Abstract
Acute myeloid leukemia (AML) represents a malignant accumulation of immature myeloid cells in the marrow, presenting with impaired hematopoiesis and its attendant complications, including bleeding, infection, and organ infiltration. Chromosomal abnormalities remain the most powerful predictors of AML prognosis and help to identify a subgroup with favorable prognosis. However, the majority of AML patients who are not in the favorable category succumb to the disease. Therefore, better efforts to identify those patients who may benefit from more aggressive and investigational therapeutic approaches are needed. Newer molecular markers aim at better characterizing the large group of intermediate-risk patients and to identify newer targets for therapy. A group that has seen little improvement over the years is the older AML group, usually defined as age ≥ 60. Efforts to develop less intensive but equally efficacious therapy for this vulnerable population are underway.
Keywords: AML, management, prognosis
Introduction
Acute myeloid leukemia (AML) is a clonal malignant proliferation of myeloid blast cells in the marrow with impaired normal hematopoiesis. AML occurs at an approximate rate of 3 cases per 100,000 individuals per year, causing 1.6% of cancer deaths. According to 2006 estimates, there were 11,930 new cases of AML and 9,040 AML-related deaths in the United States.1 The median age of individuals with newly diagnosed AML continues to increase and averages 66–68 years.1 There generally is no identifiable predisposing cause of AML. However, there are an increasing number of AML cases following exposure to cytotoxic therapy to treat carcinomas or benign conditions (termed therapy-related AML). In addition, AML can arise as the terminal phase of antecedent hematological disorders, such as myelodysplastic syndromes or myeloproliferative disorders. This fact, coupled with an increasing age at diagnosis, can pose significant challenges in the management of AML.2
AML Classification
The French-American-British (FAB) system of AML classification, proposed in the late 1970’s, relied on morphologic features, which were not consistently predictive of clinical behavior.3 The World Health Organization (WHO) proposed a more relevant classification scheme, which focused on characteristics with prognostic value.4 WHO categories include AML with recurrent cytogenetic abnormalities, those with translocation (15;17), the core binding factor leukemias [(CBF), translocation (8;21), inversion 16, or translocation (16;16)], and translocations involving the mixed lineage leukemia (MLL) gene on chromosome 11. AML with dysplasia with or without an antecedent myelodysplastic syndrome, treatment-related AML and AML not-otherwise-specified constitute the remaining categories.4 2 other major differences between the FAB and the WHO classifications are the threshold for AML diagnosis (30% vs. 20% blasts, respectively) and the fact that acute promyelocytic leukemia (APL) and CBF AML are defined as AML based on the chromosomal abnormality regardless of the blast percentage. Although the FAB classification is still utilized in some clinical trials, it has been largely supplanted by the WHO classification.
Predictors of Prognosis
Karyotype
Cytogenetic abnormalities remain the most powerful predictors of outcome and are essential in guiding AML treatment decisions. The importance of chromosomal abnormalities in AML was established by the Medical Research Council (MRC) AML 10 trial, which included cytogenetic information at diagnosis on 1,612 children and adults up to 55 years of age with newly diagnosed AML.5 This data was recently updated by Grimwade et al, based on analysis on 5876 patients treated on the MRC 10, 12, and 15 trials.6 Specifically, their report clarifies the significance of rare re-occurring chromosomal abnormalities in a large cohort of patients age 16–59.6 The South Western Oncology Group (SWOG) and the Medical Research Council (MRC) cytogenetic risk groupings are the most commonly utilized risk categories in the United States.6–8Table 1 describes the differences between these systems. The core binding factor leukemias and APL comprise the favorable risk group with an estimated 10-year overall survival (OS) of 55%–81%. Those in the intermediate risk category had a 10-year OS of 30%–40%, and 10-year OS for the adverse risk category was 8%–20%.6 In addition, the poor impact of monosomal karyotype on AML prognosis, as defined by Breems et al,9 was also demonstrated in recent trials.6,10
Table 1.
| Risk status | SWOG coding | MRC coding |
|---|---|---|
| Favorable | inv(16)/t(16;16)/del(16q), t(15;17) irrespective of additional cytogenetic abnormalities; t(8;21) lacking del(9q) or complex karyotypes | t(15;17), inv(16)/t(16;16), t(8;21); irrespective of additional cytogenetic abnormalities |
| Intermediate | Normal, +8, +6, −Y, del(12p) | Entities not classified as favorable or adverse |
| Unfavorable | abn(3q) del(5q)/−5 −7/del(7q) abn(9q) abn(11q) abn(20q) abn(17p) t(6;9) t(9;22) complex (≥3 unrelated abnormalities) |
In the abscence of favorable risk cytogenetics: abn(3q) [excluding t(3;5)], inv(3)/t(3;3) add(5q), del(5q), −5 −7, add(7q)/del(7q) t(6;11) t(10;11) t(11q23) [excluding t(9;11) and t(11;19)] t(9;22) −17/abn(17p) complex (≥4 unrelated abnormalities) |
| Unknown | All other abnormalities | Category not recognized |
Molecular markers
Much attention has recently been focused on incorporating molecular markers into the prognostic classification of AML. A few of these markers have been incorporated into the routine evaluation of newly-diagnosed AML, as knowledge of the presence or absence of these markers allows clinicians to better identify patients at increased risk of relapse who may benefit from more intensive and curative treatments, such as early allogeneic bone marrow/stem cell transplantation or inclusion onto clinical trials of molecularly targeted agents. These mutations may also play a role in the early detection of minimal residual disease during treatment and follow-up of AML. C-kit is a proto-oncogene that encodes a transmembrane tyrosine kinase receptor and has been implicated in leukemogenesis.11,12 Mutations in the c-kit gene are detected in approximately 20%–40% of CBF AML. In addition, they occur most frequently within exon 17, which encodes the activation loop in the kinase domain, and in exon 8, which encodes the extracellular portion of the kit receptor. Finally, their presence portends a poor prognosis for patients with CBF AML.13–15 Specifically, among patients with t(8;21), kit mutations were associated with higher marrow blast percentage, shorter overall survival (OS), event-free survival (EFS), and shorter time-to-relapse than those without kit mutations. The 5 year OS for adult patients with t(8;21) harboring exon 17 c-kit mutations was shorter than for those without mutations (27% vs. 61%).15 The prognostic effect of kit mutations among pediatric CBF AML has not been clearly established.15,16 Among patients with inv(16), data on the prognostic impact of c-kit mutations has been less consistent.14,15 Therefore, it is reasonable to recommend a risk-adapted approach to the management of AML with t(8;21) based on the presence or absence of c-kit (exon 17) mutations.
