Abstract
Objective
Prenatal exposure to women’s mood dysregulation is associated with variation in neurobehavioral profiles in children. Few studies have assessed these relationships during the prenatal period.
Methods
In 113 women in the 36th – 38th gestational week (mean age 26.3 ± 5.4 years), electrocardiogram, blood pressure, respiration, salivary cortisol, and fetal heart rate (HR) were measured during baseline, a psychological challenge (Stroop color–word matching task), and a standardized paced breathing protocol. Subjects underwent the Structured Clinical Interview for DSM-IV prior to testing and were grouped as: depressed, co–morbid for depression and anxiety, anxiety disorder only, and control.
Results
There was a significant main effect of maternal diagnostic group on fetal HR only during the Stroop task: fetuses of women in the co–morbid group had a greater HR increase compared to controls (p < .05). Overall, fetuses showed robust increases in HR during paced breathing (p < .0001), but there was no significant difference by maternal diagnosis. For both tasks, changes in fetal HR were independent of women’s concurrent cardiorespiratory activity. Finally, although cortisol was higher in the co-morbid group (p <.05), independent of diagnosis, there was a trend for maternal baseline cortisol to be positively associated with average fetal HR (p = .08).
Conclusions
These findings indicate that variation in fetal HR reactivity — an index of emerging regulatory capacities — is likely influenced by multiple acute and chronic factors associated with women’s psychobiology.
Keywords: Fetal heart rate, fetal neurobehavioral development, antenatal depression, maternal stress, fetal programming
The tremendous plasticity, and activity-dependency of the developing nervous system during fetal life, make this period a prime target for investigations of influences on the course of psychobiological development. Recent research across diverse disciplines has focused on identifying early life origins of adult disease (Godfrey & Barker, 2001), and has demonstrated that factors in pregnant women’s health, such as elevations in life stress, may explain some of the individual differences in children’s future risk for physical as well as psychological disorders (Barker, 2000; Davis et al., 2004; O’Connor, Heron, Golding, Beveridge, & Glover, 2002; Van den Bergh & Marcoen, 2004; Van den Bergh et al., 2005; O’Connor et al., 2005; Pawlby, Hay, Sharp, Waters, & O’Keane, 2009).
In our ongoing work, we have reasoned that if the psychological functioning of pregnant women affects offsprings’ long–term development, we should be able to identify markers of that influence when it occurs, that is, during the prenatal period. In prior reports, we showed that prenatal maternal depression, as well as high trait anxiety, predict an increase in fetal heart rate (HR) during women’s laboratory–based, acute stress experience (Stroop test) compared to fetuses of euthymic women who show no significant change in HR (Monk et al., 2000; Monk et al., 2004). Others found that maternal depression during pregnancy is associated with greater fetal movement and slower return to baseline HR following vibroaccoustic stimulation applied to the women’s abdomen (Allister, Lester, Carr, & Liu, 2001; Dieter, Emory, Johnson, & Raynor, 2008). Because increases in fetal HR are often coincident with fetal movements (DiPietro et al., 2004), these findings are consistent in suggesting that greater fetal reactivity to external stimuli maybe a characteristic of fetal neurodevelopment when women experience significant prenatal mood dysregulation.
Several studies have attempted to identify characteristics of pregnant women’s physiology that are potentially mood–based, and related to alterations in fetal neurobehavior, which would support the concept of women’s psychosocial functioning being ‘transduced to the fetus’ and shaping fetal development. The maternal HPA–axis has been a focus of this research {Wadhwa, 2005 #2514;Weinstock, 2005 #2656, as it is a major effector of psychosocial stress, and anxiety and depression are associated with elevations in resting cortisol CITES, even during pregnancy (EVANS). Using a laboratory stressor paradigm similar to ours, Fink et al. found that fetuses of women who had a cortisol increase following an arithmetic task, versus those who did not, had higher resting HR and less short–term HR variability 20 minutes after the stressor task ended. There was a trend finding for participants who had a cortisol increase to report higher levels of life stress {Fink, 2010 #4937}. In other work, higher resting maternal cortisol during the 3rd trimester of pregnancy was associated with greater amplitude and amount (time spent) of fetal movement during a 50 minute observation period (DiPietro, Kivlighan, Costigan, & Laudenslager, 2009). Sandman et al., found that higher levels of placenta–derived corticotropin releasing hormone (pCRH) (which, in contrast to CRH in the hypothalamus, shows increased synthesis in response to glucocorticoid exposure), is linked to increased fetal HR reactivity (‘arousal’) studied in a vibroaccoustic habituation paradigm (Sandman, Wadhwa, Chicz-DeMet, Porto, & Garite, 1999). Taken together, these studies raise the following question: are differences in fetal HR associated with maternal psychiatric status also related to differences in maternal cortisol levels?
So far, we have found minimal indication that women’s psychobiological experience was being ‘transduced’ to the fetus and determining the fetal HR response to women’s exposure to a laboratory stressor. Our results showed no psychiatric diagnostic group differences in women’s cardiorespiratory activity that paralleled the group differences observed in fetal HR reactivity (Monk et al., 2000; Monk et al., 2004), and, across all subjects, only a small inverse association between changes in women’s systolic blood pressure and fetal HR (Monk et al., 2004). Instead, based on our results, we have posited that women’s significant cardiorespiratory reactivity during the stressor task functions as a stimulus that triggers a HR increase in fetuses of depressed or anxious women who already have been shaped over the course of gestation to be more reactive (Monk et al., 2004). To support this interpretation, we sought in this study to manipulate an acute change in maternal cardiorespiratory activity, and investigate it as a stimulus that could differentiate reactive from non–reactive fetuses, while simultaneously considering whether maternal cortisol level is associated with fetal HR. That is, a correlation between maternal cortisol and fetal HR in the laboratory likely reflects maternal–fetal experiences outside the laboratory, and thus finding an association would support the hypothesis that women’s psychosocial functioning shapes fetal development via maternal HPA–axis functioning.
