Abstract
Background
Rapid-response extracorporeal membrane oxygenation (RR-ECMO) has been implemented at select centers to expedite cannulation for patients placed on ECMO during cardiopulmonary resuscitation (ECPR). In 2008, we established such a program and used it for all pediatric veno-arterial ECMO initiations. This study was designed to compare outcomes before and after program implementation.
Methods
Between 2003 and 2011, 144 pediatric patients were placed on veno-arterial ECMO. Records of patients placed on ECMO before (17 ECPR and 62 non-ECPR) or after (14 ECPR and 51 non-ECPR) RR-ECMO program implementation were retrospectively compared.
Results
The peak performance of the ECMO team was assessed by measuring ECMO initiation times for the ECPR patient subgroup (n=31). There was a shift towards more ECPR initiations achieved in under 40 minutes (24% pre-RR-ECMO vs. 43% RR-ECMO, P=0.25) and fewer requiring more than 60 minutes (47% pre-RR-ECMO vs. 21% RR-ECMO, P=0.14) after program implementation, although these changes did not reach statistical significance. After multivariable risk-adjustment, RR-ECMO was associated with a 52% reduction in neurologic complications for all patients (adjusted odds ratio, 0.48; confidence interval, 0.23–0.98; P=0.04), but the risk of in-hospital death remained unchanged (adjusted odds ratio, 0.99; confidence interval, 0.50–1.99; P=0.99).
Conclusion
Implementation of a pediatric RR-ECMO program for veno-arterial ECMO initiation was associated with reduced neurologic complications but not improved survival during the first three years of program implementation. These data suggest that development of a coordinated system for rapid ECMO deployment may benefit both ECPR and non-ECPR patients, but further efforts are required to improve survival.
Keywords: Extracorporeal membrane oxygenation (ECMO), outcomes, pediatric
BACKGROUND
Extracorporeal membrane oxygenation (ECMO) remains the primary therapy for pediatric patients acutely requiring mechanical circulatory support for cardiac or respiratory failure [1]. ECMO is usually initiated emergently in the intensive care unit (ICU) for severe respiratory or circulatory failure, sometimes during or in anticipation of cardiopulmonary arrest. When ECMO is used in the setting of active cardiopulmonary resuscitation (ECPR), longer deployment times have been associated with increased mortality [2]. As a result, rapid-response ECMO services for ECPR have been implemented at a small number of hospitals in attempt to improve patient outcomes through shortened ECMO initiation times [3–7].
Beginning in May 2008, a formal pediatric rapid-response ECMO program was implemented at our institution with the similar goal of improving patient outcomes through shortened ECMO initiation times and streamlined ECMO team functioning. However, at our center the rapid-response ECMO service was applied broadly to all pediatric patients requiring emergent veno-arterial (VA) ECMO and was not restricted to patients requiring ECPR. This report compares patient outcomes before and after implementation of the pediatric rapid-response ECMO program at our institution.
MATERIALS AND METHODS
Patient Population
This study was approved by the Institutional Review Board of Duke University and the need for individual patient consent was waived. We retrospectively reviewed the records of all pediatric patients (age < 18) placed on VA ECMO five years before (April 30, 2003–May 1, 2008) and three years after (May 1, 2008–March 1, 2011) implementation of the rapid-response ECMO program. Patients placed on ECMO in the operating room after failure to wean from cardiopulmonary bypass were excluded.
Rapid-Response ECMO Program
Key features of the rapid-response ECMO program included: (1) introduction of a batch paging system for notification of ECMO team members, (2) training of respiratory therapists to initiate ECMO without a perfusionist, (3) placement of surgical cannulation carts in all pediatric ICUs, and (4) maintenance of crystalloid-primed ECMO circuits dedicated to rapid deployment.
The decision to initiate the rapid-response ECMO batch paging system is typically made by the in-house cardiac, pediatric, or neonatal intensive care physician. Recipients of the rapid-response ECMO batch page are: the on-call attending cardiothoracic surgeon, pediatric perfusionist, pediatric respiratory therapist, operating room charge nurse, ICU charge nurse, attending intensive care physician, and cardiothoracic surgical resident. While the on-call pediatric perfusionist and operating room team are included in the notification, the system has been designed to allow for ECMO deployment in their absence.