Among the cytogenetically normal group of AML (CN-AML), several molecular abnormalities have prognostic implications. Those include point mutations or internal tandem duplication (ITD) in the FMS-like Tyrosine Kinase 3 (FLT3) gene, which confer a prognosis similar to that of AML with adverse risk cytogenetics.17–19 Isolated mutations in the nucleophosmin (NPM1) gene and the CCAAT/enhancer binding protein α gene (CEPBA) confer a favorable prognosis.20,21 Among CN-AML, compared to NPM1 wild type, NPM1 mutations (in the absence of FLT3 mutations) independently predict for higher CR rates (84% vs. 48%, P < 0.001), longer DFS (23% at 3 years vs. 10%, P = 0.004), and longer OS (3 year rates of 35% vs. 8%, P < 0.001).22 CEBPA mutations were also identified as independent indicators of favorable prognosis, even after adjusting for cytogenetics and FLT3 status, with an estimated 5-year OS of 53% vs. 25% for unmutated CEBPA (P = 0.04).23 Patel et al reported the prognostic relevance of integrated genetic profiling in samples from 398 patients treated on the ECOG E1900 phase 3 clinical trial.24 In this trial, when compared to standard-dose daunorubicin (45 mg/m2), high-dose daunorubicin (90 mg/m2) improved the rate of survival among patients with mutated DNA (cytosine-5)-methyltransferase 3A (DNMT3A), NPM1, or MLL translocations.24 The use of mutational profiling is leading to re-classification of the subgroup of patients defined as having intermediate-risk AML, based on chromosomal analysis, and reassigns a significant number of them to the favorable or unfavorable-risk subgroups. Such efforts aim to more precisely define the heterogeneous group of AML patients with intermediate-risk cytogenetics and to better select those who may benefit from allogeneic transplantation in 1st complete remission as curative therapy. A summary of selected reported mutations to date is in Table 2.25–27 Evaluation of these molecular markers should be incorporated into the management of newly diagnosed AML, especially among those who are potential candidates for allogeneic bone marrow/stem cell transplantation. Indeed, in 2010, the European Leukemia Net (ELN) investigators proposed a standardized system for reporting chromosomal and selected molecular abnormalities in AML trials.28 The proposed ELN system, which utilizes 4 genetic groups (favorable, intermediate I, intermediate II, and adverse) was recently applied to 818 adults aged < 60 years and 732 adults aged ≥ 60 years with AML treated on Cancer and Leukemia Group B (CALGB) first-line trials, in a study that confirmed the prognostic significance of the ELN classification. Not surprisingly, within each ELN group, older patients had worse outcomes when compared to younger patients.27
Table 2.
Molecular abnormalities with prognostic value in normal cytogenetic AML.
Minimal residual disease (MRD) monitoring in AML with specific molecular markers has been evolving over the last few years. However, except for the case of APL, where there is a standard assay and international guidelines for MRD monitoring,29,30 the findings are not widely generalizable due to the different MRD methods utilized. The MRC AML15 trial revealed that MRD monitoring by quantitative RT-PCR at specific time points in CBF AML allows identification of patients at high risk of relapse and hinted at a future role for risk-directed or preemptive therapy.31 Defining clinically relevant time points for NPM1 MRD assessment has allowed for the identification of patients with AML at high risk of relapse who may benefit from more aggressive treatments.32 The achievement of PCR negativity following induction therapy identified patients with an incidence of relapse of 6.5% at 4 years and an OS of 90%, compared to 53% and 51% for those with positive NPM1 mutations (detected by PCR) respectively (P < 0.001).32 Overexpression of the Wilms tumor (WT1) gene in AML has led to its use as a potential marker of MRD.33 However, its utility has been hindered by variability in performance of the assays evaluated to date. The European Leukemia Net (ELN) undertook a systematic evaluation of a range of published WT1 assays and established the basis for a highly sensitive assay, which could predict reduced relapse risk among subjects with greater WT1 transcript reduction after induction chemotherapy.34 Detection of MRD is likely to become a standard endpoint in clinical trials.
Age and AML prognosis
The prognostic significance of age at the time of AML diagnosis has been well described.35,36 Most trials of induction and post remission therapy have focused on patients younger than 60 years of age, with good performance status. However, given that the age at diagnosis of AML is increasing, attention is shifting toward a focus on the older AML population. The combination of poor performance status and older age identifies a group of patients with extremely high induction-related mortality, while the effect of performance status tends to be less pronounced among the younger AML population.35
Other prognostic factors
The white blood cell (WBC) count at time of AML diagnosis has variable prognostic significance. Patients with a white cell count exceeding 100,000 tend to have a higher risk of complications during induction therapy but a similar overall outcome when compared to patients with a WBC count less than 100,000 cells/mcL.37,38 In addition, hyperleukocytosis (WBC > 100,000) has been identified as a risk factor for central nervous system (CNS) involvement with AML.39,40 The significance of CNS involvement with AML has been debated, with some authors reporting a worse prognosis and others reporting no effect on AML prognosis.39,41 Pediatric regimens routinely utilize CNS prophylaxis while adult protocols do not.28 However, sampling of cerebrospinal fluid and intrathecal prophylaxis should be considered in patients with AML and hyperleukocytosis. Extramedullary disease (EMD) in general has been associated with lower rates of CR and inferior long-term prognosis in adult AML when compared to those presenting without EMD.41 The development of AML in the setting of an antecedent hematological disorder or prior exposure to chemotherapy or radiation therapy has been associated with less favorable prognosis when compared to de-novo AML; however, this group of AML patients also tends to have less favorable cytogenetic abnormalities.42
Obesity has been found to be a negative prognostic factor in pediatric AML.43 The Children’s Oncology Group (COG) found that body mass index (BMI) > 95th percentile was predictive of worse outcomes, with lower complete remission (CR), EFS and OS.43 However, the same effect has not been consistently demonstrated in adult AML. In fact, in a recent report, increased BMI was evaluated among 63 newly-diagnosed AML patients receiving induction chemotherapy. In that cohort, 33% of patients were obese (≥130% ideal body weight [IBW]). The authors found no significant effect of obesity on CR, OS, count recovery, or rates of non-hematological toxicity. In addition, CR rates were not significantly different between patients whose chemotherapy doses were not adjusted for IBW and those with dose adjustments (CR = 86% vs. 67%, P = 0.55).44 Le et al evaluated whether obesity affected outcomes of 329 adult patients who received high dose cytarabine-based regimens for induction. In this study, in which doses of chemotherapy were administered according to actual body weight, 1/3 of patients were obese, and BMI did not have a negative impact on OS or treatment-related complications.45
Treatment Strategies
The treatment of AML has not significantly evolved over the last 2 decades, with curative treatment still dependent on effective induction therapy to achieve a complete remission and subsequent risk-adapted consolidation therapy to prevent relapse. Response to induction chemotherapy is substantial, with over 2/3 of younger adults and approximately 1/2 of older adults achieving complete remission.2 The challenge remains in keeping patients in remission, as relapse is expected in most of the responding patients, especially those without favorable cytogenetic/molecular features. The increased age at diagnosis of AML and the higher likelihood of the patient population to be frail, with borderline performance status makes delivering what is considered standard induction therapy more challenging. Thus, there is a predilection to divide therapy into intensive versus non-intensive regimens when approaching the older AML patient.
