Introduction
To comprehend swallowing physiology and pathophysiology, our knowledge and understanding of the underlying neural pathways that govern swallowing is critical. As recently as the 1980s, swallowing was thought to be automatic, mediated at the brainstem level and was commonly referred to as a “reflex”. Current research of the neural underpinnings of deglutition, however, has led to the more accurate terminology of swallowing as a patterned response,1 which is mediated by much more complex neurophysiological processes than were previously understood.
For swallowing to be initiated, sensory fibers of the oropharynx that respond to temperature alone, to touch-pressure alone, or to both touch-pressure and temperature2 as well as chemoreceptors, need to send taste and sensory information to five pairs of cranial nerves (CN V, CN VII, CN IX, and fibers shared by CN X and XI).3 This information is then transferred to groups of nuclei in the brainstem, including the dorsal area within and around the Nucleus Tractus Solitarius (NTS), the ventral area around Nucleus Ambiguus (NA) and the area surrounding the reticular formation of these groups of nuclei.4-6 Recent neuroimaging findings support that supramedullary input also needs to travel to these nuclei relatively simultaneously and enable initiation of motor commands sent through six pairs of cranial nerves (CN V, CN VII, CN IX, fibers shared by CN X and XI, and CN XII) to the end organs, i.e. the oropharyngeal muscles.
Evidence of the role of supramedullary areas in the regulation of swallowing neurophysiology emerged from clinical studies of dysphagic patients with cortical or subcortical damage, as well as from recent neuroimaging research in healthy and dysphagic populations.7-22 The use of functional Magnetic Resonance Imaging (fMRI) in many of these studies has been prevalent and has proved effective in providing evidence for the complex neurophysiological control of swallowing in vivo.
What is Functional MRI (fMRI)?
Functional MRI was first introduced in human experiments in 1992,23-25 and in the years that followed, it has been widely accepted as an excellent non-invasive method for studying brain function.26 Ogawa and colleagues were the first to show the effect of Blood Oxygenation Level-Dependent (BOLD) signal with the use of an anesthetized rat model.27 Specifically, these researchers used blood as an endogenous contrast agent and showed that with rapid MR imaging sequences, temporary changes in the MR signal occur and accompany the hemodynamic events.24 By being sensitive to the BOLD effect, fMRI can use secondary hemodynamic and metabolic responses to changes in neuronal activity to inform us about it.28, 29
When a brain area is neurally active, its gray matter exhibits an increase in the metabolic rate of oxygen (CMRO2) and glucose,30 and subsequently an increase in blood flow. This increase causes two more events: an increase of oxyhemoglobin (red blood cells with an oxygen molecule attached) and a decrease of deoxyhemoglobin (red blood cells without an oxygen molecule attached).31 Deoxyhemoglobin acts as an endogenous contrast agent, since it is more paramagnetic than the tissue itself and it can enable detection of neuronal activation in the brain.26 It is this decrease that leads to an increase in the values of T2 and T2* (especially T2*) in the areas of the brain that are activated.31 Thus, the signal that is most often measured in BOLD fMRI is the T2* signal.28 In other words, fMRI measures the hemodynamic response, and as such it is an indirect measure of neural activity, and it does not measure neural activity per se.
