Summary
This is the first large-scale epidemiological study evaluating the prevalence of interictal epileptic discharges (IEDs) and photosensitivity (PS) recorded by scalp EEG in a natural nonhuman-primate model of photosensitive, generalized epilepsy. Scalp EEG was used to characterize electroclinical phenotypes in a large baboon pedigree housed at the Southwest National Primate Research Center at the Texas Biomedical Research Institute (Texas Biomed) based upon IEDs and photosensitivity. Scalp EEG studies including intermittent light stimulation (ILS) were performed in 671 baboons. Clinical histories were available for 531 (79%) of the animals. The EEG studies lasted 53 (±11) min, during which the baboons were lightly sedated with intramuscular ketamine doses of 5.6 (±0.8) mg. The animals were further classified according to electroclinical phenotypes recorded by scalp EEG: presence or absence of IEDs, seizures and photoparoxysmal or photoconvulsive responses. Effects of age, gender, and species on EEG phenotypes were compared using (Chi-square, two-sided, α < 0.05). Sensitivity and specificity of IEDs and photosensitivity to detect a history of seizures was calculated. Generalized IEDs and photosensitivity were identified in 324 (49%) and 156 (23%) pedigreed baboons, respectively. Only photosensitivity was associated with gender, significantly increased in males. Otherwise, while IEDs were marginally more prevalent among males, there were no other significant associations of IEDs or photosensitivity with age or subspecies. Photosensitivity was significantly associated with IEDs, with demonstrating a possible association with gender and subspecies. Of 531 baboons with histories of clinical events, 91 (17%) had witnessed seizures and 269 (51%) were asymptomatic. IEDs demonstrated sensitivity and specificity of 62% and 57%, and photosensitivity of 40% and 83%, for prediction of seizures, respectively. While these EEG findings mirror the high prevalence of seizures in the colony, the sensitivity and specificity of scalp EEG may have been affected by ketamine’s ability to lower the threshold for IEDs and seizures, particularly in animals predisposed to epilepsy. Photosensitivity provides a specific biological marker for epilepsy in future epidemiological, genetic, behavioral and histopathological studies.
Keywords: Baboon, Epilepsy, Electroencephalography, Photosensitivity, Animal models
Introduction
The baboon represents a natural model of photosensitive, generalized epilepsy (Killam, 1979). Spontaneous seizures have been reported in the baboon, but the interest in this model was fueled by the high prevalence of photosensitivity, or the ability to trigger seizures with intermittent light stimulation (ILS). Early studies aimed to dissect the epileptic networks underlying photosensitivity (Naquet and Meldrum, 1972). Although semiological and electroencephalographic (EEG) data were recorded by several groups (Fischer-Williams et al., 1968; Corcoran et al., 1979), data were limited on the epidemiology and electroclinical classification of the seizures in wild or captive baboon colonies.
The Southwest National Primate Research Center (SNPRC) of the Texas Biomedical Research Institute (TBRI) in San Antonio, Texas, currently sustains about 2000 baboons, including P.h. anubis (PHA), P.h. anubis/cynocephalus crosses (PHX), P.h. hamadryas, P.h. papio and other subspecies or crosses. The SNPRC is distinguished as housing the largest colony of baboons of any national primate center, and is home to the oldest and largest captive baboon pedigree, consisting of over 2000 animals and spanning as many as seven generations (Rogers and Hixson, 1997). Spontaneous seizures have been witnessed since the inception of the pedigree five decades ago (Killam et al., 1967). Because of the ability to observe baboons in large cages and detailed record-keeping, the SNPRC provides an ideal setting to study the epidemiology of seizures. Despite historical breeding across subspecies, seizures are widespread. The seizure prevalence of 26% was recently reported, and, in 15%, seizures tend to recur (Szabó et al., 2012a). This figure may underestimate the true prevalence, as the baboons are not under constant observation, and seizures, particularly if nocturnal, may go unnoticed. Furthermore, some of the baboons may exhibit predominantly eyelid myoclonia or absence seizures, which are difficult to recognize by untrained observers, while in others, craniofacial trauma may represent seizure-related injuries (Szabó et al., 2012a).
This is the first large-scale epidemiological study evaluating the prevalence of interictal epileptic discharges (IEDs) and photosensitivity (PS) in a large pedigree of baboons. A previous scalp EEG studies in a smaller group of animals selected from this pedigree, demonstrated that the natural epilepsy of the baboon model closely resembles one of the most common epilepsy syndromes in humans, juvenile myoclonic epilepsy (JME; Szabó et al., 2004, 2005). The seizures are generalized myoclonic or tonic–clonic in character, present chiefly in adolescence, occur predominantly in the morning and can be provoked by stress, handling and ketamine administration. These EEG studies documented a high prevalence of eyelid myoclonia even in baboons without other witnessed seizure types. Generalized IEDs were prevalent in the pedigree. The IEDs consisted predominantly of 4–6 Hz spike- or polyspike-and-wave discharges, even in asymptomatic baboons. A few young baboons, ages 4 years old and younger, demonstrated IEDs of 2–3 Hz frequency, while IEDs of 6–7 Hz were exhibited in a few adult animals.