Induction therapy
Early trials by the Cancer and Leukemia Group B (CALGB) in the 1980’s established the “7 + 3” regimen consisting of cytarabine at a dose of 100 mg/m2/day by continuous infusion for 7 days and daunorubicin at a dose of 45 mg/m2/day for 3 days, as the standard induction regimen for newly diagnosed AML in the United States.46,47 This regimen led to remissions in 60%–80% of younger adults and 40%–60% of older adults.48 A decade later, we learned that idarubicin at 12/mg/m2 was superior to the standard dose of daunorubicin for AML induction, especially among younger patients.49,50 The MD Anderson group performed a large retrospective analysis to compare the relative activity of combinations containing cytarabine and idarubicin without fludarabine or topotecan (IA) and those containing cytarabine with either topotecan (TA) or fludarabine (FA). This analysis included 1,279 patients treated for newly diagnosed AML, refractory anemia with excess blasts (RAEB) or RAEB in transformation (RAEB-t) at their center. Despite the limitations of a retrospective study, this large study revealed that IA combinations generally led to higher CR rates and better overall outcomes than the TA and FA combinations, albeit in a very heterogeneous population of patients.51
Different modifications of the 7 + 3 regimen have been carried out, including intensifying the dose of cytarabine, changing the type or dose of anthracycline, and adding additional chemotherapeutic agents (Table 3). The Acute Leukemia French Association (ALFA) 9000 study was a randomized comparison of control “3 + 7” induction (daunorubicin 80 mg/m2/d for 3 days and cytarabine at 200 mg/m2/d by continuous infusion for 7 days), double induction (3 + 7 followed by mitoxantrone 12 mg/m2/d on days 20–21 and cytarabine at 500 mg/m2 every 12 hours on days 20–22), and timed-sequential induction (daunorubicin 80 mg/m2/d and cytarabine at 500 mg/m2/d by continuous infusion on days 1–3, followed by mitoxantrone 12 mg/m2/d on days 8–9 and cytarabine at 500 mg/m2 every 12 hours on days 8–10). Consolidation was similar, but dose was stratified according to patient age. Among 592 subjects, 449 (76%) patients obtained complete remission. EFS at 2 and 5 years was estimated at 20.5% and 19.4% respectively and OS was estimated at 45.4% and 29.8% at 2 and 5 years, with no significant difference among the 3 treatment arms.52 The subsequent ALFA-9801 evaluated the effect of high doses of daunorubicin (DNR at 80 mg/m2/d × 3 days) or idarubicin (IDA4 at 12 mg/m2/d × 4 days) with standard doses of idarubicin (IDA3; 12 mg/m2/d × 3 days) for remission induction in patients with AML age 50–70. The complete remission rates were significantly higher for the Idarubicin groups when compared to the high dose daunorubicin group (83%, 78%, and 70% in the IDA3, IDA4, and DNR arms, respectively; P = 0.04); however, no significant differences were observed in relapse incidence, EFS, or OS among the 3 arms.53 The MRC AML 12 trial addressed the question of optimal anthracycline for induction in pediatric AML and reported a remission rate of 92% (73% CR after Course 1 and 20% after Course 2) with no difference among treatment groups when using mitoxantrone or daunorubicin with cytarabine and etoposide. 10-year EFS and OS was 54% and 63% respectively and relapse risk was estimated at 35%. There was a benefit for using mitoxantrone with regard to relapse rate (32% vs. 39%; Hazard ratio (HR) 0.73) and disease-free survival (DFS; 63% vs. 55%; HR 0.72), but this benefit did not translate into better EFS or OS.54 The ECOG investigators recently published results of the E1900 clinical trial comparing daunorubicin at 90 mg/m2 with the standard 45 mg/m2 dosing for AML induction in patients age 18–60 years. The results revealed better OS in the favorable and intermediate cytogenetic groups with the higher doses of daunorubicin, with complete remission rates in 70% versus 57% respectively (P < 0.001).55 In the unfavorable cytogenetic group, the overall survival was similar between the 2 dosing schemas. In AML patients, older than 60 years of age, escalating the dose of daunorubicin to 90 mg/m2/dose, with the entire dose administered in the first induction cycle, lead to a higher response rate (CR of 64% vs. 54%, P = 0.002) when compared to the conventional (45 mg/m2/dose) dose, without additional toxicity. However, survival outcomes did not differ between the 2 dosing groups.56
Table 3.
Treatment regimens for AML.
| Study group | Regimen | Patients | CR (%) | Survival (m) | Year |
|---|---|---|---|---|---|
| CALGB135 | ARA-C/dauno 45–60 mg/m2 | 668 | 68 | 10 | 1987 |
| ECOG136 | ARA-C/ida (7 + 3) | 214 | 70 vs. 59 | 12.9 vs. 7.8 | 1992 |
| SWOG137 | HDAC + dauno vs. DA | 665 | 55 vs. 58 | NR | 1996 |
| MRC AML 1572 | Standard induction ± GO | 1115 | 85 vs. 85 | NR | 2006 |
| ECOG 190055 | Dauno 90 vs. 45 + ARA-C | 657 | 70 vs. 57.3 | 23.7 vs. 15.7 | 2009 |
| HOVON/SAKK138 | HDAC vs. ARA-C | 860 | 82 vs. 80 | 42 vs. 40* | 2011 |
| ECOG 3489139 | Post remission HIDAC | 740 | 83 vs. 74 vs. 56 | 19 | 1998 |
| CALGB 9222140 | Post remission multiagent chemo vs. HIDAC | 474 | 72 | 13 vs. 12 | 2005 |
Abbreviations: CALGB, Cancer and Leukemia Group B; ECOG, Eastern Cooperative Oncology Group; SWOG, Southwest Oncology Group; MRC, Medical Research Council; HOVON/SAKK, Hemato Oncology Foundation for Adults in the Netherlands/Swiss Group for Clinical Cancer Research; NR, not reported.
= % survival at 5 years.
Post-remission therapy
It has been recognized since the 1970’s that additional chemotherapy is required in order to sustain initial remissions in AML.57,58 In the early 1980’s, the ECOG investigators conducted a randomized trial (EST 3483) comparing the effect of continuation of chemotherapy (consolidation or prolonged maintenance) with no further therapy in AML patients after the achievement of complete remission. Accrual to the “no further therapy” arm was halted after the interim analysis discovered that all the patients in the “no further therapy” arm had relapsed by a median of 4 months.59 Subsequently, the final analysis of the EST 3483 trial revealed that a single course of consolidation or allogeneic transplantation (patients age ≤ 40) were superior to 2 years of maintenance therapy for AML in first remission, and that consolidation with high dose cytarabine (3 gm/m2 IV over 1 hour every 12 hours for 12 consecutive doses, days 1–6) and amsacrine (100 mg/m2/d IV for 3 days, days 7–9) was extremely toxic for patients aged ≥ 60 years (early mortality of 57% for age ≥ 60 vs. 13% for <60 years old).60 Consolidation regimens have included intensive chemotherapy, non-intensive regimens, allogeneic and autologous transplantation. The choice of consolidation therapy is guided by baseline cytogenetic and molecular markers, with allogeneic transplantation in first complete remission being potentially curative for the unfavorable cytogenetic/molecular subgroup. The MRC AML8 trial randomized AML patients in complete remission following 2 courses of induction therapy with daunorubicin, cytarabine, and 6-thioguanine (DAT) to consolidation with 2 vs. 6 further courses of DAT. Overall outcomes (OS and EFS) were not different between the 2- and 6-course groups. However, 6 courses of DAT consolidation resulted in a reduction in the number of relapses that occurred beyond the first year after randomization.61 This large trial also found no significant advantage to central nervous system (CNS) prophylaxis with intrathecal cytarabine and methotrexate, and no benefit for late intensification vs. continued maintenance chemotherapy.61 The Cancer and