Advantages and Limitations of fMRI
The major advantages of fMRI are that it provides a safe, noninvasive method for investigating human brain processes, is able to detect relatively small regional signal changes with high reliability in localizing the areas of increased neuronal activity26 and offers a spatial resolution of 3-5 mm or less. 30, 32 Additionally, in contrast with studies using electrophysiological techniques to study brain function, fMRI allows for large cerebral areas to be studied with relative ease.32
The main limitation consists of the interpretation of the fMRI signal. It must be considered that task-related fMRI results are always relative to some comparison condition (which is typically ‘resting’ for swallowing studies). The fMRI contrast is not quantitative, i.e., it is simply net difference between conditions or the percent increase of one condition over the other. Other factors that can influence the accurate interpretation of the fMRI signal include medication use and effects of medication on the hemodynamic response and on neural excitability, motion related artifacts caused by motor tasks under examination (e.g. swallowing), and degree of relaxation during “rest” / baseline condition. Additionally, the quality of the neurovascular response in patient populations cannot be assumed to be normal, and thus when studying patient populations with possible neurovascular complications, additional control mechanisms and scanning procedures need to be utilized. These limitations to fMRI are well known and reviewed in detail by D’Esposito and colleagues.33
Functional MRI in the study of Normal Swallowing in Humans
The need for employing sensitive neuroimaging methodologies to study the neural control of normal swallowing in vivo has been repeatedly expressed. 4, 34, 35 As such, several studies have attempted to identify the neural correlates of swallowing using fMRI in healthy individuals. 12-19, 21, 22, 36-39
One of the first published swallowing fMRI studies attempted to identify the brain areas involved in the motor control of voluntary water and saliva swallows in eight normal adults.12 Results suggested bilateral activations in a large neural network, including the precentral gyrus (primary motor cortex) and multiple activations in the: primary sensorimotor cortex, supplementary motor cortex (SMA), prefrontal cortex, Heschl’s gyri, cingulate gurus, insula, Broca’s areas, and superior temporal gyrus. Similarly, Hamdy and colleagues found activations of the antero-rostral cingulate cortex, caudolateral sensorimotor cortex, anterior insula, frontal opercular cortex, superior premotor cortex, anteromedial temporal cortex, anterolateral somatosensory cortex, and precuneous, during water swallowing of 10 healthy adults.22 Several other studies published in the following years also aimed to elucidate the activations associated with volitional swallowing. Despite differences in methodology, these studies reported highly similar results. 13-19, 38
The plethora of neural activations led some of these researchers to state that these may not be specific to swallowing innervation, but rather may indicate innervations of the tongue, larynx, pharynx and face as well.22 Malandraki and colleagues investigated three tasks representative of different components of the swallow, in order to attempt a relative neural differentiation of components of the swallowing process.21 Results indicated that pharyngeal components of swallowing (such as laryngeal closure) rely more heavily on subcortical networks, whereas oral components of swallowing (such as tongue elevation) depend more on cortical sensorimotor cortex innervation.20, 21 (Figure 1, with permission)
Figure 1.

Areas of most significant activation during swallowing (shown in red), during throat clearing (shown in blue), and during tongue tapping (shown in yellow). Boxes report the areas. Images are shown in radiological convention (the right hemisphere is shown on the left). Coordinates are given in MNI space. (In: Malandraki et al. 2009, HBM, used with permission).
Recently, the first interleaved fMRI/ dynamic MRI sequence that enables simultaneous acquisition of fMRI data while dynamically imaging oropharyngeal swallowing (aka SimulScan) was developed by Paine et al.40 This sequence allows for joint acquisition of the cortical, subcortical and oropharyngeal areas and with the use of fast FLASH spiral sequences provides interleaved acquisition, while maintaining dynamic imaging rates of 14.5 frames per second. Paine and colleagues tested this new methodology in 3 young healthy subjects during a covert swallowing fMRI experiment and were able to validate it. All swallowing events were successfully detected during the dynamic MRI acquisition, and fMRI results revealed areas of activation during swallowing that are commonly reported by other swallowing fMRI studies. This technique is rather promising as it will enable us to simultaneously visualize neural and muscular/structural components of swallowing disorders in dysphagic patients and, therefore better understand direct clinical correlations between the two.
Functional MRI and Aging Swallowing
Increased prevalence of swallowing difficulties in healthy aging even in the absence of disease is frequently observed (aka presbyphagia).41-43 Age-related changes in lingual pressure generation,44 increased time to manipulate the food in the oral cavity,45 and slower swallowing responses43, 46 are some of the motor declines seen in swallowing with age. Regarding sensory swallowing components, older healthy adults need larger volumes of material in order to trigger the pharyngeal swallow response,47 demonstrate a delay in the initiation of this response,43 have reduced taste perception,48, 49 and need increased sensory discrimination thresholds in the mouth and the laryngopharynx.50
Despite the fact that physiological aging changes in swallowing have been extensively investigated, changes in the neural mechanisms that may contribute to these declines have received limited attention.37 In fact there are only three fMRI studies to date that have studied the neural control of swallowing and related tasks in elderly individuals. The first one examined the neural activation of swallowing in nine older healthy females over 60 years of age,37 and found activations in the lateral pericentral, perisylvian, and anterior cingulate cortex with postcentral gyrus activation being more lateralized to the left for both dry and water swallows. Interestingly, during water swallowing, a fourfold increase in the brain volume activated was seen when compared to the saliva swallow, especially in the right premotor and prefrontal cortex in this group, possibly suggesting a compensatory mechanism.37
A more recent fMRI study investigating age effects, revealed that, for all swallow types examined (i.e., saliva, water and barium), older adults showed significantly higher BOLD activity than the younger group across a large region of the cortex, including the right pre and postcentral gyri, bilateral frontal lobe, bilateral parietal regions (inferior and superior gyri), and the right superior temporal gyrus.19 However, the younger group also exhibited higher BOLD activity in selected areas, including the left pre and postcentral gyri, left supplementary motor area (SMA), and right superior frontal gyrus. The authors conclude that the additional cortical activations seen in some brain areas in their elderly sample may designate that older adults need increased effort than younger individuals to swallow the same bolus types and amounts, and that young adults may be more efficient in cortical use for the same task than elders.19
Malandraki and colleagues compared the neural activation of four tasks, swallowing, tongue tapping, throat clearing and planning of swallowing in young and older healthy adults using fMRI.21 In this study both groups showed activations in the major motor areas involved in the initiation and execution of movement; however, areas involved in sensory processing, sensorimotor integration and/or motor coordination and control, showed reduced or limited activity in the elderly.
