Abstract
Objective
The evidence for the efficacy of D-cycloserine (DCS) for augmenting cognitive behavioral therapy (CBT) for anxiety disorders has been mixed. Guided by preclinical research and initial findings from a small-scale study involving humans, we tested the hypothesis that DCS enhancement of exposure therapy would be specific to successful exposure sessions.
Method
Medication-free adults with generalized social anxiety disorder (N = 145) received 50 mg of DCS or placebo 1 hour before each of 5 exposure sessions that were part of a standardized 12-session group CBT protocol. Participants provided fear ratings at the beginning and just before the end of exposure exercises. Independent raters, blind to group assignment, administered the clinical global impression improvement and severity scales at each session and at posttreatment.
Results
Mixed-effects analyses revealed that, among patients who reported low fear at the end of an exposure session, those who had received DCS evidenced significantly greater clinical improvement at the next session, relative to those who had received placebo. In contrast, when exposure end fear was high, patients receiving DCS exhibited less clinical improvement at the following session than patients receiving placebo. Similarly, patients who had received DCS evidenced lower clinical severity at posttreatment, relative to patients who had received placebo, only when their average end fear for medication-augmented sessions had been in the low to moderate range. Finally, these moderating effects of exposure success as indexed by end fear were not better accounted for by within-session extinction.
Conclusions
The efficacy of DCS for augmenting exposure-based CBT depends on the success of exposure sessions. These findings may help guide the development of an algorithm for the effective use of DCS for augmenting exposure-based CBT.
Trial Registry
http://www.ClinicalTrials.gov, ID# NCT00633984, http://www.clinicaltrials.gov/ct2/show/NCT00633984
Keywords: CBT, Cognitive Behavioral Therapy, Exposure therapy, Fear extinction, D-cycloserine, Moderators, Social Anxiety Disorder, Social Phobia
Introduction
One particular success of translational research is the investigation of D-cycloserine (DCS), a partial agonist of the glycine recognition site of the N-Methyl-D-Aspartate receptor (NMDAr), as an augmentation strategy for exposure-based cognitive behavioral therapy for the anxiety disorders (Davis et al., 2006; Hofmann, Smits, Asnaani, Gutner, and Otto, 2011). Following a series of studies indicating that extinction learning is NMDAr dependent (see Davis et al., 2006), Davis and colleagues first demonstrated that DCS can enhance retention of fear extinction in rats and subsequently showed that DCS enhances the outcome of extinction-based therapy (i.e., virtual reality exposure therapy) for height phobia (Davis et al., 2006). These initial findings created great excitement among anxiety disorder treatment researchers who have been faced with the challenge to improve the outcomes of exposure-based CBT for anxiety disorders such as social anxiety disorder (Hofmann and Smits, 2008), which are prevalent and associated with significant personal and economic costs (Greenberg et al., 1999; Kessler et al., 2005). Not surprisingly, the last several years have seen a number of studies evaluating the efficacy of DCS for enhancing outcomes for exposure-based CBT (Hofmann et al., 2011).
The efficacy of D-cycloserine (DCS) for enhancing exposure therapy has been variable across these studies, with several evidencing strong augmentative effects of DCS (Guastella et al., 2008; Hofmann et al., 2006; Otto et al., 2010; Ressler et al., 2004), and several showing either relatively weak effects (Kushner et al., 2007; Wilhelm et al., 2008), no effects (Guastella, Dadds, Lovibond, Mitchell, and Richardson, 2007; Storch et al., 2007; Tart et al., 2013), or even detrimental effects (Litz et al., 2012). Animal research has pointed to the adequacy of extinction training, as indexed by sufficient decrement in fear responding during the training session, as a potential moderator of the augmentation effects of DCS. Indeed, in re-analyses of null findings, Weber and colleagues (Weber, Hart, and Richardson, 2007) and Bouton and colleagues (Bouton, Vurbic, and Woods, 2008) demonstrated that the efficacy of DCS for facilitating extinction retention was evident only in animals that had demonstrated a large decrement in fear responding during extinction training.
Analogous to these animal studies, we recently reanalyzed a null finding for DCS augmentation from a small-scale trial involving patients (N=29) undergoing exposure therapy for height phobia. The original analyses revealed that patients receiving 50 mg of DCS administered following each of two sessions of 30 minutes of hierarchical virtual reality exposure did not evidence better clinical outcomes than patients receiving identical exposure combined with placebo (Tart et al., 2013). In our reanalysis of these findings (Smits et al., 2013), we tested whether the effects of DCS administration on subsequent clinical improvement would be moderated by the relative success of the exposure session. Because the exposure session was delivered in a hierarchical fashion (i.e., gradually moving up a simulated glass elevator), we indexed exposure success (or decrement in fear responding) using the fear level that patients reported at the end of the session, while controlling for baseline severity, the number of floors completed in the exposure hierarchy, and the level of fear reported at the beginning of the exposure session. Consistent with the findings from animal studies (Bouton et al., 2008; Weber et al., 2007), the result of this reanalysis showed that DCS facilitated clinical improvement when patients ended their previous exposure session with low fear levels, and, conversely, inhibited clinical improvement when patients ended their previous exposure session with elevated fear levels (Smits et al., 2013). Assuming clinicians accurately targeted patients’ fears with challenging exposure assignments, low end fear provides a measure of extinction success, consistent with preclinical studies (Lee et al., 2006). Replication and extension of this potential marker for the successful use of DCS has important implications for the clinical application of DCS augmentation strategies.