At our institution, on-call perfusionists do not remain in-house after regular work hours. However, ECMO-trained respiratory therapists maintain active ECMO circuits 24-hours a day. A subset of these ECMO-trained respiratory therapists underwent additional training to perform asanguineous ECMO circuit priming and initiation of ECMO without a perfusionist.
Identical surgical supply carts with all supplies necessary for intra-thoracic, cervical or femoral cannulation were placed in the pediatric ICUs. The pediatric cardiothoracic surgeons perform all pediatric ECMO cannulations at our institution. The general cannulation strategy involves an intra-thoracic approach in patients with a recent median sternotomy and cervical (infants and small children) or femoral/iliac (larger children) access in all other children.
The ECMO circuits consist of a Maquet (Maquet Inc., Wayne, NJ) Bioline coated Quadrox D oxygenator, a BB14 Better Bladder (Circulatory Technology, Inc., Oyster Bay, NY) venous reservoir, A SMARxT coated Sorin (Sorin Group USA, Arvada, CO) Revolution centrifugal pump, and ¼ inch Carmeda (Medtronic, Inc., Minneapolis, MN) coated tubing. For patients weighing > 10 kg. the circuit tubing was upsized to 3/8 inch. The length of the tubing is minimized to decrease the prime volume. These circuits were primed in a sterile fashion with Normosol R (Hospira, Inc,. Lake Forest, IL), de-aired, and covered for a maximum of 30 days [8]. If blood products are not immediately available, the circuits are prepared for asanguineous ECMO deployment by adding 25 mEq sodium bicarbonate, 200 units heparin and 300 mg calcium gluconate to the circuit. Immediately after ECMO initiation, equal volumes of banked red blood cells and ultrafiltrate are exchanged to increase the hematocrit of the patient. Identical pump consoles and circuits are used in all pediatric ICUs to maximize familiarity for the ECMO specialists.
ECMO team members are routinely debriefed following ECMO deployment, and perception surveys are periodically administered to ICU nurses, respiratory therapists and perfusionists to allow for anonymous feedback and an additional mechanism for identifying weaknesses of the rapid-response ECMO program that can be targeted for quality improvement.
Data Collection
Diagnostic categories included pre- or non-operative heart disease (structural congenital heart disease without operation prior to ECMO, cardiomyopathy, myocarditis), post-operative congenital heart surgery (cardiac operation during the same admission prior to ECMO) and respiratory (meconium aspiration, primary pulmonary hypertension of the newborn, sepsis, viral or bacterial pneumonia, congenital diaphragmatic hernia). Non-cardiac structural malformations or chromosomal abnormalities were defined as previously described by Kane and colleagues [6]. Indications for ECMO included primary respiratory failure (hypoxemia or pulmonary hypertension), arrhythmia, or cardiogenic shock. ECPR patients were defined as those placed on ECMO during cardiopulmonary resuscitation (CPR) with chest compressions. ECMO initiation times for ECPR patients were determined by the interval between the onset of CPR and the initiation of ECMO flow. ECMO initiation times for non-ECPR patients were not compared given the lack of a documented start time for the ECMO initiation process in the pre-rapid-response ECMO era. Pre-ECMO acid/base status was determined from the most recent blood gas immediately prior to placement on the ECMO circuit. The peak lactate level prior to or within 24 hours of ECMO initiation was recorded.
Complications were defined as previously described by Kane and colleagues [6]. Circuit complications included thrombus formation in the circuit, circuit failure, and circuit or oxygenator change. Bleeding was defined as surgical or cannulation site hemorrhage requiring surgical exploration. Anti-coagulation protocols during ECMO were reviewed and were not found to have changed during the study period. Respiratory complications included pneumonia, acute respiratory distress syndrome, pneumothorax requiring tube thoracostomy, and pulmonary hemorrhage. Sepsis was defined as a positive blood culture while on ECMO. Central nervous system (CNS) injury was defined as any radiologic evidence of brain injury temporally related to the time of ECMO support, seizures documented by electroencephalography, or pronouncement of brain death. Protocols for neurologic assessment and imaging surveillance were reviewed and were not found to have changed during the study period. The rate of neurological imaging by ultrasound, computed tomography, or magnetic resonance imaging during ECMO support or prior to hospital discharge was 96% and did not differ between eras (97% pre-RR-ECMO vs. 94% RR-ECMO, P=0.28) Post-ECMO neurologic status was assessed for survivors using pediatric cerebral performance category (PCPC) scores as previously described [5–7, 9]. PCPC scores were assigned at latest follow-up if adequate neurological information was available in the medical record.
