Abstract
Background
High forefoot plantar pressure is associated with plantar ulcers in people with diabetes and peripheral neuropathy. The purpose of this pilot study is to determine safety and efficacy of botulinum toxin A injected into the gastrocnemius-soleus muscles to reduce muscle strength and plantar pressure.
Materials and Methods
This double blind, randomized clinical trial studied 17 people with diabetes mellitus, peripheral neuropathy and forefoot plantar ulcer. Subjects were randomized into one of three groups receiving gastrocnemius-soleus muscle injections on the involved side with; 1) Saline (n=5, weight = 99 ± 21 kg), 2) 200 units of Botox® (n=7, weight = 101 ± 5 kg), or 3) 300 units of Botox® (n=5, weight=129 ± 22 kg). Botox® dose was converted to units/kg, the majority received between 1.9 and 2.4 units/kg (n=11) and one 3.2 units/kg. Plantarflexor peak torque and forefoot peak plantar pressure were quantified prior and two weeks post injection.
Results
There were no complications from the injections. Plantarflexor peak torque on the involved side increased in the placebo and 300 groups (3 ± 4 Nm and 6 ± 10 Nm respectively) and decreased −8 ± 11 Nm in the 200 group. There was no relationship between units/kg of Botox® for each subject and change in plantarflexor peak torque. Forefoot peak plantar pressure did not change in the placebo and 300 groups (0 ± 11 and 0 ± 5 N/cm2 respectively) and decreased −4 ± 16 N/cm2 (4%) for the 200 group.
Conclusions
There were no adverse events associated with the Botox® injections. This study was unable to determine the dose to consistently reduce plantarflexor strength and forefoot plantar pressure. Additional research is needed to investigate diabetes mellitus specific physiological changes and their impact of BoNT-A effectiveness in order to guide appropriate dosing.
Keywords: Botulinum Toxin A, peripheral nervous system diseases, diabetes mellitus
Approximately 346 million individuals worldwide have diagnosed or undiagnosed diabetes mellitus.1 Fifteen percent of those with diabetes mellitus will develop a foot ulcer during the course of their disease.30 A non-healing or slow healing ulcer has the potential to develop an acute infection, require a lower extremity amputation and contribute to significant decrease in function and an increase risk of death.3, 10, 27
The most common cause for diabetic plantar ulcers is high plantar pressures in the presence of sensory neuropathy and foot deformity.5 Limited ankle dorsiflexion range of motion (Equinus) has been thought to contribute to high forefoot plantar pressure.4, 7, 15, 20 Mueller et al assessed the impact of a tendo-achilles lengthening on dorsiflexion range of motion, plantar pressure and ulcer recurrence.22 Those that received the tendo-achilles lengthening had a significant reduction in ulcer recurrence. However, forefoot plantar pressure was only temporarily reduced, following the pattern of change in plantarflexor strength. Change in forefoot plantar pressure was not associated with the change in dorsiflexion range of motion which remained increased throughout the follow up period.16 From this study it was hypothesized that the temporary reduction in plantarflexor strength, which reduced push-off power protected the newly healed skin. As strength returned and plantar pressure increased, the skin was gradually loaded increasing tolerance to high plantar pressure and decreasing the risk of reulceration.
The tendo-achilles surgical procedure, although relatively uncomplicated, carries the risks of infection, negative effects of sedation and local anesthesia, and the potential to over lengthen the tendon inducing hindfoot ulcers.22 The surgical risks, the association between plantar flexor muscle strength and forefoot plantar pressure, and the lack of association between dorsiflexion range of motion and ulcer recurrence led us to consider a novel use of therapeutic botulinum toxin (BoNT-A) to comparatively and temporarily weaken the plantar flexor muscles, reduce plantar forefoot pressure, and potentially reduce the risk of ulcer recurrence.
BoNT-A dosing and effectiveness in adult plantarflexors has been studied in the treatment of spasticity in stroke,14, 17, 26, 34 multiple sclerosis,25 and traumatic brain injury9 and limited dorsiflexion post Achilles tendon surgery31. BoNT-A dosing has not been studied in adults with diabetes mellitus and peripheral neuropathy who are generally obese. Additionally, it is not known how skeletal muscle insulin resistance33 and peripheral vascular disease6, all associated with complicated diabetes, might impact BoNT-A dosing and effectiveness.