The present paper represents the first reanalysis of a large-scale trial of DCS augmentation. Specifically, in the largest clinical trial of DCS augmentation published to date, Hofmann et al. (Hofmann et al., 2013) found that DCS augmentation of exposure-based CBT for social anxiety disorder resulted in faster, but not greater, treatment response than placebo augmentation. Based on the extant research, we hypothesized that the relative advantage conferred by DCS administration on clinical improvement would be moderated by the success of the exposure session, such that advantage of DCS over placebo with respect to clinical improvement would be greater following sessions characterized by low end fear levels than following sessions characterized by elevated end fear levels. Building further upon our previous study (Smits et al., 2013), we also explored in this paper the possibility that within-session extinction (i.e., peak fear minus end fear), an alternative operationalization of exposure success (Smits et al., 2013), is a more critical dimension for moderating the efficacy of DCS than end fear.
Materials and Method
Participants
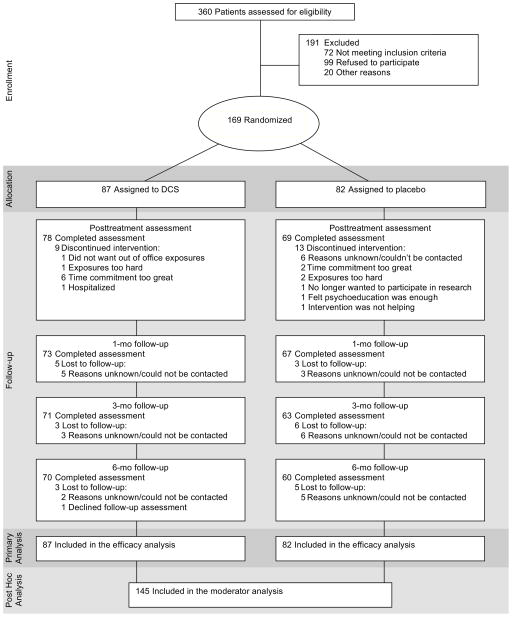
Participants in the trial were 169 adults with a diagnosis of generalized SAD utilizing the Structured Clinical Interview for DSM-IV Diagnosis (First, Spitzer, Gibbon, and Williams, 2001) and a score of 60 or higher on the Liebowitz Social Anxiety Scale (LSAS; Liebowitz, 1987). Exclusion criteria included (a) medical disorders of clinical significance; (b) lifetime history of obsessive-compulsive disorder, bipolar disorder, schizophrenia, psychosis, or delusional disorders; (c) diagnosis within the past 6 months of post-traumatic stress disorder, eating disorders, or substance abuse or dependence; (d) organic brain syndrome, mental retardation, or other cognitive dysfunction; (e) current suicidality and/or clinically significant suicidal behavior or ideation within the past 6 months; (f) concurrent psychotropic medication, concurrent psychotherapy, or prior non-response to adequately delivered exposure therapy; and (g) women who were pregnant, breastfeeding, or planning to become pregnant. This study reports on the 145 patients who provided in-session fear ratings (see Figure 1).
Figure 1.
CONSORT diagram
Study Procedures
As reported previously (Hofmann et al., in press), participants were screened for eligibility and then enrolled in the study at one of three sites: Boston Massachusetts General Hospital (MGH), Boston University (BU), or Southern Methodist University (SMU). The Institutional Review Boards at MGH, BU, and SMU approved the study protocol and all participants provided written informed consent. Participants then began a 12-session CBT protocol, during which they were randomized at session 3 to receive either DCS or pill placebo an hour prior to sessions 3–7. Randomization was performed in a double-blind fashion using a computer-generated allocation schedule which stratified participants by baseline LSAS severity ratings (≤70 or ≥70; Liebowitz et al., 1992). Self-reported ratings of beginning fear, end fear, and peak fear were obtained for each exposure session. Clinical status and improvement were assessed by an independent rater, blind to group assignment, prior to treatment sessions and pill administration (at baseline, weeks 2–8, 10, and 12), at posttreatment (week 13), and follow-up (at 1, 3, and 6 months posttreatment). This manuscript reports on data collected at baseline, during the treatment phase and at posttreatment.