Statistical Analysis
Variables were compared between patients treated before (pre-RR-ECMO) and after (RR-ECMO) implementation of the rapid-response ECMO program. Continuous and categorical variables were compared using the Mann-Whitney rank sum test or chi-square test, respectively. For outcomes with greater than ten events per group (CNS injury, renal failure, liver injury, death), the association between rapid-response ECMO and outcome was assessed using logistic regression models to adjust for differences in patient demographics and disease severity between groups. In multivariable models, odds ratios were adjusted for all variables with P < 0.20 on univariate analysis (age, weight, and pre-ECMO arterial pH). Calculations were performed using STATA 11.1 (StataCorp, College Station, TX).
RESULTS
Patient and ECMO Characteristics
Between April 2003 and March 2011, 144 pediatric patients were placed on emergent VA ECMO, including 31 (22%) ECPR initiations. Of these, 79 (55%) were placed on ECMO before and 65 (45%) were placed on ECMO after implementation of the rapid-response ECMO program. Patient demographics, baseline parameters, and ECMO characteristics are shown in Table 1. Rapid-response ECMO patients demonstrated more severe pre-ECMO acidosis when compared to pre-rapid-response ECMO patients.
Table 1.
Patient and extracorporeal membrane oxygenation characteristics
| Variable | All patients | ECPR | Non-ECPR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-RR-ECMO (n=79) | RR-ECMO (n=65) | P Value | Pre-RR-ECMO (n=17) | RR-ECMO (n=14) | P Value | Pre-RR-ECMO (n=62) | RR-ECMO (n=51) | P Value | |
| Demographics | |||||||||
| - Age (mos) | 0.13 [0.07–1.48] | 0.36 [0.07–4.24] | 0.17 | 0.92 [0.33–7.04] | 1.50 [0.76–47.0] | 0.27 | 0.09 [0.03–0.36] | 0.20 [0.03–3.65] | 0.20 |
| - Weight (kg) | 3.3 [2.7–4.0] | 3.5 [3.1–5.5] | 0.12 | 4.0 [3.3–6.9] | 3.9 [3.3–13.2] | 0.61 | 3.3 [2.7–4.0] | 3.5 [3.0–5.0] | 0.11 |
| - Female | 32 (41%) | 28 (43%) | 0.76 | 7 (41%) | 7 (50%) | 0.62 | 25 (40%) | 21 (41%) | 0.93 |
| Diagnosis | 0.32 | 0.04 | 0.72 | ||||||
| - Pre-/non-operative heart disease | 16 (20%) | 19 (29%) | 3 (18%) | 5 (36%) | 13 (21%) | 14 (27%) | |||
| - Post-operative congenital heart surgery | 27 (34%) | 16 (25%) | 14 (82%) | 6 (43%) | 13 (21%) | 10 (20%) | |||
| - Respiratory | 36 (46%) | 30 (46%) | 0 | 3 (21%) | 36 (58%) | 27 (53%) | |||
| Non-cardiac structural/chromosomal abnormality | 24 (30%) | 17 (26%) | 0.58 | 6 (35%) | 4 (29%) | 0.69 | 18 (29%) | 13 (25%) | 0.68 |
| Indication | 0.43 | 0.09 | 0.43 | ||||||
| - Respiratory | 46 (58%) | 35 (54%) | 3 (18%) | 3 (21%) | 43 (69%) | 32 (63%) | |||
| - Arrhythmia | 11 (14%) | 6 (9%) | 10 (59%) | 3 (21%) | 1 (2%) | 3 (6%) | |||