Differences in methodologies may explain the discrepancies between the findings of the aforementioned studies. Future multi-site investigations with larger sample of subjects and commonly designed methodologies are needed to further elucidate aging swallowing neural changes.
fMRI in Dysphagic Populations: Understanding the Pathophysiology of Swallowing
The use of fMRI in identifying neural correlates of swallowing in patient populations has been limited for several reasons. One is the potentially increased difficulty that dysphagic patients may face while swallowing in the supine position (necessary for MR imaging in the magnet). Additionally, accurate and adequate MR imaging requires patients to lie flat on their back for several minutes to an hour at a time, making it challenging for patients with postural restrictions and limitations to participate. Despite these difficulties a few investigators have used fMRI to study a limited number of patient populations.
Li and colleagues studied 5 right hemisphere and 5 left hemisphere stroke patients in the acute stage (3-5 days post stroke) and 10 healthy age matched controls using fMRI. The subjects were required to swallow their saliva when visually cued. Results of the within group analysis revealed greater contralesional activation of swallowing related areas for both stroke groups studied. That is, dysphagic stroke patients who suffered a stroke in the left hemisphere showed overactivation in their right cortical swallowing maps, compared to the infarcted hemisphere. Similarly, dysphagic stroke patients who had a stroke in the right hemisphere showed overactivation in their left cortical swallowing network areas. When all stroke participants were compared to the normal group, however, results revealed higher BOLD activations ipsilesionally for both the right and left hemisphere stroke participants, possibly indicating initiation of neuroplastic compensatory recruitment of neural areas neighboring the lesion even in the acute stroke phase. These results have to be interpreted cautiously given factors such as limited stroke sample size, wide variability in stroke site, volume and dysphagia severity for both stroke groups, and more importantly assumption of a normal neurovascular response for the stroke participants by the investigators.
A similar design was followed by the same research group to study 5 patients with Amyotrophic Lateral Sclerosis (ALS) with dysphagia and 5 ALS patients without dysphagia.51 Neural activation during saliva swallows of these patients was compared to 10 age matched control individuals. Diffusion tensor imaging (DTI) was also employed in this study to investigate white matter tracts orientation and integrity. Results revealed that ALS patients without dysphagia show similar activations of the swallowing neural network with the healthy normal controls, and even increased activation in the primary sensorimotor cortex. ALS patients with dysphagia, however, showed significantly reduced BOLD signal especially in the primary sensory cortex when compared with the normal control group. Similarly to the previous study, apart from sample size, other confounds include variability in ALS type and duration and assumption of normal neurovascular response. These confounds limit the generalizability of the results.
Humbert and colleagues were the first to employ videofluoroscopy and fMRI to study the physiology and neurophysiology of swallowing in 13 patients with mild Alzheimer’s disease (AD) and 11 healthy age and gender matched controls.52 Participants had to complete 10 swallows of each of three bolus types (saliva, barium and water) during their fMRI paradigm. Results showed that controls had greater BOLD responses than the AD subjects during water and saliva swallows. Within the AD group, saliva swallows evoked a greater response than barium and water in motor and premotor cortical regions. Mild differences were found in swallowing physiology as well, with AD participants showing shorter laryngeal vestibule closure duration and reduced hyolaryngeal complex elevation compared to the normal controls. The authors conclude that these findings may suggest preclinical compromise of the neural control of swallowing for the AD patients and if future validation of the results is achieved, fMRI may be beneficial in predicting which AD patients may benefit from preventative treatment during preclinical stages of swallowing dysfunction. This study did include a larger patient sample size than typically reported and made gray matter comparisons between groups to control for gray matter decreases in the AD population.
fMRI and Dysphagia Rehabilitation: Evidence of Neuroplasticity
Similar to fMRI studies in dysphagic patient populations, swallowing treatment studies that have employed fMRI paradigms are limited in numbers as well as valid designs. Despite that, an increasing need for use of treatment modalities that are evidence-based is emerging in recent years in all medical fields, including speech and language pathology. One of the highest levels of evidence that can be provided for swallowing treatments is through the use of neuroimaging methodologies employed in pre - post treatment designs.