Cognitive Behavioral Therapy
The intervention approach was based on a 12-week CBT treatment protocol involving weekly 2.5-hour sessions (Heimberg and Becker, 2002), but drew heavily from the strategies outlined by Hofmann and Otto (Hofmann and Otto, 2008) based on the model described by Hofmann (2007). The first two sessions were psychoeducational, describing the nature of SAD, providing the treatment rationale, and introducing the concept of cognitive restructuring. Participants completed exposure exercises in sessions 3–7, consisting of prolonged public speaking, with the goal of fear extinction. Given that public speaking ranks high in the hierarchy of feared situations in patients with generalized SAD, we opted to focus mostly on public speaking during the exposure sessions 3–7. This is similar to previous studies from our group (e.g., Hofmann et al., 2004, 2006), which have shown efficacy of such a protocol for reducing social anxiety disorder symptom severity and functional impairment. An additional rationale for focusing on public speaking exercises during the augmented portion of the protocol was to reduce variability in procedures across participants, thereby enhancing internal validity. Sessions 8–12 involved continued exposures combined with cognitive restructuring strategies. Treatment was provided in a group format of 4–6 participants and led by two therapists trained and supervised by SGH and JAJS.
Medication
Abrams Royal Pharmacy in Dallas, TX and Massachusetts General Hospital Pharmacy in Boston, MA compounded study capsules. DCS capsules contained 50 mg DCS (derived from Seromycin 250 mg capsules) and polyethylene glycol 3350 powder, whereas placebo capsules contained polyethylene glycol 3350 powder. DCS and placebo capsules were identical in appearance to maintain the blind.
Measures
Fear
Participants provided fear ratings at the beginning of an exposure exercise (i.e., Beginning Fear) and just prior to the conclusion of an exposure exercise (i.e., End Fear). In addition, they indicated their highest level of fear experienced during exposure after the exercise (i.e., Peak Fear). Fear ratings were assessed using the subjective units of distress scale (SUDs; Wolpe, 1958), which ranges from 0 to 100 (0=no fear, relaxed; 25=mild fear, able to cope; 50=moderate fear, trouble concentrating; 75=severe fear, thoughts of leaving; 100=very severe fear, worst ever experienced). The procedures for collecting fear ratings were similar to that in previous social anxiety disorder treatment studies from our group and other groups (e.g., Smits, Powers, et al., 2006; Smits, Rosenfield, et al., 2006; Hayes et al., 2008). Specifically, during the second session, therapists introduced patients to the SUDs scale as they worked together to develop a fear and avoidance hierarchy. Attention was given to the anchors such that patients could distinguish the different levels along the scale. By the time patients initiated exposure practice (i.e., session 3), they had had ample practice using the scale. Similar to the Smits et al. (2013) re-analysis, we used fear rating at the end of exposure as an index of exposure success.
Clinical Global Impressions Severity and Improvement scales (CGI-S and CGI-I)
Initially developed for the clinical trials evaluating the efficacy of psychotropic drugs (Guy, 1970), the CGI-S and CGI-I are widely used measures of global psychopathology severity and improvement, respectively. Our rationale for selecting the CGI-I for the present analysis was threefold: (1) The CGI-I is an index of clinical improvement rather than clinical status (unlike other measures), and thus is a natural selection for testing the study hypothesis “that the relative advantage conferred by DCS administration on clinical improvement would be moderated by the success of extinction learning during the session, such that advantage of DCS over placebo would be greater following sessions characterized by low end fear than following sessions characterized by high end fear;” (2) we attempted to replicate and extend our previous study (Smits et al., 2013), which also employed the CGI-I as the DV; and (3) the CGI-I is an established measure for assessing improvement (and response) in clinical trials of social anxiety disorder and other anxiety disorders. Therefore, we believe that selecting the CGI-I as opposed to a study-specific measure increases the relevance of the current study and its findings.
Independent evaluators, blind to condition, used the CGI-S to evaluate the participant’s severity on a scale of 1 to 7 (1=normal, not at all ill; 2=borderline mentally ill; 3=mildly ill; 4=moderately ill; 5=markedly ill; 6=severely ill; 7=extremely ill) and the CGI-I to rate the level of improvement using a 7-point scale (1=very much improved; 2=much improved; 3=minimally improved; 4=no change; 5=minimally worse; 6=much worse; 7=very much worse). Of interest for this study was the CGI improvement (CGI-I) rating at the next session following an index episode of exposure (extinction training) and the post-treatment (Week 13) CGI severity (CGI-S) ratings.
For this study, we developed specific guidelines for completing the CGI scales based on experiences in previous trials of social anxiety disorder and other related disorders. The CGI scales used in this study had specific, carefully defined, anchors and clinical assessors were instructed and trained to use scale scores on other measures of clinical severity to complete their CGI assessments. Furthermore, the seventh author (NMS), who led the quality assurance/quality control effort for this trial, periodically reviewed assessment recordings and met with clinical assessors to address potential drift.