In this study, scalp EEG findings will be presented for 671 baboons, 531 of which also have clinical information regarding seizures. The purpose of this study is to correlate EEG findings, including the presence or absence of IEDs, recorded seizures and photosensitivity, with demographic data (age, gender and subspecies) and clinical history (history of provoked or unprovoked seizures).
Methods
Six hundred seventy-one baboons belonging to P.h. anubis and hybrid subspecies were evaluated with scalp EEG, and classified according to interictal and ictal EEG traits, as well as photosensitivity. Of the 671 baboons undergoing scalp EEG studies, 452 (67%) were females, 219 (33%) were males, 425 (63%) belonged to PHA, 219 (33%) to PHX and the remaining 27 (4%) to other subspecies (Table 1). Their mean age at the time of the EEG study was 13 (range 0.5–33) years old, their mean weight was 21 (±7) kg. The selection of the baboons included a broad representation across the pedigree. However, because of some baboons, particularly younger animals, presenting with severe seizures, as well as older animals that were never witnessed to have seizures and were either ill or near to termination, were preferentially referred for EEG studies, some demographic and clinical biases were unavoidable. The baboons were treated in strict accordance with the U.S. Public Health Service’s Guide for the Care and Use of Laboratory Animals (Committee for the Update, 2011) and the Animal Welfare Act. This study was approved by the Institutional Animal Care and Use Committees of UTHSCSA and Texas Biomed.
Table 1.
EEG phenotypes and correlation with demographic findings.
| EEG findings |
Demographic and clinical findings |
Totals | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IEDs | Seizure | PS | PHA | PHX | Female | Male | <4 yo | 4–7 yo | >7 yo | |
| + | + | + | 58 | 43 | 63 | 40 | 6 | 20 | 77 | 103 |
| − | 66 | 39 | 68 | 40 | 2 | 24 | 82 | 108 | ||
| − | + | 21 | 11 | 19 | 17 | 8 | 3 | 25 | 36 | |
| − | 59 | 18 | 59 | 21 | 10 | 14 | 56 | 80 | ||
| − | + | + | 2 | 0 | 1 | 1 | 1 | 0 | 1 | 2 |
| − | 3 | 1 | 3 | 1 | 0 | 2 | 2 | 4 | ||
| − | + | 13 | 2 | 10 | 5 | 1 | 0 | 14 | 15 | |
| − | 203 | 105 | 229 | 94 | 30 | 51 | 242 | 323 | ||
| Totals | 425 | 219 | 452 | 219 | 58 | 114 | 499 | 671 | ||
| Prevalence of IEDs (%) | 48 | 51 | 46 | 54 | 44 | 54 | 48 | 49 | ||
| Prevalence of PS (%) | 22 | 26 | 21* | 29* | 28 | 20 | 23 | 23 | ||
| Prevalence of PS in IED + EEG studies (%) | 39 | 49 | 39 | 48 | 54 | 38 | 42 | 43 | ||
IED, interictal epileptic discharge; PS, photosensitivity; PHA, P.h. anubis; PHX, P.h. anubis/cynocephalus cross; yo, year old.
Statistically significant difference (Chi-square, two-sided, α < 0.05).
Clinical information was obtained from a computerized database, and complemented by the review of veterinary records, particularly in animals whose computerized records were inconclusive (Szabó et al., 2012a). Seizures were determined in animals demonstrating generalized motor activity during the ictus or based upon postictal findings of confusion reported in the chart. Spontaneous, unprovoked seizures were classified separately from seizures were provoked either by ketamine anesthesia or handling. Epilepsy was only diagnosed in baboons with at least two witnessed, unprovoked seizures.