Leukemia Group B (CALGB) evaluated 3 dosing schedules of cytarabine (3 gm/m2 twice daily on days 1, 3, and 5 vs. 400 mg/m2 or 100 mg/m2 daily for 5 days) for 4 cycles in consolidation (followed by maintenance) for AML in first remission. This trial established the commonly used high dose (3 grams) Ara-C (HiDAC) regimen62 for AML in first CR, with a higher probability of remaining in continuous CR at 4 years for the HiDAC group compared to the lower doses of Ara-C (44% vs. 24% and 29%).62 The benefit of HiDAC consolidation was greatest for younger patients with CBF AML, and to a lesser degree for those with intermediate risk cytogenetics.63 Of note, interim analysis of this trial revealed that the regimen was prohibitively toxic in patients age 60 and older and that fewer than 50% of the patients older than 60 had received the intended number of cycles of HiDAC.62 Our experience using a modified HiDAC schedule (2 gm/m2 daily for 6 days and daunorubicin 45 mg/m2 daily for 3 days) for induction and consolidation revealed promising remissions (69%) and <10% 30-day mortality in patients age 60 and older with newly-diagnosed de-novo AML.64 The US intergroup Study, E3489/S9034, compared 1 cycle of high-dose cytarabine with allogeneic and autologous transplantation for patients with AML in first CR and did not reveal a difference in OS.65 However, when analyzed according to cytogenetic grouping, patients with unfavorable cytogenetics had better outcome with allogeneic transplantation.7 Similarly, the German AML Cooperative Group study group (AMLCG) reported a landmark analysis of patients younger than 60 years with AML and high-risk cytogenetics in CR1, treated on the AMLCG 99 who underwent allogeneic transplantation as consolidation therapy. When compared to patients who received continued consolidation chemotherapy, those who received allogeneic transplantation experienced superior 5-year OS (48% vs. 18% respectively, P = 0.004) and RFS (39% vs. 10% respectively, P < 0.001).66 The MRC AML 10 trial aimed to assess the benefit of high-dose therapy followed by autologous or allogeneic bone marrow transplantation (BMT) in patients age < 56 in first CR after receiving 4 courses of intensive chemotherapy. In that trial, patients received up to 2 cycles of induction chemotherapy. Patients who achieved CR received 2 further courses of chemotherapy and those who had an HLA-matched sibling went on to allogeniec BMT after the 4th course of chemotherapy. Patients who lacked an HLA-matched sibling donor were randomized to either autologous BMT or no further treatment. There was no significant survival advantage for autologous BMT when compared to the no-further-treatment arm.67 There was a significant reduction in relapses for the autologous BMT group when compared to the no-further-treatment arm (37% vs. 58%, P < 0.001), but this did not translate into a benefit on OS, likely due to an excess of death in CR for the autologous BMT group vs. the no-further-treatment arm.67 Similarly, in that trial, the relapse risk was reduced in the donor arm (36%) when compared to the no donor arm (52%; P = 0.001) and the disease-free survival (DFS) was also improved for the donor vs. no donor arm (50% vs. 42% respectively; P = 0.01). However, OS was not different (55% vs. 50%) at 7 years.68
Gemtuzumab ozogamicin (GO) is an anti-CD33 immunoconjugate that is worth mentioning due to its recent media coverage. GO was granted approval in 2000 under the FDA’s accelerated approval program, for patients ≥ 60 years of age with AML in first relapse, not considered candidates for standard cytotoxic therapy, based on its activity in the relapsed setting.69,70 Subsequently, GO was voluntarily withdrawn from the US market in 2010 after the confirmatory study designed to assess improvement in survival for the addition of GO to standard chemotherapy in previously untreated AML showed no improvement in clinical benefit, as well as an increased 30-day mortality for patients who received GO compared to those who received chemotherapy alone. The phase III SWOG “S0106” trial randomized patients aged 18–60 with newly diagnosed AML to induction with daunorubicin and cytarabine (“3 + 7”, AD) chemotherapy +/− GO at 6 mg/m2 on day 4. Patients who achieved CR received 3 cycles of consolidation with high dose cytarabine, and those who remained in CR after consolidation were eligible for a second randomization between either 3 doses of GO at 5 mg/m2 every 28 days, or observation. The interim analysis revealed almost identical CR rates (66% for AD + GO vs. 69% for AD), no difference in relapse-free or overall survival, and a significantly higher rate of fatal adverse events for the AD + GO group (5.8%) vs. the AD group (0.8%, P = 0.002).71
These results led to the closure of the S0106 trial by the SWOG DSMC on August 11, 2009 and the voluntary withdrawal of GO from the market. It is postulated that the non-fractionated dosing schema that was used may have led to the increase in GO-associated toxicity. European investigators have explored fractionated, lower doses of GO in an attempt to reduce GO-associated toxicity, and have found promising results. The randomized MRC AML15 trial showed that the addition of GO at 3 mg/m2 on day 1 of the first and third courses of chemotherapy did not add any advantage over chemotherapy alone in terms of relapse, relapse-free survival, or overall survival. However, a prespecified analysis according to cytogenetic grouping revealed a significant benefit of GO in the younger, favorable-risk patients, with a 79% OS for GO vs. a 51% OS for the control group, with no added toxicity.72 Recently, investigators from the Acute Leukemia French Association conducted a study (ALFA-0701) designed to compare the addition of low, fractionated doses of GO to standard front-line chemotherapy in 280 patients age 50–70 years with previously untreated de novo AML. Patients were randomized 1:1 to standard chemotherapy (“3 + 7”) +/− 5 doses of GO administered at 3 mg/m2/dose on days 1, 4 and 7 during induction and on day 1 of each of 2 consolidation chemotherapy cycles. They found no difference in rates of CR (81% for GO and 75% for control); but at 2 years, the estimated EFS was 40.8% in the GO group vs. 17.1% in the control group (hazard ratio [HR] 0.58; P = 0.0003), OS was 53.2% vs. 41.9% (GO vs. control, respectively; HR 0.69; P = 0.0368), and RFS 50.3% for GO vs. 22.7% for the control arm (HR 0.52; P = 0.0003) all in benefit of the GO group. Hematological toxicity, particularly persistent thrombocytopenia, was more common in the GO group than in the control group (16% vs. 3%; P < 0.0001), without an increase in toxic deaths (9/139 [6%] for GO vs. 5/139 [4%] for control).73 The MRC AML16 trial showed that the addition of GO to low dose Ara-C, in older patients with AML, doubled the CR rate but did not improve OS.74 However, in the AML16-intensive trial, adding GO at 3 mg/m2 on day 1 of the first course of chemotherapy in older patients with AML did not affect the CR rate, but a benefit was seen in terms of cumulative incidence of relapse (CIR; 3-year CIR 68% for GO vs. 76% for control arm; P = 0.007), RFS (21% vs. 16% at 3 years; P = 0.04), and OS at 3 years (25% vs. 20% for GO vs. control arm respectively, P = 0.05).75 A meta-analysis of the 2 large trials (AML 15 and AML 16) confirmed the benefit of adding GO to upfront AML therapy, with a reduction in relapse (HR, 0.82; 95% CI, 0.72 to 0.93; P = 0.002) and improved survival (HR, 0.88; 95% CI, 0.79 to 0.98; P = 0.02. These trials support a role for GO administered at 3 mg/m2 in combination chemotherapy for the upfront treatment of AML.