Data Analysis
Data were analyzed using multilevel models (MLM), which is the recommended analytic approach for psychiatric data (Hamer and Simpson, 2009). To determine if End Fear moderated the effect of condition (DCS vs. Placebo) on CGI-I at the next session, our model included the following predictors: End Fear (as a level 1 time-varying predictor of CGI-I at the next session), Condition, and End Fear x Condition. Because End Fear was an observed variable, we included several variables to control for potential third variables (Cook and Steiner, 2010). Based on previous work (Smits et al., Under Review), we included sex, age, race (African American vs. not), cohabiting vs. not, initial severity on CGI-S, CGI-I rating at the previous session, Beginning Fear, and Beginning Fear x Condition. This analysis included only the sessions in which DCS (or Placebo) was administered (sessions 3–7).
To determine whether lower End Fear across the five augmented sessions interacted with Condition to predict clinical status at posttreatment, we modeled CGI-S scores at posttreatment (week 13) as well as slopes of improvement on the CGI-S over entire course of treatment (sessions 3–13) as a function of participants’ average End Fear ratings over the five augmented sessions (3–7). This MLM model included linear time (centered at week 13), Condition, average End Fear rating, and their interactions as predictors of CGI-S at each assessment week. We also included the control variables used in the above analysis, except in this case, Beginning Fear was averaged over the 5 augmented sessions (just like End Fear) and we did not use CGI-I ratings as a control (since the outcome in this analysis was CSI-S, not CGI-I).
For both analyses, we used maximum likelihood estimation, an unstructured covariance matrix for the covariances of the errors of the repeated measures, and the Satterthwaite technique for calculating degrees of freedom of the regression coefficient t-test.
Results
The sample of 145 participants was 41.8% female and 9.2% African American. Their mean age was 32.5 (SD=10.5) and 77.9% were cohabitating with a partner. End Fear ratings ranged from 0 to 100 (M=49.5; SD=19.2), and Beginning Fear ranged from 10 to 100 (M=66.7; SD=17.8). Average End Fear across the 5 augmented sessions ranged from 5 to 83.8 (M=49.2; SD=15.5) and Average Beginning Fear across the 5 augmented sessions ranged from 30 to 100 (M=66.7; SD = 14.5).
End Fear Moderating the Effect of DCS on Clinical Improvement at the Next Session
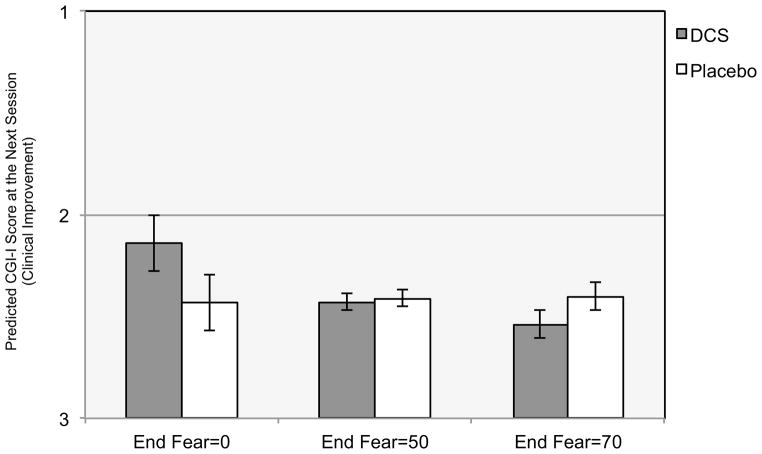
The analysis revealed a significant End Fear x Condition interaction predicting CGI-I at the next session (b=.006, t(163)=2.30; p=.023). To elucidate the form of the interaction, we utilized the approach developed by Aiken and West (1991), which involves calculating model-based predicted differences between DCS and Placebo for participants who have different levels of End Fear, using a clinically-relevant range observed in the sample. These predicted differences are based on the full MLM model including all participants. As can be seen in Figure 2, for those with End Fear=0, participants receiving DCS were rated .29 points more improved on the CGI-I at the next session relative to those receiving Placebo (b=.29; p=.039). Conversely, for those with End Fear=70, participants receiving DCS were rated .14 points less improved on the CGI-I than those given Placebo (b=−.14; p=.041). There were no between-group differences for those with End Fear=50 (b=−.02; p=.678). Finally, the MLM analysis showed that End Fear was significantly related to CGI-I at the next session for those given DCS (b=.001; p=.002), but it was not related to next session CGI-I for those given Placebo, (b=.000; p=.827).
Figure 2. End Fear Moderating the Effect of D-cycloserine Augmentation of Cognitive Behavioral Therapy for Social Anxiety Disorder on Clinical Improvement at the Next Session.