Scalp EEG
The methods for the scalp EEG studies were described by our group previously (Szabó et al., 2004, 2005). The surface electrodes are placed according to the standard international 10–20 electrode placement system at FP1, FP2, T8, C4, Cz, C3, T7, O1, O2, A1 and A2 positions. Bipolar montages were utilized for both the recording and review of the EEG data, and included the longitudinal chains FP1-C3-O1, FP1-T7-O1, FP2-C4-O2, and FP2-T8-O2, as well as the transverse chain T7-C3-Cz-C4-T8. Electrodes were also placed bilaterally beside the eyes to monitor lateral eye movements (EOG1, EOG2), over the deltoids or paraspinal muscles to monitor skeletal muscle activity, and a single electrode monitored the heart rhythm (ECG). EEG studies were acquired on a laptop-based acquisition machine (Grass-Telefactor, USA, XLTEK, Canada, and Nihon-Kohden, Japan). Electrode impedances were below 10 kW. All but four baboon infants were restrained in a primate chair for the scalp EEG study (the four were infants, small enough to be held by one of the investigators). Ketamine was used to sedate animals in order to transfer them to a primate chair for electrode placement and EEG recording. Ketamine was injected intramuscularly using a mean dose of 5.6 (±0.8) mg/kg, transiently sedating the animal without suppression their respiratory drive. At these doses, ketamine can activate interictal and ictal epileptic discharges, with minimal effect on photosensitivity (Szabó et al., 2004). ILS was performed at least 15 min apart, the first time while the baboons were still sedated, and the second time after they were able to track movement in their environment. The same dose of ketamine was administered prior to the baboons’ removal from the primate chair. The baboons were recorded for 10 min after the second injection for ictal or interictal epileptic discharges acutely provoked by the ketamine (peak dose effect). Ketamine’s maximal effect on the EEG is visible between 2 and 15 min from the time of the intramuscular injection, and is characterized by a burst suppression pattern, with myoclonus associated with the bursts of high amplitude slow waves (Szabó et al., 2004). The EEG studies lasted a mean of 53 (±11) min.
The EEG studies were interpreted and classified by a single investigator, who is board certified in clinical neurophysiology (CAS). This investigator was blinded to the animals’ previous history while interpreting the scalp EEG study. IEDs typically consisted of 4–6 Hz generalized spike-and-wave discharges, characterized on the basis of their morphology and distribution (Chartrian et al., 1974). Seizures were classified as myoclonic, absence or tonic–clonic (Commission on Classification and Terminology, 1981). Myoclonic seizures are characterized by sudden, brief, contractions of periorbital facial, truncal or appendicular muscles, in association with an epileptic discharge. Myoclonic seizures were differentiated from nonepileptic myoclonus on the basis of the somatotopic distribution of the motor activity and EEG correlation (Ménini et al., 1994). Photosensitivity is characterized by photoparoxysmal or photoconvulsive responses. A photoparoxysmal response was defined as an IED activated by ILS, with the discharges appearing time-locked with the stimulus onset or if their doubled during the stimulus compared to the resting EEG activity in the 60-s period prior to stimulation. Slow wave activity during the stimulus did not constitute a photoparoxysmal response. Photoconvulsive episodes included myoclonic, absence and generalized tonic–clonic seizures (GTCS) that were activated by ILS in a time-locked fashion. Photomyogenic activity in the eyes, trunks, or limbs of the baboons that occurred time-locked to stimulus frequency was not classified as a seizure unless it extended beyond the stimulus.
The prevalence of IEDs or photoparoxysmal or photoconvulsive responses were compared between genders, subspecies (PHA vs PHX baboons), and for clinical features, including for provoked or unprovoked seizures (Chi-Square, two-sided, α < 0.05; Table 2). Furthermore, the sensitivity and specificity of IEDs and PS were calculated for the diagnosis of seizures or epilepsy.
Table 2.
Electroclinical correlation.
| EEG findings |
Clinical (seizure) status |
Totals | ||||||
|---|---|---|---|---|---|---|---|---|
| IEDs | Seizures | PS | Single unprovoked seizure (+H/K) |
Epilepsy (+H/K) | H/K alone | Seizures (total) | None | |
| + | + | + | 11 (5) | 11(3) | 4 | 26 | 54 | 80 |
| − | 5 (1) | 7 (2) | 0 | 12 | 68 | 80 | ||
| − | + | 7 (1) | 5 (3) | 2 | 14 | 17 | 31 | |
| − | 5 (1) | 5 (1) | 3 | 13 | 51 | 64 | ||
| − | + | + | 0 | 0 | 0 | 0 | 2 | 2 |
| − | 0 | 1 | 0 | 1 | 1 | 2 | ||
| − | + | 0 | 1 | 0 | 1 | 11 | 12 | |
| − | 17 (2) | 16 (4) | 5 | 38 | 222 | 260 | ||
| IEDs | 28 (8) (62%) | 28 (9) (61%) | 9 (64%) | 65 (62%)* | 190 (45%)* | 255 | ||
| PS | 18 (3) (40%) | 17(6) (37%) | 6 (43%) | 41 (39%)* | 84 (20%)* | 125 | ||
| Totals | 45 (10) | 46 (13) | 14 | 105 | 426 | 531 | ||
IED, interictal epileptic discharge; PS, photosensitivity; H or K, history of handling- or ketamine-induced seizures; (+H/K), number of animals with additional handling- or ketamine-induced seizures are contained within the brackets, Asym asymptomatic.
Statistically significant difference (Chi-square, two-sided, α < 0.05).