AML adults older than 60 years
Reasons for the poorer prognosis of AML in older adults relate to patient-related factors such as advanced age, comorbidities, and poor performance status, leading to early induction-mortality. In addition, disease-related factors, such as lower incidence of favorable cytogenetics, higher incidence of unfavorable cytogenetics,35 higher incidence of secondary AML,76 and higher expression of the multi-drug resistance gene77 lead to a more resistant disease when compared to younger AML. There may also be physician-related factors that limit treatment options in the aging AML population. Indeed, among over 2500 AML patients of medicare age, chemotherapy was given to only 30 percent of the population. The median survival for treated patients was 7 months versus 1 month for the untreated patients.78
Enrollment of patients older than 60 years onto AML clinical trials was curtailed in the early 1990s due to high induction-mortality, despite the fact that intensive induction regimens have led to higher rates of complete remission (Table 4). The search for less intensive chemotherapy regimens is ongoing. Current non-intensive regimens include hypomethylating agents such as azacitidine and decitabine. These agents appear to have a favorable toxicity profile and promising remission rates in the range of 20%–50%.79–83 Clofarabine, a next generation nucleoside analogue, has shown promising results that await phase III confirmation.84,85 In a randomized comparison, low dose cytarabine (20 mg subcutaneously twice daily for 10 days on 4–6 week cycles showed a better remission rate (18% vs. 1%) and better overall survival compared to hydroxyurea in AML patients considered unfit for induction chemotherapy.86 Low-dose cytarabine has since become a standard comparator arm for randomized trials for newly diagnosed, older, unfit, AML patients. Clinical trials with combination regimens of non-intensive agents are ongoing.
Table 4.
Treatment of Older AML patients ≥ 60 years of age.64
| Study group | Regimen | Patients | CR (%) | Early death (%) (within 30 days) |
|---|---|---|---|---|
| AMLCG | TAD9 induction and consolidation +/− maintenance | 511 | 51 | 27–34 |
| BMRC | DAT induction and consolidation | 636 | 46–48 | 30–52 |
| CALGB | Standard or intensified 7 + 3 +/− maintenance | 556 | 41–47 | 31–54 |
| SECSG | Idarubicin vs. daunorubicin + cytarabine | 111 | 53 | 20 |
| EORTC/LCG HOVON | Mitoxantrone vs. daunorubicin +/− LDAC3 maintenance | 489 | 38–47 | 6–15 |
| MDAC | Cytarabine-based intensive induction | 430 | 45 | 36 |
| Clofarabine | Clofarabine monotherapy | 112 | 38 | 10 |
| Emory | Modified HiDAC induction/consolidation | 59 | 69 | 10 |
Abbreviations: AMLCG, AML Cooperative Group; BMRC, Behavioral Medicine Research Center; CALGB, Cancer and Leukemia Group B; SECSG, Southeastern Cancer Study Group; EORTC, European Organisation for Research and Treatment of Cancer; HOVON, Hemato Oncology Foundation for Adults in the Netherlands; MDAC, MD Anderson Cancer Center.
Acute promyelocytic leukemia
APL is a distinct subtype of AML characterized by a unique reciprocal translocation, t(15;17)(q22;q11-12), leading to a fusion between the promyelocytic leukemia (PML) gene on chromosome 15 and the retinoic acid receptor-α (RARA) gene on chromosome 17. This results in a blockade in the differentiation of granulocytic precursors. The PML-RARA fusion gene is detectable in over 95% of APL cases, while variant rearrangements have been detected in the remainder of cases.87 APL cases with these variant rearrangements do not have the same clinical behavior as typical APL and are treated similar to non-APL AML due to their resistance to all-trans retinoic acid and arsenic trioxide.
Due to the profound bleeding diathesis that is characteristic of APL, the mere suspicion of APL diagnosis by morphologic evaluation should trigger a reflexive mechanism of treatment and supportive care strategies, including frequent transfusions with fresh frozen plasma, cryoprecipitate and platelets to treat disseminated intravascular coagulation88 and prompt initiation of all-trans-retinoic acid (ATRA), due to its ability to control APL-associated coagulopathy and to decrease the risk of bleeding.89 The following step should be confirmation of the APL diagnosis in bone marrow or peripheral blood at the genetic level, by demonstration of the t(15;17) or the PML/RARA gene by chromosomal analysis, fluorescence in-situ hybridization (FISH), or reverse transcriptase polymerase chain reaction (PCR) since the efficacy of ATRA and arsenic trioxide depends on its presence.90,91
Early recognition of the retinoic acid or differentiation syndrome, which presents with dyspnea, fever, acute renal failure, and pulmonary infiltrates, together with a rising white blood cell count, usually after initiation of targeted or cytotoxic therapy, should prompt the rapid institution of dexamethasone (10 mg twice daily). Some authors advocate preemptive dexamethasone in APL patients at risk for the syndrome due to presenting WBC > 5–10,000 cells/mcL.88
APL therapy
Once a diagnosis of APL has been confirmed, induction therapy should be promptly started. While ATRA alone induced remissions in the majority of APL patients, the risk of relapse was high.92 Subsequently, several groups worldwide have confirmed the superiority of ATRA in combination with anthracycline-based chemotherapy for APL, with remissions in approximately 95% of patients.93–96 Three APL risk groups have been identified, based on the presenting WBC count and platelet count at diagnosis, with those presenting with WBC > 10,000 cells/mcL being considered high risk, those with presenting WBC ≤ 10,000 cells/mcL and platelets ≤ 40,000 cells/mcL being intermediate, and those presenting with WBC ≤ 10,000 cells/mcL and platelets > 40,000 cells/mcL considered to be at low risk of relapse.97 Based on this distinction, risk-adapted therapy has become the standard, providing more therapy for those who are at high risk of relapse, and less therapy and therefore potentially fewer treatment-related complications for those who are at lower risk of relapse. The most recent update from the Programa Espaňol de Tratamientos en Hematología (PETHEMA) and Hemato-Oncologie voor Volwassenen Nederland (HOVON) investigators, the LPA2005 trial, found a significantly lower 3-year relapse rate (11% in the LPA2005 vs. 26% in the LPA99 trial) with the addition of cytarabine for high-risk patients and reduced duration of cytopenias and hospital stay for low- and intermediate-risk patients after a reduced dose of mitoxantrone in the second consolidation without sacrificing efficacy.98 The European Acute Promyelocytic Leukemia Group (APL93 and 2000 trials) showed a benefit to escalating the dose of cytarabine and adding intrathecal therapy to the ATRA–anthracycline combination, especially for high-risk patients, in addition to confirming the role of maintenance therapy in APL. Of note, the APL 93 and 2000 trials utilized daunorubicin, instead of idarubicin, which was used in the PETHEMA trials.96,99 The role of low-dose oral maintenance therapy has also become standard, although authors have questioned the utility of maintenance therapy in low-risk APL. It is not advisable to adapt or mix and match portions or different regimens. Indeed, the expert panel on behalf of the LeukemiaNet support the use of maintenance therapy with protocols in which maintenance was shown to confer a benefit.29
The role of arsenic trioxide (ATO) is being expanded from the relapsed setting into the upfront setting.100–103 With a median follow-up of 5 years, the EFS, disease-free survival, and OS were 69%, 80%, and 74% respectively for 72 patients with APL treated with single-agent ATO for induction, consolidation, and maintenance.103 It should be noted that anthracycline was allowed at induction for patients with rapidly rising WBC.102 Data on 197 newly diagnosed APL patients from Tehran treated with single-agent ATO daily until CR, followed by 1–4 courses of ATO for consolidation, was promising, with an 86% CR rate, 67% DFS and 64% OS at 5 years.104 At the recent 2012 annual meeting of the American Society of Hematology, Lo-Coco and colleagues presented remarkable results of a phase III trial that randomized newly-diagnosed patients with low- and intermediate-risk APL to ATRA + ATO vs. ATRA + idarubicin (IDA). In this non-inferiority trial, the ATRA + ATO combination not only proved “not inferior” but it also was superior to ATRA + idarubicin with respect to 2-year EFS (97% vs. 86.7% for ATRA + ATO vs. ATRA + IDA respectively; P = 0.03) and OS (98.7% vs. 91.1%; P = 0.03), with fewer episodes of fever and less prolonged cytopenia in the ATRA + ATO arm.105 ATO has been combined with chemotherapy, ATRA, and/or gemtuzumab ozogamicin (GO) in newly diagnosed APL106,107 and is currently being tested in combination with GO, ATRA, and chemotherapy for newly-diagnosed high-risk APL in an intergroup trial.