Note. CGI-I is the Clinical Global Impressions Improvement scale that uses a 7-point scale, with lower scores indicating greater improvement (1=very much improved; 2=much improved; 3=minimally improved; 4=no change; 5=minimally worse; 6=much worse; 7=very much worse). End Fear is the fear rating provided just prior to concluding the exposure exercises. Fear ratings were assessed using the subjective units of distress scale (SUDs; Wolpe, 1958), which ranges from 0 to 100 (0=no fear, relaxed; 25=mild fear, able to cope; 50=moderate fear, trouble concentrating; 75=severe fear, thoughts of leaving; 100=very severe fear, worst ever experienced), and thus low ratings indicate exposure success and higher ratings indicate less exposure success.
Average End Fear Moderating the Effect of DCS on Clinical Severity at Posttreatment
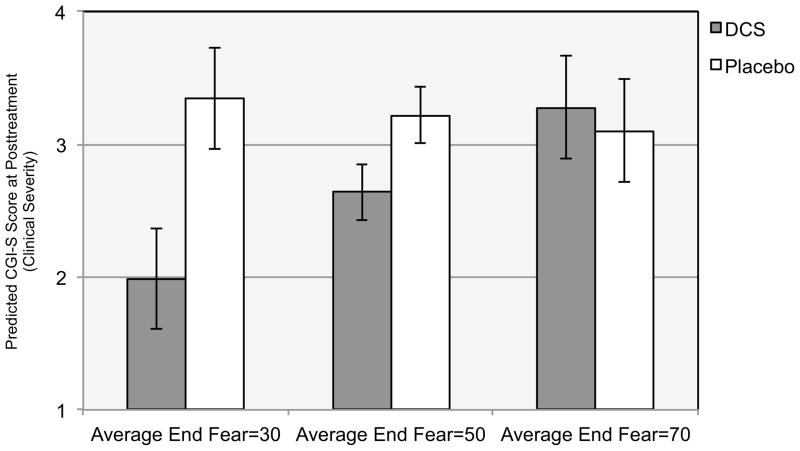
The average level of End Fear during augmented sessions (3–7) moderated the effect of DCS condition on CGI-S at posttreatment (intercept; b=−.04, t (134)=2.41; p=.017). Similar to the previous analyses, we calculated model-based predicted differences between DCS and Placebo for participants with different average levels of End Fear, again using a clinically-relevant range observed in the sample. As shown in Figure 3, among participants with an average End Fear=30, those receiving DCS had significantly lower posttreatment CGI-S scores (by 1.35 units on the 1–7 scale) than those receiving Placebo (b=1.35; p=.001). Among participants with average End Fear=50, those receiving DCS also had lower posttreatment CGI-S scores than those receiving Placebo (b=.58; p=.007), but the difference was much smaller (.58 units). Finally, among participants with average level of End Fear=70, there were no significant between-group differences on posttreatment CGI-S scores (b=−.18, p=.635).
Figure 3. Average End Fear Moderating the Effect of D-cycloserine Augmentation of Cognitive Behavioral Therapy for Social Anxiety Disorder on Clinical Severity at Posttreatment.
Note. CGI-S is the Clinical Global Impressions Severity scale that uses a 7-point scale, with higher scores indicating greater severity (1=normal, not at all ill; 2=borderline mentally ill; 3=mildly ill; 4=moderately ill; 5=markedly ill; 6=severely ill; 7=extremely ill). Average End Fear is the mean fear rating provided just prior to concluding the exposure exercises during medication-augmented sessions (sessions 3–7). Fear ratings were assessed using the subjective units of distress scale (SUDs; Wolpe, 1958), which ranges from 0 to 100 (0=no fear, relaxed; 25=mild fear, able to cope; 50=moderate fear, trouble concentrating; 75=severe fear, thoughts of leaving; 100=very severe fear, worst ever experienced), and thus low ratings indicate exposure success and higher ratings indicate less exposure success.
Similar results were obtained for the relation between average level of End Fear and the slope of improvement in CGI-S scores over the course of the acute treatment (3–13). Specifically, average level of End Fear moderated the relation between Condition and slope over time (b=−.003, t(133)=2.18; p=.031). Among participants with an average End Fear=30, the slope of CGI-S over time varied by Condition (b=.14; p<.001), and the slope of improvement for those receiving DCS was steeper than for those receiving Placebo (b=−.28; p<.001 vs. b=−.14; p<.001, respectively). Among participants with an average End Fear=70, the slopes of improvement did not differ between DCS and Placebo (b=.00; p=.922). Finally, these analyses also revealed that average level of End Fear was only significantly related to slope of improvement in CGI-S over time among participants receiving DCS (b=.003; p =.009), and not for those receiving Placebo (b=−.001; p=.590).