Specifically, the purpose of the present study was to replicate our prior results showing a greater fetal HR increase during maternal laboratory stress associated with women’s diagnosis of clinical depression (Monk et al., 2004), and to determine whether higher levels of women’s cortisol is associated with higher fetal HR. We also used a paced breathing task, in which participants increase and decrease their respiration rate according to a standardized protocol (Wilhelm, Grossman, & Coyle, 2004), to manipulate a specific aspect of women’s physiological activity (rapid changes in breathing) to determine its effects on fetal HR, and as a stimulus that reveals differences in fetal HR reactivity.
METHODS
Subjects
Two–hundred and one women (ages 18 – 40) in the late 3rd trimester (weeks 36 – 38), carrying singleton fetuses were recruited from clinics affiliated with Columbia University Medical Center between July 2001 and March 2006. Women were excluded from entering the study if they smoked during pregnancy, were taking any medications, or if there were any maternal or fetal medical complications such as hypertension, diabetes mellitus, or suspected fetal growth restriction. Forty–six women were dropped from the study for the following reasons: failure to attend sessions, delivery prior to fetal testing session, fetal anomaly, fetal demise, and other serious health problems. Because earlier birth is potentially indicative of a medically compromised intrauterine period, women who gave birth before 37 weeks gestation (n = 8) were excluded from all analyses, as were women whose babies’ gestational age could not be confirmed from electronic medical records (n = 28). Four subjects were removed from analyses because poor quality maternal respiratory signals produced implausible values (4 SD below the mean) and two subjects were removed from analyses because maternal diastolic BP was three SD above the mean. For the remaining sample (n = 113), analyses were based on data for variable numbers of data points due to equipment failure, poor quality of fetal data collection, and inadequate saliva sample to determine cortisol concentration. The smallest n for any given variable was 105. (Considered across tasks and variables, missing data for subjects by diagnostic group was as follows: 0 – 3 for anxiety only, 0 for depressed, 0 – 1 for co–morbid, and 0 – 4 for controls.)
The sample was made up of 60% Latina women, 19% Caucasian, 10% African American, 5% Asian, and 6% of another or mixed ethnicity. Women’s age was 26.3 ± 5.4 years (mean ± SD). With respect to education, 71.87 % of the sample had completed either high school or two–four years of college and 18.75% had received an advanced degree. Forty percent of the women were married and 37 % of the unmarried were cohabitating. This study was approved by the New York State Psychiatric Institute Institutional Review Board. All subjects gave written, informed consent.
Average gestational age at birth was 39 weeks (SD = 1 week, range 37 to 43 weeks). The average weight at birth was 3405 grams (SD=433) (range 1984 – 4440 grams). Fifty–seven percent of the babies were male.
Procedure
During the 2nd trimester, subjects completed demographic questionnaires and were interviewed by a licensed mental health practitioner using the Scheduled Clinical Interview for DSM-IV (SCID) for Axis I disorders (First, Spitzer, Gibbon, & Williams, 1997).
At approximately 36 weeks gestation (35.9 ± 0.8 weeks), subjects participated in a psychophysiology session. Most sessions were scheduled to begin between 10:30 and 11:30 AM to control for diurnal variations in salivary cortisol levels (though due to scheduling difficulties, some sessions were begun between 1 – 2 pm after 12 noon; we controlled for this variation in our analyses). Women completed self–report questionnaires and took part in two laboratory tasks. Subjects were told that they would be asked to rest quietly and then to participate in a “challenging color-word matching task” on the computer (computerized version of the Stroop task) as well as a paced breathing task. Women were instrumented for electrocardiogram, respiration and BP collection. An ultrasound transducer was placed on the subject’s abdomen to record fetal HR. Maternal cardiorespiratory and fetal HR data were obtained during five periods: while the subjects rested quietly for a 5-minute baseline, during the 5–minute Stroop color-word matching task, during a first 5-minute recovery period, during the 6–minute paced breathing task, and during a second 5–minute recovery period. Estimates of baseline salivary cortisol were made from samples obtained just before the psychophysiology recording session started, but after a brief (2–3 minutes) practice of the forthcoming tasks. Although some procedures can increase cortisol in pregnant women (de Weerth, Wied, Jansen, & Buitelaar, 2007), we used baseline cortisol, as opposed to a reactivity measure, because the neuroendocrine adaptations of late pregnancy, which are marked by hypercortisolemia, make it relatively difficult to induce a significant cortisol change to stress in pregnant women (Evans, Myers, & Monk, 2008; Kammerer, Adams, Castelberg, & Glover, 2002; Slattery & Neumann, 2008).
The Stroop Task
The Stroop task presents subjects with color words in either congruently or incongruently colored letters and asks the subject to identify the color of the letters. It is commonly used to induce stress in a laboratory setting (Renaud & Blondin, 1997) or as a cognitive assessment of executive function (Bugg, Jacoby, & Toth, 2008). In our version, the subject was required to push keys that corresponded to the correct color responses as fast as possible. If responses were incorrect or too slow, the computer displayed the message “incorrect” on the screen. To augment the cognitive performance demands, the experimenter prompted subjects to work faster throughout the task.