Finally, we evaluated the stability of scalp EEG findings, such as IEDs and photosensitivity, over time. The principal investigator (CAS) interpreted 35 (21 female, 14 male; 29 PHA, 6 PHX) studies that were performed at an interval of at least one year (mean 3 (±1) years) after the index study. For the purpose of this study, the principal investigator was blinded with respect to the initial EEG diagnosis. The mean age of the baboons at the first study was 9 (±6) years old (5 preadolescent baboons were younger than four years old, 13 adolescents ages 4 to 7 years old, and 17 were adults eight years or older).
Results
EEG classification
The majority of the EEG studies (350 of 671, 52%) showed IEDs, seizures and/or photosensitivity. Spontaneous IEDs were identified in 327 (49%) baboons, and seizures and photosensitivity were reported in 23 (3%) studies without IEDs (Table 1). Even among the 323 (48%) baboons with normal studies, 56 (17%) had IEDS or seizures that were detected after the second ketamine administration (peak-dose effect). Nevertheless, IEDs were induced by the second ketamine injection in 96% of the studies already positive for IEDs. IEDs were more common in males than in females (χ2 = 3.45, df = 1, p = 0.06), but the difference was only marginally significant. IEDs were similarly distributed between the PHA and PHX baboons. Furthermore, the prevalence of IEDs did not significantly differ between preadolescent (44%), adolescent (54%) or adult (48%) baboons. The diagnosis, based upon the presence or absence of IEDs, remained constant in 25 of 35 (71%) baboons with repeated EEG studies. IEDs persisted in 14 (78%) baboons. Six of ten baboons demonstrating changes in IED status were seven years or younger at the time of their first study.
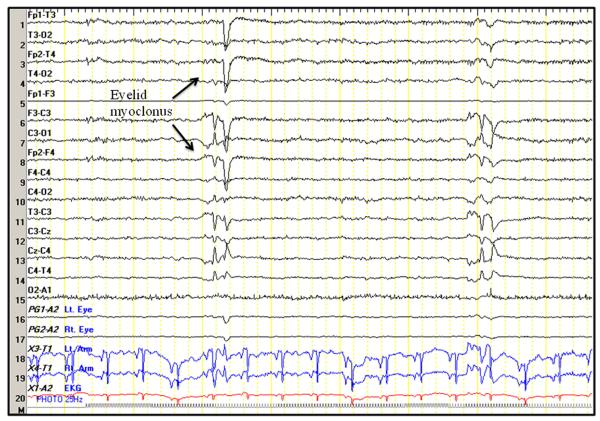
Seizures were recorded by scalp EEG in 217 (32%) baboons (Table 1). While seizures were more prevalent in adolescents, males, and PHX baboons, the differences were not statistically significant. The predominant seizure type was an eyelid or facial myoclonus in over two thirds of the baboons, and more myoclonic seizures affecting the trunk or extremities in about one third of the cases (Fig. 1). Absence seizures consisting of an unresponsive stare with partial eye closure and decreased responsiveness to noxious stimuli were noted in only 3 baboons, all with 2–3 Hz spike-and-wave discharges (Fig. 2). Generalized tonic or tonic–clonic seizures were recorded were recorded in 9 baboons, all associated with a generalized transient or spike followed by suppression with secondary evolution of a generalized rhythmical discharge in the theta frequency. In 3 animals, the GTCS were recorded only after the second ketamine administration (peak-dose effect). Ketamine may have triggered nonconvulsive status epilepticus in 3 preadolescent baboons, characterized by persistent decrease in responsiveness throughout the study, associated with continuous rhythmical 2–3 Hz slowing intermixed with intermittent or continuous 1–2 Hz periodic or semiperiodic generalized spike-and-wave complexes, and (Fig. 3). Nonetheless, runs of generalized interictal epileptic discharges spikes were also noted young epileptic baboons without exposure to ketamine (Fig. 4).
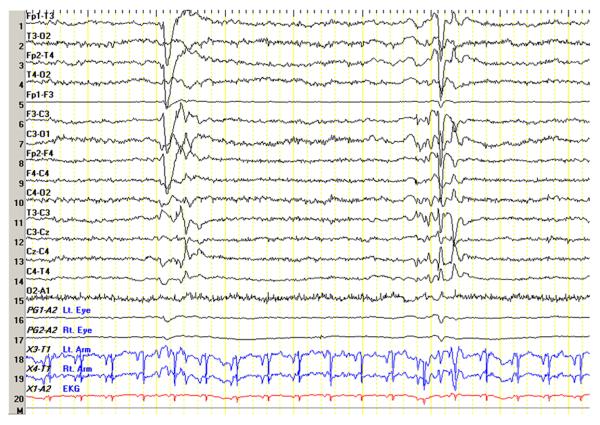
Figure 1.
Eyelid myoclonus. Nine-year-old female with a recent history of craniofacial trauma but no witnessed seizures.