Exploratory Analyses
It is possible that the moderating effects of End Fear reflect the effects of within-session extinction (i.e., Peak Fear minus End Fear). However, when within-session extinction and its interactions with other variables were added to our two MLM models predicting CGI-I at the next session and CGI-S over time, none of the terms involving within-session extinction were significantly related to improvement (p’s>.257). The relative importance of within-session extinction can also be examined by simultaneously including both individual components of within-session extinction (i.e., Peak Fear and End Fear) in the MLM models. Indeed, if within-session extinction is important for predicting outcome, Peak Fear should be positively related to outcome when controlling for End Fear. However, neither Peak Fear nor its interactions with the other variables in the models were significantly related to outcome (p’s>.246). Importantly, End Fear and its interactions with Condition and Time remained significantly related to outcome in both MLM analyses (p’s<.046), further supporting the notion that End Fear is related to outcome regardless of level of Peak Fear. Together, these results provide no support for the notion that End Fear is related to outcome merely because it is related to within-session extinction or that within-session extinction is a stronger moderator of DCS efficacy than End Fear.
Discussion
The present study reported on a re-analysis of the largest clinical trial of DCS enhancement of exposure therapy to date (Hofmann et al., in press). Guided by preclinical research (Bouton et al., 2008; Lee et al., 2006; Weber et al., 2007) and initial findings from a small-scale study involving humans (Smits et al., 2013), we tested the hypothesis that DCS enhancement of fear extinction would be specific to successful exposure sessions. Consistent with predictions, we found that patients who received DCS exhibited significantly greater clinical improvement than patients receiving placebo when their session ended with low fear. Conversely, when end fear was higher, patients receiving DCS showed significantly less subsequent clinical improvement than those receiving placebo. We extended the results from our previous study (Smits et al., 2013) by examining whether the per-session effects (i.e., relation between the interaction of condition and end fear levels in one session and clinical improvement measured at the following session) would accumulate to predict outcomes at posttreatment. Consistent with predictions, we found that DCS enhancement was only evident among patients who, on average, exhibited lower end fear during the augmented sessions (i.e., during sessions 3 to 7 of a 12-session protocol). Of note, the difference between DCS and placebo for such patients was 1.35 points on the CGI-S, which reflects a very large effect. In contrast to the findings of the per-session effect analysis, we did not observe a detrimental effect of DCS on outcomes at posttreatment for patients who, on average, exhibited higher end fear during the augmented sessions. Collectively, these findings suggest that augmenting five sessions with DCS when these sessions are relatively successful significantly improves the outcome of a 12-session cognitive-behavioral treatment protocol for social anxiety disorder. When these augmented sessions are relatively less successful, DCS appears to interfere with subsequent improvement measured at the next session. However, this detrimental effect, observed during weeks 3 to 7 of this protocol, was fully attenuated during the subsequent weeks (8–12) that involved sessions without augmentation, resulting in no net detrimental effect at posttreatment.
We also found that the moderating effect of exposure success was specific to the way in which this success was measured. It is noteworthy that we fully replicated the predictive significance of end-of-exposure fear levels on the DCS augmentation effects in our previous study (Smits et al., 2013) but that an alternative measure of exposure success, the change in fear across the exposure (i.e., peak fear minus end fear), was not a useful predictor over and above end fear. It is not unusual for there to be disagreement between the outcome prediction offered by end-of-exposure vs. change-in-fear ratings of exposure success (Berry, Rosenfield, and Smits, 2009; Craske et al., 2008; Meuret, Seidel, Rosenfield, Hofmann, and Rosenfield, 2012). Indeed, consistent with the broader literature on a dissociation between fear expression and fear learning in exposure (Craske et al., 2008), neither of these measures were useful predictors in patients receiving augmentation with placebo. Yet, under conditions of DCS augmentation, end-of-exposure fear ratings do appear to be important predictors of subsequent clinical outcome. Given that DCS appears to aid the consolidation of fear extinction (i.e., extinction retention) rather than fear extinction learning (i.e., within-session extinction; Davis, Ressler, Rothbaum, and Richardson, 2006), this finding may indicate that it is the final memory trace of the exposure experience that is a central feature of this consolidation. If this is true, judicious use of DCS appears warranted. As we have argued previously (Smits et al., 2013; Tart et al., 2013), one option for the effective application of DCS may be post-session instead of pre-session dosing, as this timing of administration would provide the clinician the opportunity to limit DCS use to sessions that are deemed successful (i.e., low end fear). Indeed, initial evidence for this strategy comes from the Smits et al. (2013) study that showed that post-session augmentation with DCS is (only) effective when it follows sessions that conclude with low levels of fear. Further research into what constitutes a meaningful end fear level could guide clinician timing on when to conclude or prolong an exposure session based on end fear ratings.