| - Cardiogenic shock | 22 (28%) | 24 (37%) | 4 (24%) | 8 (57%) | 18 (29%) | 16 (31%) | |||
| ECPR | 17 (22%) | 14 (22%) | 1 | 17 (100%) | 14 (100%) | 1 | 0 | 0 | 1 |
| - Duration of CPR (min) | 68 ± 45 (31–187) | 51 ± 19 (30–99) | 0.32 | ||||||
| - CPR duration < 60 minutes | 9 (53%) | 11 (79%) | 0.14 | ||||||
| Cannulation site | 0.29 | 0.09 | 0.98 | ||||||
| - Neck | 51 (65%) | 43 (66%) | 5 (29%) | 5 (36%) | 46 (74%) | 38 (75%) | |||
| - Chest | 26 (33%) | 17 (26%) | 12 (71%) | 6 (43%) | 14 (23%) | 11 (22%) | |||
| - Groin | 2 (3%) | 5 (8%) | 0 | 3 (21%) | 2 (3%) | 2 (4%) | |||
| pre-ECMO acidosis | |||||||||
| - Arterial pH | 7.31 ± 0.18 (6.81–7.85) | 7.23 ± 0.19 (6.74–7.67) | 0.007 | 7.26 ± 0.30 (6.81–7.85) | 7.15 ± 0.20 (6.80–7.39) | 0.16 | 7.32 ± 0.13 (7.01–7.57) | 7.26 ± 0.18 (6.74–7.67) | 0.02 |
| - Base excess | −3.1 ± 7.9 (−25, +29) | −4.9 ± 8.2 (−30, +14) | 0.20 | −4.3 ± 12.9 (−25, +29) | −5.3 ± 10.7 (−28, +11) | 0.94 | −2.8 ± 5.9 (−22, +9) | −4.8 ± 7.5 (−30, +14) | 0.12 |
| Peak lactate (mmol/L) | 11.2 ± 6.8 (0.9–27) | 11.5 ± 6.4 (1.9–36) | 0.63 | 16.9 ± 6.1 (5.3–27) | 17.8 ± 6.5 (10–36) | 0.89 | 9.6 ± 6.1 (0.9–25) | 9.8 ± 5.2 (1.9–23) | 0.59 |
| Duration of ECMO (days) | 8.6 ± 5.1 (0.7–22.3) | 10.6 ± 8.9 (0.4–39.6) | 0.70 | 7.8 ± 5.3 (0.7–20.0) | 8.6 ± 10.1 (0.4–35.0) | 0.38 | 8.8 ± 5.1 (1.5–22.3) | 11.2 ± 8.6 (0.5–39.6) | 0.37 |
Values expressed as median [interquartile range], mean ± standard deviation (range), or number (percent). ECPR = extracorporeal cardiopulmonary resuscitation; RR-ECMO = rapid-response extracorporeal membrane oxygenation.
ECPR Response Times
The coordinated performance and peak speed of the ECMO team was assessed by measuring the duration of CPR with chest compressions prior to ECMO initiation in ECPR patients. The mean duration of CPR before and after implementation of the rapid-response ECMO program shifted from 68 to 51 minutes (P=0.32). When viewed as categorical data, there was also a shift towards more ECPR initiations achieved in under 40 minutes (24% pre-rapid-response ECMO vs. 43% rapid-response ECMO, P=0.25) and fewer ECPR initiations requiring more than 60 minutes (47% pre-rapid-response ECMO vs. 21% rapid-response ECMO, P=0.14) with rapid-response ECMO, although these changes did not reach statistical significance (Figure). Eleven of 31 (35%) ECPR patients survived to hospital discharge, and mean ECPR initiation time was similar between survivors and non-survivors (56 ± 41 minutes vs. 63 ± 35 minutes, P=0.32).
Figure.
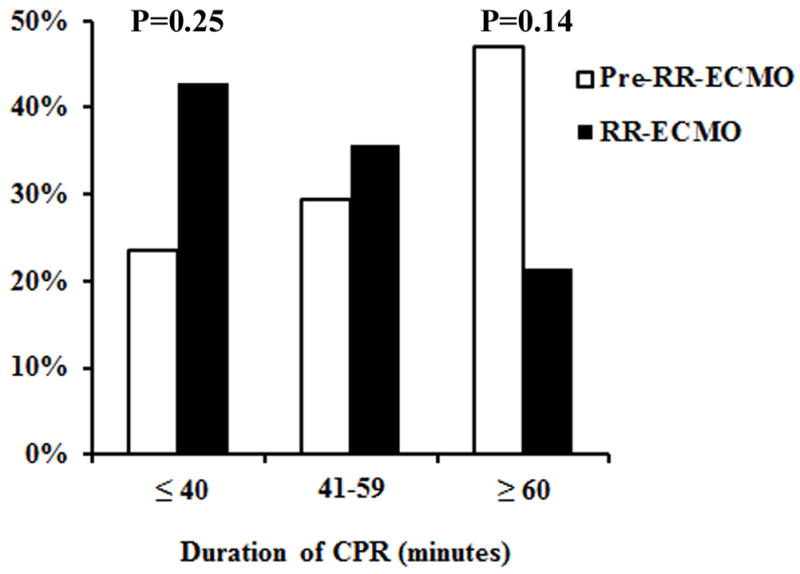
Duration of cardiopulmonary resuscitation with chest compressions for extracorporeal cardiopulmonary resuscitation patients before (Pre-RR-ECMO, n=17) and after (RR-ECMO, n=14) rapid-response extracorporeal membrane oxygenation program implementation.