Treatments of oropharyngeal dysphagia can be behavioral, medical or surgical. In most cases behavioral treatments are preferred (due to lower cost and risk) and are the ones that are attempted first.53 These are either compensatory or rehabilitative. Compensatory treatments are interventions designed to reduce, avoid or bypass the effects of impaired structures and physiology and redirect the biomechanics of bolus flow, e.g., use of thickened liquids or postural changes during swallowing. Rehabilitative or neurorehabilitative interventions include exercise programs that aim at directly improving the neuromuscular anatomy, physiology, or the neural circuitry, thus providing a direct influence on the biological underpinnings of swallowing.54
Compensatory strategies have been investigated recently with the use of fMRI for healthy young adults. Peck et al. studied the effects of dry swallows, effortful swallows and the Mendelsohn maneuver in the neural activation of 10 healthy young adults.55 As expected, effortful swallows and swallows with the use of Mendelsohn maneuver elicited higher BOLD activity in many swallowing related neural areas compared to dry swallows of these participants. Significant limitations of this study include the lack of controlling for the excess movements associated with these techniques, use of a fixed interstimulus interval during fMRI scanning, limited number of stimuli presentations, as well as lack of a task compliance verification method in the magnet. Without verifying task compliance and onset of swallowing occurrences, timing of signal activity cannot be accurately calculated. Consideration of these confounds in a future study may provide important input on the neurophysiological underpinnings of these swallowing strategies.
Kawai and colleagues investigated the effects of auditory, visual and audiovisual swallowing related biofeedback on the neural activity of 12 young adults.56 These researchers had their participants watch swallowing related laryngeal movements and control images, and listen to swallowing sounds and control sounds in different combinations during a block-design fMRI experiment. Results revealed different swallowing network areas being activated during each of the biofeedback modalities that were specific to swallowing (swallowing movies and sounds), leading the authors to suggest the use of biofeedback techniques to stimulate swallowing related brain areas during treatment of dysphagia. Statistical analysis and design limitations as well as omission of investigating direct effects of these biofeedback techniques on the actual swallowing act are confounds of this study.
In a study by Babaei and colleagues, different tastants (in liquid form) were tested during an fMRI paradigm to explore taste and sensory enhancement effects on the neural activity of 14 healthy right handed adults.57 These researchers presented their taste stimuli (dry swallows, water, lemon, popcorn and chocolate flavored liquids) with simultaneous representative visual and scent stimulation to their subjects. Intensity and extent of activation in the swallowing related neural areas of the left hemisphere were investigated. Results revealed significantly greater BOLD signal and extent of activation for all enhanced stimuli compared to dry swallows. Areas with consistently higher signal included the prefrontal cortex, the cingulate gyrus, and the primary sensory/motor cortices.
No research study to date has used this advanced neuroimaging modality to study effects of rehabilitative treatments of swallowing. Such interventions, however, have the potential of providing unique neuroplastic effects especially in patients with neurogenic dysphagia.
Neuroplasticity is the mechanism by which the damaged brain relearns “lost behavior” in response to rehabilitation. Understanding and systematically manipulating basic principles of exercise and their influence on neuroplasticity58 may permit identification of approaches that drive recovery. While effects of rehabilitative interventions in the form of strengthening the swallowing mechanism are gaining attention at the muscle level, rehabilitation strategies to address the neural underpinnings are less clear.
In an effort to increase our understanding of the potential neuroplastic effects of rehabilitative treatments, we have employed the use of fMRI before and after an 8-week lingual strengthening paradigm in a chronic stroke patient. Results of this preliminary case study follow herein.
Preliminary Study: Case study of a chronic right hemisphere stroke patient with dysphagia
A chronic ischemic stroke male patient (60 yoa, 9 months post right hemisphere stroke in the MCA area) participated in a lingual strengthening protocol, as well as in a baseline and post-treatment fMRI experiment. The patient also participated in a supine videofluoroscopic swallow protocol followed by a functional MRI experiment at two time points, at baseline and at week 8 (post-treatment).
