Abstract
Microglia were described by Pio del Rio-Hortega (1932) as being the ‘third element’ distinct from neurons and astrocytes. Decades after this observation, the function and even the very existence of microglia as a distinct cell type was a topic of intense debate and conjecture. However, considerable advances have been made towards understanding the neurobiology of microglia resulting in a radical shift in our view of them as being passive bystanders that have solely immune and supportive roles, to being active principal players that contribute to central nervous system pathologies caused by disease or following injury. Converging lines of evidence implicate microglia as being essential in the pathogenesis of neuropathic pain, a debilitating chronic pain condition that can occur after peripheral nerve damage caused by disease, infection, or physical injury. A key molecule that modulates microglial activity is ATP, an endogenous ligand of the P2-purinoceptor family consisting of P2X ionotropic and P2Y metabotropic receptors. Microglia express several P2 receptor subtypes, and of these the P2X4, P2X7, and P2Y12 receptor subtypes have been implicated in neuropathic pain. The P2X4 receptor has emerged as the core microglia-neuron signaling pathway: activation of this receptor causes release of brain-derived neurotrophic factor (BDNF) which causes disinhibition of pain-transmission neurons in spinal lamina I. The present review highlights recent advances in understanding the signaling and regulation of P2 receptors expressed in microglia and the implications for microglia-neuron interactions for the management of neuropathic pain.
Keywords: Microglia, P2 purinoceptors, Neuropathic pain, Nerve injury, ATP, BDNF, spinal cord
Pain is a double-edged sword that can be protective or cause considerable suffering. Acute nociceptive pain warns against imminent or existing tissue damage, whereas chronic pain has no known defensive or beneficial function and is unremitting for those who suffer from this condition. Acute pain is produced by physiological functioning of the normal peripheral and central nervous systems. However, the processes initiated during acute pain can sometimes progress to chronic pain that is characterized as persisting long after the initiating event has healed. This transition to chronicity is highly variable between individuals and the degree of injury is not necessarily predictive of the severity or chronicity of the pain. There is mounting evidence that the transition from acute to chronic pain involves discrete pathophysiological steps that alter the cellular, molecular, and anatomical organization of nociceptive neural networks in the spinal dorsal horn (Latremoliere and Woolf, 2009;Scholz and Woolf, 2002;Voscopoulos and Lema, 2010;Woolf and Salter, 2000). In this pathologically altered system, the balance of inhibitory and excitatory control is shifted such that inhibitory mechanisms are weakened while excitatory mechanisms are strengthened. A subtle shift in this balance can have a profound effect that results in both a pathological amplification and a change in modality of sensory input and output from the spinal cord which leads to the exaggerated pain responses seen in chronic pain conditions (Costigan et al., 2009b).
Neuropathic pain is among the most debilitating type of chronic pain, which typically develops because of injury to a nerve caused by trauma, infection, or pathology (Scholz and Woolf, 2002;Zimmermann, 2001;Gwak and Hulsebosch, 2009). The cardinal symptom of neuropathic pain is hypersensitivity that can manifest spontaneously in the absence of an overt stimulus (spontaneous pain), or it can be evoked, such as in the case of allodynia (pain resulting from an innocuous stimulus) and hyperalgesia (an exaggerated pain response to a noxious stimulus). The sequelae of neuropathic pain are difficult to treat and often refractory to the current available pharmacological treatments, which are typically directed against neuronal molecular targets. The failure of these neuron-targeted drugs to alleviate neuropathic pain is consistent with accruing evidence that non-neuronal mechanisms are also major contributors to chronic neuropathic pain. Thus, a new framework for understanding the etiology of neuropathic pain is forming from a rapidly growing body of evidence that glia in the central nervous system are critical in establishing and maintaining neuropathic pain (Beggs and Salter, 2010;Grace et al., 2011;Inoue and Tsuda, 2006;Milligan and Watkins, 2009;Gwak and Hulsebosch, 2010). Microglia, in particular, have emerged as key players in the etiology of neuropathic pain (Inoue and Tsuda, 2006;Tsuda et al., 2003;Tsuda et al., 2005;Watkins et al., 2001;Watkins and Maier, 2003;Tsuda et al., 2005). The present article highlights the recent advances in our understanding of the role microglia, and particularly microglia-neuron interactions mediated through ATP-gated P2-receptors, play in the pathogenesis of nerve injury-induced neuropathic pain.
Microglial response to peripheral nerve injury
Microglia are resident cells in the central nervous system that respond to adverse physiological conditions such as trauma, ischemia, inflammation, and infection. Recent evidence suggests that almost the entire population of microglia originate from embryonic macrophages derived from the yolk sac (Ginhoux et al., 2010). In the adult central nervous system, microglia comprise 5–10% of the total glial population and they are roughly equal in number to neurons (Kreutzberg, 1996;Lawson et al., 1990;Nakajima and Kohsaka, 2001). Under normal homeostatic conditions, microglia display a small soma bearing thin ramified processes that cover large non-overlapping territories throughout the brain and spinal cord (Bushong et al., 2002;Kreutzberg, 1996). Microglia with this phenotype – the surveillance mode of activity – continuously scan their local environment by actively extending and retracting their processes (Davalos et al., 2005;Nimmerjahn et al., 2005). This dynamic process reorganization is thought to enable otherwise stationary microglia to rapidly detect potential stimuli that threaten to disrupt homeostasis in the central nervous system. When injury is detected, microglia respond within seconds to minutes by extending their processes toward the site of neural damage (Davalos et al., 2005;Nimmerjahn et al., 2005). This targeted movement enables the microglia to converge and form a barrier between healthy and injured cells, limiting the extent of damage in the central nervous system. Being able to adopt a phagocytic phenotype, the microglia also remove debris from degenerating primary afferent terminals and apoptotic cells at the site of injury (Aldskogius, 2001;Rotshenker, 2003).
The rapid microglia responsiveness is coupled to a more slowly developing series of changes in morphology, gene expression, function, and proliferation (Hanisch and Kettenmann, 2007;Kettenmann et al., 2011;Kreutzberg, 1996;Nakajima and Kohsaka, 2001). A progressive series of morphological changes in spinal microglia has been reported in rodent models of nerve injury caused by compression, ligation, or transection (Liu et al., 1995;Tsuda et al., 2003;Zhang and De, 2006). After peripheral nerve injury, microglia withdraw their thin processes and adopt an amoeboid-like structure (Eriksson et al., 1993). These morphological changes are often followed by an increase in the number and density of microglia in the ipsilateral spinal dorsal horn (Calvo and Bennett, 2011;Calvo et al., 2011;Echeverry et al., 2008;Gehrmann and Banati, 1995;Perry, 1994;Stoll and Jander, 1999). The stereotypical microglial response also entails the upregulation of a number of surface marker proteins belonging to the complement cascade: complement receptor 3 (CR3), Toll-like receptor 4 (TLR4), CD14, CD4, and major histocompatibility complex (MHC) class I and II (Coyle, 1998;Liu et al., 1995;Sweitzer et al., 2002b;Tanga et al., 2004;Tsuda et al., 2003). In addition, spinal microglia produce and secrete cytokines, chemokines, and neurotrophic factors that signal to neurons in the spinal cord to alter neuronal excitability. This microglia-neuron communication is increasingly recognized as being essential in the etiology of neuropathic pain (Clark et al., 2010;DeLeo and Yezierski, 2001;Inoue and Tsuda, 2006;Tsuda et al., 2005;Watkins et al., 2001;Zhang and De, 2006).
In addition to their functional and morphological plasticity, microglia are characterized by their low threshold for reactivity and respond to a wide range of challenges, even a remote stimulus in the periphery is a sufficient trigger for microglial response. That microglia respond to damage in the periphery suggests signaling from primary afferents contributes to the microglial response. In accord, it is known that discharge activity of primary afferents initiated by injury to peripheral nerves has considerable consequences in the nervous system that can lead to chronic pain (Woolf and Salter, 2000). Indeed, afferent barrage after the nerve injury is thought to trigger neuropathic pain by activating p38 in spinal microglia (Wen et al., 2007). One such consequence of peripheral nerve injury is the entry of blood bourn immune cells into the central nervous system (Sweitzer et al., 2002a), specifically, these cells include monocytes (Zhang et al., 2007), and T-lymphocytes (Cao and DeLeo, 2008;Costigan et al., 2009a). Transit of these cells is likely aided by increased permeability of the blood brain barrier and the blood spinal cord barrier (Beggs et al., 2010;Echeverry et al., 2011;Gordh and Sharma, 2006). The increased vascular permeability can be triggered by injury to a peripheral nerve, electrical stimulation or application of capsaicin to primary afferent C-fibers (Beggs et al., 2010). It is uncertain whether circulating monocytes that infiltrate into the spinal cord proceed to differentiate into microglia and contribute to the local microglial response.
Microglia increase and transform the output of pain transmission neurons
A major ascending nociceptive (pain-related) pathway arises from neurons in lamina I of the spinal dorsal horn (Bester et al., 2000). The action potential discharge of these neurons, that is to say the output of these neurons, is normally evoked only in response to noxious peripheral stimulation, such as pinch to the skin (Keller et al., 2007) (Figure 1). However, after peripheral nerve injury the output of these neurons is transformed such that innocuous stimulus, such as brush or touch, evoke action potential discharges. Moreover, the response to noxious stimulation is greatly exaggerated and there is a pronounced after-discharge in lamina I neurons after peripheral nerve injury. Also, after peripheral nerve injury lamina I neurons exhibit spontaneous bursting in the absence of overt stimulation whereas in naïve animals these neurons are normally silent. The change in the output of the lamina I neurons may be readily understood to provide a neural substrate for the three cardinal signs of neuropathic pain in humans (Woolf and Salter, 2000): mechanical allodynia (discharge in response to innocuous stimulation), hyperalgesia (increased response to noxious stimulation), and spontaneous pain (spontaneous bursting). Importantly, in otherwise naïve animals, delivering microglia which have been stimulated with ATP phenocopies the changes in lamina I neurons induced by peripheral nerve injury. As such, ATP-stimulated microglia are sufficient to transform the output of this major pain transmission pathway and hence provide a neural basis for microglia involvement in neuropathic pain.
Figure 1. Peripheral nerve injury and microglia stimulation alters sensory output of spinal lamina I neurons underlying neuropathic pain.
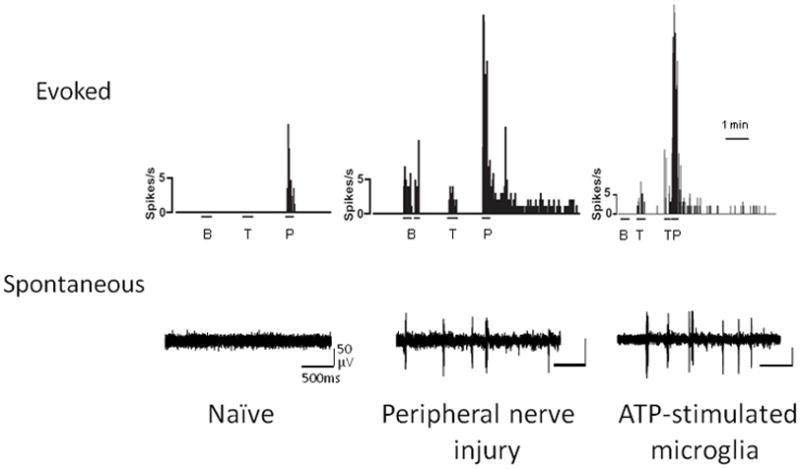
Rate meter records of representative responses evoked by brush (B), touch (T), or pinch (P) (top traces), and continuous extracellular single-unit records of spontaneous bursts of activity (bottom traces) in lamina I projection neurons in naive, after peripheral nerve injury or local spinal administration of ATP-stimulated microglia. The action potential discharge of these neurons is normally only evoked by noxious peripheral stimulation. However, after peripheral nerve injury the output of these neurons is transformed so that innocuous stimulus, such as brush or touch, evokes action potential discharges. In addition, after nerve injury, response to noxious stimulation is greatly exaggerated, and lamina I neurons exhibit spontaneous bursting in the absence of overt stimulation whereas in naïve animals these neurons are normally silent. This change in the output of lamina I neurons is one of the mechanisms that contributes to neuropathic pain. Moreover, local administration of ATP stimulated microglia on the surface of the lumbar spinal cord recapitulates the changes in lamina I neurons induced by peripheral nerve injury. Modified from Keller et al. 2007.
P2 receptor expression in microglia
Microglia-neuron communication is bidirectional and considerable evidence implicates ATP as being a critical molecular substrate for interaction between these cells (Coull et al., 2005;Jarvis, 2010;Maeda et al., 2010). ATP is an endogenous ligand of the P2 purinergic family of receptors, which consists of P2Y metabotropic receptors and P2X ionotropic receptors. P2Y receptors (P2Y1,2,4,6,11,12,13 and 14) are G-protein coupled, whereas P2X receptors (P2X1–P2X7) are non-selective cation channels permeable to Ca2+, K+, and Na+ (Burnstock, 2007;Burnstock et al., 2011;Inoue, 2002;Inoue and Tsuda, 2006;North, 2002). There is a wide expression of P2YRs on microglia, including P2Y1,2,4,6, and 12 receptor subtypes (Boucsein et al., 2003;Farber and Kettenmann, 2005;Kobayashi et al., 2008;Tozaki-Saitoh et al., 2008). By contrast, microglia expression of P2X receptors is restricted to the P2X4 and P2X7 receptor subtypes (Collo et al., 1997;Ferrari et al., 1996;Inoue and Tsuda, 2006;Tsuda et al., 2003). In microglia, stimulation of P2X or P2Y receptors evokes current responses, increases intracellular [Ca2+], and causes release of signaling molecules that explicitly affect neuronal function (Illes et al., 1996;Moller et al., 2000;Norenberg et al., 1994;Walz et al., 1993). However, only recently have microglial P2 receptors been causally implicated in nerve injury-induced pain behaviours (Chessell et al., 2005;Coull et al., 2005;Kobayashi et al., 2008;Tozaki-Saitoh et al., 2008;Tsuda et al., 2003;Tsuda et al., 2009a;Tsuda et al., 2009c;Ulmann et al., 2008). Much of our conceptual understanding of the role microglial P2 receptors play in neuropathic pain stems from recent advances made in elucidating the cellular and molecular mechanisms that regulate P2 receptor function, as well as the identification of signaling pathways downstream from these receptors. The significance of these findings for microglia involvement in neuropathic pain will be discussed in this article.
Role of microglial P2X4 receptors in neuropathic pain
The essential role of P2X4 receptors in neuropathic pain was first reported by Tsuda et al. (2003)(Tsuda et al., 2003). It was demonstrated that intrathecal injection of 2′,3′-O-(2,4,6-trinitrophenyl)adenosine 5′-triphosphate (TNP-ATP), an antagonist of P2X1-4Rs, reversed tactile allodynia in nerve-injured rats. By contrast, treatment with pyridoxalphosphate-6-azophenyl-2′,4′-disulphonoic acid (PPADs), an antagonist of P2X1-3,5,7Rs, but not the P2X4 receptor, had no effect on tactile allodynia. Based on the pharmacological profiles of these antagonists, it was inferred that the essential P2X receptor subtype involved in central responses to peripheral nerve injury is the P2X4 receptor, and that ongoing expression of tactile allodynia requires tonic P2X4 receptor activation. Directly targeting P2X4 receptors with P2X4 receptor antisense oligonucleotide (Tsuda et al., 2003) or genetically deleting the P2rx4 gene (Ulmann et al., 2008) significantly attenuated nerve injury induced pain behaviours. The development of tactile allodynia correlated with a progressive increase in spinal P2X4 receptor expression, which normally is at a low level in the naïve central nervous system. On closer examination, it was evident that the cells expressing P2X4 receptors were microglia rather than neurons or astrocytes. This observation was recently confirmed in CX3CR1+/GFP mice, in which induction of spinal P2X4 receptor expression following nerve injury occurred specifically in the eGFP microglia (Ulmann et al., 2008). The increase in P2X4 receptor expression correlated temporally with the development of tactile allodynia. However, preventing the increase in P2X4 receptor did not affect expression of protein markers of microglia response, such as the complement receptor 3 (Tsuda et al., 2003) or the ionized calcium binding adaptor molecule-1 (Ulmann et al., 2008). Thus, the upregulation of these microglial markers after peripheral nerve injury is not dependent upon P2X4 receptors. Definitive evidence that stimulation of P2X4 receptors expressed on microglia is sufficient to elicit pain hypersensitivity comes from the finding that intrathecal injection of P2X4 receptor-stimulated cultured microglia elicits robust mechanical allodynia in non-nerve injured animals (Coull et al., 2005;Tsuda et al., 2003;Tsuda et al., 2008b). Taken together, the pharmacological, genetic, and behavioral findings indicate that activity of P2X4 receptors expressed on spinal microglia is both necessary and sufficient, and therefore logically causative, to induce tactile allodynia after peripheral nerve injury. Although these mechanisms are clearly a major component of the functional alterations in the spinal dorsal horn that result in ongoing pain following peripheral nerve injury, they are one of many mechanisms thought to contribute in multiple sites both peripheral and central (Beggs and Salter, 2010;Clark et al., 2007;Hulsebosch et al., 2009;Inoue and Tsuda, 2009;Watkins et al., 2001). How each of these mechanisms overlap and their relative importance is yet to be determined, however changes such as these, occurring potentially beyond the reach of effective endogenous spinal cellular inhibitory control, are likely to have a relatively profound role within the overall etiology of peripherally mediated neuropathic pain.
Significant advances have recently been made towards understanding how injury to a nerve signals to the dorsal horn to cause increased P2X4 receptor expression in microglia. Several signaling molecules have been implicated in the upregulation of P2X4 receptors, including CCL21, a chemokine released from injured neurons (Biber et al., 2011;de Jong et al., 2005), interferon γ, a cytokine that transforms resting spinal microglia into an activated state (Tsuda et al., 2009b), and tryptase, a protease released from mast cells that activates proteinase-activated receptor 2 in microglia (Yuan et al., 2010). Also critical for upregulating expression of P2X4 receptors is the extracellular matrix molecule fibronectin, which through the Lyn kinase signaling pathway modulates the transcriptional and post-transcriptional levels of P2X4 receptor expression in microglia (Nasu-Tada et al., 2006;Tsuda et al., 2008a;Tsuda et al., 2008b;Tsuda et al., 2009c). Similarly, activation of mu-opioid receptors by morphine can drive P2X4 receptor expression in states of opioid tolerance (Horvath and DeLeo, 2009;Horvath et al., 2010) and opioid-induced hyperalgesia (Ferrini et al., 2010). Thus, several critical elements of the molecular machinery required for upregulation of P2X4 receptors in microglia following peripheral nerve injury have recently been identified, but whether these elements converge on a common pathway that controls P2X4 receptor expression or how they are causally connected is not known.
Cell surface expression of P2X4 receptors is regulated by constitutive internalization and reinsertion(Bobanovic et al., 2002;Fujii et al., 2011;Royle et al., 2002;Toulme et al., 2006). In microglia, internalized P2X4 receptors are targeted to lysosomes and constitutive retrieval of these receptors from the plasma membrane regulates the proportion of P2X4 receptors on the cell surface (Qureshi et al., 2007). Internalization is controlled by the C-terminus of the P2X4 receptor (Fujii et al., 2011;Qureshi et al., 2007;Royle et al., 2002), this region is also important for agonist-induced desensitization (Fountain and North, 2006) and phosphoinositide PIP2 modulation of P2X4 receptor function (Bernier et al., 2008). The recent elucidation of the crystal structure of the zebrafish P2X4 receptor revealed critical details about the extracellular domain, putative ATP binding site, transmembrane regions, and ion permeation pathway (Kawate et al., 2009). Time-lapse imaging using fast-scanning atomic force microscopy added further to our understanding of the P2X4 receptor channel function, revealing that ATP-stimulation induces structural changes that result in two distinct conformations: in the presence of extracellular Ca2+, ATP stimulation causes the P2X4 receptor to open a non-selective cation permeable channel, but in the absence of extracellular Ca2+ the receptor forms a macropore (i.e. a process known as pore dilation) that allows passage of large molecules (Shinozaki et al., 2009). P2X4 receptors expressed in microglia and macrophages have been reported to possess this ability to function both as a cation channel and as a macropore (Bernier et al., 2010;Seil et al., 2010); however, the implications of this dual mode of functioning, particularly in the context of neuropathic pain remains an intriguingly open question.
In microglia, influx of Ca2+ through the P2X4 receptor is a critical step linking stimulation of these receptors to the synthesis and release of brain-derived neurotrophic factor (BDNF) (Trang et al., 2009). The requirement for P2X4 receptors in the release of BDNF is supported by findings that P2X4 receptor-deficient mice have impaired microglial BDNF release and altered BDNF signaling in the spinal cord (Ulmann et al., 2008). Moreover, P2X4 receptor-deficient mice do not develop mechanical allodynia following peripheral nerve injury, indicating BDNF release driven by P2X4 receptor activation is necessary for the development of neuropathic pain. Indeed, considerable evidence supports a critical role for BDNF in the initiation of central sensitization associated with neuropathic pain (Biggs et al., 2010;Lever et al., 2003;Lu et al., 2007;Obata et al., 2011).
The previous framework of thinking was based on the hypothesis that BDNF derived from primary afferent neurons is responsible for spinal nociceptive hypersensitivity. This source of BDNF, however, has been brought into question because of evidence indicating that there is a lack of primary afferent evoked BDNF release in the spinal cord after nerve injury (Lever et al., 2003), and that eliminating BDNF from primary afferent neurons has no effect on nerve injury induced mechanical allodynia (Zhao et al., 2006). A study by Coull et al. (2005) (Coull et al., 2005) provided the first evidence that in neuropathic pain, it is BDNF from microglia that affects nociceptive processing in the spinal cord. It was demonstrated that intrathecal administration of P2X4 receptor-stimulated microglia causes a depolarizing shift in EGABA in spinal lamina I neurons that reduces inhibition and in about one-third of neurons the GABAA-ergic responses are converted to excitation. The altered inhibitory responses produce a phenotypic switch in spinal lamina I neurons such that they relay innocuous mechanical input, increase discharge to a noxious stimulus, and display spontaneous activity (Coull et al., 2003;Keller et al., 2007). Several key findings implicate BDNF as the cellular substrate in aberrant pain signaling between microglia and neurons in the spinal cord: 1) intrathecal application of BDNF mimicks the mechanical allodynia and alteration in EGABA caused by peripheral nerve injury or by administering P2X4 receptor-stimulated microglia; 2) blocking BDNF-trkB signaling prevents mechanical allodynia evoked by P2X4 receptor-stimulated microglia; and, 3) siRNA knockdown of BDNF expression abrogates the effects of intrathecally administered microglia on lamina I neurons and on the microglia-elicited pain behaviours (Coull et al., 2005). Collectively, these findings suggest that BDNF from microglia is a critical signaling molecule in aberrant spinal nociceptive processing, and that microglia-neuron signaling through BDNF plays a causal role in the sequelae of neuropathic pain.
A major gap in understanding how activation of P2X4 receptors causes BDNF release was recently resolved with the identification of p38 mitogen-activated protein kinase (MAPK) as a cellular intermediary in the P2X4 receptor-BDNF signaling pathway (Trang et al., 2009). Pharmacological and biochemical interrogation of this pathway revealed that influx of Ca2+ through the P2X4 receptor activates p38 MAPK: activation of this kinase is permissive for the release and synthesis of BDNF in microglia. This type of Ca2+-dependent activation of p38-MAPK has been reported in a number of cell types (Blanquet, 2000;Dehez et al., 2001;Elzi et al., 2001;Kreideweiss et al., 1999;Shigemoto-Mogami et al., 2001), including peripheral macrophages which express functional P2X4 receptor (Brone et al., 2007;Qureshi et al., 2007;Ulmann et al., 2010). In macrophages, P2X4 receptor activation of p38 MAPK is upstream from the production and release of prostaglandin E2 (Ulmann et al., 2010), a principal substrate for inflammation that sensitizes peripheral nociceptors (Portanova et al., 1996;Samad et al., 2002). Thus, the findings in microglia and macrophages place p38 MAPK in the core P2X4 receptor signaling pathway. p38 MAPK therefore appears to gate the release of distinct signaling molecules from microglia (BDNF) and peripheral macrophages (prostaglandin E2) that alter both the afferent input and the processing of such input. These discoveries highlight a novel role for P2X4 receptors in inflammation and represent a significant conceptual advance that extends involvement of P2X4 receptors in chronic pain beyond neuropathic pain to encompass the pathoetiology of inflammatory pain. Identification of the upstream and downstream components of the P2X4 receptor signaling pathway has provided important new information about the fundamental central and peripheral mechanisms that underlie pain hypersensitivity subsequent to nerve injury and inflammation. The P2X4 receptor is therefore a promising therapeutic target for the treatment of neuropathic pain and inflammatory pain.
Role of microglial P2X7 receptors in neuropathic pain
In addition to P2X4 receptors, microglia express functional P2X7 receptors. Stimulation of P2X7 receptors is implicated in the microglia response to inflammation (Collo et al., 1997), microglial proliferation (Bianco et al., 2005;Monif et al., 2009), and release of proinflammatory cytokines (Brough et al., 2002;Chakfe et al., 2002;Clark et al., 2010;Ferrari et al., 1997a;Ferrari et al., 1997b). A role for P2X7 receptors in neuropathic pain is also suggested on the basis of reduced pain sensitivity in P2X7 receptor-deficient mice (Chessell et al., 2005) and after pharmacological blockade of the receptor (Broom et al., 2008;Dell’Antonio et al., 2002;Honore et al., 2006;Honore et al., 2009;McGaraughty et al., 2007;Perez-Medrano et al., 2009). Although P2X7 receptors are expressed on a wide variety of cell types, evidence for involvement of microglial P2X7 receptors in neuropathic pain stems from findings that activating microglial P2X7 receptors causes the release of interleukin-1β and cathepsin S, which in the spinal cord contributes to the maintenance of mechanical hypersensitivity (Clark et al., 2007;Clark et al., 2010). Like P2X4 receptor-mediated release of BDNF, the release of interleukin-1β and cathepsin S from microglia involves P2X7 receptor signaling to p38 MAPK (Clark et al., 2010). Thus, activation of p38 MAPK may be a critical mechanistic step of convergence in the P2X7 and P2X4 receptor signaling pathways in neuropathic pain. P2X7 and P2X4 receptors also interact to form complexes with unique channel properties (Antonio et al., 2009;Boumechache et al., 2009;Casas-Pruneda et al., 2009;Guo et al., 2007;Nicke, 2008). This raises the interesting possibility that structural and functional interactions, as well as converging signaling pathways, might be critical cellular mechanisms that underlie involvement of microglial P2X7 and P2X4 receptors in neuropathic pain.
Role of microglial P2Y receptors in neuropathic pain
Microglia express a wide range of P2Y receptors (P2Y1, 2, 4, 6, and 12), with P2Y6 and P2Y12 receptors mediating chemotaxis and migration of microglia towards the site of damage (Haynes et al., 2006;Honda et al., 2001;Maeda et al., 2010;Ohsawa et al., 2007). To date, only the P2Y12 receptor subtype has been explicitly implicated in the development of neuropathic pain. In response to peripheral nerve injury, P2Y12 receptor expression is upregulated on microglia and activation of these receptors is critical for microglial engulfment of myelinated axons in the spinal dorsal horn (Kobayashi et al., 2008;Maeda et al., 2010;Tozaki-Saitoh et al., 2008). Inhibition of these receptors by pharmacological blockade, antisense knockdown of P2Y12 receptor expression, or genetic deletion of the P2ry12 gene, suppresses both mechanical allodynia and thermal hyperalgesia in nerve injured rats (Kobayashi et al., 2008;Tozaki-Saitoh et al., 2008). Conversely, intrathecal administration of the P2Y12 receptor agonist 2Me-SADP elicits pain behaviours in naïve rats that mimic those observed in nerve-injured rats (Kobayashi et al., 2008). The involvement of microglial P2Y12 receptors in neuropathic pain is critically dependent upon p38 MAPK activation (Kobayashi et al., 2008); however, the signaling events downstream from P2Y12 receptor activation of p38 MAPK remain a critical open question.
Conclusion and future directions
Several advances in recent years have emphasized the essential role of microglial P2 receptors in the sequelae of neuropathic pain arising from peripheral nerve injury. In particular, P2X4, P2X7 and P2Y12 receptors expressed on microglia have emerged as new molecular players that are critically involved in the etiology of neuropathic pain. Activation of these P2 receptor subtypes engages distinct intracellular signaling pathways in microglia that converge onto p38 MAPK (Figure 2). Thus, p38 MAPK is a cellular intermediary and its activation is a key point of convergence for P2X4, P2X7, and P2Y12 receptor signaling in neuropathic pain. The next major challenges are to determine the significance of this convergence in signaling for microglia function and how the upstream, as well as, downstream components contribute to aberrant spinal nociceptive processing following nerve injury. In addition to converging signaling pathways, P2 receptor subtypes can interact at the structural level to form unique receptor complexes possessing distinct biophysical properties (Dubyak, 2007;Guo et al., 2007;Jarvis and Khakh, 2009;Nicke, 2008). The potential functional consequences of this physical interaction add another layer of complexity that may have important implications for microglial P2 receptors in neuropathic pain.
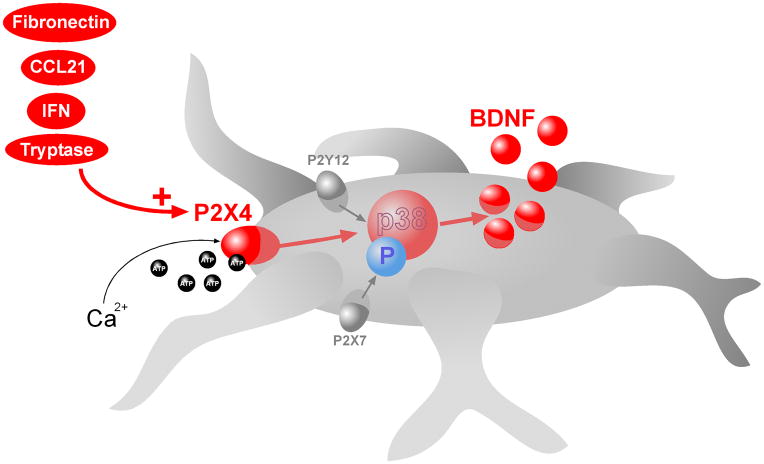
Figure 2. P2X4, P2X7 and P2Y12 receptor signaling converge on p38 MAPK in microglia.
Activity of P2X4, P2X7, and P2Y12 receptors expressed on microglia is critically involved in neuropathic pain arising from peripheral nerve injury. In response to peripheral nerve injury, P2X4 receptors are upregulated in spinal microglia. Several mechanisms have recently been implicated in the upregulation of P2X4 receptors including: CCL21, interferon γ, tryptase, fibronectin, and the activation of mu-opioid receptors. ATP stimulation of P2X4 receptors leads to influx of extracellular Ca2+ which causes activation of p38 MAPK leading to SNARE-dependent release of BDNF from the microglia. BDNF is a key microglia-neuron signaling molecule that causes disinhibition of nociceptive dorsal horn neurons by disrupting intracellular Cl− homeostasis. Disinhibition by microglia-neuron signaling underlies the development of tactile allodynia, a hallmark symptom of neuropathic pain. Activation of P2X7 or P2Y12 receptors also signals through p38 MAPK. P2X7 receptors-p38 MAK signaling drives the release of interleukin-1β and cathepsin S, which in the spinal cord contributes to the maintenance of mechanical hypersensitivity. Although it is known that P2Y12 receptor expression is upregulated in microglia and activation of these receptors is involved in neuropathic pain, the signaling steps downstream from p38 MAPK activation has yet to be elucidated.
The P2X4 receptor has emerged as a core microglia-neuron signaling pathway that is necessary for ongoing expression of tactile allodynia following nerve injury. There have been major advances in understanding the essential molecular components that regulate P2X4 receptor expression and the cellular machinery that drives BDNF release from microglia. These discoveries have built a complete framework for understanding microglia-neuron signaling in neuropathic pain. Based on this new framework, the current model is that activating microglial P2X4 receptors drives the release of BDNF, which causes disinhibition of nociceptive dorsal horn neurons by disrupting intracellular Cl− homeostasis. Disinhibition by microglia-neuron signaling enhances excitatory synaptic transmission in the dorsal horn to transform the output of the nociceptive network. The consequent altered inhibitory responses increases discharge output, unmasks responses to innocuous peripheral inputs and spontaneous activity in neurons that otherwise only transmit nociception(Coull et al., 2003;Coull et al., 2005;Keller et al., 2007). These changes in electrophysiological phenotype of lamina I neurons are the cellular underpinnings that may account for the cardinal signs (allodynia, hyperalgesia, and spontaneous pain) of neuropathic pain. Recent studies have identified an unexpected commonality in mechanisms between neuropathic pain (Coull et al., 2005), morphine-induced hyperalgesia (Ferrini et al., 2010), and the sequelae of spinal cord injury (Boulenguez et al., 2010). Thus, the P2X4R-p38 MAPK-BDNF pathway from microglia to neurons defines a fundamental signaling ensemble that may underlie hyperexcitability across a range central nervous system disorders.
Acknowledgments
MWS is supported by grants from the Canadian Institutes of Health Research (CIHR; grant number MT-11219) and the Neuroscience Canada Brain Repair Program. MWS is an International Research Scholar of the Howard Hughes Medical Institute and holds a Canada Research Chair (Tier I) in Neuroplasticity and Pain. TT was supported by a CIHR fellowship.
Reference List
- Aldskogius H. Regulation of microglia - potential new drug targets in the CNS. Expert Opin Ther Targets. 2001;5:655–668. doi: 10.1517/14728222.5.6.655. [DOI] [PubMed] [Google Scholar]
- Antonio LS, Costa RR, Gomes MD, Varanda WA. Mouse Leydig cells express multiple P2X receptor subunits. Purinergic Signal. 2009;5:277–287. doi: 10.1007/s11302-008-9128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S, Liu XJ, Kwan C, Salter MW. Peripheral nerve injury and TRPV1-expressing primary afferent C-fibers cause opening of the blood-brain barrier. Mol Pain. 2010;6:74. doi: 10.1186/1744-8069-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S, Salter MW. Microglia-neuronal signalling in neuropathic pain hypersensitivity 2.0. Curr Opin Neurobiol. 2010;20:474–480. doi: 10.1016/j.conb.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier LP, Boue-Grabot E, Seguela P. Functional modulation of P2X4 receptor-channels by UDP-activated P2Y6 receptors 2010 [Google Scholar]
- Bernier LP, Ase AR, Chevallier S, Blais D, Zhao Q, Boue-Grabot E, Logothetis D, Seguela P. Phosphoinositides regulate P2X4 ATP-gated channels through direct interactions. J Neurosci. 2008;28:12938–12945. doi: 10.1523/JNEUROSCI.3038-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bester H, Chapman V, Besson JM, Bernard JF. Physiological properties of the lamina I spinoparabrachial neurons in the rat. J Neurophysiol. 2000;83:2239–2259. doi: 10.1152/jn.2000.83.4.2239. [DOI] [PubMed] [Google Scholar]
- Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- Biber K, Tsuda M, Tozaki-Saitoh H, Tsukamoto K, Toyomitsu E, Masuda T, Boddeke H, Inoue K. Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J. 2011 doi: 10.1038/emboj.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs JE, Lu VB, Stebbing MJ, Balasubramanyan S, Smith PA. Is BDNF sufficient for information transfer between microglia and dorsal horn neurons during the onset of central sensitization? Mol Pain. 2010;6:44. doi: 10.1186/1744-8069-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanquet PR. Identification of two persistently activated neurotrophin-regulated pathways in rat hippocampus. Neuroscience. 2000;95:705–719. doi: 10.1016/s0306-4522(99)00489-3. [DOI] [PubMed] [Google Scholar]
- Bobanovic LK, Royle SJ, Murrell-Lagnado RD. P2X receptor trafficking in neurons is subunit specific. J Neurosci. 2002;22:4814–4824. doi: 10.1523/JNEUROSCI.22-12-04814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucsein C, Zacharias R, Farber K, Pavlovic S, Hanisch UK, Kettenmann H. Purinergic receptors on microglial cells: functional expression in acute brain slices and modulation of microglial activation in vitro. Eur J Neurosci. 2003;17:2267–2276. doi: 10.1046/j.1460-9568.2003.02663.x. [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med. 2010;16:302–307. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- Boumechache M, Masin M, Edwardson JM, Gorecki DC, Murrell-Lagnado R. Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells. J Biol Chem. 2009;284:13446–13454. doi: 10.1074/jbc.M901255200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brone B, Moechars D, Marrannes R, Mercken M, Meert T. P2X currents in peritoneal macrophages of wild type and P2X4 −/− mice. Immunol Lett. 2007;113:83–89. doi: 10.1016/j.imlet.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Broom DC, Matson DJ, Bradshaw E, Buck ME, Meade R, Coombs S, Matchett M, Ford KK, Yu W, Yuan J, Sun SH, Ochoa R, Krause JE, Wustrow DJ, Cortright DN. Characterization of N-(adamantan-1-ylmethyl)-5-[(3R-amino-pyrrolidin-1-yl)methyl]-2-chloro-ben zamide, a P2X7 antagonist in animal models of pain and inflammation. J Pharmacol Exp Ther. 2008;327:620–633. doi: 10.1124/jpet.108.141853. [DOI] [PubMed] [Google Scholar]
- Brough D, Le Feuvre RA, Iwakura Y, Rothwell NJ. Purinergic (P2X7) receptor activation of microglia induces cell death via an interleukin-1-independent mechanism. Mol Cell Neurosci. 2002;19:272–280. doi: 10.1006/mcne.2001.1054. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Krugel U, Abbracchio MP, Illes P. Purinergic signalling: From normal behaviour to pathological brain function. Prog Neurobiol. 2011 doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo M, Bennett DL. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Calvo M, Zhu N, Grist J, Ma Z, Loeb JA, Bennett DL. Following nerve injury neuregulin-1 drives microglial proliferation and neuropathic pain via the MEK/ERK pathway. Glia. 2011;59:554–568. doi: 10.1002/glia.21124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, DeLeo JA. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur J Immunol. 2008;38:448–458. doi: 10.1002/eji.200737485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas-Pruneda G, Reyes JP, Perez-Flores G, Perez-Cornejo P, Arreola J. Functional interactions between P2X4 and P2X7 receptors from mouse salivary epithelia. J Physiol. 2009;587:2887–2901. doi: 10.1113/jphysiol.2008.167395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakfe Y, Seguin R, Antel JP, Morissette C, Malo D, Henderson D, Seguela P. ADP and AMP induce interleukin-1beta release from microglial cells through activation of ATP-primed P2X7 receptor channels. J Neurosci. 2002;22:3061–3069. doi: 10.1523/JNEUROSCI.22-08-03061.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Clark AK, Wodarski R, Guida F, Sasso O, Malcangio M. Cathepsin S release from primary cultured microglia is regulated by the P2X7 receptor. Glia. 2010;58:1710–1726. doi: 10.1002/glia.21042. [DOI] [PubMed] [Google Scholar]
- Clark AK, Yip PK, Grist J, Gentry C, Staniland AA, Marchand F, Dehvari M, Wotherspoon G, Winter J, Ullah J, Bevan S, Malcangio M. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:10655–10660. doi: 10.1073/pnas.0610811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology. 1997;36:1277–1283. doi: 10.1016/s0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- Costigan M, Moss A, Latremoliere A, Johnston C, Verma-Gandhu M, Herbert TA, Barrett L, Brenner GJ, Vardeh D, Woolf CJ, Fitzgerald M. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci. 2009a;29:14415–14422. doi: 10.1523/JNEUROSCI.4569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009b;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- Coyle DE. Partial peripheral nerve injury leads to activation of astroglia and microglia which parallels the development of allodynic behavior. Glia. 1998;23:75–83. [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- de Jong EK, Dijkstra IM, Hensens M, Brouwer N, van AM, Liem RS, Boddeke HW, Biber K. Vesicle-mediated transport and release of CCL21 in endangered neurons: a possible explanation for microglia activation remote from a primary lesion. J Neurosci. 2005;25:7548–7557. doi: 10.1523/JNEUROSCI.1019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y. Altered chloride homeostasis in neurological disorders: a new target. Curr Opin Pharmacol. 2007;7:93–99. doi: 10.1016/j.coph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Dehez S, Daulhac L, Kowalski-Chauvel A, Fourmy D, Pradayrol L, Seva C. Gastrin-induced DNA synthesis requires p38-MAPK activation via PKC/Ca(2+) and Src-dependent mechanisms. FEBS Lett. 2001;496:25–30. doi: 10.1016/s0014-5793(01)02396-1. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- Dell’Antonio G, Quattrini A, Cin ED, Fulgenzi A, Ferrero ME. Relief of inflammatory pain in rats by local use of the selective P2X7 ATP receptor inhibitor, oxidized ATP. Arthritis Rheum. 2002;46:3378–3385. doi: 10.1002/art.10678. [DOI] [PubMed] [Google Scholar]
- Dubyak GR. Go it alone no more--P2X7 joins the society of heteromeric ATP-gated receptor channels. Mol Pharmacol. 2007;72:1402–1405. doi: 10.1124/mol.107.042077. [DOI] [PubMed] [Google Scholar]
- Echeverry S, Shi XQ, Rivest S, Zhang J. Peripheral nerve injury alters blood-spinal cord barrier functional and molecular integrity through a selective inflammatory pathway. J Neurosci. 2011;31:10819–10828. doi: 10.1523/JNEUROSCI.1642-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverry S, Shi XQ, Zhang J. Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. Pain. 2008;135:37–47. doi: 10.1016/j.pain.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Elzi DJ, Bjornsen AJ, MacKenzie T, Wyman TH, Silliman CC. Ionomycin causes activation of p38 and p42/44 mitogen-activated protein kinases in human neutrophils. Am J Physiol Cell Physiol. 2001;281:C350–C360. doi: 10.1152/ajpcell.2001.281.1.C350. [DOI] [PubMed] [Google Scholar]
- Eriksson NP, Persson JK, Svensson M, Arvidsson J, Molander C, Aldskogius H. A quantitative analysis of the microglial cell reaction in central primary sensory projection territories following peripheral nerve injury in the adult rat. Exp Brain Res. 1993;96:19–27. doi: 10.1007/BF00230435. [DOI] [PubMed] [Google Scholar]
- Farber K, Kettenmann H. Physiology of microglial cells. Brain Res Brain Res Rev. 2005;48:133–143. doi: 10.1016/j.brainresrev.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal SM, Melchiorri L, Baricordi OR, Di VF. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997a;159:1451–1458. [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di VF. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997b;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Villalba M, Chiozzi P, Falzoni S, Ricciardi-Castagnoli P, Di VF. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J Immunol. 1996;156:1531–1539. [PubMed] [Google Scholar]
- Ferrini F, Mattioli TAM, Lorenzo LE, Godin A, Wiseman PW, Ribeiro-Da-Silva A, Cahill CM, Milne B, DeKoninck Y. Morphine-induced pain hypersensitivity, but not opioid tolerance, depends on microglia-mediated alteration of Cl− homeostasis in the spinal dorsal horn. 2010:678.9/PP14. [Google Scholar]
- Fountain SJ, North RA. A C-terminal lysine that controls human P2X4 receptor desensitization. J Biol Chem. 2006;281:15044–15049. doi: 10.1074/jbc.M600442200. [DOI] [PubMed] [Google Scholar]
- Fujii K, Young MT, Harris KD. Exploiting powder X-ray diffraction for direct structure determination in structural biology: The P2X4 receptor trafficking motif YEQGL. J Struct Biol. 2011 doi: 10.1016/j.jsb.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann J, Banati RB. Microglial turnover in the injured CNS: activated microglia undergo delayed DNA fragmentation following peripheral nerve injury. J Neuropathol Exp Neurol. 1995;54:680–688. doi: 10.1097/00005072-199509000-00010. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordh T, Sharma HS. Chronic spinal nerve ligation induces microvascular permeability disturbances, astrocytic reaction, and structural changes in the rat spinal cord. Acta Neurochir Suppl. 2006;96:335–340. doi: 10.1007/3-211-30714-1_70. [DOI] [PubMed] [Google Scholar]
- Grace PM, Rolan PE, Hutchinson MR. Peripheral immune contributions to the maintenance of central glial activation underlying neuropathic pain. Brain Behav Immun. 2011;25:1322–1332. doi: 10.1016/j.bbi.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD. Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol. 2007;72:1447–1456. doi: 10.1124/mol.107.035980. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience. 2009;161:895–903. doi: 10.1016/j.neuroscience.2009.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. “Gliopathy” maintains persistent hyperexcitability of spinal dorsal horn neurons after spinal cord injury: substrate of central neuropathic pain. In: Costa A, Villalba E, editors. Horizons in Neuroscience Research. Vol. 1. Nova Science Publishers, Inc; 2010. pp. 195–224. [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Donnelly-Roberts D, Namovic M, Zhong C, Wade C, Chandran P, Zhu C, Carroll W, Perez-Medrano A, Iwakura Y, Jarvis MF. The antihyperalgesic activity of a selective P2X7 receptor antagonist, A-839977, is lost in IL-1alphabeta knockout mice. Behav Brain Res. 2009;204:77–81. doi: 10.1016/j.bbr.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P, Harris R, Medrano AP, Carroll W, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006;319:1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- Horvath RJ, DeLeo JA. Morphine enhances microglial migration through modulation of P2X4 receptor signaling. J Neurosci. 2009;29:998–1005. doi: 10.1523/JNEUROSCI.4595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath RJ, Landry RP, Romero-Sandoval EA, DeLeo JA. Morphine tolerance attenuates the resolution of postoperative pain and enhances spinal microglial p38 and extracellular receptor kinase phosphorylation. Neuroscience. 2010;169:843–854. doi: 10.1016/j.neuroscience.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes P, Nieber K, Norenberg W. Electrophysiological effects of ATP on brain neurones. J Auton Pharmacol. 1996;16:407–411. doi: 10.1111/j.1474-8673.1996.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Inoue K. Microglial activation by purines and pyrimidines. Glia. 2002;40:156–163. doi: 10.1002/glia.10150. [DOI] [PubMed] [Google Scholar]
- Inoue K, Tsuda M. The role of microglia and ATP receptors in a mechanism of neuropathic pain. Nippon Yakurigaku Zasshi. 2006;127:14–17. doi: 10.1254/fpj.127.14. [DOI] [PubMed] [Google Scholar]
- Inoue K, Tsuda M. Microglia and neuropathic pain. Glia. 2009;57:1469–1479. doi: 10.1002/glia.20871. [DOI] [PubMed] [Google Scholar]
- Jarvis MF. The neural-glial purinergic receptor ensemble in chronic pain states. Trends Neurosci. 2010;33:48–57. doi: 10.1016/j.tins.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Khakh BS. ATP-gated P2X cation-channels. Neuropharmacology. 2009;56:208–215. doi: 10.1016/j.neuropharm.2008.06.067. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, Mount DB. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol. 2008;4:490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AF, Beggs S, Salter MW, De Koninck Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain. 2007;3:27. doi: 10.1186/1744-8069-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K. P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci. 2008;28:2892–2902. doi: 10.1523/JNEUROSCI.5589-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreideweiss S, Ahlers C, Nordheim A, Ruhlmann A. Ca2+-induced p38/SAPK signalling inhibited by the immunosuppressant cyclosporin A in human peripheral blood mononuclear cells. Eur J Biochem. 1999;265:1075–1084. doi: 10.1046/j.1432-1327.1999.00830.x. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Lever I, Cunningham J, Grist J, Yip PK, Malcangio M. Release of BDNF and GABA in the dorsal horn of neuropathic rats. Eur J Neurosci. 2003;18:1169–1174. doi: 10.1046/j.1460-9568.2003.02848.x. [DOI] [PubMed] [Google Scholar]
- Liu L, Tornqvist E, Mattsson P, Eriksson NP, Persson JK, Morgan BP, Aldskogius H, Svensson M. Complement and clusterin in the spinal cord dorsal horn and gracile nucleus following sciatic nerve injury in the adult rat. Neuroscience. 1995;68:167–179. doi: 10.1016/0306-4522(95)00103-p. [DOI] [PubMed] [Google Scholar]
- Lu VB, Ballanyi K, Colmers WF, Smith PA. Neuron type-specific effects of brain-derived neurotrophic factor in rat superficial dorsal horn and their relevance to ‘central sensitization’. J Physiol. 2007;584:543–563. doi: 10.1113/jphysiol.2007.141267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M, Tsuda M, Tozaki-Saitoh H, Inoue K, Kiyama H. Nerve injury-activated microglia engulf myelinated axons in a P2Y12 signaling-dependent manner in the dorsal horn. Glia. 2010;58:1838–1846. doi: 10.1002/glia.21053. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Namovic MT, Donnelly-Roberts DL, Harris RR, Zhang XF, Shieh CC, Wismer CT, Zhu CZ, Gauvin DM, Fabiyi AC, Honore P, Gregg RJ, Kort ME, Nelson DW, Carroll WA, Marsh K, Faltynek CR, Jarvis MF. P2X7-related modulation of pathological nociception in rats. Neuroscience. 2007;146:1817–1828. doi: 10.1016/j.neuroscience.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller T, Kann O, Verkhratsky A, Kettenmann H. Activation of mouse microglial cells affects P2 receptor signaling. Brain Res. 2000;853:49–59. doi: 10.1016/s0006-8993(99)02244-1. [DOI] [PubMed] [Google Scholar]
- Monif M, Reid CA, Powell KL, Smart ML, Williams DA. The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore. J Neurosci. 2009;29:3781–3791. doi: 10.1523/JNEUROSCI.5512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Kohsaka S. Microglia: activation and their significance in the central nervous system. J Biochem (Tokyo) 2001;130:169–175. doi: 10.1093/oxfordjournals.jbchem.a002969. [DOI] [PubMed] [Google Scholar]
- Nasu-Tada K, Koizumi S, Tsuda M, Kunifusa E, Inoue K. Possible involvement of increase in spinal fibronectin following peripheral nerve injury in upregulation of microglial P2X4, a key molecule for mechanical allodynia. Glia. 2006;53:769–775. doi: 10.1002/glia.20339. [DOI] [PubMed] [Google Scholar]
- Nicke A. Homotrimeric complexes are the dominant assembly state of native P2X7 subunits. Biochem Biophys Res Commun. 2008;377:803–808. doi: 10.1016/j.bbrc.2008.10.042. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Norenberg W, Langosch JM, Gebicke-Haerter PJ, Illes P. Characterization and possible function of adenosine 5′-triphosphate receptors in activated rat microglia. Br J Pharmacol. 1994;111:942–950. doi: 10.1111/j.1476-5381.1994.tb14830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Obata N, Mizobuchi S, Itano Y, Matsuoka Y, Kaku R, Tomotsuka N, Morita K, Kanzaki H, Ouchida M, Yokoyama M. Decoy strategy targeting the brain-derived neurotrophic factor exon I to attenuate tactile allodynia in the neuropathic pain model of rats. Biochem Biophys Res Commun. 2011;408:139–144. doi: 10.1016/j.bbrc.2011.03.137. [DOI] [PubMed] [Google Scholar]
- Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55:604–616. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- Perez-Medrano A, Donnelly-Roberts DL, Honore P, Hsieh GC, Namovic MT, Peddi S, Shuai Q, Wang Y, Faltynek CR, Jarvis MF, Carroll WA. Discovery and biological evaluation of novel cyanoguanidine P2X(7) antagonists with analgesic activity in a rat model of neuropathic pain. J Med Chem. 2009;52:3366–3376. doi: 10.1021/jm8015848. [DOI] [PubMed] [Google Scholar]
- Perry VH. Modulation of microglia phenotype. Neuropathol Appl Neurobiol. 1994;20:177. [PubMed] [Google Scholar]
- Portanova JP, Zhang Y, Anderson GD, Hauser SD, Masferrer JL, Seibert K, Gregory SA, Isakson PC. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi OS, Paramasivam A, Yu JC, Murrell-Lagnado RD. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J Cell Sci. 2007;120:3838–3849. doi: 10.1242/jcs.010348. [DOI] [PubMed] [Google Scholar]
- Rotshenker S. Microglia and macrophage activation and the regulation of complement-receptor-3 (CR3/MAC-1)-mediated myelin phagocytosis in injury and disease. J Mol Neurosci. 2003;21:65–72. doi: 10.1385/JMN:21:1:65. [DOI] [PubMed] [Google Scholar]
- Royle SJ, Bobanovic LK, Murrell-Lagnado RD. Identification of a non-canonical tyrosine-based endocytic motif in an ionotropic receptor. J Biol Chem. 2002;277:35378–35385. doi: 10.1074/jbc.M204844200. [DOI] [PubMed] [Google Scholar]
- Samad TA, Sapirstein A, Woolf CJ. Prostanoids and pain: unraveling mechanisms and revealing therapeutic targets. Trends Mol Med. 2002;8:390–396. doi: 10.1016/s1471-4914(02)02383-3. [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci. 2002;5(Suppl):1062–1067. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- Seil M, El OM, Fontanils U, Etxebarria IG, Pochet S, Dal MG, Marino A, Dehaye JP. Ivermectin-dependent release of IL-1beta in response to ATP by peritoneal macrophages from P2X(7)-KO mice. Purinergic Signal. 2010;6:405–416. doi: 10.1007/s11302-010-9205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Koizumi S, Tsuda M, Ohsawa K, Kohsaka S, Inoue K. Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. J Neurochem. 2001;78:1339–1349. doi: 10.1046/j.1471-4159.2001.00514.x. [DOI] [PubMed] [Google Scholar]
- Shinozaki Y, Sumitomo K, Tsuda M, Koizumi S, Inoue K, Torimitsu K. Direct Observation of ATP-Induced Conformational Changes in Single P2X4 Receptors. PLoS Biol. 2009;7:e103. doi: 10.1371/journal.pbio.1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Hickey WF, Rutkowski MD, Pahl JL, DeLeo JA. Focal peripheral nerve injury induces leukocyte trafficking into the central nervous system: potential relationship to neuropathic pain. Pain. 2002a;100:163–170. doi: 10.1016/s0304-3959(02)00257-9. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, White KA, Dutta C, DeLeo JA. The differential role of spinal MHC class II and cellular adhesion molecules in peripheral inflammatory versus neuropathic pain in rodents. J Neuroimmunol. 2002b;125:82–93. doi: 10.1016/s0165-5728(02)00036-x. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Toulme E, Soto F, Garret M, Boue-Grabot E. Functional properties of internalization-deficient P2X4 receptors reveal a novel mechanism of ligand-gated channel facilitation by ivermectin. Mol Pharmacol. 2006;69:576–587. doi: 10.1124/mol.105.018812. [DOI] [PubMed] [Google Scholar]
- Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci. 2008;28:4949–4956. doi: 10.1523/JNEUROSCI.0323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci. 2009;29:3518–3528. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain. 2009a;5:28. doi: 10.1186/1744-8069-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Masuda T, Kitano J, Shimoyama H, Tozaki-Saitoh H, Inoue K. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci U S A. 2009b;106:8032–8037. doi: 10.1073/pnas.0810420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Toyomitsu E, Komatsu T, Masuda T, Kunifusa E, Nasu-Tada K, Koizumi S, Yamamoto K, Ando J, Inoue K. Fibronectin/integrin system is involved in P2X(4) receptor upregulation in the spinal cord and neuropathic pain after nerve injury. Glia. 2008a;56:579–585. doi: 10.1002/glia.20641. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Toyomitsu E, Kometani M, Tozaki-Saitoh H, Inoue K. Mechanisms underlying fibronectin-induced up-regulation of P2X4R expression in microglia: distinct roles of PI3K-Akt and MEK-ERK signalling pathways. J Cell Mol Med. 2009c;13:3251–3259. doi: 10.1111/j.1582-4934.2009.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Tozaki-Saitoh H, Masuda T, Toyomitsu E, Tezuka T, Yamamoto T, Inoue K. Lyn tyrosine kinase is required for P2X(4) receptor upregulation and neuropathic pain after peripheral nerve injury. Glia. 2008b;56:50–58. doi: 10.1002/glia.20591. [DOI] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmann L, Hirbec H, Rassendren F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J. 2010;29:2290–2300. doi: 10.1038/emboj.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voscopoulos C, Lema M. When does acute pain become chronic? Br J Anaesth. 2010;105(Suppl 1):i69–i85. doi: 10.1093/bja/aeq323. [DOI] [PubMed] [Google Scholar]
- Walz W, Ilschner S, Ohlemeyer C, Banati R, Kettenmann H. Extracellular ATP activates a cation conductance and a K+ conductance in cultured microglial cells from mouse brain. J Neurosci. 1993;13:4403–4411. doi: 10.1523/JNEUROSCI.13-10-04403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Wen YR, Suter MR, Kawasaki Y, Huang J, Pertin M, Kohno T, Berde CB, Decosterd I, Ji RR. Nerve conduction blockade in the sciatic nerve prevents but does not reverse the activation of p38 mitogen-activated protein kinase in spinal microglia in the rat spared nerve injury model. Anesthesiology. 2007;107:312–321. doi: 10.1097/01.anes.0000270759.11086.e7. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Yuan H, Zhu X, Zhou S, Chen Q, Zhu X, Ma X, He X, Tian M, Shi X. Role of mast cell activation in inducing microglial cells to release neurotrophin. J Neurosci Res. 2010;88:1348–1354. doi: 10.1002/jnr.22304. [DOI] [PubMed] [Google Scholar]
- Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Seereeram A, Nassar MA, Levato A, Pezet S, Hathaway G, Morenilla-Palao C, Stirling C, Fitzgerald M, McMahon SB, Rios M, Wood JN. Nociceptor-derived brain-derived neurotrophic factor regulates acute and inflammatory but not neuropathic pain. Mol Cell Neurosci. 2006 doi: 10.1016/j.mcn.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]