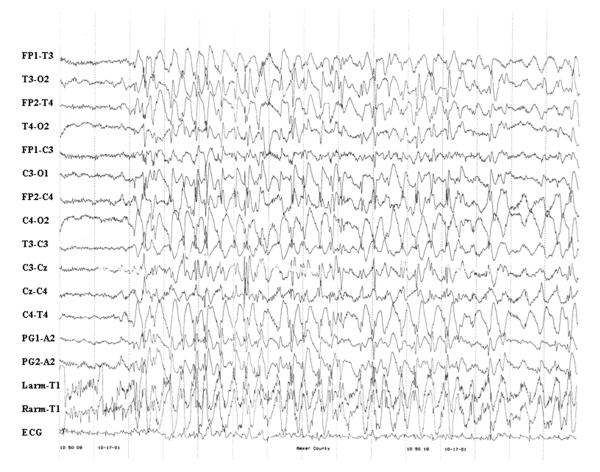
Figure 2.
2–3 Hz spike-and-wave discharge. Fourteen-month-old female with a history of new onset GTCS, staring spells and prolonged confusional episodes. EEG sample demonstrates a paroxysm of 2–3 Hz spike-and-wave complexes associated with decreased responsiveness.
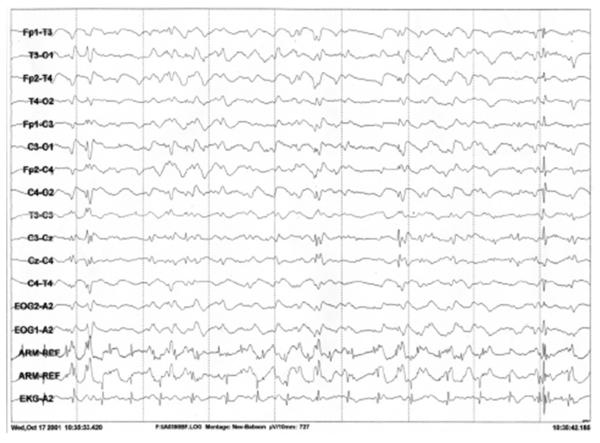
Figure 3.
Nonconvulsive status epilepticus. EEG sample demonstrates periodic generalized discharges during a period of decreased responsiveness consistent with nonconvulsive status epilepticus in the same baboon as depicted in Fig. 2.
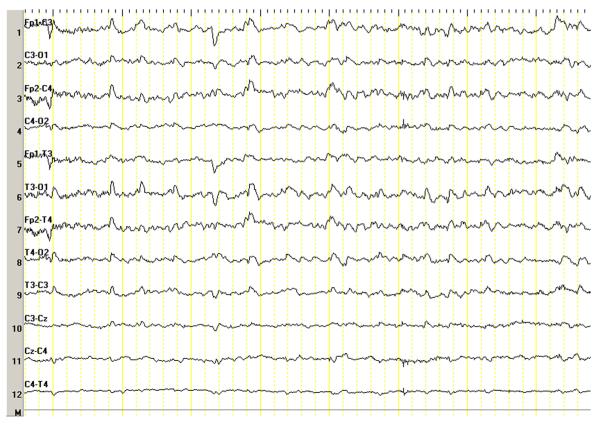
Figure 4.
Repetitive generalized interictal epileptic discharges in an infant baboon. Interictal epileptic discharges in a 4-month-old male with new onset seizures. This EEG was performed without ketamine.
Photosensitivity was detected in 156 (23%) baboons overall, but in 43% of baboons with IEDs (Table 1 and Fig. 5). PS was significantly increased in baboons with IEDs compared baboons without IEDs (χ2 = 130.5, df = 1, p < 0.0001). Conversely, IEDs were recorded in 96% of photosensitive baboons, whereas only 5% of the baboons without IEDs were photosensitive. The prevalence for photosensitivity was significantly increased in males than in females (χ2 = 5.6, df = 1, p < 0.02), but was equally distributed among age groups, though increased in preadolescent baboons compared to adolescent and adults, and subspecies. The absence or presence of photosensitivity remained constant in 25 (71%) repeat studies (6 with and 19 without and photoparoxysmal or photoconvulsive responses at baseline). Photosensitivity persisted in 6 of 12 (50%) baboons. Five of ten baboons with altered status of photosensitivity were either preadolescent or adolescents at the time of their first study.
Figure 5.
Photoparoxysmal response. Eyelid myoclonus time-locked with ILS onset (see arrow), occurring repetitively, in baboon depicted in Fig. 1.