Intervention consisted of an 8-week lingual exercise program including compressing an air-filled bulb between the tongue and hard palate using the IOPI (Iowa Oral Performance Instrument) and has been described in detail by Robbins et al.59 The IOPI measures the tongue pressure using an intra-orally placed air-filled plastic bulb. The subject exercised the tongue blade for 3 sets of 10 repetitions 3 times per day on 3 days a week as recommended for strength training by the American College of Sport Medicine.60 Before beginning the exercise program, a baseline 1-repetition maximum pressure was identified. After intervention started, every two weeks clinicians re-measured the patient’s maximum lingual pressures to determine target values for the following two weeks of the regimen.
The fMRI experiment employed an event-related design and included a total of 33 swallows each in 5 ml amounts (13 barium, 13 water, and 7 saliva) presented in pseudo-random order. All fMRI acquisition parameters were the same as the ones reported in previous published work from our laboratory.19 Functional images were processed using components of the Analysis of Functional NeuroImages (AFNI, Medical College of Wisconsin, USA) software package. First-level analysis was performed for each time point, baseline and post-treatment. Water and barium swallows were combined to obtain higher power in the data. The task-related responses were analyzed using multiple linear regression with a single regressor for each task convolved with a canonical hemodynamic response function and motion parameter estimates were used as regressors of no interest in the analysis.
The T statistic images shown in Figure 2 present preliminary results of week 1 and 8 for the water and barium swallows (combined) at an uncorrected significance threshold of P = 0.05. These preliminary results showed that the stroke subject exhibited some perilesional activity in the primary motor cortex and the premotor cortex of the ipsilesional hemisphere at week 1. However, post-treatment, more areas in both the ipsi and the contralesional hemisphere were active. These include the primary motor cortex, the primary sensory cortex, the premotor area, and the insula.
Figure 2.
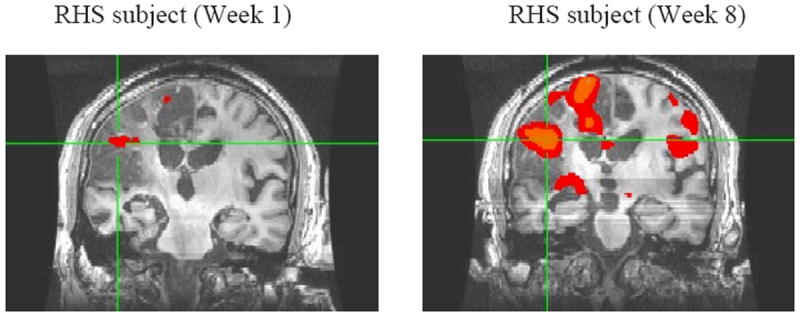
t=1.97 (uncorrected p = 0.05) [x= 69; y= 145; z = 182]
Neural activation during liquid swallows at week 1 (baseline) and at week 8 (post-treatment). Increased amplitude and extent of activation ipsi- and contralesionally are observed at week 8. Images are shown in MNI convention.
These results also should be interpreted with caution. This is only a single stroke subject observation; controlling for lesion site and size as well as atypical neurovascular response has not been attempted at this stage of analysis. Nevertheless, the dramatic increase in BOLD signal post-treatment in this chronic ischaemic patient, who had no other apparent changes in treatment or medication use during this 8-week period, likely indicates treatment-related plasticity. To further support this preliminary conclusion, these neural changes were accompanied by significant increases in lingual isometric pressures (200%), swallowing pressures, improved swallow kinematics and reduced residue reflecting improved bolus clearance, and swallowing safety as reflected in improved Penetration/Aspiration Scale score.61, 62 Future research with larger sample sizes and more in-depth signal analysis is needed to further validate these preliminary results.
Conclusions
Functional MRI is an advanced neuroimaging methodology that constitutes a useful and powerful tool for investigating the neurophysiology of sensorimotor human processes in vivo. The use of fMRI in the study of human swallowing has been popular in the last 10 to 15 years and has provided greater insight on the role of supramedullary areas in swallowing function. Design considerations and patient related restrictions have limited the use of this technique with dysphagic patients and/or as a treatment outcome measurement. Careful research design planning, controlling for differences in neurovascular responses in patient populations, and supplementing fMRI paradigms with other imaging and behavioral modalities, however, can significantly reduce fMRI confounds and increase the utility of this technique in patient and treatment studies. The appropriate and accurate use of fMRI to study the neuroplastic effects of swallowing treatments may enable us not only to better understand the effects of our treatments on our patients’ swallowing function, but also to design even more effective remedies that will target the underlying neural mechanisms of dysphagia in addition to the peripheral (muscular or sensory) processes that contribute to their difficulties.