Paced breathing
This task requires subjects to control their breathing by inhaling when a bar on a computer screen rises and to exhale when it falls (Wilhelm et al., 2004). During the task, subjects alternate among periods of breathing at a faster than normal rate (approximately 30 breaths per minute), a slower than normal rate (about 10 breaths per minute), and at an approximately normal rate (20 breaths per minute). As paced by the computer, the breathing rates change every 30 seconds.
Salivary cortisol
Subjects were instructed to suck and chew on a cotton roll for one minute or until saturated. These samples were stored at −20°C until time of assay. Saliva extracted from these cotton rolls was analyzed at the Analytical Psychopharmacology Laboratories at the Nathan Kline Institute. Cortisol was measured by radioimmunoassay using primary antibodies and I125 labeled cortisol purchased from ICN Biomedicals. The cortisol standards used were from Sigma Chemical Co. Samples were assayed in duplicate. The intra– and inter–assay coefficients of variation were 3.0% and 6.0% at the 3.1 ug/dL level.
Acquisition and processing of maternal and fetal signals
Maternal electrocardiogram and respiration impedance signals from a Hewlett Packard 78292A monitor were digitized at 500 and 50 samples/sec respectively using a 16-bit A/D card (National Instruments 16XE50). Software written by Ledano Solutions, Inc., was used to mark R-waves and create files of RR-intervals. R–wave markings were visually inspected and corrected where necessary. Peaks in the impedance respiratory waveform also were marked. These marks were verified by visual inspection and then were used to calculate respiratory rate. BP was acquired on a beat-to-beat basis by an Ohmeda Finapres 2300 monitor. The analog pressure waveform was digitized at 250 Hz. Systolic BP and diastolic BP values were marked by peak/trough detection software and errors in marking were corrected interactively. Fetal HR was recorded via an ultrasound transducer (Advanced Medical Systems, IM76) and digitized at 50 Hz. (For details, see Monk et al., (Monk et al., 2004)).
Women’s psychiatric status
Based on results from the SCID interview conducted in the 2nd trimester, women with a current major depressive disorder and/or dysthymia were classified as depressed (n = 7). None of the depressed women had bipolar disorder. Women who currently had a social phobia, a simple phobia, generalized anxiety disorder, or agoraphobia without panic disorder were classified as having only an anxiety disorder (n = 25). Women with depression and an anxiety disorder, including Post Traumatic Stress Disorder, were classified as co–morbid (n = 13). Women free of Axis I pathology were classified as controls (n = 68). There were no diagnostic group differences in the distribution of women’s ethnicity and/or race.
Self–report of depression
Current symptoms of depression were measured at the time of the fetal study using the Center for Epidemiological Studies – Depression Scale (CES-D). The CES–D is a 20–item questionnaire with a possible score range from 0–60. A score of 16 or more is considered an indication of depression. The validation and use of the CES–D in community samples has been well established (Radloff, 1977) (Boyd, Weissman, Thompson, & Myers, 1982; Roberts, 1980).
Data analyses
Analytic plan
Mean values for fetal HR and women’s HR, BP, and respiration rate were computed for each of the discrete periods (baseline, Stroop, 1st recovery, paced breathing, and 2nd recovery). Fetal HR during the Stroop and paced-breathing tasks was modeled using separate linear mixed models with random intercepts. All analyses were carried out using SAS 9.1.3 PROC MIXED procedure. Women’s diagnostic group, study period (i.e. baseline, Stroop, recovery, etc.) and their interaction were the primary predictors in both models. Cortisol explain Maternal cortisol level, time of cortisol collection (morning versus after 12 noon), fetal sex, and gestational age at time of testing were included in the models because they were either a primary variable of interest (cortisol, time of cortisol collection) or other studies (Buss et al., 2009; Weinberg, Sliwowska, Lan, & Hellemans, 2008) supported their relevance to the outcome variables, or their addition corrected for possible bias related to non random group assignment. Other potential covariates were additively introduced into the original models and the resulting −2 restricted log–likelihood values were obtained. The difference between the −2 restricted log–likelihood values for the original and the current model was used to approximate the chi-square distribution with degrees of freedom equal to the difference in parameters estimated. The covariate was kept in the model if the deviance analysis yielded statistically significant result and also that it was clinically and statistically significant. Interactions were tested using the same techniques discussed above. However, interactions were kept in the model only if the parameter estimates yielded significant results. Table 1 below presents lists of covariates that were tested to obtain the final working model.
Table 1.
Maternal cardiorespiratory activity and fetal HR during baseline, Stroop, and paced breathing periods
| Baseline (± sd) | Stroop (± sd) | 1st recovery (± sd) | Paced breathing (± sd) | 2nd recovery (±sd) | Change from baseline to Stroop (± sd)b | Change from 1st recovery to paced breathing (± sd)b | |
|---|---|---|---|---|---|---|---|
| HR (bpm) | 89.4a (±13.3) | 91.7 (±12.3) | 89.1a (±11.6) | 90.0 (±12.5) | 87.6 (±12.8) | 2.3 (±6.5)**** | 0.4 (±4.8) |
| Systolic BP (mmHg) | 111.1 (±14.0) | 119.8 (±16.1) | 113.7 (±14.7) | 111.1 (±16.1) | 116.6 (±15.2) | 8.7 (±9.0)**** | − 2.6 (±7.3)**** |
| Diastolic BP (mmHg) | 64.1 (±9.9) | 68.9 (±10.8) | 65.3 (±10.5) | 64.1 (±11.2) | 68.2 (±13) | 4.8 (±6.0)**** | − 1.5 (±5.4)** |
| Respiration (breaths pm) | 19.8 (±5.0) | 24.1 (±5.5) | 20.1 (±5.1) | 21.1 (±4.9) | 18.3 (±6.6) | 4.0 (±7.2)**** | 1.1 (±5.2)* |
| Stress rating (scale1–10) | 2.1 (±1.8) | 4.8 (±2.2) | 2.2 (±1.7) | 3.6 (±2.2) | N/A | 2.7 (±2.2)**** | 1.3 (±2.1) **** |
| Fetal HR (bpm) | 146.7 (±9.1) | 147.3 (±8.9) | 146.4 (±9.6) | 150.4 (±9.7) | 151.5 (±10.6) | .6 (± 5.5) | 3.9 (±7.2)**** |
ANOVAs revealed no diagnostic group differences in maternal HR, BP, and respiration rate during any of the 4 periods (baseline, Stroop, 1st recovery, paced breathing) except for maternal HR during baseline and 1st recovery (ps < .05); post hoc analyses indicated women in the anxiety only group compared to controls had higher HR during baseline (96.6 bpm versus 86.7 bpm, LSD, p = .002) and during the 1st recovery (95.1 bpm versus 86.7; LSD, p = .004).
Paired t-tests were used to evaluate significance of reactivity (baseline to Stroop, and 1st recovery to paced breathing.
p < .0001;
p < .001;
p < .01;
p < .05.
N/A = not applicable; stress rating not determined following the 2nd recovery period.
With regard to maternal diagnosis, the control group was the reference category. With regard to effects of period, for the first model, the baseline was the reference period while for the second model, the period immediately prior to paced breathing (i.e. the 1st recovery period) was the reference period. Fetal sex, gestational age at time of testing, maternal HR, diastolic and systolic BP and respiration rate during each period, maternal age, cortisol values, time of cortisol collection, and self–reported depression (CES–D) at time of laboratory session were examined as potential covariates in the fetal HR models. The majority of these proposed covariates were not maintained in the final models because they did not significantly alter the results (p values for effects on model deviances all > .10). However,
The magnitude of maternal cardiorespiratory reactivity during the Stroop and paced breathing periods were computed as a within–subject change (e.g., average value during Stroop – average value during baseline). Student’s t-tests were used to make comparisons of diagnostic group differences in women’s physiological reactivity to the tasks, and to characterize the significance of the physiologic reactivity.
RESULTS
Women’s cardiorespiratory activity: Baseline & Stroop
Women’s cardiorespiratory activity during baseline and the Stroop task is summarized in Table 1. ANOVAs revealed no diagnostic group differences in maternal HR, BP, and respiration rate during baseline or Stroop except for maternal HR during baseline (p < .05); post hoc analyses indicated only women in the anxiety group differed from controls; they had higher HR during baseline (96.6 bpm versus 86.7 bpm, LSD, p = .002). There also were no diagnostic group differences in the changes in women’s cardiorespiratory reactivity to the Stroop task (all ps > .30). In the absence of diagnostic group differences, women’s cardiorespiratory reactivity during the Stroop period was analyzed across all subjects using single–sample t tests. On average, women showed significant HR, BP, and respiration rate changes to the Stroop task compared to baseline values (all ps <.05) (see Table 1).
Women’s cardiorespiratory activity: 1st recovery & paced breathing
Similar to the findings for the Stroop challenge, ANOVAS revealed that, except for maternal HR during 1st Recovery, there were no diagnostic group differences in the women’s cardiorespiratory activity values for the 1st recovery or paced breathing periods (all ps > .5) (see Table 1). For the 1st recovery, post hoc analyses (least squares differences, LSD) indicated women in the anxiety only group compared to controls had higher HR (95.1 bpm versus 86.7; p = .004). There also were no diagnostic group differences in the changes in women’s cardiorespiratory reactivity to the paced breathing task (all ps > .11). In the absence of diagnostic group differences, women’s cardiorespiratory reactivity during the paced breathing period was analyzed across all subjects using one–sample t tests. On average, women showed significant BP and respiration rate changes to the paced breathing task compared to values during the prior recovery period (1st recovery) (ps<.05), but there was no significant change in HR.
Women’s cortisol levels
Table 2 reports women cortisol levels by diagnostic group as an ANOVA showed significant group difference in women’s resting cortisol level (F (3, 101) = 2.72, p = .05); post hoc analysis indicated that women in the co–morbid group had significantly higher cortisol levels compared to controls (p = .01).
Table 2.
Women’s cortisol levels by diagnostic group
| Anxiety Disorders only (n = 17) | Co–Morbid (n = 12) | Depression (n= 7) | Control (n = 61) | |
|---|---|---|---|---|
| Cortisol (ng/mL) | 1.9 ± 3.2 | 2.8 ± 3.2*a | .8 ± 1.3 | 1.1 ± 1.5 |
There was a significant difference in cortisol levels (F (3, 101) = 2.72, p = .05); post hoc analysis indicated that women in the co–morbid group had significantly higher cortisol levels compared to controls (LSD, p = .01).
Modeling fetal heart rate: Stroop and adjacent resting periods
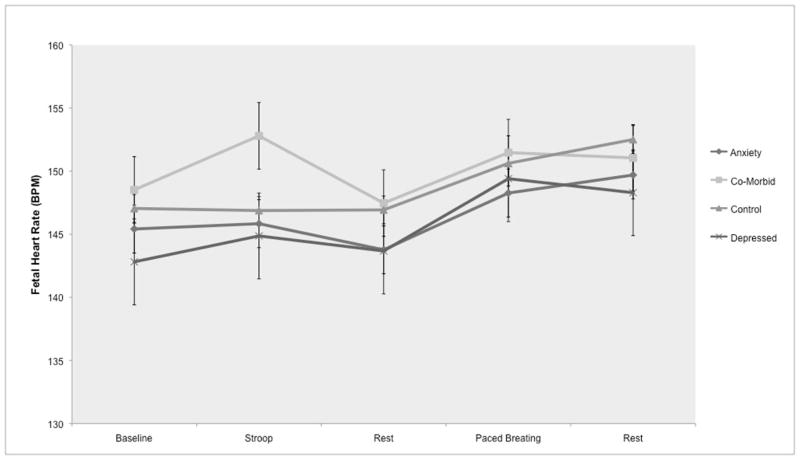
A linear model with random intercept showed that, across all subjects, fetal HR did not change during the Stroop task; however, there was an interaction effect of diagnostic group and period such that fetuses of women in the co–morbid group (i.e. depression and an anxiety disorder) had a significantly larger increase in HR during the Stroop task compared to controls (p < .05) (Table 3 & Fig 1). In this model, there also was a trend for maternal cortisol to be positively associated with fetal HR (p = .08), such that, across all subjects, greater maternal cortisol at baseline was associated with overall higher fetal HR across all periods. There were no significant main effects of diagnostic group on fetal HR at resting baseline before the Stroop task or during the recovery period following the Stroop task, or interaction effects with diagnostic group and period. (Other possible variables (described in the methods section, i.e., maternal cardiorespiratory activity) were not included in the model due to non significance.)
Table 3.
Linear mixed model with random intercept: Fetal HR during Stroop and adjacent baseline and recovery periods. Reference group effects=controls. Reference time period=baseline.
| Variables
| |||
|---|---|---|---|
| Estimate (β) | Standard error | p value | |
| Group effects | |||
| Co–morbid vs Controls | −1.3 | 3.0 | 0.7 |
| Depressed vs Controls | −5.1 | 3.6 | 0.2 |
| Anxiety disorders vs Controls | −3.6 | 2.5 | 0.2 |
| Time effects | |||
| Baseline to 1st recovery | −0.9 | 0.8 | 0.3 |
| Baseline to Stroop | −0.7 | 0.8 | 0.4 |
| Time x group effects | |||
| Baseline to 1st recovery: co–morbid vs controls | 0.1 | 2.0 | 0.9 |
| Baseline to 1st recovery: depressed vs controls | 1.8 | 2.5 | 0.5 |
| Baseline to 1st recovery: anxiety vs controls | 0.5 | 1.7 | 0.8 |
| Baseline to Stroop: co–morbid vs controls | 4.9 | 2.0 | 0.01 |
| Baseline to Stroop: depressed vs controls | 2.7 | 2.5 | 0.3 |
| Baseline to Stroop: anxiety vs controls | 2.3 | 1.7 | 0.2 |
| Covariates | |||
| Sex (male = 0, female = 1) | −1.9 | 1.8 | 0.3 |
| Gestational age | 1.1 | 1.0 | 0.3 |
| Cortisol sample time (AM = 0, PM = 1) | 2.0 | 2.0 | 0.3 |
| Cortisol | 1.0 | 0.6 | 0.08 |
Figure 1.
Fetal HR during laboratory protocol by women’s diagnostic status
Modeling fetal heart rate: paced breathing and adjacent resting periods
A linear model with random effects showed that fetal HR significantly increased during the paced breathing task (p < .0001) and during the subsequent recovery period (p < .001) relative to the resting period before the breathing task (see Fig 1). However, there was no significant interaction between diagnostic groups and these changes in fetal HR. No other variables (i.e., maternal cortisol, HR) were significant (See Table 4, and Methods section for description of criteria for inclusion in the model). Removal of 2 Low Birth Weight infants did not affect the results.
Table 4.
Linear mixed model with random intercept: Fetal HR during paced breathing and adjacent rest periods. Reference group effects=controls. Reference time period = 1st recovery.
| Variables
| |||
|---|---|---|---|
| Estimate (β) | Standard error | p value | |
| Group effects | 75 | 39 | 0.06 |
| Co–morbid | 0.0 | 3.3 | 0.9 |
| Depressed | −2.8 | 4.0 | 0.5 |
| Anxiety disorders | −3.0 | 2.8 | 0.3 |
| Time effects | |||
| 1st recovery to 2nd recovery | 6.3 | 1.1 | 0.0001 |
| 1st recovery to paced breathing | 4.1 | 1.1 | 0.0002 |
| Time x group effects | |||
| 1st recovery to 2nd recovery: co–morbid vs controls | −2.5 | 2.7 | 0.4 |
| 1st recovery to 2nd recovery: depressed vs controls | −1.6 | 3.4 | 0.6 |
| 1st recovery to 2nd recovery: anxiety vs controls | −0.5 | 2.4 | 0.8 |
| 1st recovery to paced breathing: co–morbid vs controls | −0.1 | 2.7 | 0.9 |
| 1st recovery to paced breathing: depressed vs controls | 1.6 | 3.4 | 0.6 |
| 1st recovery to paced breathing: anxiety vs controls | 0.7 | 2.4 | 0.8 |
| Covariates | |||
| Sex (male = 0, female = 1) | −2.3 | 1.9 | 0.2 |
| Gestational age | 1.9 | 1.1 | 0.1 |
| Cortisol sample time (AM = 0, PM = 1) | 0.1 | 2.1 | 0.6 |
| Cortisol | 0.5 | 0.6 | 0.4 |
However, during paced breathing, women were asked to alter their breathing rate a total of 5 times over the course of the task. A 5–minute average of women’s respiration rate or HR may mask acute changes associated with fetal HR. Thus, in addition to associations between 5–minute averages of maternal cardiorespiratory and fetal HR examined in the model, we considered associations between maternal cardiorespiratory and fetal HR during each of the 1–minute intervals of paced breathing. An examination of maternal HR, systolic BP, diastolic BP, and respiration rate with fetal HR for each minute of paced breathing revealed only one significant correlation: during Minute 4 of paced breathing there was a positive association between women’s respiration rate and fetal HR (r = .28, p < .01).
Stroop versus paced breathing: Comparing maternal cardiorespiratory reactivity
The fetal HR response during maternal exposure to the Stroop versus paced breathing tasks differed (i.e, only fetuses of co–morbid women showed a response to the Stroop task while, on average, all fetuses showed a HR increase during paced breathing), yet women’s contemporaneously collected cardiorespiratory activity was not associated with fetal HR during these periods (see Methods for model description and Tables 3 & 4). To consider if variation in the magnitude in women’s cardiorespiratory reactivity between the tasks functioned as divergent stimuli to the fetuses such that greater maternal cardiorespiratory activity during paced breathing could account for the consistent fetal HR increase during this period, we compared maternal reactivity between Stroop and paced breathing. Student t tests comparing women’s cardiorespiratory reactivity indicated that maternal HR, BP, and respiration rate responses were greater during Stroop compared to paced breathing (and in the opposite directions for BP; increased during Stroop and decreased during paced breathing) (all t tests p <.05); (see Table 1).
However, as indicated, during paced breathing, women were asked to increase and decrease their breathing rate frequently so that the period average respiration rate change from 1st recovery to paced breathing does not reflect the magnitude of these multiple changes in breathing rate during this period. To better characterize the magnitude and frequency of maternal respiration rate changes for the paced breathing task, we calculated minute–by– prior minute changes in respiration rate (e.g., average breathing rate during the 2nd minute of the task compared to the 1st, etc.) for both paced breathing and the Stroop task. Women’s average minute–by–prior minute changes in respiration rate were 6–9 times larger during paced breathing than the spontaneous changes occurring during the Stroop task (all t tests all p < .0001).
DISCUSSION
In this study of 3rd trimester pregnant women undergoing a psychophysiology protocol consisting of two laboratory tasks, there were no diagnostic group differences in women’s cardiorespiratory reactivity to the tasks based on their psychiatric classification. Except for HR during baseline and the 1st recovery period, when women with anxiety disorders had a higher HR than controls, there were no other differences in cardiorespiratory activity in these rest periods. In contrast, pregnant women co–morbid for depression and anxiety had higher resting cortisol than women with depression only, an anxiety disorder, or who were controls.
There were, however, diagnostic group differences in fetal HR reactivity to the Stroop task. Fetuses of women co–morbid for depression and an anxiety disorder showed a greater HR increase during women’s exposure to the Stroop task compared to fetuses of women with only depression, an anxiety disorder, or no psychiatric illness. There were no diagnostic group differences in fetal HR responses to the paced breathing task, when, on average, all subjects showed a HR increase.
In modeling fetal HR, none of the maternal cardiorespiratory variables from the baseline or task periods was retained, as they did not reach significance. However, cortisol was included in the final model since there was a trend for women with higher levels of salivary cortisol to have fetuses with higher HR during the Stroop task and the two adjacent rest periods (p<.08). Cortisol failed to reach statistical significance in the model of fetal HR for paced breathing and the associated rest periods.
Fetal HR activity during the Stroop task, distinguished by a HR increase unique to fetuses of women co–morbid for depression and anxiety, is consistent with our hypothesis and prior results (though see below for a discussion of the discrepancy in results regarding which psychiatric condition shows the effect). In the absence of correlations between contemporaneously collected maternal and fetal cardiorespiratory activity, and in the context of similar maternal cardiorespiratory reactivity to the Stroop task across groups, the data suggest the possibility that fetuses of women with co-morbid depression and anxiety are more reactive to stimuli than those of women with only an anxiety disorder, or a control. The marginally significant positive association between maternal cortisol and fetal HR does not clearly support or challenge the hypothesis that mood–based alterations in women’s HPA–axis activity may influence fetal neurobehavioral development. DiPietro’s recent study showed that higher maternal cortisol was associated with greater amplitude and amount (time spent) of fetal movement (DiPietro et al., 2009); though we did not measure fetal movement, HR changes and movement are often coincident (DiPietro et al., 2004; DiPietro, Hodgson, Costigan, & Johnson, 1996).
Contrary to our hypothesis, there was no main effect of diagnostic group on fetal HR during the paced breathing task; fetuses of women in all groups demonstrated similar increases in HR during the paced breathing task. Furthermore, maternal cortisol levels were not associated with fetal HR during paced breathing and the associated rest periods, nor was maternal cardiorespiratory activity, examined as 5–minute averages, or in smaller, 1–minute intervals for acute associations with fetal HR. In the absence of associations between maternal and fetal physiology, at least as tested here, it seems possible that the changes in women’s respiratory activity during paced breathing, which were far greater than during the Stroop task, function as a suprathreshold in utero sensory stimulus to the fetus, and thereby elicited equivalent robust increases in fetal HR across all groups. Rapid changes in women’s respiration may activate multiple fetal senses, including auditory, proprioceptive, and vestibular. When the stimuli are multimodal, frequent, and of great magnitude (i.e. during paced breathing), fetuses in all groups respond. Alternatively, because the tasks were not counterbalanced, it may be that fetal exposure to a second maternal task (and associated changes in the intrauterine environment) are of sufficient duration to affect most fetuses and elicit a HR increase.
The finding that fetuses from the group of women co–morbid for depression and anxiety showed greater fetal HR response during women’s exposure to the Stroop task varies from our prior results in which fetus of depressed women showed this effect (Monk et al., 2004). The small number of subjects in both studies in the depressed and co–morbid groups, and the associated increased influence of outliers, could account for the discrepancies between the prior and current findings. In the 2004 sample, for fetuses of women in the depressed group, the average fetal HR change to Stroop was 3.2 ± 1.1 bpm; in the current sample, 3.8 ± 6.5 bpm and 2.1 ± 3.1 bpm, for the depressed and co–morbid groups, respectively, indicating greater variability in the current results.
It also is possible that these results are similar to other non–specific findings in psychiatry in which risk factors and biological markers (here, fetal HR) are associated with more than one category of DSM–IV psychiatric illness, particularly when the diagnostic categories share clinical features and are often co–morbid (Gottesman & Hanson, 2005; Kendler, 2005; Rommelse, Van der Stigchel, & Sergeant, 2008). Future investigations are needed to further explore the reliability of the original finding with respect o maternal depression, and to replicate our current results1.
Similar to our prior study (Monk et al., 2004), in this work, fetuses of women with only anxiety disorders did not show a HR response during women’s exposure to the Stroop task. This finding continues to be unexpected, and warrants further exploration, in particular, examination of any possible acute effects.
SUMMARY
When women were exposed to the Stroop task, fetuses of women with co–morbid depression and anxiety had a HR increase, independent of women’s cardiovascular reactivity. Contrary to our hypothesis, women’s cortisol levels showed only a trend association with fetal HR. Though the pathway remains unspecified, the results are consistent with the hypothesis that prolonged exposure to physiological factors associated with women’s mood dysregulation alters fetal neurobehavioral development, resulting in greater reactivity to environmental perturbation in affected fetuses. During paced breathing, externally–driven rapid and dramatic increases in women’s respiration rate was associated with acute changes in fetal HR regardless of diagnostic group status and independent of associations with women’s contemporaneously–collected cardiorespiratory activity, indicating fetal HR response to in utero stimulation. Emerging processes of neurobiological regulation, as well as individual variation in these developing capacities, can be identified in the prenatal period.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Mental Health (MH001928–01A1) and NARSAD (the National Alliance for Research on Schizophrenia & Depression).
Acronyms
- HR
heart rate
- ANS
Autonomic nervous system
- CRH
corticotropin releasing hormone
- SCID
Structured Clinical Interview for DSM–IV
- CES–D
Center for Epidemiological Studies –Depression Scale
Footnotes
Although it was not an aim of this study to investigate associations between the level of self– reported anxiety in control women and fetal HR reactivity, we note that unlike our prior results, there was no such relationship in these data. Again, we find a possible reason to be inconsistency in diagnosing between the studies. Fifteen percent were classified in the anxiety disorder group in the 2004 study (Monk et al., 2004), whereas in this sample, the proportion is 22%. We hypothesize that the greater identification of women as belonging in the anxiety disorder group in the recent sample reduced the number of women scoring high on trait anxiety in the control group — some of whose fetuses might have shown a HR increase to the Stroop task. This hypothesis is supported by a comparison of fetal HR change in the Anxiety Disorder groups between the two studies: In the 2004 sample, the average fetal HR change during the Stroop task for those in the anxiety disorder group was −.03 ± .8 bpm versus +.65 ± 5.2 bmp in this current sample.
Contributor Information
Catherine Monk, Email: cem31@columbia.edu, Department of Psychiatry, Columbia University, 1150 St Nicholas Ave., Suite 1–121, New York, NY 10032, 212.851.5576 phone, 212.851.5580 fax
William P. Fifer, Department of Psychiatry, Columbia University
Michael M. Myers, Department of Psychiatry, Columbia University
Emilia Bagiella, Biostatistics, Columbia University
Jimmy K. Duong, Irving Institute for Clinical & Translational Research, Columbia University Medical Center
Ivy S. Chen, Irving Institute for Clinical & Translational Research, Columbia University Medical Center
Lauren Leotti, Department of Psychology, Rutgers University
References
- Allister L, Lester BM, Carr S, Liu E. The effects of maternal depression on fetal heart rate responce to vibroacoustic stimulation. Developmental Neuropsychology. 2001;20(3):639–651. doi: 10.1207/S15326942DN2003_6. [DOI] [PubMed] [Google Scholar]
- Barker DJ. In utero programming of cardiovascular disease. Theriogenology. 2000;53:555–574. doi: 10.1016/s0093-691x(99)00258-7. [DOI] [PubMed] [Google Scholar]
- Boyd JH, Weissman MM, Thompson WD, Myers JK. Screening for depression in a community sample. Understanding the discrepancies between depression symptom and diagnostic scales. Arch Gen Psychiatry. 1982;39(10):1195–1200. doi: 10.1001/archpsyc.1982.04290100059010. [DOI] [PubMed] [Google Scholar]
- Bugg JM, Jacoby LL, Toth JP. Multiple levels of control in the Stroop task. Mem Cognit. 2008;36(8):1484–1494. doi: 10.3758/MC.36.8.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Class QA, Gierczak M, Pattillo C, Glynn LM, et al. Maturation of the human fetal startle response: evidence for sex-specific maturation of the human fetus. Early Hum Dev. 2009;85(10):633–638. doi: 10.1016/j.earlhumdev.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class QA, Buss C, Davis EP, Gierczak M, Pattillo C, Chicz-DeMet A, et al. Low levels of corticotropin-releasing hormone during early pregnancy are associated with precocious maturation of the human fetus. Dev Neurosci. 2008;30(6):419–426. doi: 10.1159/000191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PE, Snidman N, Wadhwa P, Glynn LM, Schetter CD, Sandman CA. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy. 2004;6(3):319–331. [Google Scholar]
- de Weerth C, Wied CC, Jansen LM, Buitelaar JK. Cardiovascular and cortisol responses to a psychological stressor during pregnancy. Acta Obstet Gynecol Scand. 2007:1–12. doi: 10.1080/00016340701547442. [DOI] [PubMed] [Google Scholar]
- Dieter J, Emory E, Johnson K, Raynor B. Maternal depression and anxiety effects on the human fetus: Preliminary findings and clinical implications. Infant Mental Health Journal. 2008;29(5):420–441. doi: 10.1002/imhj.20192. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Caulfield L, Costigan KA, Merialdi M, Nguyen RH, Zavaleta N, et al. Fetal neurobehavioral development: a tale of two cities. Dev Psychol. 2004;40(3):445–456. doi: 10.1037/0012-1649.40.3.445. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Hodgson KA, Costigan SC, Johnson TRB. Developmental of fetal movement – fetal heart rate coupling from 20 weeks through term. Early Human Development. 1996;44:139–151. doi: 10.1016/0378-3782(95)01704-6. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Kivlighan KT, Costigan KA, Laudenslager ML. Fetal motor activity and maternal cortisol. Dev Psychobiol. 2009;51(6):505–512. doi: 10.1002/dev.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LM, Myers MM, Monk C. Pregnant women’s cortisol is elevated with anxiety and depression - but only when comorbid. Arch Womens Ment Health. 2008 doi: 10.1007/s00737-008-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM–IV axis I disorders–non-patient edition. Biometrics Research Department/NYSPI 1997 [Google Scholar]
- Godfrey KM, Barker DJP. Fetal programming and adult health. Public Health Nutrition. 2001;4(2B):611–624. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Hanson DR. Human development: biological and genetic processes. Annu Rev Psychol. 2005;56:263–286. doi: 10.1146/annurev.psych.56.091103.070208. [DOI] [PubMed] [Google Scholar]
- Kammerer M, Adams D, Castelberg B, Glover V. Pregnant women become insensitive to cold stress. BMC Pregnancy Childbirth. 2002;2(1):8. doi: 10.1186/1471-2393-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. “A gene for...”: the nature of gene action in psychiatric disorders. Am J Psychiatry. 2005;162(7):1243–1252. doi: 10.1176/appi.ajp.162.7.1243. [DOI] [PubMed] [Google Scholar]
- Monk C, Fifer WP, Sloan RP, Myers MM, Trien L, Hurtado A. Maternal stress responses and anxiety during pregnancy: Effects on fetal heart rate. Developmental Psychobiology. 2000;36:67–77. [PubMed] [Google Scholar]
- Monk C, Myers MM, Sloan RP, Werner L, Jeon J, Tager F, et al. Fetal heart rate reactivity differs by women’s psychiatric status: an early marker for developmental risk? Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(3):283–290. doi: 10.1097/00004583-200403000-00009. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58(3):211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioral/emotional problems at 4 years: Report from the Avon Longitudinal Study of Parents and Children. British Journal of Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- Pawlby S, Hay DF, Sharp D, Waters CS, O’Keane V. Antenatal depression predicts depression in adolescent offspring: prospective longitudinal community-based study. J Affect Disord. 2009;113(3):236–243. doi: 10.1016/j.jad.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES–D scale: A self–report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Renaud P, Blondin JP. The stress of Stroop performance: physiological and emotional responses to color-word interference, task pacing, and pacing speed. Int J Psychophysiol. 1997;27(2):87–97. doi: 10.1016/s0167-8760(97)00049-4. [DOI] [PubMed] [Google Scholar]
- Roberts RE. Reliability of the CES-D Scale in different ethnic contexts. Psychiatry Res. 1980;2(2):125–134. doi: 10.1016/0165-1781(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Rommelse NN, Van der Stigchel S, Sergeant JA. A review on eye movement studies in childhood and adolescent psychiatry. Brain Cogn. 2008 doi: 10.1016/j.bandc.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa PD, Chicz-DeMet A, Porto M, Garite TJ. Maternal corticotropin-releasing hormone and habituation in the human fetus. Developmental Psychobiology. 1999;34:163–173. doi: 10.1002/(sici)1098-2302(199904)34:3<163::aid-dev1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol. 2008;586(2):377–385. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems and anxiety in 8–9 year olds. Child Dev. 2004;75(4):1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Mennes M, Oosterlaan J, Stevens V, Stiers P, Marcoen A, et al. High antenatal maternal anxiety is related to impulsivity during performance on cognitive tasks in 14- and 15-year-olds. Neurosci Biobehav Rev. 2005;29(2):259–269. doi: 10.1016/j.neubiorev.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20(4):470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm FH, Grossman P, Coyle MA. Improving estimation of cardiac vagal tone during spontaneous breathing using a paced breathing calibration. Biomed Sci Instrum. 2004;40:317–324. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.