Finally, the primary outcomes for BoNT-A studies have been change in spasticity, pain and function,9, 14, 17, 25, 26 or range of motion31. Muscle strength is difficult to quantify in the presence of spasticity and has rarely been reported as an outcome measure. Mancini et al measured strength reductions of one muscle grade in the injected muscles that received total lower limb doses of 540 units of Botox (Botox®, Allergan, P.O. Box 19534, Irvine, CA 92623-9534, USA). 17 Plantarflexor strength changes associated with BoNT-A injections have not been measured in a quantitative manner to assist in determining the appropriate and safe dose to induce a reduction in strength comparable to Mueller et al (30%).22
The purpose of this double-blinded, placebo-controlled prospective randomized clinical trial was to collect pilot data to determine safety and proof of concept of two doses of BoNT-A 200 or 300 units, injected into the gastrocnemius-soleus muscles to reduce muscle strength and plantar pressure, similar to that experienced with the tendo-achilles lengthening surgery. If the decrease in plantar pressure was similar, BoNT-A injections may be an alternative for surgical intervention to reduce ulcer recurrence rate.
METHODS
Study Design Overview
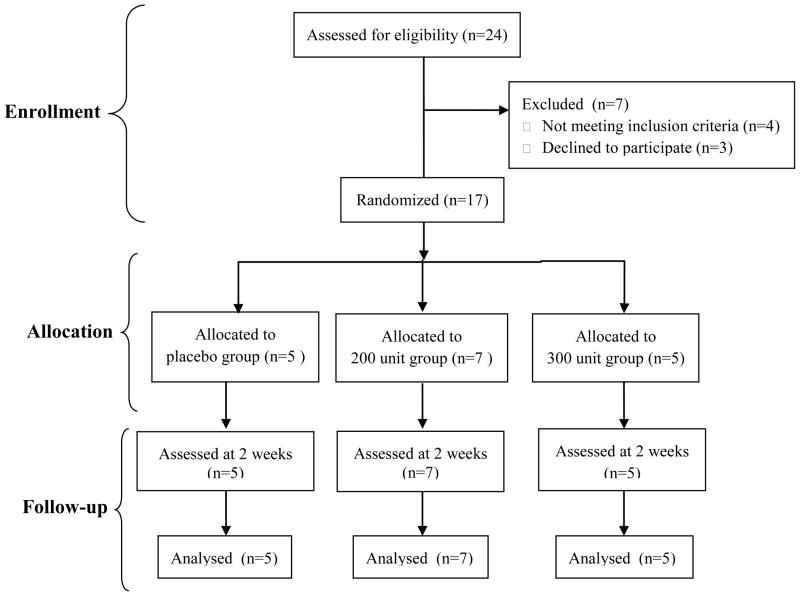
This study was a double blind, placebo controlled, prospective randomized clinical trial. Seventeen subjects provided informed consent, signed the consent form approved by the (Blinded) and participated in the study. Subjects were remunerated for their participation in the study. All subjects were randomly assigned, using a computer generated randomization list, to one of three treatment groups: 1) placebo group (n=5) that received 3cc of normal saline, 2) 200 unit group (n=7) that received 200 units of Botox®, and 3) 300 unit group (n=5) receiving 300 units of BoNT-A (Botox®, Allergan, P.O. Box 19534, Irvine, CA 92623-9534, USA) (Figure 1). All study personnel and subjects were blinded to group assignment except a study nurse that maintained the randomization list and prepared the injections. Forefoot plantar pressure, plantarflexor muscle strength, and dorsiflexion range of motion measurements were collected at baseline and two weeks after the injections. The two week time frame for post injection measurements was chosen because maximum reduction in strength was expected at this time.25
Figure 1.
Enrollment, randomization, and follow-up of study sample
Inclusion Criteria
Patients were included in the study if they had a diagnosis of diabetes mellitus, inability to feel 5.07 Semmes-Weinstein monofilament on at least 1 location on the plantar foot,8 a recurrent forefoot plantar ulcer, and were ambulatory with or without an assistive device. A recurrent forefoot ulcer was defined as at least one previous occurrence of a plantar ulcer or failure to heal a plantar ulcer with reasonable treatment intervention (e.g. total contact cast, off loading boot, or therapeutic footwear modifications)
Exclusion Criteria
Patients were excluded if they had an active infection in the involved foot, ulcers on the dorsal surface of the foot, ankle-brachial index <0.45, history of cerebral vascular accident or other neurological problems complicating their rehabilitation, or history of a neuromuscular disease, except peripheral neuropathy as a result of diabetes mellitus. Additionally, patients were excluded if they had received BoNT-A injections before, were on current drug therapy that included the anticoagulant warfarin, and were women of childbearing years unless pregnancy test was agreed upon, completed and negative and patients agreed to use some form of contraception during the study.