AML salvage therapy
Despite major advances in the upfront management of AML, relapse remains the leading cause of death for most patients. Among the total number of AML patients who relapse, about 10% survive long term.108 While there is no standard salvage regimen for relapsed AML, what is well accepted is that relapsed AML patients should be considered for inclusion onto clinical trials of salvage therapy and that for those who achieve a subsequent remission, allogeneic transplantation can be curative. Several groups have attempted to define prognostic factors for success/failure to facilitate treatment decisions for relapsed AML.108–111 Despite the promise of cure with high-dose therapy and allogeneic transplantation, this approach carries significant treatment-related morbidity and mortality, and until recently there was no reproducible way to predict who is most likely to benefit from this approach.
Chevallier et al published a prognostic scoring system based on an analysis of 138 adult patients with relapsed/refractory AML undergoing treatment with an intensive salvage regimen of chemotherapy + GO.112 3 poor prognostic markers were identified: early relapse (<1 year after complete remission), unfavorable cytogenetics, and the presence of FLT3-ITD. Age was not found to be a significant prognostic factor. The prognostic scoring system was validated on an independent cohort of 111 patients and a clear-cut difference existed between relapsed AML patients with 0–1 factors (2-year OS 23%–35%), and those with 2–3 adverse factors (2-year OS 0%) in the validation group.112 In addition, a study by the Center for International Blood and Marrow Transplant Research (CIBMTR) focused on AML patients not in CR at the time of transplantation (with myeloablative conditioning) and identified 5 adverse factors: first CR < 6 months, circulating blasts, donor other than matched sibling, Karnofsky performance status < 90%, and adverse risk cytogenetics, with 3-year survival of 42% for those with none of those factors, 28% for those with 1 adverse factor, 15% for 2 factors and 6% if ≥3 adverse factors were present.113 These are important studies to keep in mind when tailoring intensive therapy for relapsed AML patients.
Clofarabine is a novel purine nucleoside analogue that is structurally similar to fludarabine and cladribine. Clofarabine has shown activity against AML in phase I/II studies.114,115 Recent trials have shown efficacy of clofarabine (CLO) in combination with high-dose cytarabine and granulocyte colony-stimulating factor (G-CSF) priming (GCLAC), in the treatment of patients with relapsed or refractory acute myeloid leukaemia, where 46% of patients achieved CR (61% CR + CRi) and their median survival was 9 months.114 In addition, the CLASSIC I trial, a phase III trial comparing ara-C alone to CLO+ ara-C in patients with relapsed/refractory AML age ≥ 55 showed a superior CR rate for the combination (35% vs. 22.9% respectively; P < 0.01), but no difference in OS was seen.116 An interesting combination included temsirolimus, a mammalian target of rapamycin (mTOR) inhibitor, in combination with lower-dose clofarabine as salvage therapy for older patients with AML. In this phase II trial reported by the Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) cooperative group, CLO was administered at 20 mg/m2 on days 1–5 with temsirolimus at 25 mg on days 1, 8, and 15, followed by monthly maintenance with temsirolimus for those who achieved CR. The overall response rate (ORR) was 21% (8% CR and 13% CRi), with a median DFS and OS of 3.5 months and 4 months, respectively, and 30-day induction mortality of 13%. Perhaps the most interesting finding was that .50% in vivo inhibition of S6 ribosomal protein phosphorylation was highly correlated with responses (75% response with inhibition and 0% without inhibition).117 The role of CLO alone and in combination for AML continues to be investigated (see Tables 5 and 6).
Table 5.
Selected clinical trials in older AML ≥ 60.
| Population | Treatment | Phase |
|---|---|---|
| AML > 60 | Cladribine + LDAC | II |
| AML > 60 | Temozolomide + vorinostat | II |
| AML > 60 | Entinostat, AZA | I |
| AML > 65 | Len, AZA or combo | II |
| AML > 65 | Tipifarnib | II |
| AML > 70 | Sapacitabine + decitabine | III |
| AML > 60 | Plerixafor + clofarabine | I |
| AML > 60 | Midostaurin + decitabine | I |
| AML > 60 | Everolimus, mito, VP16, ARA-C, Ida | I |
| AML > 60 | TKI AC220, plerixafor w SI | I |
| AML > 60 | Clofarabine + ARA-C | I |
| AML > 65 | Panobinostat | I |
| AML > 60 | Clofarabine or DA followed by decitabine | III |
| AML > 65 | Panobinostat | I |
| AML > 60 | LDAC + AZD1152 | II |
| AML > 60 | Sorafenib | II |
| AML > 60 | Clofarabine + ARA-C + decitabine | II |
Table 6.
Selected clinical trials for AML 18–60 years of age.
| Population | Treatment | Phase |
|---|---|---|
| AML | Plerixafor, mitoxantrone, VP16, ARA-C | I |
| AML | Pazopanib | I |
| AML | Decitabine + bexarotene | I |
| AML | Temsirolimus | II |
| AML | Plerixafor, mitoxantrone, VP16, ARA-C | I |
| AML | Tosedostat + ARA-C or decitabine | II |
| AML | Alvocidib + mitoxantrone + DA | II |
| FLT3 AML | Midostaurin + DA | I |
| AML | Clofarabine vs. fludarabine with IA | I |
| AML | Panobinostat +DA | I |
| AML | Dasatinib + DA | II |
| AML | Plerixafor + DA | I |
| AML | Vorinostat + AZA | II |
| AML | Clofarabine vs. HDAC | II |
| CBF AML | Dasatinib +/− DA | I |
| FLT3 AML | Plerixafor, sorafenib, GCSF | I |
| AML | SGI-110 | I |
| AML | Clofarabine + temsirolimus | II |
| AML | Fludarabine + IA + GCSF | II |
| AML | GO, mitoxantrone, VP16 | I |
Mitoxantrone and etoposide (ME)-based salvage regimens alone (ME) or with intermediate dose cytarabine (MEC) have been successfully used for the treatment of primary induction failure or relapsed AML,118,119 but the activity of ME vs. MEC has not been directly compared. Recently, Trifilio et al reported outcomes of 65 patients with relapsed/refractory AML who received either MEC or ME as salvage. They found a CR rate of 59% for MEC treated patients and 34% for ME treated patients (P = NS) with a similarly poor median OS at 5.2 months of follow-up.120 The MEC regimen is commonly used as the comparator arm in randomized trials of salvage therapy.