Acknowledgments
This material is the result of work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service, VA Merit Grant C4796R. This is GRECC Manuscript #2011-09.
Disclaimer: The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Kahrilas PJ, Dodds WJ, Dent J, Logemann JA, Shaker R. Upper esophageal sphincter function during deglutition. Gastroenterology. 1988;95:52–62. doi: 10.1016/0016-5085(88)90290-9. [DOI] [PubMed] [Google Scholar]
- 2.Dubner R, Sessle B, Storey A. The Neural Basis of Oral and Facial Function. Plenum. 1978 [Google Scholar]
- 3.Perlman AL. Neuroanatomy and neurophysiology: implications for swallowing. Topics in Stroke Rehabilitation. 1996;3:1–13. doi: 10.1080/10749357.1996.11754118. [DOI] [PubMed] [Google Scholar]
- 4.Miller AJ. Neurophysiological basis of swallowing. Dysphagia. 1986;1:91–100. [Google Scholar]
- 5.Bieger D. Neuropharmacologic correlates of deglutition: lessons from fictive swallowing. Dysphagia. 1991;6:147–64. doi: 10.1007/BF02493518. [DOI] [PubMed] [Google Scholar]
- 6.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–69. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 7.Robbins JA, Levine RL. Swallowing after unilateral stroke of the cerebral cortex: Preliminary experience. Dysphagia. 1988;3:11–17. doi: 10.1007/BF02406275. [DOI] [PubMed] [Google Scholar]
- 8.Celifarco A, Gerard G, Faegenburg D, Burakoff R. Dysphagia as the sole manifestation of bilateral strokes. Am J Gastroenterol. 1990;85:610–3. [PubMed] [Google Scholar]
- 9.Alberts MJ, Horner J, Gray L, Brazer SR. Aspiration after stroke: lesion analysis by brain MRI. Dysphagia. 1992;7:170–3. doi: 10.1007/BF02493452. [DOI] [PubMed] [Google Scholar]
- 10.Levine R, Robbins JA, Maser A. Periventricular white matter changes and oropharyngeal swallowing in normal individuals. Dysphagia. 1992;7:142–7. doi: 10.1007/BF02493446. [DOI] [PubMed] [Google Scholar]
- 11.Daniels SK, Foundas AL. The role of the insular cortex in dysphagia. Dysphagia. 1997;12:146–56. doi: 10.1007/PL00009529. [DOI] [PubMed] [Google Scholar]
- 12.Mosier KM, Liu WC, Maldjian JA, Shah R, Modi B. Lateralization of cortical function in swallowing: a functional MR imaging study. AJNR American journal of neuroradiology. 1999;20:1520–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Kern M, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R. Swallow-related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol. 2001;280:G531–8. doi: 10.1152/ajpgi.2001.280.4.G531. [DOI] [PubMed] [Google Scholar]
- 14.Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol. 2001;280:G354–60. doi: 10.1152/ajpgi.2001.280.3.G354. [DOI] [PubMed] [Google Scholar]
- 15.Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001;85:938–50. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki M, Asada Y, Ito J, Hayashi K, Inoue H, Kitano H. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia. 2003;18:71–7. doi: 10.1007/s00455-002-0088-x. [DOI] [PubMed] [Google Scholar]
- 17.Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, Menon RS. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J Neurophysiol. 2004;92:2428–43. doi: 10.1152/jn.01144.2003. [DOI] [PubMed] [Google Scholar]
- 18.Toogood JA, Barr AM, Stevens TK, Gati JS, Menon RS, Martin RE. Discrete functional contributions of cerebral cortical foci in voluntary swallowing: a functional magnetic resonance imaging (fMRI) “Go, No-Go” study. Exp Brain Res. 2005;161:81–90. doi: 10.1007/s00221-004-2048-1. [DOI] [PubMed] [Google Scholar]
- 19.Humbert IA, Fitzgerald ME, McLaren DG, Johnson S, Porcaro E, Kosmatka K, Hind J, Robbins J. Neurophysiology of swallowing: effects of age and bolus type. Neuroimage. 2009;44:982–91. doi: 10.1016/j.neuroimage.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malandraki GA, Perlman AL, Sutton BP, Karampinos DC. Age-related changes in laterality of cortical activations in swallowing. Dysphagia. doi: 10.1007/s00455-009-9250-z. In Press. [DOI] [PubMed] [Google Scholar]