Treatment Description
All subjects received treatment for their wounds, which included debridement of callus and other devitalized tissues and use of a removable walker boot to unload the ulcer and protect the limb after the injections. All subjects attended a single injection visit, with the neurologist. During this visit subjects received a total of six injections using electromyographic guidance. The medial gastrocnemius, lateral gastrocnemius, and soleus muscle bellies, on the side with the forefoot ulcer, each received 2 injections utilizing a 0.5 cc dilution. The placebo group was injected with normal saline while those subjects that randomized into the BoNT-A groups received either 200 or 300 units of BoNT-A, reconstituted with normal saline into a total volume of 3cc to preserve blinding.(Botox®, Allergan, P.O. Box 19534, Irvine, CA 92623-9534, USA) The 200 and 300 unit doses were chosen because they fall within tested doses that produced a clinical effect without significant adverse events.17, 26
Testing Methods
Concentric plantar flexor torque was assessed using the Biodex System 3 Pro Orthopedic Testing & Rehabilitation dynamometer (Biodex Medical Systems, 20 Ramsay Rd, Shirley, New York 11967-4704, USA). Plantar flexor peak torque was measured at 60o/sec, which is comparable to the angular velocity of the ankle joint during the stance phase of walking.21 Three trials, each with 3 repetitions, were completed. Peak torque was calculated as the average of the two highest torque values across trials.
Barefoot plantar pressure was assessed on all subjects while walking at their preferred speed, over an embedded EMED-ST P-2 pressure platform (sampling frequency=50Hz, 2 sensors/cm2) (Novel Inc., 964 Grand Avenue, St. Paul, MN, 55105 USA). A two-step method of data collection was used19 and a minimum of two trials of each foot were recorded. The plantar pressure map was divided into 3 horizontal masks using Percent Mask software (Percent Mask software, 964 Grand Avenue, St. Paul, MN, 55105 USA) and peak plantar pressure was determined for the forefoot region.
Dorsiflexion range of motion was measured in two positions with a universal full-circle goniometer as previously described.11, 12 Subjects were positioned in prone with the hip and knee extended with the feet and ankles off the end of the table and prone with the hip extended and the knee flexed. The axis of the goniometer was placed over the lateral malleolus with one arm aligned with the fifth metatarsal head and the other aligned with the fibula.
All people were assessed for adverse events at the 2 weeks follow up. The assessment included questions and examination of pain/redness/edema at the injection sites, presences of systemic weakness, nausea or fever, or decline in gait and/or function.
Statistical Analysis
The pilot study was not powered to determine conclusive group differences in main outcome measures. The statistical examination of the data using a one-way ANOVA for continuous variables (age, duration of DM, height, weight, body mass index and hemoglobin A1c), a Chi-square test for gender and type of DM and a repeated measures ANOVA for the intervention outcome measures yielded no significant group differences (p>.05) except subject weight. Thus, to prevent over-interpretation of the data, only descriptive statistics (mean and standard deviation) are reported for all data collected.
RESULTS
Subjects were predominately male and obese with chronic, type 2 diabetes mellitus (See Table 1). Groups were similar in all characteristics except that the 300 unit group weighed more (129 kg ± 22) than the placebo and 200 unit group (100 ± 21and 101 ± 5 kg, respectively, p<.05). No adverse events occurred in any subject following injection, regardless of dose/group.
Table 1.
Subject Characteristics
| Placebo Group | 200 Unit Group | 300 Unit Group | |
|---|---|---|---|
| No. of patients | 5 | 7 | 5 |
| Age (yrs) | 48(9) | 56(10) | 57(9) |
| Male/female | 4/1 | 6/1 | 4/1 |
| Type 1/type 2 diabetes mellitus (no. of patients) | 2/3 | 1/6 | 0/5 |
| Duration of diabetes mellitus (yrs) | 21.2(15.0) | 18.6(10.3) | 15.6(9.7) |
| Height (m) | 1.79 (0.01) | 1.78 (0.12) | 1.84(0.04) |
| Weight (kg) | 99.7(20.7) | 100.7(4.5) | 129.2(21.7) |
| Body-mass index | 30.9 (5.4) | 32.0(4.4) | 38.1(5.5) |
| HbA1c (%) | 7.7(2.6) | 8.0(2.7) | 6.7(0.27) |
| Ankle Brachial index | 1.18(0.28) | 1.07(0.27) | 1.3(0.18) |
Values given as the mean and standard deviation.
Plantarflexor peak torque on the involved side increased 3 ± 4 Nm for the placebo group, decreased −8 ± 11 Nm in the 200 unit group, and increased 6 ± 10 Nm in the 300 unit group (See Table 2). Two of the 12 subjects injected with Botox® had a decrease in plantarflexor peak torque above the variability measured in the placebo group (subjects 6 and 11). Three subjects in the 300 unit group experienced an increase in plantarflexion peak torque that was greater than the changes experienced in the placebo group (subjects 13, 15, and 17).