Fludarabine-based combinations provided an important advance in the management of AML.121 Fludarabine plus cytarabine (FA) was active in newly-diagnosed AML and MDS, with 53% CRs. The addition of G-CSF (FLAG) did not add to the activity of the regimen, although it accelerated the time to neutrophil recovery (34 days for FA vs. 21 days for FLAG; P < 0.001).122 In a prospective multi-center phase II trial, FLAG achieved CRs in 17/21 (81%) of patients with late relapse AML and 13/44 (30%) in those with early relapse/refractory AML, with the main toxicity being severe myelosuppression.123 The addition of Idarubicin (FLAG-Ida) did not add to the response rate.124–126 Adding GO to FLAG-Ida in the salvage setting does not appear to improve response rates.127 In a randomized setting, a large study by the MRC (MRC-HR) found that fludarabine and high-dose cytarabine (FLA) may be inferior to their standard cytarabine, daunorubicin, and etoposide (ADE) combination for high-risk (relapsed, refractory, or adverse cytogenetic) AML, with no differences in CR and DFS, but worse survival with FLA vs. ADE (16% vs. 27% at 4 years respectively; P = 0.05). In addition, the trial found no benefit to the addition of G-CSF or ATRA to either regimen.128 This study highlighted the difficulty in defining a standard treatment for AML salvage and clarifies some of the questions raised in small, single-center studies, which comprise the majority of data on relapsed/refractory AML.
Newer approaches for AML salvage include the addition of targeted agents to chemotherapy combinations. Due to the poor prognosis associated with FLT3 ITD and point mutations in both newly diagnosed and relapsed AML, targeting FLT-3 became the subject of intense study. Several FLT3 inhibitors in the form of monoclonal antibodies and tyrosine kinase inhibitors have been developed.129,130 Among those, lestaurtinib (CEP 701) has been evaluated in Phase III, in a randomized multi-center trial of 224 adult patients with FLT3-mutant AML in first relapse. Patients were randomized prior to the start of salvage chemotherapy to either receive or not receive lestaurtinib starting 2 days after completion of salvage chemotherapy (day 7). Salvage chemotherapy was selected based on duration of 1st CR (CR1), with either MEC (CR1 of 1–6 months) or high-dose cytarabine (HiDAC; CR1 of 6–24 months). This large, randomized trial failed to demonstrate improvement in CR rate or survival, with 29/112 (26%) patients on the lestaurtinib arm achieving CR + CRp compared to 23/112 (21%) for the control arm (P = 0.35). There was, however, a difference in the level of toxicity, with discontinuation due to toxicity in 24% of patients on the lestaurtinib arm compared to 7% of control arm. In addition, there was a lack of correlation between high lestaurtinib drug levels and in vivo FLT3 inhibition. Although there was a hint that FLT3 inhibition may correlate with activity, this conclusion could not be drawn due to the small number of responders.131 Results of a Phase II trial of quizartinib (AC220), an effective FLT3 inhibitor, were presented to the 2012 ASH meeting. This drug showed single-agent activity against both FLT3-ITD+ (CRc [CR + CRp + CRi] = 44/99 or 44%) and FLT3-ITD-(CRc = 13/38 or 34%) relapsed/refractory AML.132 Approximately 1/3 of the patients in this trial were bridged to allogeneic transplantation. This level of single-agent activity in relapsed/refractory AML is unprecedented and warrants further development of quizartinib for AML.
Other available agents registered on trial are seen in Tables 5 and 6. The absence of a standard for relapsed/refractory AML makes patients with relapsed/refractory AML potential candidates for clinical trials. For those who achieve subsequent remissions, allogeneic transplantation may be curative.
Summary
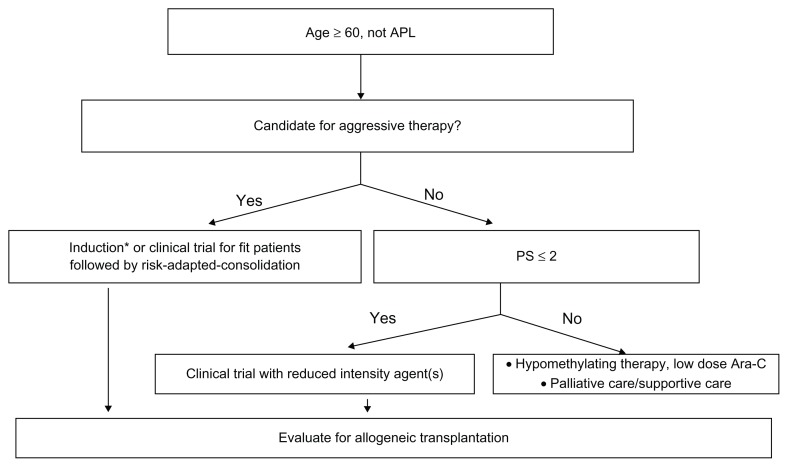
Advances in diagnostic technology leading to better classification and risk stratification in AML have led to improved outcomes for younger patients. However, more efforts are being focused on the older AML population. Treatment decisions should take into account patient and disease-related factors in order to improve responses and limit treatment-related toxicity (see Fig. 1 for a proposed algorithm for older AML patients). Inclusion of patients into clinical trials remains critical if we hope to improve AML outcomes in the future. Among APL patients, efforts continue to focus on improving the incorporation of targeted therapy into multi-agent regimens.
Figure 1.
Proposed algorithm for newly-diagnosed AML.
Note: *= 7 + 3 or modified HiDAC induction.
Footnotes
Author Contributions
Conceived and designed the experiments: FER, MA. Analyzed the data: FER, MA. Wrote the first draft of the manuscript: FER, MA. Agree with manuscript results and conclusions: FER, MA. Jointly developed the structure and arguments for the paper: FER, MA. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
Funding
Author(s) disclose no funding sources.
References
- 1.National Cancer Institute. SEER Stat Fact Sheets: Acute Myeloid Leukemia, 1975–2009. [Accessed Apr 16, 2013]. Available at http://seer.cancer.gov/statfacts/html/amyl.html#incidence-mortality.