Complications and Outcomes
Unadjusted rates of ECMO complications and patient outcomes before and after rapid-response ECMO implementation are shown in Table 2. Overall, 65 (45%) patients experienced CNS injury, 50 (35%) experienced renal failure, and 28 (19%) experienced liver injury. One-hundred-five (73%) patients were successfully weaned from ECMO support and 73 (51%) survived to hospital discharge. Survival by diagnostic category was 46% (16 of 35) for pre-/non-operative heart disease patients, 42% (18 of 43) for post-operative congenital heart surgery patients, and 59% (39 of 66) for respiratory patients. PCPC scores were assigned to 71 of 73 survivors, of which 53 (75%) had no to mild neurological injury (PCPC score ≤ 2). After multivariable risk-adjustment, rapid-response ECMO was associated with a 52% reduced risk of CNS injury relative to the immediate pre-rapid-response ECMO era, but the risk of renal failure, liver injury, and in-hospital death were unchanged (Table 3).
Table 2.
Complications and outcomes
| Variable | All patients | ECPR | Non-ECPR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-RR-ECMO (n=79) | RR-ECMO (n=65) | P Value | Pre-RR-ECMO (n=17) | RR-ECMO (n=14) | P Value | Pre-RR-ECMO (n=62) | RR-ECMO (n=51) | P Value | |
| Circuit complication | 6 (8%) | 9 (14%) | 0.22 | 1 (6%) | 0 | 0.36 | 5 (8%) | 9 (18%) | 0.12 |
| Bleeding | 12 (15%) | 4 (6%) | 0.09 | 3 (18%) | 0 | 0.10 | 9 (15%) | 4 (8%) | 0.27 |
| Respiratory complication | 5 (6%) | 9 (14%) | 0.13 | 1 (6%) | 1 (7%) | 0.89 | 4 (6%) | 8 (16%) | 0.11 |
| Sepsis | 9 (11%) | 6 (9%) | 0.67 | 2 (12%) | 2 (14%) | 0.84 | 7 (11%) | 4 (8%) | 0.54 |
| CNS injury | 40 (51%) | 25 (38%) | 0.14 | 9 (53%) | 6 (43%) | 0.58 | 31 (50%) | 19 (37%) | 0.18 |
| - Seizures | 5 (6%) | 1 (2%) | 1 (6%) | 0 | 4 (6%) | 1 (2%) | |||
| - Radiological evidence of | 34 (43%) | 21 (32%) | 8 (47%) | 5 (36%) | 26 (42%) | 16 (31%) | |||
| CNS injury | |||||||||
| - Brain death | 6 (8%) | 3 (5%) | 2 (12%) | 1 (7%) | 4 (6%) | 2 (4%) | |||
| Renal failure | 27 (34%) | 23 (35%) | 0.97 | 6 (35%) | 6 (43%) | 0.67 | 21 (34%) | 17 (33%) | 0.95 |
| - Serum creatinine ≥ 1.5 mg/dl | 26 (33%) | 23 (35%) | 6 (35%) | 6 (43%) | 20 (32%) | 17 (33%) | 0.90 | ||
| - Dialysis use (PD or CVVHD) | 7 (9%) | 5 (8%) | 3 (18%) | 0 | 4 (6%) | 5 (10%) | 0.51 | ||
| Liver injury (ALT or AST ≥ 500 IU/dL) | 13 (16%) | 15 (23%) | 0.32 | 5 (29%) | 7 (50%) | 0.24 | 8 (13%) | 8 (16%) | 0.67 |
| ECMO weaned | 62 (78%) | 43 (66%) | 0.10 | 12 (71%) | 6 (43%) | 0.12 | 50 (81%) | 37 (73%) | 0.31 |
| Survival to hospital discharge | 41 (52%) | 32 (49%) | 0.75 | 7 (41%) | 4 (29%) | 0.47 | 34 (55%) | 28 (55%) | 0.99 |
| Length of stay (days) | 28 [16–64] | 34 [15–70] | 0.87 | 32 [15–52] | 23 [4–46] | 0.45 | 28 [16–64] | 36 [15–74] | 0.55 |
| PCPC score ≤ 2 | 32/40 (80%) | 21/31 (68%) | 0.24 | 4/7 (57%) | 2/4 (50%) | 0.82 | 28/33 (85%) | 19/27 (70%) | 0.18 |
Values expressed as median [interquartile range] or number (percent). ALT = alanine aminotransferase; AST = aspartate aminotransferase; CNS = central nervous system; CVVHD = continuous veno-venous hemodialysis; RR-ECMO = rapid-response extracorporeal membrane oxygenation; PCPC = pediatric cerebral performance category, PD = peritoneal dialysis.