Table 2.
Pre and post-test results of plantarflexor peak torque, peak plantar pressure, and dorsiflexion range of motion
| Dose/kg | PFT Pre (Nm) | PFT Post (Nm) | Change | PPP Pre (N/cm2) | PPP Post (N/cm2) | Change | DFROM pre Knee Extended/Flexed (degrees) | DFROM post Knee Extended/Flexed (degrees) | Change | |
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo Group | ||||||||||
| 1 | 0 | 25 | 22 | −3 | 41 | 41 | 0 | −4/6 | −4/0 | 0/−6 |
| 2 | 0 | 27 | 29 | 2 | 27 | 36 | 9 | −4/8 | −10/4 | −6/−4 |
| 3 | 0 | 22 | 29 | 7 | 60 | 63 | 3 | 0/7 | 0/0 | 0/−7 |
| 4 | 0 | 23 | 28 | 5 | 53 | 34 | −19 | −4/−3 | −2/8 | 2/10 |
| 5 | 0 | 56 | 58 | 2 | 31 | 36 | 5 | 0/8 | 2/10 | 2/2 |
| Average ± SD | 3 ± 4 | 0 ± 11 | 0 ± 3/−1 ± 7 | |||||||
| 200 Unit Group | ||||||||||
| 6 | 2.0 | 81 | 52 | −29 | 34 | 31 | −3 | 4/10 | 3/6 | −1/−4 |
| 7 | 2.2 | 17 | 10 | −7 | 31 | 29 | −2 | 0/0 | 3/2 | 3/2 |
| 8 | 2.0 | 14 | 12 | −2 | 84 | 93 | 9 | 0/4 | 9/6 | 9/2 |
| 9 | 1.9 | 25 | 23 | −2 | 24 | 31 | 7 | −5/10 | 2/0 | 7/−10 |
| 10 | 2.0 | 22 | 26 | 4 | 60 | 53 | −7 | −4/2 | −2/2 | 2/0 |
| 11 | 2.0 | 70 | 59 | −11 | 59 | 20 | −39 | −8/8 | −5/10 | 3/2 |
| 12 | 1.9 | 30 | 23 | −7 | 51 | 55 | 4 | −6/6 | 0/0 | 6/−6 |
| Average ± SD | −8 ± 11 | −4 ± 16 | 4 ± 3/−2 ± 5 | |||||||
| 300 Unit Group | ||||||||||
| 13 | 2.0 | 17 | 25 | 8 | 23 | 29 | 6 | 4/10 | 0/2 | −4/−8 |
| 14 | 3.2 | 13 | 10 | −3 | 9 | 11 | 2 | 2/2 | 8/14 | 6/12 |
| 15 | 2.1 | 30 | 43 | 13 | 38 | 39 | 1 | −4/4 | 3/11 | 7/7 |
| 16 | 2.3 | 22 | 17 | −5 | 34 | 27 | −7 | 0/2 | −4/2 | −4/0 |
| 17 | 2.4 | 36 | 53 | 17 | 32 | 31 | −1 | 8/16 | 5/14 | −3/−2 |
| Average ± SD | 6 ± 10 | 0 ± 5 | 0 ± 6/2 ± 8 | |||||||
PFT=plantarflexor peak torque, PPP=peak plantar pressure, DFROM=dorsiflexion range of motion
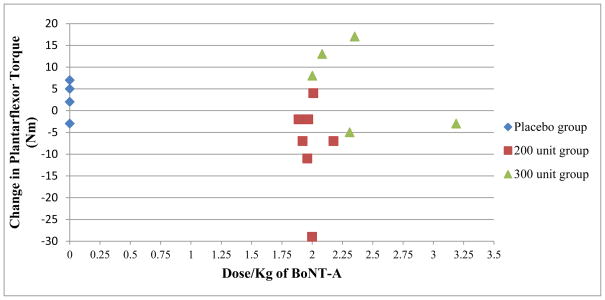
Botox® dose was converted to units/kg for each subject (See Table 2). Eleven of 12 subjects received a dose of between 1.9 and 2.4 units/kg and one subject received a dose of 3.2 units/kg. There was no apparent relationship between dose/kg of Botox® for each subject and change in plantarflexor peak torque (See Figure 2).
Figure 2.