- 21.Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: An attempt to separate the components of deglutition. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol. 1999;277:G219–25. doi: 10.1152/ajpgi.1999.277.1.G219. [DOI] [PubMed] [Google Scholar]
- 23.Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675–9. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89:5951–5. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med. 1992;25:390–7. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa S, Menon RS, Kim SG, Ugurbil K. On the characteristics of functional magnetic resonance imaging of the brain. Annu Rev Biophys Biomol Struct. 1998;27:447–74. doi: 10.1146/annurev.biophys.27.1.447. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- 28.Heeger DJ, Ress D. What does fMRI tell us about neuronal activity? Nat Rev Neurosci. 2002;3:142–51. doi: 10.1038/nrn730. [DOI] [PubMed] [Google Scholar]
- 29.Pekar JJ. A brief introduction to functional MRI. IEEE Eng Med Biol Mag. 2006;25:24–6. doi: 10.1109/memb.2006.1607665. [DOI] [PubMed] [Google Scholar]
- 30.Webb AG. Introduction to Biomedical Imaging: IEEE Press Series on Biomedical Engineering. Wiley-IEEE Press; 2003. [Google Scholar]
- 31.Ugurbil K, Adriany G, Andersen P, Chen W, Gruetter R, Hu X, Merkle H, Kim DS, Kim SG, Strupp J, Zhu XH, Ogawa S. Magnetic resonance studies of brain function and neurochemistry. Annu Rev Biomed Eng. 2000;2:633–60. doi: 10.1146/annurev.bioeng.2.1.633. [DOI] [PubMed] [Google Scholar]
- 32.Ugurbil K, Toth L, Kim DS. How accurate is magnetic resonance imaging of brain function? Trends Neurosci. 2003;26:108–14. doi: 10.1016/S0166-2236(02)00039-5. [DOI] [PubMed] [Google Scholar]
- 33.D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–72. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- 34.Martin RE, Sessle BJ. The role of the cerebral cortex in swallowing. Dysphagia. 1993;8:195–202. doi: 10.1007/BF01354538. [DOI] [PubMed] [Google Scholar]
- 35.Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clin Neurophysiol. 2003;114:2226–44. doi: 10.1016/s1388-2457(03)00237-2. [DOI] [PubMed] [Google Scholar]
- 36.Hartnick CJ, Rudolph C, Willging JP, Holland SK. Functional magnetic resonance imaging of the pediatric swallow: imaging the cortex and the brainstem. Laryngoscope. 2001;111:1183–91. doi: 10.1097/00005537-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Martin R, Barr A, MacIntosh B, Smith R, Stevens T, Taves D, Gati J, Menon R, Hachinski V. Cerebral cortical processing of swallowing in older adults. Exp Brain Res. 2007;176:12–22. doi: 10.1007/s00221-006-0592-6. [DOI] [PubMed] [Google Scholar]
- 38.Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Exp Brain Res. 2001;140:280–9. doi: 10.1007/s002210100813. [DOI] [PubMed] [Google Scholar]
- 39.Malandraki GA, Sutton BP, Perlman AL, Karampinos DC. Reduced somatosensory activations in swallowing with age. Hum Brain Mapp. doi: 10.1002/hbm.21062. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paine T, Conway C, Malandraki GA, Sutton BP. Simultaneous Dynamic and Functional MRI Scanning (SimulScan) of Natural Swallows. Magn Reson Med. doi: 10.1002/mrm.22824. In Press. [DOI] [PubMed] [Google Scholar]
- 41.Leslie P, Drinnan MJ, Ford GA, Wilson JA. Swallow respiratory patterns and aging: presbyphagia or dysphagia? J Gerontol A Biol Sci Med Sci. 2005;60:391–5. doi: 10.1093/gerona/60.3.391. [DOI] [PubMed] [Google Scholar]
- 42.Ney DM, Weiss JM, Kind AJ, Robbins J. Senescent swallowing: impact, strategies, and interventions. Nutr Clin Pract. 2009;24:395–413. doi: 10.1177/0884533609332005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103:823–9. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]
- 44.Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. The journals of gerontology Series A, Biological sciences and medical sciences. 1995;50:M257–62. doi: 10.1093/gerona/50a.5.m257. [DOI] [PubMed] [Google Scholar]