- 2.Rowe JM. Optimal induction and post-remission therapy for AML in first remission. Hematology Am Soc Hematol Educ Program. 2009:396–405. doi: 10.1182/asheducation-2009.1.396. [DOI] [PubMed] [Google Scholar]
- 3.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33(4):451–8. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 4.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100(7):2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 5.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92(7):2322–33. [PubMed] [Google Scholar]
- 6.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–65. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 7.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075–83. [PubMed] [Google Scholar]
- 8.Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100(13):4325–36. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 9.Breems DA, Van Putten WL, De Greef GE, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008;26:4791–7. doi: 10.1200/JCO.2008.16.0259. [DOI] [PubMed] [Google Scholar]
- 10.Medeiros BC, Othus M, Fang M, Roulston D, Appelbaum FR. Prognostic impact of monosomal karyotype in young adult and elderly acute myeloid leukemia: the Southwest Oncology Group (SWOG) experience. Blood. 2010;116(13):2224–8. doi: 10.1182/blood-2010-02-270330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birg F, Rosnet O, Carbuccia N, Birnbaum D. The expression of FMS, KIT and FLT3 in hematopoietic malignancies. Leuk Lymphoma. 1994;13(3–4):223–7. doi: 10.3109/10428199409056285. [DOI] [PubMed] [Google Scholar]
- 12.Tsao AS, Kantarjian H, Thomas D, et al. C-kit receptor expression in acute leukemias-association with patient and disease characteristics and with outcome. Leuk Res. 2004;28(4):373–8. doi: 10.1016/j.leukres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Boissel N, Leroy H, Brethon B, et al. Incidence and prognostic impact of c-Kit, FLT3, and Ras gene mutations in core binding factor acute myeloid leukemia (CBF-AML) Leukemia. 2006;20(6):965–70. doi: 10.1038/sj.leu.2404188. [DOI] [PubMed] [Google Scholar]
- 14.Paschka P, Marcucci G, Ruppert AS, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study. J Clin Oncol. 2006;24(24):3904–11. doi: 10.1200/JCO.2006.06.9500. [DOI] [PubMed] [Google Scholar]
- 15.Park SH, Chi HS, Min SK, Park BG, Jang S, Park CJ. Prognostic impact of c-KIT mutations in core binding factor acute myeloid leukemia. Leuk Res. 2011;35(10):1376–83. doi: 10.1016/j.leukres.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Shimada A, Taki T, Tabuchi K, et al. KIT mutations, and not FLT3 internal tandem duplication, are strongly associated with a poor prognosis in pediatric acute myeloid leukemia with t(8;21): a study of the Japanese Childhood AML Cooperative Study Group. Blood. 2006;107(5):1806–9. doi: 10.1182/blood-2005-08-3408. [DOI] [PubMed] [Google Scholar]
- 17.Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111(5):2776–84. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- 18.Whitman SP, Ruppert AS, Radmacher MD, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008;111(3):1552–9. doi: 10.1182/blood-2007-08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kayser S, Schlenk RF, Londono MC, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood. 2009;114(12):2386–92. doi: 10.1182/blood-2009-03-209999. [DOI] [PubMed] [Google Scholar]
- 20.Dohner K, Schlenk RF, Habdank M, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005;106(12):3740–6. doi: 10.1182/blood-2005-05-2164. [DOI] [PubMed] [Google Scholar]
- 21.Tenen DG. Abnormalities of the CEBP alpha transcription factor: a major target in acute myeloid leukemia. Leukemia. 2001;15(4):688–9. doi: 10.1038/sj.leu.2402088. [DOI] [PubMed] [Google Scholar]
- 22.Becker H, Marcucci G, Maharry K, et al. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene- and microRNA-expression signatures: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28(4):596–604. doi: 10.1200/JCO.2009.25.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preudhomme C, Sagot C, Boissel N, et al. Favorable prognostic significance of CEBPA mutations in patients with de novo acute myeloid leukemia: a study from the Acute Leukemia French Association (ALFA) Blood. 2002;100(8):2717–23. doi: 10.1182/blood-2002-03-0990. [DOI] [PubMed] [Google Scholar]
- 24.Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–89. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 26.Marcucci G, Haferlach T, Dohner H. Molecular genetics of adult acute myeloid leukemia: prognostic and therapeutic implications. J Clin Onc. 2011;29(5):475–86. doi: 10.1200/JCO.2010.30.2554. [DOI] [PubMed] [Google Scholar]
- 27.Mrozek K, Marcucci G, Nicolet D, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30(36):4515–23. doi: 10.1200/JCO.2012.43.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 29.Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113(9):1875–91. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 30.Reiter A, Lengfelder E, Grimwade D. Pathogenesis, diagnosis and monitoring of residual disease in acute promyelocytic leukaemia. Acta Haematol. 2004;112(1–2):55–67. doi: 10.1159/000077560. [DOI] [PubMed] [Google Scholar]
- 31.Yin JA, O’Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. 2012;120(14):2826–35. doi: 10.1182/blood-2012-06-435669. [DOI] [PubMed] [Google Scholar]
- 32.Kronke J, Schlenk RF, Jensen KO, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011;29(19):2709–16. doi: 10.1200/JCO.2011.35.0371. [DOI] [PubMed] [Google Scholar]
- 33.Inoue K, Sugiyama H, Ogawa H, et al. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood. 1994;84(9):3071–9. [PubMed] [Google Scholar]
- 34.Cilloni D, Renneville A, Hermitte F, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009;27(31):5195–201. doi: 10.1200/JCO.2009.22.4865. [DOI] [PubMed] [Google Scholar]
- 35.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–5. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Estey E. AML in older patients: are we making progress? Best Pract Res Clin Haematol. 2009;22(4):529–36. doi: 10.1016/j.beha.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Dutcher JP, Schiffer CA, Wiernik PH. Hyperleukocytosis in adult acute nonlymphocytic leukemia: impact on remission rate and duration, and survival. J Clin Oncol. 1987;5(9):1364–72. doi: 10.1200/JCO.1987.5.9.1364. [DOI] [PubMed] [Google Scholar]
- 38.Greenwood MJ, Seftel MD, Richardson C, et al. Leukocyte count as a predictor of death during remission induction in acute myeloid leukemia. Leuk Lymphoma. 2006;47(7):1245–52. doi: 10.1080/10428190600572673. [DOI] [PubMed] [Google Scholar]
- 39.Johnston DL, Alonzo TA, Gerbing RB, Lange BJ, Woods WG. The presence of central nervous system disease at diagnosis in pediatric acute myeloid leukemia does not affect survival: a Children’s Oncology Group study. Pediatr Blood Cancer. 2010;55(3):414–20. doi: 10.1002/pbc.22511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pui CH, Dahl GV, Kalwinsky DK, et al. Central nervous system leukemia in children with acute nonlymphoblastic leukemia. Blood. 1985;66(5):1062–7. [PubMed] [Google Scholar]
- 41.Chang H, Brandwein J, Yi QL, Chun K, Patterson B, Brien B. Extramedullary infiltrates of AML are associated with CD56 expression, 11q23 abnormalities and inferior clinical outcome. Leuk Res. 2004;28(10):1007–11. doi: 10.1016/j.leukres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Larson RA. Is secondary leukemia an independent poor prognostic factor in acute myeloid leukemia? Best Pract Res Clin Haematol. 2007;20(1):29–37. doi: 10.1016/j.beha.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a children’s oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children’s oncology group. Blood. 2008;111(3):1044–53. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin A, Othus M, McQuary A, Chi M, Estey E. Influence of obesity on efficacy and toxicity of induction chemotherapy in patients with newly diagnosed acute myeloid leukemia. Leuk Lymphoma. 2013;54(3):541–6. doi: 10.3109/10428194.2012.717278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee HJ, Licht AS, Hyland AJ, et al. Is obesity a prognostic factor for acute myeloid leukemia outcome? Ann Hematol. 2012;91(3):359–65. doi: 10.1007/s00277-011-1319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yates J, Glidewell O, Wiernik P, et al. Cytosine arabinoside with daunorubicin or adriamycin for therapy of acute myelocytic leukemia: a CALGB study. Blood. 1982;60(2):454–62. [PubMed] [Google Scholar]
- 47.Rai KR, Holland JF, Glidewell OJ, et al. Treatment of acute myelocytic leukemia: a study by cancer and leukemia group B. Blood. 1981;58(6):1203–12. [PubMed] [Google Scholar]
- 48.Estey E, Smith TL, Keating MJ, McCredie KB, Gehan EA, Freireich EJ. Prediction of survival during induction therapy in patients with newly diagnosed acute myeloblastic leukemia. Leukemia. 1989;3(4):257–63. [PubMed] [Google Scholar]
- 49.Vogler WR, Velez-Garcia E, Weiner RS, et al. A phase III trial comparing idarubicin and daunorubicin in combination with cytarabine in acute myelogenous leukemia: a Southeastern Cancer Study Group Study. J Clin Oncol. 1992;10(7):1103–11. doi: 10.1200/JCO.1992.10.7.1103. [DOI] [PubMed] [Google Scholar]
- 50.Reiffers J, Huguet F, Stoppa AM, et al. A prospective randomized trial of idarubicin vs. daunorubicin in combination chemotherapy for acute myelogenous leukemia of the age group 55 to 75. Leukemia. 1996;10(3):389–95. [PubMed] [Google Scholar]
- 51.Estey EH, Thall PF, Cortes JE, et al. