Abstract
In the past decade, community-associated (CA-) infections with methicillin-resistant Staphylococcus aureus (MRSA) have emerged throughout the world. Different CA-MRSA strains dominate in different geographical locations. Many CA-MRSA lineages contain genes coding for the Pantón-Valentine leukocidin. However, the role of this leukotoxin in CA-MRSA pathogenesis is still controversial. The genome sequences of two key PVL-positive CA-MRSA strains (USA300, USA400) have been reported, but we lack information on the more recently found PVL-negative CA-MRSA strains. One such strain is the PVL-negative ST72, the main cause of CA-MRSA infections in Korea. Here, we report the entire genome sequence of CA-MRSA ST72 and analyze its gene content with a focus on virulence factors. Our results show that this strain does not have considerable differences in virulence factor content compared to other CA-MRSA strains (USA300, USA400), indicating that other toxins do not substitute for the lack of PVL in ST72. This finding is in accordance with the notion that differential expression of widespread virulence determinants, rather than the acquisition of additional virulence factors on mobile genetic elements, such as PVL, is responsible for the increased virulence of CA- compared to hospital-associated MRSA.
Introduction
Staphylococcus aureus is a dangerous human pathogen and S. aureus infections are among the most frequent causes of deaths in hospitals around the globe [1]. Antibiotic resistance severely complicates the treatment of such infections [2]. After the worldwide spread of penicillin-resistant strains in the mid of the last century, methicillin became the treatment option of choice for S. aureus infections. However, methicillin resistance developed quickly, and nowadays methicillin-resistant S. aureus (MRSA) is pandemic, with many countries reporting methicillin resistance rates among hospital-associated S. aureus isolates that exceed 50% [3].
In the 1990s, MRSA infections – previously limited to predisposed patients in hospitals – started occurring in otherwise healthy people in the community without connections to the hospital setting [4]. These community-associated (CA-) MRSA infections are on a worldwide surge, with the United States so far seeing the most pronounced CA-MRSA epidemic. Most CA-MRSA infections are moderately severe infections of the skin and soft tissues, but more severe and sometimes fatal infections, such as necrotizing pneumonia, are also seen with CA-MRSA. The rise of CA-MRSA is due to the development of strains that combine methicillin resistance with a high level of aggressive virulence not commonly present in hospital-associated (HA-) MRSA [5]. Globally, CA-MRSA infections are caused by different lineages that are not genetically related [6]. In addition to pronounced virulence, which they all share, some strains may express specific additional factors that further promote pathogenic success. For example, the epidemic U.S. strain, USA300, harbors a mobile genetic element (MGE), called arginine catabolic element (ACME), containing a gene, speG, which abrogates the unique hypersensitivity of S. aureus to host-produced polyamines, thereby increasing survival on the human skin and during skin abscesses [7].
Soon after the first cases of CA-MRSA infections, researchers started determining the genetic composition of CA-MRSA isolates, including by whole-genome sequencing [8,9]. The most important initial finding was that genes encoding a specific leukotoxin, the Pantón-Valentine leukocidin (PVL), were present in virtually all CA-MRSA isolates, while these genes, called lukS and lukF, are much less frequent among HA-MRSA [10]. However, further investigation using animal infection models indicated that the extraordinary virulence of CA-MRSA is only in part, and in comparatively rare types of infections such as severe lung infection, due to PVL [5,11,12]. Rather, high expression of core genome-encoded virulence determinants, such as phenol-soluble modulins (PSMs) and α-toxin, appears to have played a preeminent role in the evolution of CA-MRSA virulence, especially as it relates to skin infections [12–14].
In addition to animal experiments casting doubt on the key role of PVL and the acquisition of the prophage ΦSLT containing the lukSF genes during the evolution of CA-MRSA virulence, several CA-MRSA strains have been isolated in the meantime that do not harbor lukSF and therefore do not produce PVL [5]. One such strain is the CA-MRSA clone of sequence type (ST) 72 that is the premier cause of CA-MRSA infections in Korea [15]. Here, to gain insight in the genetic composition as a basis for the extraordinary, PVL-independent virulence of that strain, we determined the whole genome sequence of a CA-MRSA ST72 isolate and analyzed its composition with a focus on virulence factors.
Materials and Methods
DNA extraction
Total bacterial DNA was isolated from an overnight culture of HL1 (ST72) using lysostaphin digestion and the method of Marmur [16]. The pellet of a 1-ml overnight culture was resuspended with 400 µl buffer P1 (Qiagen), to which 20 µl lysostaphin were added. The sample was incubated at 37°C for 15 min. Then 20 µl of a saturated sodium dodecylsulfate solution (in 45% ethanol) was added, the sample was vortexed, and incubated at 37°C for 5 min. Then 130 µl of a 5 M sodium perchlorate solution were added, and the sample was vortexed again. Six hundred µl of phenol/chloroform/isoamylalcohol (25:24:1 by volume) were added, the sample was vortexed, and the bottom phase transferred to a new tube. This extraction was repeated twice, then 800 µl ice-cold ethanol was added to precipitate the DNA, which was harvested by centrifugation for 15 min at 13,000 rpm in a tabletop centrifuge. The DNA was washed twice with 70% ethanol and resuspended in water.
Genome sequencing, annotation and comparative analysis
DNA sequencing was performed using fragment and paired-end libraries on a Roche 454 FLX genome sequencer using Titanium chemistry (454 Life Sciences [a Roche company], Branford, CT), Coverage of 40-60× or higher was obtained according to the manufacturer’s recommendations. Reads were assembled using the GS Assembler Version 2.5 software program. 454 reads were re-aligned to the contigs to check for assembly accuracy, and misassembled portions were corrected All gaps between contigs were closed by oligonucleotide primer design, PCR fragment generation, and Sanger sequencing of the PCR products on an Applied Biosystems 3730XL DNA sequencer (Applied Biosystems, Foster City, CA), Primer walking of large gaps or correction of ambiguous base calls was performed by PCR and Sanger sequencing. Open reading frame (ORF) calling was performed using public and proprietary algorithms, with a minimum length cutoff of 40 amino acids, as previously described [17,18]. The genome sequence and annotation of HL1 (ST72) are deposited in DDBJ/EMBL/GenBank under the NCBI accession numbers CP003979 (chromosome), CP003980 (pHL1), CP003981 (pHL2). ORFs displaying evidence of frameshifts or mutations leading to premature stop codons were identified by proprietary algorithms and were manually verified. Genome comparisons were performed using ClustalW alignments.
Genome analysis
To compare genome contents, we used a proprietary algorithm (Integrated Genomics) and compared every ORF from the HL1 genome with the ORFs from the two selected S. aureus genomes (FPR3757, MW2). Each ORF was compared using three metrics: similarity score (1e-10), functional annotation, and protein length. For ORFs to be considered for inclusion the following criteria had to be satisfied. The considered ORFs must have a P-Score similarity to an ORF from the HL1 genome of 1e-10 or less. In addition, the ORF in consideration should either have same functional annotation as of the HL1 genome’s ORF or have at least 80% matching protein length with that of the corresponding ORF in the HL1 genome. Further genome analyses were performed using ERGO (Integrated Genomics).
Results and Discussion
Overview
The CA-MRSA isolate HL1, previously also termed CN1 [13], was obtained from the pus of a necrotizing fasciitis infection in an 80-year old patient in the Seoul area of South Korea [15]. The isolate was then determined to be PVL-negative, resistant to clindamycin and erythromycin, belong to ST72 and spa type t324, and harbor SCCmec type IVa. Furthermore, we previously showed that it has high virulence in a rabbit model of skin infection and promotes neutrophil lysis to an extent almost as pronounced as seen with strain USA300 and within the range of other global CA-MRSA isolates [13].
The isolate has a genome of 2,757,070 base pairs (bp), with 2,726 assigned ORFs, of which 1970 have assigned functions, 53 tRNAs, and 9 rRNAs. The overall GC content is 32.79%. The HL1 genome is thus a little shorter than those of the prominent CA-MRSA strains USA300 (strain FPR3757, 2,917,469 bp, 2672 ORFs) and USA400 (strain MW2, 2,820,462 bp, 2644 ORFs) [4,8], which will serve as comparison in this study. HL1 also harbors two plasmids, which we named pHL1 and pHL2, of 3332 and 2472 bp, respectively.
Virulence factors and pathogenicity islands
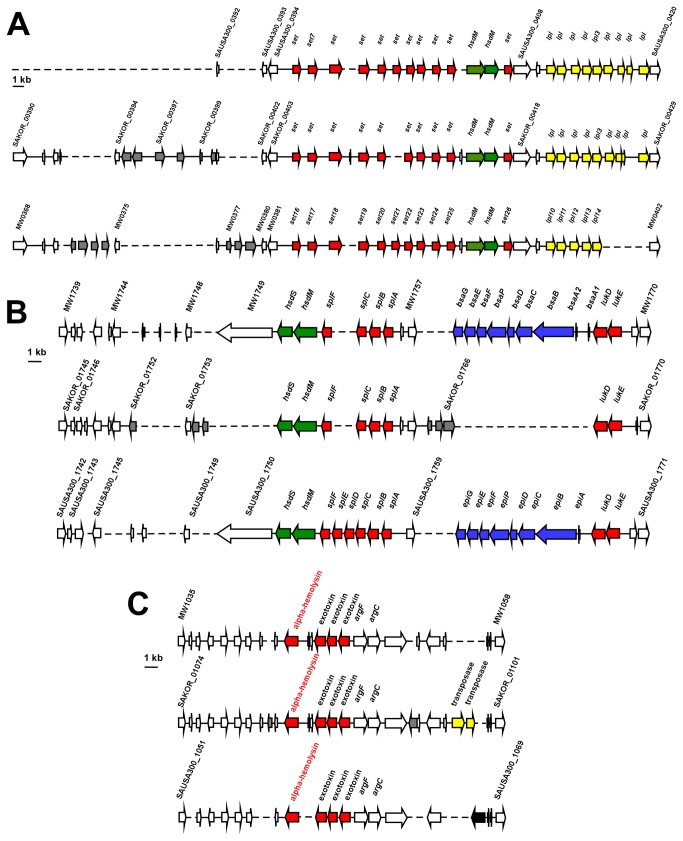
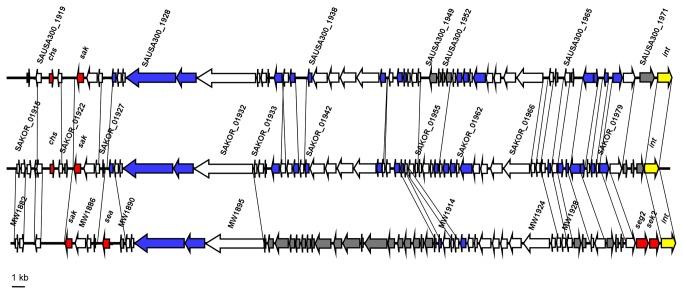
HL1 harbors the vSaα, vSaβ, and vSaγ genomic islands and the ΦSa3 prophage (Tab. 1). The vSaα, vSaβ, and vSaγ genomic islands occur in most S. aureus strains and harbor a series of virulence factors. For example, vSaα encodes a series of exotoxins and a restriction/modification system. vSaβ encodes another restriction/modification system, four serine proteases, and the bicomponent leukocidin LukDE. vSaγ encodes exotoxins, fibrinogen-binding proteins, a formyl peptide receptor 1 inhibitory protein, α-toxin, and the phenol-soluble modulins (PSMs) PSMβ1 and PSMβ2. The ΦSa3 prophage is not always present in S. aureus; for example, it is absent from the HA-MRSA strain COL, representing the archaic MRSA lineage. It contains genes encoding immune evasion factors, namely the chemotaxis inhibitory protein CHIPS, the complement inhibitor SCIN, and staphylokinase. Of note, ΦSa3 splits the gene encoding the sphingomyelinase β-toxin into two non-functional parts.
Table 1. Genomic and pathogenicity islands.
| HL1 (ST72) | MW2 (USA400) | FPR3757 (USA300) | |
|---|---|---|---|
| vSa1 | - | - | - |
| vSa2 | - | - | - |
| vSa3 | - | + | + |
| vSa4 | - | + | - |
| vSaα | + | + | + |
| vSaβ | + | + | + |
| vSaγ | + | + | + |
| ΦSa1 | - | - | - |
| ΦSa2 | - | + | + |
| ΦSa3 | + | + | + |
These islands and the ΦSa3 prophage are also present in the USA300 and USA400 CA-MRSA strains. Although the overall genetic composition differs, there are no considerable differences in known virulence determinants (Figs. 1, 2). As a notable exception, HL1 vSaβ does not contain the bsa operon coding for the biosynthesis of the epidermin-like lantibiotic aureodermin. It has been shown that this operon encodes a functional lantibiotic [19]. However, production levels are very low under all conditions tested so far, and the role of aureodermin in S. aureus physiology is unclear [20]. Importantly, HL1 does not contain virulence factors encoded on genomic islands or prophages that are absent from USA300 and USA400. Vice versa, both USA300 and USA400 contain the ΦSa2 (ΦSLT) prophage harboring the lukSF genes coding for PVL. USA300 and USA400 also contain the vSa3, and USA400 the vSa4 pathogenicity islands, which are absent from HL1. vSa3 contains two enterotoxin genes with unknown function in virulence, and vSa4 does not comprise known virulence factors. Finally, USA300 contains ACME, harboring the recently described virulence and colonization factor speG [21].
Figure 1. Genomic islands in ST72 HL1 compared to USA300 and USA400.
A, vSaα genomic island. B, vSaβ genomic island. C, vSaγ genomic island. Broken lines, missing ORFs; red, virulence determinants; green, type I restriction-modification system specificity subunit; yellow, lipoproteins; blue, bacteriocin-related genes; yellow, determinants; grey= ORF not identical to other strains.
Figure 2. ΦSa3 prophage in ST72 HL1 compared to USA300 and USA400.
Broken lines, missing ORFs; red, virulence determinants; blue, phage gene or phage-related gene; grey, ORF not identical to other strains.
A genome-wide analysis on 103 virulence genes to determine whether virulence factors present in HL1 are also present in USA300 and USA400 further confirmed that the overall composition of virulence genes in HL1, USA300, and USA400 is almost identical (Tab. 2). USA300 is missing two enterotoxin genes on vSaβ present in USA400 and HL1 (Fig. 1); and USA400 lacks the chemotaxis-inhibiting protein (CHIPs, gene chs), present in HL1 and USA300 on the ΦSa3 prophage (Fig. 2). The gene coding for the staphylococcal complement inhibitor SCIN is truncated in HL1, but not in USA300 or USA400. The gene crtN coding for the biosynthesis of the carotenoid staphyloxanthin immune evasion factor is annotated as truncated in HL1, because it appears to be split in a very short gene and a larger gene. However, the larger gene likely codes for the functional, previously described CrtN protein [22], while the shorter ORF may be a pseudo-gene. Similarly, HL1 and USA400 have a split coagulase gene, whereas it is not split in USA300. Finally, 28 surface proteins, identified by the sortase substrate LPXTG motif [23], did not show differences in composition between the three analyzed strains (Tab. 3).
Table 2. Presence/absence of important virulence factor genes found in HL1 in comparison to USA300 and USA400.
| Annotation | Gene number | HL1 | USA 400 | USA300 |
|---|---|---|---|---|
| Immunoglobulin G binding protein A precursor | SAKer_00085 | + | + | + |
| Lipase (EC 3.1.1.3) | SAKer_00314 | + | + | + |
| Exotoxin | SAKer_00368 | + | + | + |
| Exotoxin | SAKer_00404 | + | + | + |
| Exotoxin | SAKer_00405 | + | + | + |
| Exotoxin | SAKer_00406 | + | + | + |
| Exotoxin | SAKer_00408 | + | + | + |
| Exotoxin | SAKer_00409 | + | + | + |
| Exotoxin | SAKer_00410 | + | + | + |
| Exotoxin | SAKer_00411 | + | + | + |
| Exotoxin | SAKer_00412 | + | + | + |
| Exotoxin | SAKer_00413 | + | + | + |
| Exotoxin | SAKer_00417 | + | + | + |
| Phenol-soluble modulin α4 | SAKer_05000 | + | + | + |
| Phenol-soluble modulin α3 | SAKer_05001 | + | + | + |
| Phenol-soluble modulin α2 | SAKer_05002 | + | + | + |
| Phenol-soluble modulin α1 | SAKer_05003 | + | + | + |
| Staphylococcal accessory regulator SarA | SAKer_00611 | + | + | + |
| Lipase (EC 3.1.1.3) | SAKer_00649 | + | + | + |
| Staphylococcal accessory regulator homolog (SarX) | SAKer_00663 | + | + | + |
| Sensory transduction protein kinase SaeS (EC 2.7.13.3) | SAKer_00702 | + | + | + |
| Two-component response regulator SaeR | SAKer_00703 | + | + | + |
| Staphylocoagulase precursor | SAKer_00791 | ×1 | × | + |
| Thermonuclease (EC 3.1.31.1) | SAKer_00795 | + | + | + |
| Staphostatin B | SAKer_00967 | + | + | + |
| Staphopain (EC 3.4.22.48) | SAKer_00968 | + | + | + |
| Glutamyl endopeptidase precursor (EC 3.4.21.19) | SAKer_00969 | + | + | + |
| Heme uptake protein IsdB | SAKer_01050 | + | + | + |
| Heme uptake heme-iron binding protein IsdA | SAKer_01051 | + | + | + |
| Heme uptake cell surface protein IsdC | SAKer_01052 | + | + | + |
| Heme uptake ABC transporter, membrane component IsdD | SAKer_01053 | + | + | + |
| Heme uptake ABC transporter, heme-binding protein IsdE | SAKer_01054 | + | + | + |
| Heme uptake ABC transporter, permease protein IsdF | SAKer_01055 | + | + | + |
| Sortase B family protein | SAKer_01056 | + | + | + |
| Formyl peptide receptor family 1 inhibitory protein Flipr | SAKer_01077 | + | + | + |
| Alpha-hemolysin | SAKer_01084 | + | + | + |
| Exotoxin | SAKer_01087 | + | + | + |
| Exotoxin | SAKer_01088 | + | + | + |
| Exotoxin | SAKer_01089 | + | + | + |
| Phenol-soluble modulin β1 | SAKer_01099 | + | + | + |
| Phenol-soluble modulin β2 | SAKer_01100 | + | + | + |
| Peptide deformylase (EC 3.5.1.88) | SAKer_01142 | + | + | + |
| Thermonuclease (EC 3.1.31.1) | SAKer_01259 | + | + | + |
| Lysyltransferase (EC 2.3.2.3) MprF | SAKer_01297 | + | + | + |
| Two-component sensor kinase ArlS (EC 2.7.13.3) | SAKer_01351 | + | + | + |
| Two-component response regulator ArlR | SAKer_01352 | + | + | + |
| Superoxide dismutase (EC 1.15.1.1) | SAKer_01500 | + | + | + |
| Enterotoxin | SAKer_01549 | + | + | + |
| Enterotoxin | SAKer_01550 | + | + | + |
| Heme uptake receptor for hemoglobin-haptoglobin complexes IsdH | SAKer_01672 | + | + | + |
| Staphylococcal accessory regulator homolog (Rot) | SAKer_01705 | + | + | + |
| Serine protease (EC 3.4.21. -) SplD | SAKer_01758 | + | + | + |
| Serine protease (EC 3.4.21. -) SplC | SAKer_01759 | + | + | + |
| Serine protease (EC 3.4.21. -) SplB | SAKer_01760 | + | + | + |
| Serine protease (EC 3.4.21. -) SplA | SAKer_01761 | + | + | + |
| Leukocidin F subunit (LukD) | SAKer_01767 | + | + | + |
| Leukocidin S subunit (LukE) | SAKer_01768 | + | + | + |
| Enterotoxin | SAKer_01776 | + | + | - |
| Enterotoxin | SAKer_01777 | + | + | + |
| Enterotoxin | SAKer_01778 | + | + | - |
| Enterotoxin | SAKer_01779 | + | + | + |
| Enterotoxin | SAKer_01780 | + | + | + |
| Enterotoxin | SAKer_01781 | + | + | + |
| Staphopain (EC 3.4.22.48) | SAKer_01871 | + | + | + |
| Phenol-soluble modulin export ABC transporter permease PmtD | SAKer_01904 | + | + | + |
| Phenol-soluble modulin export ABC transporter ATP-binding protein PmtC | SAKer_01905 | + | + | + |
| Phenol-soluble modulin export ABC transporter permease PmtB | SAKer_01906 | + | + | + |
| Phenol-soluble modulin export ABC transporter ATP-binding protein PmtA | SAKer_01907 | + | + | + |
| Phospholipase C (beta-hemolysin) (EC 3.1.4.3), truncated | SAKer_01914 | × | × | × |
| Staphylococcal complement inhibitor SCIN | SAKer_01918 | × | + | + |
| Chemotaxis-inhibiting protein | SAKer_01920 | + | - | + |
| Staphylokinase precursor | SAKer_01923 | + | + | + |
| Leukocidin F subunit | SAKer_01986 | + | + | + |
| Leukocidin S subunit | SAKer_01987 | + | + | + |
| Delta-hemolysin | SAKer_01999 | + | + | + |
| Accessory gene regulator protein B AgrB | SAKer_02000 | + | + | + |
| Autoinducing peptide precursor AgrD | SAKer_02001 | + | + | + |
| Sensory transduction histidine kinase AgrC (EC 2.7.3. -) | SAKer_02002 | + | + | + |
| Accessory gene regulator protein A AgrA | SAKer_02003 | + | + | + |
| RNA polymerase sigma-B factor | SAKer_02029 | + | + | + |
| Staphylococcal accessory regulator homolog (SarV) | SAKer_02230 | + | + | + |
| Staphylococcal accessory regulator homolog (SarR) | SAKer_02260 | + | + | + |
| Staphylococcal accessory regulator homolog (SarY) | SAKer_02262 | + | + | + |
| Esterase (EC 3.1.1. -) | SAKer_02324 | + | + | + |
| Gamma-hemolysin subunit A | SAKer_02399 | + | + | + |
| Gamma-hemolysin subunit C | SAKer_02401 | + | + | + |
| Gamma-hemolysin subunit B | SAKer_02402 | + | + | + |
| Staphylococcal accessory regulator homolog (SarT/SarH3) | SAKer_02485 | + | + | + |
| Staphylococcal accessory regulator homolog (SarU/SarH2) | SAKer_02486 | + | + | + |
| Sortase A | SAKer_02517 | + | + | + |
| Esterase (EC 3.1.1. -) | SAKer_02525 | + | + | + |
| Staphyloxanthin biosynthesis protein CrtN (dehydrosqualene dehydrogenase) | SAKer_02552 | + | + | + |
| Staphyloxanthin biosynthesis protein CrtM (dehydrosqualene synthase) | SAKer_02554 | + | + | + |
| Staphyloxanthin biosynthesis protein CrtQ | SAKer_02555 | + | + | + |
| Staphyloxanthin biosynthesis protein CrtP | SAKer_02556 | + | + | + |
| Staphyloxanthin biosynthesis protein CrtO | SAKer_02557 | + | + | + |
| Esterase/Lipase (EC 3.1. -. -) | SAKer_02575 | + | + | + |
| Zinc metalloproteinase aureolysin (EC 3.4.24.29) | SAKer_02638 | + | + | + |
| N-acetylglucosaminyltransferase (EC 2.4.1. -) IcaA | SAKer_02668 | + | + | + |
| N-acetylglucosaminyltransferase, accessory part IcaD | SAKer_02669 | + | + | + |
| Polysaccharide intercellular adhesin deacetylase IcaB | SAKer_02670 | + | + | + |
| Putative polysaccharide intercellular adhesin exporter IcaC | SAKer_02671 | + | + | + |
| Lipase (EC 3.1.1.3) | SAKer_02673 | + | + | + |
truncated.
Table 3. LPXTG-motif surface proteins.
| Annotation | Gene number | HL1 | USA 400 | USA 300 |
|---|---|---|---|---|
| 5'-nucleotidase (EC 3.1.3.5) | SAKer_00023 | + | + | + |
| Immunoglobulin G binding protein A precursor | SAKer_00085 | + | + | + |
| Hypothetical protein | SAKer_00110 | + | + | + |
| Fibronectin-binding protein SdrC | SAKer_00549 | + | + | + |
| Fibronectin-binding protein SdrD | SAKer_00550 | + | + | + |
| Fibronectin-binding protein SdrE | SAKer_00551 | + | + | + |
| Fibronectin-binding protein ClfA | SAKer_00790 | + | + | + |
| Extracellular matrix binding protein / Fibrinogen-binding protein | SAKer_00793 | + | + | + |
| Peptidoglycan endo-beta-N-acetylglucosaminidase (EC 3.2.1.96) / N-acetylmuramoyl-L-alanine amidase (EC 3.5.1.28) | SAKer_00974 | + | + | + |
| Heme uptake protein IsdB | SAKer_01050 | + | + | + |
| Heme uptake heme-iron binding protein IsdA | SAKer_01051 | + | + | + |
| Heme uptake cell surface protein IsdC | SAKer_01052 | + | + | + |
| Extracellular fibrinogen-binding protein | SAKer_01076 | + | + | + |
| Extracellular fibrinogen-binding protein | SAKer_01079 | + | + | + |
| Extracellular matrix binding protein | SAKer_01373 | + | + | + |
| Elastin-binding protein EbpS | SAKer_01425 | + | + | + |
| Heme uptake receptor for hemoglobin-haptoglobin complexes IsdH | SAKer_01672 | + | + | + |
| Extracellular matrix binding protein | SAKer_01698 | + | + | + |
| Outer membrane protein | SAKer_01913 | + | + | + |
| Extracellular matrix binding protein | SAKer_02127 | + | + | + |
| Para-nitrobenzyl esterase (EC 3.1.1. -) | SAKer_02435 | + | + | + |
| Beta-N-acetylhexosaminidase (EC 3.2.1.52) | SAKer_02484 | + | + | + |
| Fibronectin-binding protein FnbB | SAKer_02488 | + | + | + |
| Fibronectin-binding protein FnbA | SAKer_02489 | + | + | + |
| Clumping factor B | SAKer_02630 | + | + | + |
| Hypothetical protein | SAKer_02647 | + | + | + |
| Serine-rich adhesion for platelets SraP | SAKer_02648 | + | + | + |
| Extracellular matrix binding protein | SAKer_02655 | + | + | + |
PSMs, short, amphipathic, α-helical peptides have recently been recognized as key determinants of CA-MRSA virulence [24]. They are produced by all S. aureus strains, except in naturally occurring Agr-defective mutants, in which no PSMs are detectable. HL1, USA300, and USA400 produce comparably high amounts of PSMs, while HA-MRSA often lack pronounced production of PSMs owing to low activity or mutation of the Agr system [13]. Accordingly, all three strains harbor the genetic loci encoding the PSMα peptides PSMα1 through PSMα4, PSMβ1 and PSMβ2, and the δ-toxin, which is encoded within RNAIII of the Agr quorum-sensing virulence regulator. Notably, the psmα genes are often not annotated in S. aureus genomes, due to their short length, but we annotated them in the HL1 genome and ascertained presence in USA300 and USA400.
Altogether, these findings are in good accordance with the comparable virulence of HL1 and USA400 in the rabbit model of skin infection that we performed previously [13]. The slightly higher virulence of USA300 in that model may be due to ACME-encoded speG, which a recent report indicates promotes virulence during skin infection [7]. Furthermore, they are in line with previous epidemiological studies and infection models performed in the PVL-sensitive rabbit indicating that PVL, as the only major virulence determinant that is absent from HL1 and present in USA300 and USA400, does not have a significant impact on virulence during CA-MRSA skin infection [12,13,25].
SCCmec
The comparison of the HL1 genome with those of USA300 and USA400 only revealed pronounced differences, encompassing a series of genes, in a very limited number of locations. The strongest difference was seen in and surrounding the SCCmec IVa element (Fig. 3). As previously described by Park et al., the class B mec cassette of ST72 SCCmec IVa element contains a tnp20 IS element and a pUB110 region in addition to the SCCmec IVa element of USA300 and USA400 [26]. The tnp20 IS element is also found at another location in the genome of USA300, but not USA400. The pUB110 region comprises genes involved in kanamycin and bleomycin resistance, in addition to plasmid replication and recombination enzymes. In the left extremity (L-C) region of HL1 SCCmec, there are four genes with high similarity to genes found in the S. epidermidis ATCC12228 genome, indicating that they may have been acquired from S. epidermidis – similar to SCCmec IV in general, which is believed to have originated from S. epidermidis at an earlier time [27]. One of the genes in this region has a homologue at another location in the USA400 genome, but not in USA300, while the others lack homologues in those strains. The tnp20 IS element and the USA400 homologue are in vicinity of the nifR3 gene, encoding a putative nitrogen regulatory protein, in the respective USA300 and USA400 genomes, while the nifR3 gene is found close to the J1 region of HL1 SCCmec, indicating that recombination between these two genomic sites occurred.
Figure 3. SCCmec IVa of ST72 HL1.
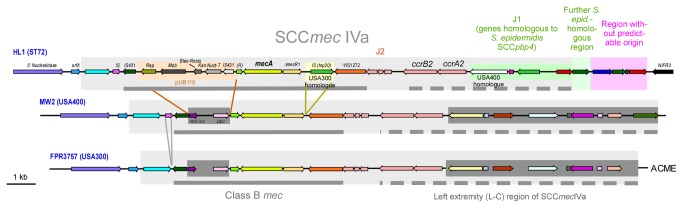
The HL1 SCCmec IVa element and the adjacent unique downstream region are compared to the SCCmec IVa elements of USA300 and USA400. SCCmec regions are shaded in grey boxes. Colored internal boxes in the case of HL1 show differing regions. Regions present in USA300 and USA400, but absent from HL1 are in dark grey, internal boxes. Solid grey bar, class B mec region; dotted grey bar, left extremity (L–C) region. Rep, replication protein; Mob, plasmid mobilization protein; Bleo-Resis, bleomycin resistance protein; Kan Nucl-T, kanamycin nucleotidyltransferase; (R), (R)-specific enoyl-CoA-hydratase; HMGCoA, hydroxymethylglutaryl CoA reductase; ACME, arginine catabolic mobile element (downstream of SCCmec in USA300).