Electroclinical correlation
Reliable clinical histories were available in 531 (79%) baboons undergoing at least one scalp EEG study (Table 2). The breakdown by gender and subspecies was similar to above. Seizures were witnessed in 100 (19%) baboons, unprovoked and spontaneous in 91 animals. Handling or ketamine anesthesia provoked additional seizures in 23 (25%) baboons with unprovoked seizures. Ketamine or handling provoked seizures in only 14 baboons without unprovoked seizures. Of the baboons with unprovoked seizures, 45 had a single witnessed seizure, while 46 had a diagnosis of epilepsy, with at least one witnessed seizure (Table 2). The time of the first seizure was the same in both groups (mean 7 ± 5 years, mean of approximately 21 years in humans), and the baboons with epilepsy had a mean of 4 ± 2 witnessed seizures. The prevalence of IEDs was 62, 61 and 64%, for baboons with single seizures, epilepsy, or provoked seizures, respectively; for photosensitivity the prevalence was 40, 37 and 43%, respectively. The baboons with epilepsy were more likely to be male and PHX baboons. IEDs were significantly increased in baboons with witnessed seizures as compared to asymptomatic baboons (χ2 = 12.4, df = 1, p < 0.0005). While photosensitivity was also encountered in almost 20% of asymptomatic baboons, it was also significantly associated with witnessed seizures (χ2 = 17.8, df = 1, p < 0.0001).
The sensitivity and specificity for scalp EEG to detect IEDs in baboons with witnessed seizures is 62% and 57%, respectively. The sensitivity and specificity of photoparoxysmal or photoconvulsive responses to confirm the diagnosis of seizures was 40% and 83%, respectively.
Discussion
This is the first epidemiological study correlating clinical findings and scalp electroencephalography (EEG) assessments in a large baboon pedigree. While previous studies in baboons reported on the prevalence of photosensitivity, there is little knowledge about the prevalence of interictal epileptic discharges (IEDs) or clinical seizures in this species. Spontaneous generalized spike-and-wave discharges were recorded in less than half the baboons. Photosensitivity less prevalent, but is specifically associated with IEDs and a history of seizures. While IEDs appear to be similarly distributed among age groups, genders and subspecies, there is a statistically significant increase in prevalence of photosensitivity in male baboons. The prevalence of IEDs and photosensitivity was surprisingly constant when comparing single unprovoked seizures, epilepsy or provoked seizures. As most ketamine- or handling-induced seizures were witnessed by veterinarians who were more apt to appropriately diagnose seizures, it is likely that this group was clinically well-defined. Furthermore, it is likely that seizures are underreported in the baboons as they are not under constant observation, and because spontaneous seizures are relatively rare events in most baboons. Incorporating other markers, such as craniofacial trauma typically associated with seizures, may improve the characterization and true estimate of the size of the epilepsy group.
EEG is an important instrument in determining the risk for epilepsy and aids in the classification of the epilepsy. In humans with epilepsy, an initial scalp EEG study is able to detect IEDs in 29–55%, the sensitivity improving to 80–90% with serial studies (Walczak and Jayakar, 1997). In IGE, the sensitivity of EEG is higher in particular age groups, as IEDs are easy to recognize due to their bihemispheric distribution. Furthermore, in certain syndromes, IEDs can be reliably provoked by sleep deprivation, hyperventilation and intermittent light stimulation. In people with JME the sensitivity of scalp EEG to detect generalized 4–6 Hz polyspike-and-wave complexes is 52% (Delgado-Escueta et al., 1996). The natural model of epilepsy in the baboon model closely resembles JME (Dreifuss, 1989), and not surprisingly, scalp EEG had a similar sensitivity and specificity for the diagnosis of seizures in baboons housed at the SNPRC.
Photosensitivity was less prevalent than IEDs in the baboons. It occurred in 40% EEG studies demonstrating IEDs, which is similar prevalence in EEG studies of photosensitive human subjects with generalized IEDs (Kasteleijn-Nolst Trenité, 1989). In baboons, the photoparoxysmal responses appear to be closely associated with the expression of IEDs, suggesting common genetic etiologies. In red baboons (Papio hamadryas papio), the prevalence of photosensitivity was estimated as high as 60% in baboons from the Salamance region (Killam, 1979). It is possible the prevalence of photosensitivity in our study may have been reduced due to ketamine’s effect on cortical responsiveness, but these earlier studies relied mainly upon observation instead of EEG for diagnostic confirmation of photoparoxysmal or photoconvulsive responses. Even so, photosensitivity’s prevalence of 40% in seizure baboons at the TBRI is similar to that of JME patients (Dreifuss, 1989). Most importantly, photosensitivity in our study provided a specific marker for a clinical history of seizures, suggesting that baboons without photosensitivity on EEG did not have seizures or epilepsy.