Change in plantarflexor torque relative to dose/kg of botulinum A (BoNT-A)
Forefoot peak plantar pressure stayed essentially the same for the placebo and 300 unit groups (0 ± 11 and 0 ± 5 N/cm2 respectively, average 1% change) and decreased −4 ± 16 N/cm2 (average of 4% change) for the 200 unit group. Across groups only 3 subjects had a decrease in peak forefoot plantar pressure pre to post injection greater than 19% (Subjects 4, 11, and 16). In all subjects the change in forefoot peak plantar pressure was not consistently related to group assignment or change in plantarflexor muscle strength.
Dorsiflexion range of motion with the knee extended and flexed remained fairly stable for the placebo and 300 unit groups (0 ± 3 to 2 ± 8 degrees). The results for the 200 unit group were mixed; dorsiflexion range of motion with the knee extended increased 4 ± 3 degrees and decreased −2 ± 8 degree with the knee flexed. There was no clear relationship between change in dorsiflexion range of motion and plantarflexor torque or peak plantar pressure.
DISCUSSION
This study reports the results of a novel use of BoNT-A to attempt to reduce plantarflexor strength and plantar pressure in individuals with diabetes mellitus and peripheral neuropathy. Our pilot results provide evidence that BoNT-A can be safely administered. However, patient response was highly variable and did not produce a consistent reduction in plantarflexor muscle peak torque or forefoot peak plantar pressure. We were unable to determine a BoNT-A dose that resulted in a predictable reduction in plantarflexor strength or plantar pressure. Additionally, the increase in plantarflexion strength in some individuals was unexpected and we believe, requires additional exploration.
In our group of 12 subjects who received Botox® injections, only two individuals (subjects 6 and 11, both in the 200 unit group), had a reduction in plantarflexor torque beyond the variation measured in the placebo group. Only subject 11 had a concurrent decrease in forefoot peak plantar pressure beyond the variation measured in placebo group. Additionally, three individuals had plantarflexor torque gains beyond the test-retest variability experienced by the placebo group. These results highlight the inconsistent response of the plantarflexor muscles to injected Botox® in our subjects with DPN and the inability to determine the association between plantarflexor strength and forefoot plantar pressure.
Botox® Dosage
There are a number of possible reasons for the lack of measured change in plantarflexor muscle strength and forefoot plantar pressure in the Botox® injected groups. One likely possibility is that the overall Botox® doses chosen for this study, 200 and 300 units, were too small. The dosing paradigm chosen for this study falls within the recommended and tested doses for the gastrocnemius and soleus muscles in adults.9, 17, 25, 26, 31 Previous studies have injected the gastrocnemius/soleus muscles with doses typically ranging from 50–320 units of Botox®.9, 17, 25, 31, 34 (Dysport conversions used 1 unit of Botox®=3 units of Dysport).23, 32 Pittock et al,26 examined a higher dose of 500 units, but this group had a slightly higher incidence of adverse event rate of 33% compared with two other dosing groups of 167 units (29%) and 333 units (25%). Reuter et al studied a plantarflexor
Botox® dosing paradigm of three to four cycles of injections, 3 months apart (total dose per cycle was 200–400 units) for patients with equines deformity and abnormal plantarflexor muscle activity following surgical repair of complete Achilles tendon rupture. This dosing strategy successfully decreased muscle activity, allowed physical therapy intervention to increase dorsiflexion range of motion, and no adverse events were experienced.
It is also possible that the dose relative to body weight was too small. Pittock et al utilized doses of 2.2, 4.5 or 6.5 units/kg26, while Turkel et al used doses ranging from 1.8–3.3 units/kg in a low dose group and 3.6–5.5 units/kg in a high dose group.34 For our study, the dose relative to body weight was in the low range, 1.9 to 2.4 units/kg and may have been inadequate to induce the strength loss we had desired. We were cautious with dosing given that therapeutic BoNT-A injections are performed for neurologic conditions in which the muscle is centrally denervated making extrapolation of dosing to muscle in people with diabetes and peripheral neuropathy more challenging. Future work should take into consideration the higher body mass of individuals with DM when determining an effective dosing strategy.
If the dose was inadequate, it is not clear from our pilot data what would be the appropriate dose for future study. The response to BoNT-A was variable at the same dose/kg and there was no indication of a dose/response relationship in our small sample of individuals. The variability of the response of our subjects to BoNT-A has led us to consider other mechanisms that might be important in the treatment of individuals with diabetes mellitus and peripheral neuropathy.
