitations to our study. First, because only hospitalizations for HF were studied, it is likely that earlier stages of HF were underestimated. Second, although we found a strong association between obesity and hospitalization for HF, we do not know if higher risk is reversible with weight loss. Strengths of the study are the large sample size and population basis and information regarding cardiovascular comorbidities and risk factors for diabetes complications, which we were able to control for in our statistical models.
In conclusion, obesity, particularly severe obesity, is a strong risk factor for hospitalization for HF in patients with type 1 diabetes, whereas overweight and low body weight shows no similar relation. A greater clinical focus on reducing body weight in obese patients with type 1 diabetes may be essential to reduce the increased risk of cardiovascular disease in this population, as well as to potentially improve the effects of intensive glycemic control by reducing insulin resistance.
Acknowledgments
This study was supported by grants from the Region of Västra Götaland in Sweden.
M.O. is partially employed by AstraZeneca. M.L. has received honoraria or served as a consultant for Bayer, Eli Lilly, Novartis, Novo Nordisk, Medtronic, Pfizer, and Sanofi. M.L. has been a member of the advisory board of Novo Nordisk. The department that M.L. is affiliated with has received grants from AstraZeneca, Novo Nordisk Scandinavia, and Abbot Scandinavia. No other potential conflicts of interest relevant to this article were reported.
D.V. wrote the manuscript, contributed to discussion, and reviewed and edited the manuscript. A.R. contributed to discussion and reviewed the manuscript. M.O. conducted the statistical modeling and analyses, designed the statistical analysis, contributed to discussion, and reviewed and edited the manuscript. S.G. and A.-M.S. researched data and contributed to discussion. M.L. designed the statistical analysis, contributed to discussion, and reviewed and edited the manuscript. D.V. and M.L. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors thank Mervete Miftaraj, Centre of Registers of Västra Götaland, for help with delivering data from the Centre of Registers in Region of Västra Götaland, Gothenburg, Sweden, and also Josh Murphy, an independent editorial consultant in Kansas City, Missouri, for language editing.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012;125:e2–e220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA 2004;292:344–350 [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med 2002;347:1397–1402 [DOI] [PubMed] [Google Scholar]
- 4.Schaufelberger M, Swedberg K, Köster M, Rosén M, Rosengren A. Decreasing one-year mortality and hospitalization rates for heart failure in Sweden; Data from the Swedish Hospital Discharge Registry 1988 to 2000. Eur Heart J 2004;25:300–307 [DOI] [PubMed] [Google Scholar]
- 5.Rydén-Bergsten T, Andersson F. The health care costs of heart failure in Sweden. J Intern Med 1999;246:275–284 [DOI] [PubMed] [Google Scholar]
- 6.Stewart S, Jenkins A, Buchan S, McGuire A, Capewell S, McMurray JJ. The current cost of heart failure to the National Health Service in the UK. Eur J Heart Fail 2002;4:361–371 [DOI] [PubMed] [Google Scholar]
- 7.Lind M, Bounias I, Olsson M, Gudbjörnsdottir S, Svensson AM, Rosengren A. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet 2011;378:140–146 [DOI] [PubMed] [Google Scholar]
- 8.Wilhelmsen L, Rosengren A, Eriksson H, Lappas G. Heart failure in the general population of men—morbidity, risk factors and prognosis. J Intern Med 2001;249:253–261 [DOI] [PubMed] [Google Scholar]
- 9.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med 2002;347:305–313 [DOI] [PubMed] [Google Scholar]
- 10.Schaufelberger M, Rosengren A. Heart failure in different occupational classes in Sweden. Eur Heart J 2007;28:212–218 [DOI] [PubMed] [Google Scholar]
- 11.Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Glycemic control and cardiovascular disease in 7,454 patients with type 1 diabetes: an observational study from the Swedish National Diabetes Register (NDR). Diabetes Care 2010;33:1640–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingelsson E, Arnlöv J, Sundström J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail 2005;7:787–791 [DOI] [PubMed] [Google Scholar]
- 13.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lind M, Odén A, Fahlén M, Eliasson B. A systematic review of HbA1c variables used in the study of diabetic complications. Diabetes and Metabolic Syndrome: Clinical Research and Reviews 2008;2:282–293 [Google Scholar]
- 15.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med 2001;161:996–1002 [DOI] [PubMed] [Google Scholar]
- 16.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983;67:968–977 [DOI] [PubMed] [Google Scholar]