The effects of age, gender and subspecies on EEG phenotypes were also evaluated. IEDs appeared to be more closely associated male gender and adolescence, while photosensitivity was significantly associated with male gender, and appeared to be more prevalent in preadolescent than adolescent baboons. The increased prevalence of IEDs in adolescent baboons seems to parallel the average age of onset of epilepsy (Szabó et al., 2012a), which are characteristics similar to JME in humans (Delgado-Escueta et al., 1997). However, the high prevalence of IEDs and photosensitivity in male, preadolescent baboons contrasts JME, with increased prevalence of IEDs and photosensitivity in adolescent females with epilepsy (Delgado-Escueta et al., 1997). Nonetheless, about one quarter of JME patients, present with absence seizures in childhood (Delgado-Escueta et al., 1997), and these children, can even exhibit motor seizures with certain triggers, such as fever or stress. As most of the baboons referred for EEG studies because of acute seizures were predominantly preadolescent males, in this demographic, the presence of IEDs and photosensitivity tracks increased susceptibility to spontaneous seizures in baboons and humans alike (Delgado-Escueta et al., 1997; Szabó et al., 2005). The presentation of more early onset seizures and photosensitivity may reflect gender-based differences in the baboon phenotypes.
Despite the overall consistency of the electroclinical findings between epileptic baboons and people with JME, it is concerning that IEDs were so prevalent in baboons even without a history of seizures. This may in part be due to the inherited predisposition to seizures within the pedigree (Szabó et al., 2012a). However, low-dose ketamine is also known to activate IEDs with epilepsy. The scalp EEGs utilized a subanesthetic dose of ketamine of 5–6 mg/kg, which is similar to the dose facilitating the expression of IEDs in humans (Ferrer-Allado et al., 1973; Celesia et al., 1975), and one which does not suppress the photoparoxysmal response (Szabó et al., 2004). Ketamine was preferred because of the potential for intramuscular administration, reliable and brief duration of its sedative activity, transient effect on the EEG signal, and lack of respiratory suppression. Without sedation, it is extremely difficult to place or maintain electrodes (Killam et al., 1967). While ketamine may have increased the sensitivity of scalp EEG by lowering the IED and seizure thresholds, we recorded similar spontaneous ictal and interictal epileptic discharges on intracranial EEG recordings in unmedicated baboons as on their prior scalp EEG studies (Szabó et al., 2012b). Furthermore, medication effects also hamper EEG studies in human epilepsies as both IEDs and photosensitivity can be suppressed, even occasionally activated, by antiepileptic medications. It is reassuring, however, that IEDs, and to lesser degree photosensitivity, were reproducible even under the effect of ketamine, whether after a week (Szabó et al., 2004), six months (Szabó et al., 2005), or several years in this study, irregardless of age. After longer intervals, it is expected that EEG phenotypes associated with IGE are more likely to change in preadolescent and adolescent than in adult baboons, particularly with regards to photosensitivity (Pedley, 1997). Nonetheless, this study needs to be repeated in baboon populations derived from different subspecies and geographical areas, or younger colonies which have not been subject to crossbreeding and, ideally, in baboons that are not sedated.
In summary, the baboon pedigree housed at the Texas Biomed offers an excellent resource to study genetic causes and pathophysiological mechanisms underlying idiopathic generalized epilepsies and photosensitivity. The availability of a large colony that has been, in a large part, electroclinically phenotyped, offers an opportunity to evaluate comorbidities of epilepsy, such as social, behavioral effects as well as SUDEP. There is a need to further improve the scalp EEG techniques in order to perform longitudinal studies to evaluate the age-dependent presentation and evolution of the EEG phenotypes in awake, unmedicated baboons.
Acknowledgements
We are grateful for Sarah Williams-Blangero’s review of our manuscript. This study was supported by the National Institute of Neurological Disorders and Stroke (NIH/NINDS 1 R21 NS065431 to CAS and 1 R01 NS047755 to JTW). This research used primate resources supported by P51 RR013986, and was conducted in facilities constructed with support from Research Facilities Improvement Grants C06 RR013556, C06 RR014578, and C06 RR015456. This research was presented at the 2010 American Epilepsy Society Meeting in San Antonio, Texas.
Footnotes
Conflict of interest None of the authors has any conflict of interest to disclose.
References
- Celesia GG, Chen R-C, Bamforth BJ. Effects of ketamine in epilepsy. Neurology. 1975;25:169–172. doi: 10.1212/wnl.25.2.169. [DOI] [PubMed] [Google Scholar]
- Chartrian G, Bergamini L, Dondey M, Klass EW, Lennox-Buchtal M, Petersén I. A glossary of terms most commonly used by clinical electroencephalographers. Electroencephalogr. Clin. Neurophysiol. 1974;37:538–548. doi: 10.1016/0013-4694(74)90099-6. [DOI] [PubMed] [Google Scholar]
- Commission on Classification and Terminology (International League Against Epilepsy) Proposal for revised clinical and EEG classification for epileptic seizures. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- Committee for the Update . Guide for the Care and Use of Laboratory Animals. eighth ed National Academy Press; Washington, DC: 2011. [Google Scholar]
- Corcoran ME, Cain DP, Wada JA. Photically induced seizures in the yellow baboon, Papio cynocephalus. Can. J. Neurol. Sci. 1979;6:129–131. doi: 10.1017/s0317167100119511. [DOI] [PubMed] [Google Scholar]
- Delgado-Escueta AV, Serratosa JM, Medina MT. Juvenile myoclonic epilepsy. In: Wyllie E, editor. The Treatment of Epilepsy: Principles and Practice. second ed Williams & Wilkins; Baltimore: 1996. pp. 484–501. [Google Scholar]
- Delgado-Escueta AV, Serratosa JM, Medina MT. Juvenile myoclonic epilepsy. In: Wyllie E, editor. The Treatment of Epilepsy: Principles and Practice. second edition Williams & Wilkins; Baltimore: 1997. pp. 484–501. [Google Scholar]
- Dreifuss FE. Juvenile myoclonic epilepsy: characteristics of a primary generalized epilepsy. Epilepsia. 1989;30(Suppl. 4):S1–S7. doi: 10.1111/j.1528-1157.1989.tb05832.x. [DOI] [PubMed] [Google Scholar]
- Ferrer-Allado T, Brechner VL, Dymond A, Cozen H, Crandall P. Ketamine-induced electroconvulsive phenomena in the human limbic and thalamic regions. Anesthesiology. 1973;38:333–344. doi: 10.1097/00000542-197304000-00006. [DOI] [PubMed] [Google Scholar]
- Fischer-Williams M, Poncet M, Riche D, Naquet R. Light-induced epilepsy in the baboon, Papio papio: cortical and depth recordings. Electroencephalogr. Clin. Neurophysiol. 1968;25:557–569. doi: 10.1016/0013-4694(68)90235-6. [DOI] [PubMed] [Google Scholar]
- Kasteleijn-Nolst Trenité DGA. Photosensitivity in epilepsy: electrophysiological and clinical correlates. Acta Neurol. Scand. 1989;125(Suppl.):3–149. [PubMed] [Google Scholar]
- Killam EK, Stark LG, Killam KF. Photic stimulation in three species of baboons. Life Sci. 1967;6:1569–1574. doi: 10.1016/0024-3205(67)90165-8. [DOI] [PubMed] [Google Scholar]
- Killam EK. Photomyoclonic seizures in the baboon, Papio papio. Fed. Proc. 1979;38:2429–2433. [PubMed] [Google Scholar]
- Ménini C, Silva-Barrat C, Naquet R. The epileptic and nonepileptic myoclonus of the Papio papio baboon. In: Malafosse A, editor. Idiopathic Generalized Epilepsies. John Libbey; London: 1994. pp. 331–348. [Google Scholar]
- Naquet R, Meldrum BS. Photogenic seizures in the baboon. In: Purpura DP, Penry JK, Tower DB, Woodbury DM, Walter RD, editors. Experimental Models of Epilepsy. Raven Press; New York: 1972. pp. 373–406. [Google Scholar]
- Pedley TA. EEG Traits. In: Engel J Jr., Pedley TA, editors. Epilepsy: A Comprehensive Textbook. Lippincott-Raven Publishers; Philadelphia: 1997. pp. 185–196. [Google Scholar]
- Rogers J, Hixson JE. Insights from model systems: baboons as an animal model for genetic studies of common human disease. Am. J. Hum. Genet. 1997;61:489–493. doi: 10.1086/515527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó CÁ, Leland MM, Sztonák L, Restrepo S, Haines R, Mahaney MC, Williams JT. Scalp EEG for the diagnosis of epilepsy and photosensitivity in the baboon. Am. J. Primatol. 2004;62:95–106. doi: 10.1002/ajp.20018. [DOI] [PubMed] [Google Scholar]
- Szabó CÁ, Leland MM, Knape KD, Elliott JJ, Haines VL, Williams JT. Clinical and EEG phenotypes of epilepsy in the baboon (Papio hamadryas spp.) Epilepsy Res. 2005;65:71–80. doi: 10.1016/j.eplepsyres.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Szabó CÁ, Knape KD, Leland MM, Cwikla DJ, Williams-Blangero S, Williams JT. Epidemiology and characterization of seizures in a pedigreed baboon colony. Comp. Med. 2012a;62:535–538. [PMC free article] [PubMed] [Google Scholar]
- Szabó CÁ, Salinas FS, Leland MM, Caron JL, Narayana S, Hanes MA, Knape KD, Xie D, Williams JT. Baboon model of generalized epilepsy: continuous intracranial video-EEG monitoring with subdural electrodes. Epilepsy Res. 2012b;101:46–55. doi: 10.1016/j.eplepsyres.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak TS, Jayakar P. Interictal EEG. In: Engel J Jr., Pedley TA, editors. Epilepsy: A Comprehensive Textbook. Lippincott-Raven Publishers; Philadelphia: 1997. pp. 831–848. [Google Scholar]