Table 3.
Risk-adjusted associations between rapid-response extracorporeal membrane oxygenation and select outcomes.
| RR-ECMO vs. pre-RR-ECMO | OR | 95% CI | P Value |
|---|---|---|---|
| CNS injury | 0.48 | 0.23–0.98 | 0.04 |
| Renal failure | 0.93 | 0.45–1.95 | 0.86 |
| Liver injury | 1.16 | 0.47–2.90 | 0.75 |
| In-hospital death | 0.99 | 0.50–1.99 | 0.99 |
CI = confidence interval; CNS = central nervous system; OR = odds ratio; RR-ECMO = rapid-response extracorporeal membrane oxygenation.
COMMENT
We retrospectively compared ECPR initiation times, complications, and outcomes before and after implementation of a pediatric rapid-response ECMO program that was applied broadly to a heterogeneous group of pediatric patients placed on VA ECMO for cardiopulmonary arrest, or near arrest. Our findings demonstrated a trend towards shortened ECPR initiation times and a reduced risk of CNS injury following program implementation. However, other complications and survival to hospital discharge remained unchanged.
The goal of reducing ECMO initiation time for patients experiencing cardiac arrest appears self-evident, as the duration of conventional CPR directly correlates with mortality and adverse neurologic outcomes [10]. In addition, a recent single-institution study of 37 ECPR patients [7], as well as a meta-analysis of 288 ECPR patients [2], reported CPR duration greater than 30 minutes was an independent risk factor for mortality [2]. In our study, an encouraging trend was observed towards reduced mean ECPR initiation time and fewer ECPR initiations requiring greater than 60 minutes with rapid-response ECMO. In addition, our mean ECPR initiation time of 51 minutes is comparable to ECPR initiation times achieved by other centers shortly after rapid-response ECMO program implementation [3, 5, 11]. However, further improvement remains possible, as the Boston group has shown median ECPR initiation times fell steadily from 50 to 25 minutes over the 13-year interval following implementation of their rapid-response ECMO program [6]. One major area for further process improvement identified by perception surveys (data not shown) was the lack of operating room staff availability, oftentimes requiring other personnel to fulfill circulating and scrub nurse duties while awaiting arrival of the full operating room team. This appears to be a rate-limiting step to ECMO initiation at our institution, and in response we have proposed training a select group of ICU charge nurses or physician extenders to perform operating room staff duties to allow for streamlined ECMO initiation in their absence. At present, we view our current ECPR initiation times as a work in progress, and ideally ECPR initiation times less than 30 minutes can routinely be achieved with further team training and process refinements. In addition, an emphasis on performance of high-quality CPR is necessary, as our study and others have not shown a survival benefit from reduced CPR duration [6, 11, 12], suggesting the quality of pre-ECMO CPR may be as important as the duration of CPR.