Whereas findings from the current study have the potential to guide development of an algorithm for enhancing the efficacy of DCS augmentation, they offer minimal insight regarding the mechanism underlying the relation between session end fear and the efficacy of DCS for enhancing exposure therapy outcomes. Among other therapeutic change processes that are potentially important to exposure therapy outcome, increased implementation of emotion regulation strategies such as cognitive reappraisal may be important to consider in future studies that aim to enhance the understanding of why DCS may only exert positive effects when sessions are characterized by low end fear (Goldin et al., 2012; Smits et al., 2012). This line of work would further benefit from employing a multi-method approach to the assessment of these and other relevant therapeutic change processes as well as the inclusion of the assessment of potential biomarkers of extinction (or exposure success) and the efficacy of DCS, respectively.
In summary, the finding that the success of exposure sessions moderates the augmentation effects of DCS offers a possible explanation for the mixed findings of studies examining DCS enhancement of exposure therapy for the anxiety disorders (Hofmann et al., 2011). The degree of benefit from DCS augmentation appears to be moderated by how well exposure leads to low fear levels at the end of the session; as such, differential levels of DCS success should emerge from protocols that differentially achieve end-of-exposure comfort with the phobic stimulus being presented. Furthermore, whereas end-of-exposure fear has little predictive significance under standard exposure conditions (Craske et al., 2008), it may be crucially important when DCS is influencing how the memory is being consolidated. Attention to this metric may lead to more consistent benefit from the use of DCS in conjunction with exposure-based CBT.
Acknowledgments
Role of Funding Source
This study was funded by NIH grants R01MH078308 and R01MH075889 from the National Institute of Mental Health. The sponsor (NIH) had no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript. Drs. Smits, Pollack, and Hofmann had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflict of Interest
Dr. Smits receives royalties from various book publishers. Dr. Rosenfield, Ms. Davis. Dr. Marques and Dr. Simon report no financial relationships with commercial interests. Dr. Otto has served, in the last three years, as consultant for Micro Transponder, Inc. and receives royalties from various book publishers. Dr. Meuret reported serving as a consultant for Palo Alto Health Sciences Inc. Dr. Pollack’s disclosures over the last three years include: Advisory Boards and Consultation: Eli Lilly, Medavante, Otsuka, Targia Pharmaceuticals Research Grants: Bristol Myers Squibb, Euthymics, Forest Laboratories, GlaxoSmithKline, NCCAM, NIDA, NIMH Equity: Medavante, Mensante Corporation, Mindsite, Targia Pharmaceutical Royalty/patent: SIGH-A, SAFER interviews. Dr. Hofmann has served as a consultant for Merck and Schering-Plough and receives book royalties from various book publishers.
Contributors
JAJS, DR, MWO, LM, SG designed the study, conducted the literature searches, participated in the data analyses, and wrote a first draft of the manuscript. MLD, AEM, NMS, and MHP assisted with the design and implementation of the study, interpretation of the data and the writing of the manuscript. All authors contributed to and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage Publications; 1991. [Google Scholar]
- Berry AC, Rosenfield D, Smits JA. Extinction retention predicts improvement in social anxiety symptoms following exposure therapy. Depression and Anxiety. 2009;26:22–7. doi: 10.1002/da.20511. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Vurbic D, Woods AM. D-cycloserine facilitates context-specific fear extinction learning. Neurobiology of Learning and Memory. 2008;90:504–10. doi: 10.1016/j.nlm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook TD, Steiner PM. Case matching and the reduction of selection bias in quasi-experiments: The relative importance of pretest measures of outcome, of unreliable measurement, and of mode of data analysis. Psychological Methods. 2010;15:56–68. doi: 10.1037/a0018536. [DOI] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behavour Research and Therapy. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biological Psychiatry. 2006;60:369–75. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis-I Disorders, Clinician Version. Arlington, VA: American Psychiatric Publishing, Inc; 2001. [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Werner K, Kraemer H, Heimberg RG, Gross JJ. Cognitive reappraisal self-efficacy mediates the effects of individual cognitive-behavioral therapy for social anxiety disorder. Journal of Consulting and Clinical Psychology. 2012;80(6):1034–40. doi: 10.1037/a0028555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JR, Ballenger JC, Fyer AJ. The economic burden of anxiety disorders in the 1990s. Jounal of Clinical Psychiatry. 1999;60:427–35. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson RA. A randomized controlled trial of the effect of D-cycloserine on exposure therapy for spider fear. Journal of Psychiatric Research. 2007;41:466–71. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds MR. A randomized controlled trial of the effect of D-cycloserine on enhancement of exposure therapy for social anxiety disorder. Biological Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Guy W. Manual for the ECDEU Assessment Battery. 1970. Clinical global impression. [Google Scholar]
- Hamer RM, Simpson PM. Last observation carried forward versus mixed models in the analysis of psychiatric clinical trials. American Journal of Psychiatry. 2009;166:639–41. doi: 10.1176/appi.ajp.2009.09040458. [DOI] [PubMed] [Google Scholar]
- Heimberg RG, Becker RE. Cognitive-behavioral group therapy for social phobia: Basic mechanisms and clinical strategies. New York, NY: Guilford Press; 2002. [Google Scholar]
- Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Archives of General Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Otto MW. Cognitive behavioral therapy for social anxiety disorder: Evidence-based and disorder-specific treatment techniques. London: Routledge; 2008. [Google Scholar]
- Hofmann SG, Smits JA, Asnaani A, Gutner CA, Otto MW. Cognitive enhancers for anxiety disorders. Pharmacology Biochemestry and Behavior. 2011;99:275–84. doi: 10.1016/j.pbb.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Smits JA, Rosenfield D, Simon NM, Otto MW, Meuret AE, Marques L, Fang A, Tart CD, Pollack MH. D-cycloserine as augmentation strategy of cognitive behavioral therapy for social anxiety disorder. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2013.12070974. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, McCabe J, Peterson J, Foa EB. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biological Psychiatry. 2007;62:835–8. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. Journal of Neuroscience. 2006;26:10051–6. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz MR. Social phobia. Modern problems in pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Schneier F, Campeas R, Hollander E, Hatterer J, Fyer A, Gorman J, Papp L, Davies S, Gully R, et al. Phenelzine vs atenolol in social phobia. A placebo-controlled comparison. Archives of General Psychiatry. 1992;49:290–300. doi: 10.1001/archpsyc.49.4.290. [DOI] [PubMed] [Google Scholar]
- Litz BT, Salters-Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, Hofmann SG. A randomized placebo-controlled trial of d-cycloserine and exposure therapy for posttraumatic stress disorder. Journal of Psychiatric Research. 2012;46:1184–90. doi: 10.1016/j.jpsychires.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Seidel A, Rosenfield B, Hofmann SG, Rosenfield D. Does fear reactivity during exposure predict panic symptom reduction? Journal of Consulting and Clinical Psychology. 2012;80:773–85. doi: 10.1037/a0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, Hofmann SG, Eisenmenger K, Krystal JH, Pollack MH. Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biological Psychiatry. 2010;67:365–70. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Archives of General Psychiatry. 2004;61:1136–44. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Smits JA, Hofmann SG, Rosenfield D, DeBoer LD, Costa PT, Simon NM, O’Cleirigh CM, Meuret AE, Marques L, Otto MW, Pollack MH. D-Cycloserine augmentation of cognitive behavioral therapy of social anxiety disorder: Prognostic and prescriptive variables. doi: 10.1037/a0034120. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Powers MB, Buxkamper R, Telch MJ. The efficacy of videotape feedback for enhancing the effects of exposure-based treatment for social anxiety disorder: a controlled investigation. Behaviour Research and Therapy. 2006;44(12):1773–85. doi: 10.1016/j.brat.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Smits JA, Julian K, Rosenfield D, Powers MB. Threat reappraisal as a mediator of symptom change in cognitive-behavioral treatment of anxiety disorders: a systematic review. Journal of Consulting and Clininical Psychology. 2012;80(4):624–35. doi: 10.1037/a0028957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Rosenfield D, McDonald R, Telch MJ. Cognitive mechanisms of social anxiety reduction: an examination of specificity and temporality. Journal of Consulting and Clinicall Psychology. 2006;74(6):1203–12. doi: 10.1037/0022-006X.74.6.1203. [DOI] [PubMed] [Google Scholar]
- Smits JA, Rosenfield D, Otto MW, Powers MB, Hofmann SG, Telch MJ, Pollack MH, Tart CD. D-cycloserine enhancement of fear extinction is specific to successful exposure sessions: Evidence from the treatment of height phobia. Biological Psychiatry. 2013;73:1054–8. doi: 10.1016/j.biopsych.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Merlo LJ, Bengtson M, Murphy TK, Lewis MH, Yang MC, Jacob ML, Larson M, Hirsh A, Fernandez M, Geffken GR, Goodman WK. D-cycloserine does not enhance exposure-response prevention therapy in obsessive-compulsive disorder. International Clinical Psychopharmacology. 2007;22:230–7. doi: 10.1097/YIC.0b013e32819f8480. [DOI] [PubMed] [Google Scholar]
- Tart CD, Handelsman PR, DeBoer LB, Rosenfield D, Pollack MH, Hofmann SG, Powers MB, Otto MW, Smits JA. Augmentation of exposure therapy with post-session administration of d-cycloserine. Journal of Psychiatric Research. 2013;47:168–74. doi: 10.1016/j.jpsychires.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Hart J, Richardson R. Effects of D-cycloserine on extinction of learned fear to an olfactory cue. Neurobiology of Learning and Memory. 2007;87:476–82. doi: 10.1016/j.nlm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, Cannistraro P, Jenike MA, Rauch SL. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. American Journal of Psychiatry. 2008;165:335–41. doi: 10.1176/appi.ajp.2007.07050776. [DOI] [PubMed] [Google Scholar]
- Wolpe J. Psychotherapy by reciprocal inhibition. Palo Alto, CA: Stanford University Press; 1958. [Google Scholar]