- 45.Feldman RS, Kapur KK, Alman JE, Chauncey HH. Aging and mastication: Changes in performance and in the swallowing threshold with natural dentition. Am Geriatr Soc. 1980;28:97–103. doi: 10.1111/j.1532-5415.1980.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 46.Cook IJ, Weltman MD, Wallace K, Shaw DW, McKay E, Smart RC, Butler SP. Influence of aging on oral-pharyngeal bolus transit and clearance during swallowing: scintigraphic study. Am J Physiol. 1994;266:G972–7. doi: 10.1152/ajpgi.1994.266.6.G972. [DOI] [PubMed] [Google Scholar]
- 47.Shaker R, Ren J, Zamir Z, Sarna A, Liu J, Sui Z. Effect of aging, position, and temperature on the threshold volume triggering pharyngeal swallows. Gastroenterology. 1994;107:396–402. doi: 10.1016/0016-5085(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 48.Calhoun KH, Gibson B, Hartley L, Minton J, Hokanson JA. Age-related changes in oral sensation. Laryngoscope. 1992;102:109–16. doi: 10.1288/00005537-199202000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Fukunaga A, Uematsu H, Sugimoto K. Influences of aging on taste perception and oral somatic sensation. J Gerontol A Biol Sci Med Sci. 2005;60:109–13. doi: 10.1093/gerona/60.1.109. [DOI] [PubMed] [Google Scholar]
- 50.Aviv JE. Effects of aging on sensitivity of the pharyngeal and supraglottic areas. Am J Med. 1997;103:74S–76S. doi: 10.1016/s0002-9343(97)00327-6. [DOI] [PubMed] [Google Scholar]
- 51.Li S, Luo C, Yu B, Yan B, Gong Q, He C, He L, Huang X, Yao D, Lui S, Tang H, Chen Q, Zeng Y, Zhou D. Functional magnetic resonance imaging study on dysphagia after unilateral hemispheric stroke: a preliminary study. J Neurol Neurosurg Psychiatry. 2009;80:1320–9. doi: 10.1136/jnnp.2009.176214. [DOI] [PubMed] [Google Scholar]
- 52.Humbert IA, McLaren DG, Kosmatka K, Fitzgerald ME, Johnson S, Porcaro E, Kays S, Robbins J. Early deficits in cortical control of swallowing in Alzheimer’s disease. Journal of Alzheimer’s Disease. 2010;19:1185–97. doi: 10.3233/JAD-2010-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Logemann JA. Swallowing disorders. Best Pract Res Clin Gastroenterol. 2007;21:563–73. doi: 10.1016/j.bpg.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Robbins J, Butler SG, Daniels SK, Diez Gross R, Langmore S, Lazarus CL, Martin-Harris B, McCabe D, Musson N, Rosenbek J. Swallowing and dysphagia rehabilitation: translating principles of neural plasticity into clinically oriented evidence. J Speech Lang Hear Res. 2008;51:S276–300. doi: 10.1044/1092-4388(2008/021). [DOI] [PubMed] [Google Scholar]
- 55.Peck KK, Branski RC, Lazarus C, Cody V, Kraus D, Haupage S, Ganz C, Holodny AI, Kraus DH. Cortical activation during swallowing rehabilitation maneuvers: a functional MRI study of healthy controls. Laryngoscope. 2010;120:2153–9. doi: 10.1002/lary.21125. [DOI] [PubMed] [Google Scholar]
- 56.Kawai T, Watanabe Y, Tonogi M, Yamane GY, Abe S, Yamada Y, Callan A. Visual and auditory stimuli associated with swallowing: an FMRI study. Bull Tokyo Dent Coll. 2009;50:169–81. doi: 10.2209/tdcpublication.50.169. [DOI] [PubMed] [Google Scholar]
- 57.Babaei A, Kern M, Antonik S, Mepani R, Ward BD, Li SJ, Hyde J, Shaker R. Enhancing effects of flavored nutritive stimuli on cortical swallowing network activity. Am J Physiol Gastrointest Liver Physiol. 2010;299:G422–9. doi: 10.1152/ajpgi.00161.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225–39. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 59.Robbins J, Gangnon R, Theis S, Kays SA, Hind J. The effects of lingual exercise on swallowing in older adults. Journal of the American Geriatrics Society. 2005;53:1483–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- 60.American College of Sports M. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness in healthy adults. Medicine and Science in Sports and Exercise. 1990;22:265–274. [PubMed] [Google Scholar]
- 61.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–8. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 62.Robbins J, Coyle J, Roecker E, Rosenbek J, Wood J. Differentiation of normal and abnormal airway protection during swallowing using the penetration-aspiration scale. Dysphagia. 1999;14:228–232. doi: 10.1007/PL00009610. [DOI] [PubMed] [Google Scholar]