The 45% incidence of CNS injury in our study was similar to the 52% incidence of CNS injury reported in the Boston ECPR experience, from which we used the same broad clinical and radiographic definitions to capture neurologic insults [6]. In addition, follow-up PCPC scores appeared favorable, with 75% of survivors demonstrating no to mild neurologic injury [5, 6]. Perhaps the most encouraging finding of our study was a 52% reduced risk of CNS injury following rapid-response ECMO implementation relative to the immediate pre-rapid-response ECMO era, after multivariable risk-adjustment. While shortened ECMO initiation time should reduce neurologic events in ECPR patients experiencing fulminant cardiopulmonary arrest [13], the majority of patients in our study were not placed on ECMO during CPR and were presumably less susceptible to neurologic injury as a result of delayed ECMO deployment. We therefore suspect other components of the rapid-response program besides initiation time may have contributed to the reduced risk of CNS injury, such as a more controlled transition to extracorporeal circulation or an increased willingness to use ECMO prior to cardiac arrest. It is also possible that practice changes unrelated to ECMO initiation contributed to reduced CNS injury, such as use of controlled hypothermia or changes in the management of the ECMO circuit. Nonetheless, the significant positive association between reduced CNS injury and implementation of the rapid-response ECMO program suggests some facet of the streamlined ECMO initiation process may lead to reduced neurologic injury, and further study to identify the precise contributors is warranted.
The overall survival rate of 51% in our study was comparable to the pediatric ECMO survival rates of 38–52% reported to the ELSO database [13]. Survival by diagnostic category also appeared consistent with published reports, with the highest and lowest survival observed for respiratory patients (59%) and ECPR patients (35%), respectively [14]. When interpreting our survival numbers it is important to emphasize that veno-venous ECMO patients, in whom the best outcomes are usually observed, were excluded [14]. Although our survival rates were acceptable, rapid-response ECMO was not associated with improved survival. For ECPR patients, it may be that initiation times less than 30 minutes are required to positively affect survival, as shown by Tajik and colleagues, a threshold our program has yet to consistently achieve [2]. For non-ECPR patients, the largest determinants of survival are likely the severity and reversibility of the underlying disease process, and rapid ECMO deployment may be less influential. However, the rapid-response ECMO patients in our study were at a higher risk of ECMO complications and death due to more severe acidosis at the time of ECMO initiation [6, 13, 15, 16], and risk-adjustment may not have fully accounted for the baseline differences in disease severity between the two patient cohorts. Although it initially seems paradoxical that the recent patients demonstrated worse acidosis prior to ECMO initiation despite rapid ECMO deployment, we speculate that the more recent rapid-response ECMO patients may have been a higher risk cohort due to more liberal use of ECMO in patients who previously would not have been considered candidates for ECMO. In support of this possibility, prior studies have shown trends towards the use of ECMO in more patients and sicker patients over time [14, 17].
Limitations
Rapid-response ECMO patients were compared to historical controls from the era immediately preceding program implementation. Unmeasured confounders related to patient selection and ECMO management may influence the comparison of outcomes between eras. Retrospective risk adjustment models are also unable to fully account for differences in patient groups in the absence of randomization. The study is prone to type II error and is underpowered to detect small differences in outcomes between groups. Retrospective assignment of PCPC scores from follow-up notes represents a crude and imperfect tool for assessing neurologic status, although this method has been used in prior ECMO reports [5–7].
Conclusion
Implementation of a pediatric rapid-response ECMO program for veno-arterial ECMO initiation was associated with reduced neurologic complications but did not improve survival or reduce other complications during the first three years of program implementation. These data suggest that development of a coordinated system for rapid ECMO deployment may benefit both ECPR and non-ECPR patients, but additional research and continued attention to process improvements are needed to improve survival following emergent ECMO deployment.
Acknowledgments
Support was received by a Thoracic Surgery Foundation for Research and Education Research Fellowship to Dr. Andersen.
Footnotes
Presented at the 59th Annual Meeting of The Southern Thoracic Surgical Association, Naples, Florida, November 7–10, 2012
DISCLOSURES
None
References
- 1.Duncan BW. Mechanical circulatory support for infants and children with cardiac disease. Ann Thorac Surg. 2002;73:1670–1677. doi: 10.1016/s0003-4975(01)03027-2. [DOI] [PubMed] [Google Scholar]
- 2.Tajik M, Cardarelli MG. Extracorporeal membrane oxygenation after cardiac arrest in children: What do we know? Eur J Cardiothorac Surg. 2008;33:409–417. doi: 10.1016/j.ejcts.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Duncan BW, Ibrahim AE, Hraska V, et al. Use of rapid-deployment extracorporeal membrane oxygenation for the resuscitation of pediatric patients with heart disease after cardiac arrest. J Thorac Cardiovasc Surg. 1998;116:305–311. doi: 10.1016/s0022-5223(98)70131-x. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs JP, Ojito JW, McConaghey TW, et al. Rapid cardiopulmonary support for children with complex congenital heart disease. Ann Thorac Surg. 2000;70:742–749. doi: 10.1016/s0003-4975(00)01562-9. discussion 749–750. [DOI] [PubMed] [Google Scholar]
- 5.Huang SC, Wu ET, Chen YS, et al. Extracorporeal membrane oxygenation rescue for cardiopulmonary resuscitation in pediatric patients. Crit Care Med. 2008;36:1607–1613. doi: 10.1097/CCM.0b013e318170b82b. [DOI] [PubMed] [Google Scholar]
- 6.Kane DA, Thiagarajan RR, Wypij D, et al. Rapid-response extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in children with cardiac disease. Circulation. 2010;122:S241–248. doi: 10.1161/CIRCULATIONAHA.109.928390. [DOI] [PubMed] [Google Scholar]
- 7.Sivarajan VB, Best D, Brizard CP, et al. Duration of resuscitation prior to rescue extracorporeal membrane oxygenation impacts outcome in children with heart disease. Intensive Care Med. 2011;37:853–860. doi: 10.1007/s00134-011-2168-6. [DOI] [PubMed] [Google Scholar]
- 8.Walczak R, Lawson DS, Kaemmer D, et al. Evaluation of a preprimed microporous hollow-fiber membrane for rapid response neonatal extracorporeal membrane oxygenation. Perfusion. 2005;20:269–275. doi: 10.1191/0267659105pf819oa. [DOI] [PubMed] [Google Scholar]
- 9.Fiser DH, Long N, Roberson PK, et al. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 10.Slonim AD, Patel KM, Ruttimann UE, Pollack MM. Cardiopulmonary resuscitation in pediatric intensive care units. Crit Care Med. 1997;25:1951–1955. doi: 10.1097/00003246-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Huang SC, Wu ET, Wang CC, et al. Eleven years of experience with extracorporeal cardiopulmonary resuscitation for paediatric patients with in-hospital cardiac arrest. Resuscitation. 2012;83:710–714. doi: 10.1016/j.resuscitation.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Morris MC, Wernovsky G, Nadkarni VM. Survival outcomes after extracorporeal cardiopulmonary resuscitation instituted during active chest compressions following refractory in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2004;5:440–446. doi: 10.1097/01.pcc.0000137356.58150.2e. [DOI] [PubMed] [Google Scholar]
- 13.Cengiz P, Seidel K, Rycus PT, Brogan TV, Roberts JS. Central nervous system complications during pediatric extracorporeal life support: Incidence and risk factors. Crit Care Med. 2005;33:2817–2824. doi: 10.1097/01.ccm.0000189940.70617.c3. [DOI] [PubMed] [Google Scholar]
- 14.Laussen PC, Fynn-Thompson F. Surgical approaches, cardiopulmonary bypass, and mechanical circulatory support in children. In: Sellke FW, del Nido PJ, Swanson SJ, editors. Sabiston & spencer surgery of the chest. Philadelphia, PA: Saunders Elsevier; 2010. pp. 1735–1747. [Google Scholar]
- 15.Barrett CS, Bratton SL, Salvin JW, et al. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr Crit Care Med. 2009;10:445–451. doi: 10.1097/PCC.0b013e318198bd85. [DOI] [PubMed] [Google Scholar]
- 16.Thiagarajan RR, Laussen PC, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation. 2007;116:1693–1700. doi: 10.1161/CIRCULATIONAHA.106.680678. [DOI] [PubMed] [Google Scholar]
- 17.Schaible T, Hermle D, Loersch F, et al. A 20-year experience on neonatal extracorporeal membrane oxygenation in a referral center. Intensive Care Med. 2010;36:1229–1234. doi: 10.1007/s00134-010-1886-5. [DOI] [PubMed] [Google Scholar]