Botox® Pharmacokinetics
It is also possible that skeletal muscle in individuals with diabetes mellitus and peripheral neuropathy does not respond similarly to BoTN-A as spastic or healthy muscle. Skeletal muscle of individuals with obesity and diabetes mellitus and peripheral neuropathy has been reported to have 2.2 times more fat located around the plantarflexor muscle belly than age matched controls without obesity, diabetes mellitus, or peripheral neuropathy.13 The impact that excessive fat accumulation has on BoNT-A efficacy is unknown but we suspect it may attenuate the ability of BoNT-A to diffuse through the muscle and bind to the muscle’s motor end plates.
The skeletal muscle of individuals with diabetes mellitus is also insulin resistant and there is a high prevalence of peripheral vascular disease.6, 33 BoNT-A has been associated with an increased intramuscular blood flow in the elbow flexors of humans with epicondylitis for up to 12 months24 and to increase glucose uptake, blood flow and blood volume in the masseter muscle of rabbits for up to 8 weeks.18 Additionally, botulinum toxin A has also been associated with an increase in liver tumor oxygenation and perfusion in mice.2 The improvement in glucose uptake and vascular perfusion may outweigh the neurotoxin effects in some individuals with diabetes mellitus and peripheral neuropathy. Incorporating imaging techniques to measure muscle metabolism and vascular perfusion may provide critical data to interpret future studies in this population.
There were no consistent changes in dorsiflexion range of motion with the Botox® injections. Although no effect on dorsiflexion range of motion would be expected because of the limited changes on muscle strength induced by the injections in this study in general, we did not expect dorsiflexion range of motion to change in subjects with diabetes and peripheral neuropathy. BoNT-A has been used to increase joint range of motion restricted by surrounding muscle that is spastic9, 14 or demonstrating an abnormal increase in activity31. The subjects included in this study did not have evidence or complaints of abnormal calf muscle activity. The dorsiflexion range of motion limitation measured in individuals with diabetes20, 28 is associated with diabetes related changes in connective tissue29 and would not be expected to change with injection of BoTN-A.
The Botox® intervention was intended to reduce forefoot plantar pressure by decreasing plantarflexor strength and the ability to push off during gait through the use of Botox®. The lack of a consistent response to the injection suggests that the dosing strategy for individuals with diabetes mellitus and peripheral neuropathy will not follow a straight forward, standard dosing strategy (e.g. based on body weight). Future research must examine how diabetes specific changes in muscle including: fatty infiltration, insulin insensitivity, and peripheral vascular disease change Botox® effectiveness. At this time, the tendo-achilles lengthening remains the intervention of choice for temporarily reducing plantar pressure and ulcer recurrence.
There are a number of possible study limitations that may explain the unexpected response to the BoNT-A injections. There is measurement error associated with plantarflexor muscle peak torque methods that could blunt the ability to detect change over time. The test requires volitional effort and motivation, which can vary between test occasions. Standard error of the measurement for plantarflexor isokinetic testing has been reported between 5.811 and 8.935 Nm for older adults. The placebo group in our study provides evidence that plantarflexor muscle torque at 60°/sec, in obese subjects with diabetes mellitus, peripheral neuropathy, and forefoot ulcer varies between test occasions by 12% or on average 3 Nm. Additionally, our study, although strong in design, was limited by a small sample size and inequality in weight between groups.
CONCLUSIONS
The plantarflexor muscles of individuals with diabetes mellitus, peripheral neuropathy, and a forefoot plantar ulcer respond in a variable and non-predictable manner when injected with BoNT-A. Overall, 200 or 300 units (1.9 to 2.2 units/kg) of Botox® do not consistently produce muscle weakness in the calf muscle nor was there a subsequent reduction in forefoot peak plantar pressures. Additional research is needed to investigate diabetes mellitus specific physiological changes and their impact of BoNT-A effectiveness in order to guide appropriate dosing.
Acknowledgments
The authors would like to thank Kathryn L. Bohnert, MS and Sandy Sagitto, RN for their assistance in study implementation and data collection.