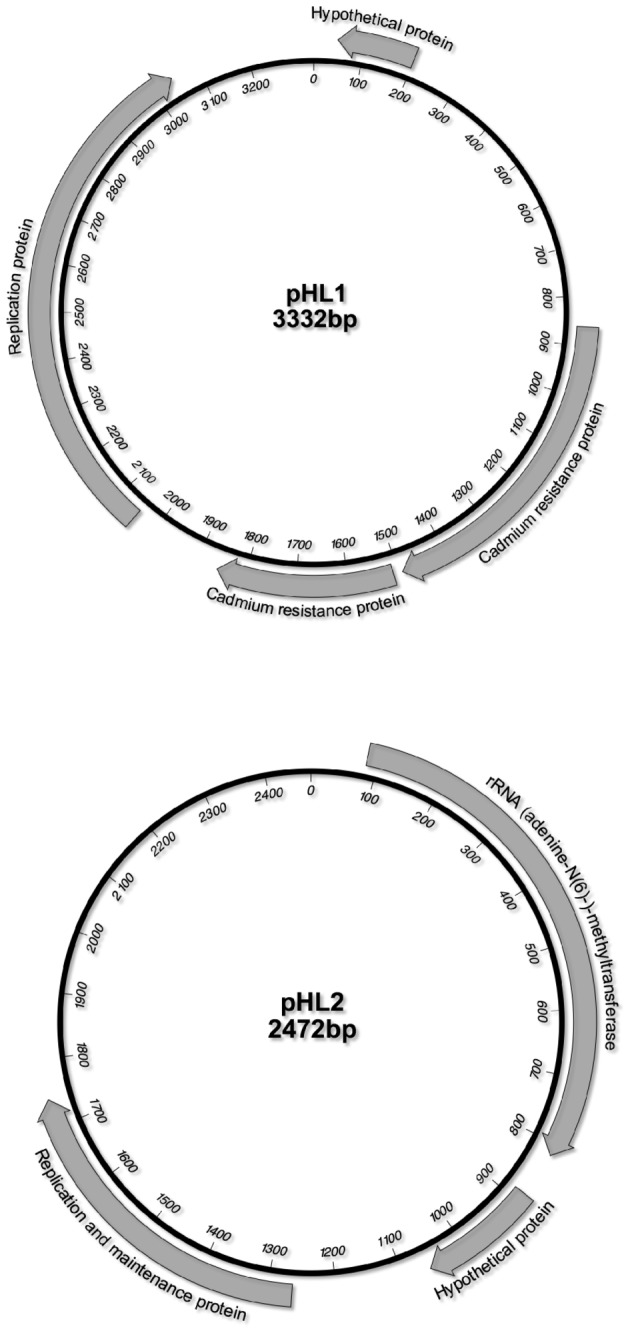
Plasmids
Plasmid pHL1 (3332 bp) contains four ORFs, encoding a plasmid replication protein, a hypothetical protein, and two cadmium resistance proteins (Figure 4). Plasmid pHL2 (2472 bp) encodes a plasmid replication protein, a hypothetical protein, and an rRNA (adenine-N6-) methyltransferase (Figure 4). No virulence factors are encoded on the HL1 plasmids.
Figure 4. Plasmids in ST72 HL1.
Concluding remarks
Our analysis of a CA-MRSA genome of a previously not analyzed ST advances our understanding of the CA-MRSA pandemic, especially as it represents the first sequenced genome of a PVL-negative CA-MRSA strain. It further strengthens the hypothesis that the success of CA-MRSA as pathogens is multi-factorial rather than dependent on the acquisition of specific, CA-MRSA-characteristic virulence determinants. In particular, we did not find virulence determinants in ST72 with an apparent role of substituting for the absence of PVL, such as, especially, other leukotoxins. While further detailed functional and gene expression analyses will be necessary, these findings suggest that the virulence of ST72 CA-MRSA is mainly dependent, as previously suggested for CA-MRSA in general [5,14], on gene regulatory adaptations enhancing the expression of core genome-encoded virulence determinants.
Funding Statement
Funding sources: Intramural Program of the NIAID (ZIA AI000904-12); Ministry of Health of the People’s Republic of China (no. 201002021). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339: 520-532. doi:10.1056/NEJM199808203390806. PubMed: 9709046. [DOI] [PubMed] [Google Scholar]
- 2. Lowy FD (2003) Antimicrobial resistance: the example of Staphylococcus aureus . J Clin Invest 111: 1265-1273. doi:10.1172/JCI18535. PubMed: 12727914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Otto M (2012) MRSA virulence and spread. Cell Microbiol 14: 1513-1521. doi:10.1111/j.1462-5822.2012.01832.x. PubMed: 22747834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeLeo FR, Otto M, Kreiswirth BN, Chambers HF (2010) Community-associated meticillin-resistant Staphylococcus aureus . Lancet 375: 1557-1568. doi:10.1016/S0140-6736(09)61999-1. PubMed: 20206987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Otto M (2010) Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus . Annu Rev Microbiol 64: 143-162. doi:10.1146/annurev.micro.112408.134309. PubMed: 20825344. [DOI] [PubMed] [Google Scholar]
- 6. Diep BA, Otto M (2008) The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol 16: 361-369. doi:10.1016/j.tim.2008.05.002. PubMed: 18585915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thurlow LR, Joshi GS, Clark JR, Spontak JS, Neely CJ et al. (2013) Functional modularity of the arginine catabolic mobile element contributes to the success of USA300 methicillin-resistant Staphylococcus aureus . Cell Host Microbe 13: 100-107. doi:10.1016/j.chom.2012.11.012. PubMed: 23332159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K et al. (2002) Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359: 1819-1827. doi:10.1016/S0140-6736(02)08713-5. PubMed: 12044378. [DOI] [PubMed] [Google Scholar]
- 9. Diep BA, Gill SR, Chang RF, Phan TH, Chen JH et al. (2006) Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus . Lancet 367: 731-739. doi:10.1016/S0140-6736(06)68231-7. PubMed: 16517273. [DOI] [PubMed] [Google Scholar]
- 10. Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR et al. (2003) Community-acquired methicillin-resistant Staphylococcus aureus carrying Pantón-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 9: 978-984. doi:10.3201/eid0908.030089. PubMed: 12967497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR et al. (2010) Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A 107: 5587-5592. doi:10.1073/pnas.0912403107. PubMed: 20231457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ et al. (2011) Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis 204: 937-941. doi:10.1093/infdis/jir441. PubMed: 21849291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li M, Cheung GY, Hu J, Wang D, Joo HS et al. (2010) Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J Infect Dis 202: 1866-1876. doi:10.1086/657419. PubMed: 21050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X et al. (2009) Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus . Proc Natl Acad Sci U S A 106: 5883-5888. doi:10.1073/pnas.0900743106. PubMed: 19293374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee SS, Kim YJ, Chung DR, Jung KS, Kim JS (2010) Invasive infection caused by a community-associated methicillin-resistant Staphylococcus aureus strain not carrying Pantón-Valentine leukocidin in South Korea. J Clin Microbiol 48: 311-313. doi:10.1128/JCM.00297-09. PubMed: 19889903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marmur J (1961) A method for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol 3: 208-218. doi:10.1016/S0022-2836(61)80047-8. [Google Scholar]
- 17. DelVecchio VG, Kapatral V, Redkar RJ, Patra G, Mujer C et al. (2002) The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc Natl Acad Sci U S A 99: 443-448. doi:10.1073/pnas.221575398. PubMed: 11756688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kapatral V, Anderson I, Ivanova N, Reznik G, Los T et al. (2002) Genome sequence and analysis of the oral bacterium Fusobacterium nucleatum strain ATCC 25586. J Bacteriol 184: 2005-2018. doi:10.1128/JB.184.7.2005-2018.2002. PubMed: 11889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Daly KM, Upton M, Sandiford SK, Draper LA, Wescombe PA et al. (2010) Production of the Bsa lantibiotic by community-acquired Staphylococcus aureus strains. J Bacteriol 192: 1131-1142. doi:10.1128/JB.01375-09. PubMed: 20023032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joo HS, Cheung GY, Otto M (2011) Antimicrobial Activity of Community-associated Methicillin-resistant Staphylococcus aureus Is Caused by Phenol-soluble Modulin Derivatives. J Biol Chem 286: 8933-8940. doi:10.1074/jbc.M111.221382. PubMed: 21278255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Joshi GS, Spontak JS, Klapper DG, Richardson AR (2011) Arginine catabolic mobile element encoded speG abrogates the unique hypersensitivity of Staphylococcus aureus to exogenous polyamines. Mol Microbiol 82: 9-20. doi:10.1111/j.1365-2958.2011.07809.x. PubMed: 21902734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wieland B, Feil C, Gloria-Maercker E, Thumm G, Lechner M et al. (1994) Genetic and biochemical analyses of the biosynthesis of the yellow carotenoid 4,4'-diaponeurosporene of Staphylococcus aureus . J Bacteriol 176: 7719-7726. PubMed: 8002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mazmanian SK, Liu G, Ton-That H, Schneewind O (1999) Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285: 760-763. doi:10.1126/science.285.5428.760. PubMed: 10427003. [DOI] [PubMed] [Google Scholar]
- 24. Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY et al. (2007) Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13: 1510-1514. doi:10.1038/nm1656. PubMed: 17994102. [DOI] [PubMed] [Google Scholar]
- 25. Bae IG, Tonthat GT, Stryjewski ME, Rude TH, Reilly LF et al. (2009) Presence of genes encoding the panton-valentine leukocidin exotoxin is not the primary determinant of outcome in patients with complicated skin and skin structure infections due to methicillin-resistant Staphylococcus aureus: results of a multinational trial. J Clin Microbiol 47: 3952-3957. doi:10.1128/JCM.01643-09. PubMed: 19846653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park C, Shin HH, Kwon EY, Choi SM, Kim SH et al. (2009) Two variants of staphylococcal cassette chromosome mec type IVA in community-associated meticillin-resistant Staphylococcus aureus strains in South Korea. J Med Microbiol 58: 1314-1321. doi:10.1099/jmm.0.009688-0. PubMed: 19574415. [DOI] [PubMed] [Google Scholar]
- 27. Otto M (2013) Coagulase-negative staphylococci as reservoirs of genes facilitating MRSA infection: Staphylococcal commensal species such as Staphylococcus epidermidis are being recognized as important sources of genes promoting MRSA colonization and virulence. Bioessays 35: 4-11. doi:10.1002/bies.201200112. PubMed: 23165978. [DOI] [PMC free article] [PubMed] [Google Scholar]