References
- 1.World Health Organization. 2011 http://www.who.int/diabetes/en/ Available at: URL: http://www.who.int/diabetes/en/
- 2.Ansiaux R, Baudelet C, Cron GO, et al. Botulinum toxin potentiates cancer radiotherapy and chemotherapy. Clin Cancer Res. 2006;12:1276–83. doi: 10.1158/1078-0432.CCR-05-1222. [DOI] [PubMed] [Google Scholar]
- 3.Apelqvist J, Ragnarson-Tennvall G, Larsson J, Persson U. Long-term costs for foot ulcers in diabetic patients in a multidisciplinary setting. Foot Ankle Int. 1995;16:388–94. doi: 10.1177/107110079501600702. [DOI] [PubMed] [Google Scholar]
- 4.Barry DC, Sabacinski KA, Habershaw GM, Giurini JM, Chrzan JS. Tendo Achillis procedures for chronic ulcerations in diabetic patients with transmetatarsal amputations. J Am Podiatr Med Assoc. 1993;83:96–100. doi: 10.7547/87507315-83-2-96. [DOI] [PubMed] [Google Scholar]
- 5.Boulton AJ, Hardisty CA, Betts RP, et al. Dynamic foot pressure and other studies as diagnostic and management aids in diabetic neuropathy. Diabetes Care. 1983;6:26–33. doi: 10.2337/diacare.6.1.26. [DOI] [PubMed] [Google Scholar]
- 6.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88:1322–35. doi: 10.2522/ptj.20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delbridge L, Perry P, Marr S, et al. Limited joint mobility in the diabetic foot: relationship to neuropathic ulceration. Diabet Med. 1988;5:333–7. doi: 10.1111/j.1464-5491.1988.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 8.Diamond JE, Mueller MJ, Delitto A, Sinacore DR. Reliability of a diabetic foot evaluation. Phys Ther. 1989;69:797–802. doi: 10.1093/ptj/69.10.797. [DOI] [PubMed] [Google Scholar]
- 9.Fock J, Galea MP, Stillman BC, Rawicki B, Clark M. Functional outcome following Botulinum toxin A injection to reduce spastic equinus in adults with traumatic brain injury. Brain Inj. 2004;18:57–63. doi: 10.1080/0269905031000149498. [DOI] [PubMed] [Google Scholar]
- 10.Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The Health Care Costs of Diabetic Peripheral Neuropathy in the U.S. Diabetes Care. 2003;26:1790–5. doi: 10.2337/diacare.26.6.1790. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann A, Knols R, Murer K, de Bruin ED. Reproducibility of an isokinetic strength-testing protocol of the knee and ankle in older adults. Gerontology. 2009;55:259–68. doi: 10.1159/000172832. [DOI] [PubMed] [Google Scholar]
- 12.Hastings MK, Mueller MJ, Sinacore DR, Salsich GB, Engsberg JR, Johnson JE. Effects of a tendo-Achilles lengthening procedure on muscle function and gait characteristics in a patient with diabetes mellitus. J Orthop Sports Phys Ther. 2000;30:85–90. doi: 10.2519/jospt.2000.30.2.85. [DOI] [PubMed] [Google Scholar]
- 13.Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive Adipose Tissue Infiltration in Skeletal Muscle in Individuals With Obesity, Diabetes Mellitus, and Peripheral Neuropathy: Association With Performance and Function. Physical Therapy. 2008;88:1336–44. doi: 10.2522/ptj.20080079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirazli Y, On AY, Kismali B, Aksit R. Comparison of phenol block and botulinus toxin type A in the treatment of spastic foot after stroke: a randomized, double-blind trial. [see comment] American Journal of Physical Medicine & Rehabilitation. 1998;77:510–5. doi: 10.1097/00002060-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Lin SS, Lee TH, Wapner KL. Plantar forefoot ulceration with equinus deformity of the ankle in diabetic patients: the effect of tendo-Achilles lengthening and total contact casting. Orthopedics. 1996;19:465–75. doi: 10.3928/0147-7447-19960501-18. [DOI] [PubMed] [Google Scholar]
- 16.Maluf KS, Mueller MJ, Strube MJ, Engsberg JR, Johnson JE. Tendon Achilles lengthening for the treatment of neuropathic ulcers causes a temporary reduction in forefoot pressure associated with changes in plantar flexor power rather than ankle motion during gait. J Biomech. 2004;37:897–906. doi: 10.1016/j.jbiomech.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Mancini F, Sandrini G, Moglia A, Nappi G, Pacchetti C. A randomised, double-blind, dose-ranging study to evaluate efficacy and safety of three doses of botulinum toxin type A (Botox) for the treatment of spastic foot. Neurological Sciences. 2005;26(1):26–31. doi: 10.1007/s10072-005-0378-9. [DOI] [PubMed] [Google Scholar]
- 18.Matic DB, Lee TY, Wells RG, Gan BS. The effects of botulinum toxin type A on muscle blood perfusion and metabolism. Plast Reconstr Surg. 2007;120:1823–33. doi: 10.1097/01.prs.0000287135.17291.2f. [DOI] [PubMed] [Google Scholar]
- 19.Meyers-Rice B, Sugars L, McPoil T, Cornwall MW. Comparison of three methods for obtaining plantar pressures in nonpathologic subjects. J Am Podiatr Med Assoc. 1994;84:499–504. doi: 10.7547/87507315-84-10-499. [DOI] [PubMed] [Google Scholar]
- 20.Mueller MJ, Diamond JE, Delitto A, Sinacore DR. Insensitivity, Limited Joint Mobility, and Plantar Ulcers in Patients with Diabetes-Mellitus. Physical Therapy. 1989;69:453–9. doi: 10.1093/ptj/69.6.453. [DOI] [PubMed] [Google Scholar]
- 21.Mueller MJ, Minor SD, Sahrmann SA, Schaaf JA, Strube MJ. Differences in the gait characteristics of patients with diabetes and peripheral neuropathy compared with age-matched controls. Phys Ther. 1994;74:299–308. doi: 10.1093/ptj/74.4.299. [DOI] [PubMed] [Google Scholar]
- 22.Mueller MJ, Sinacore DR, Hastings MK, Strube MJ, Johnson JE. Effect of Achilles tendon lengthening on neuropathic plantar ulcers. A randomized clinical trial. J Bone Joint Surg Am. 2003;85-A:1436–45. [PubMed] [Google Scholar]
- 23.Odergren T, Hjaltason H, Kaakkola S, et al. A double blind, randomised, parallel group study to investigate the dose equivalence of Dysport and Botox in the treatment of cervical dystonia. J Neurol Neurosurg Psychiatry. 1998;64:6–12. doi: 10.1136/jnnp.64.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oskarsson E, Piehl AK, Gustafsson BE, Pettersson K. Improved intramuscular blood flow and normalized metabolism in lateral epicondylitis after botulinum toxin treatment. Scandinavian Journal of Medicine & Science in Sports. 2009;19:323–8. doi: 10.1111/j.1600-0838.2008.00804.x. [DOI] [PubMed] [Google Scholar]
- 25.Pauri F, Boffa L, Cassetta E, Pasqualetti P, Rossini PM. Botulinum toxin type-A treatment in spastic paraparesis: a neurophysiological study. J Neurol Sci. 2000;181:89–97. doi: 10.1016/s0022-510x(00)00439-1. [DOI] [PubMed] [Google Scholar]
- 26.Pittock SJ, Moore AP, Hardiman O, et al. A double-blind randomised placebo-controlled evaluation of three doses of botulinum toxin type A (Dysport) in the treatment of spastic equinovarus deformity after stroke. Cerebrovasc Dis. 2003;15:289–300. doi: 10.1159/000069495. [DOI] [PubMed] [Google Scholar]
- 27.Ramsey SD, Newton K, Blough D, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22:382–7. doi: 10.2337/diacare.22.3.382. [DOI] [PubMed] [Google Scholar]
- 28.Rao SR, Saltzman CL, Wilken J, Yak HJ. Increased passive ankle stiffness and reduced dorsiflexion range of motion in individuals with diabetes mellitus. Foot Ankle Int. 2006;27:617–22. doi: 10.1177/107110070602700809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy GK. Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit achilles tendon. Experimental Diabesity Research. 2004;5:143–53. doi: 10.1080/15438600490277860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputations in individuals with diabetes. In: Harris MI, Cowie CC, Stern MP, Boyko EJ, Reiber GE, Bennett PH, editors. Diabetes in America. 2. Washington DC: US Government Printing Office; 1995. pp. 408–28. [Google Scholar]
- 31.Reuter I, Lorbach O, Mehnert S, Kaps M, Engelhardt M. Botulinum toxin improves reduced dorsiflexion after Achilles tendon surgery. Knee Surg Sports Traumatol Arthrosc. 2010;18:265–8. doi: 10.1007/s00167-009-0948-0. [DOI] [PubMed] [Google Scholar]
- 32.Simpson DM, Gracies J-M, Graham HK, et al. Assessment: Botulinum neurotoxin for the treatment of spasticity (an evidence-based review): Report of Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1691–8. doi: 10.1212/01.wnl.0000311391.00944.c4. [DOI] [PubMed] [Google Scholar]
- 33.Turcotte LP, Fisher JS. Skeletal muscle insulin resistance: roles of fatty acid metabolism and exercise. Phys Ther. 2008;88:1279–96. doi: 10.2522/ptj.20080018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turkel CC, Bowen B, Liu J, Brin MF. Pooled Analysis of the Safety of Botulinum Toxin Type A in the Treatment of Poststroke Spasticity. Archives of Physical Medicine and Rehabilitation. 2006;87:786–92. doi: 10.1016/j.apmr.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Webber SC, Porter MM. Reliability of ankle isometric, isotonic, and isokinetic strength and power testing in older women. Physical Therapy. 2010;90:1165–75. doi: 10.2522/ptj.20090394. [DOI] [PubMed] [Google Scholar]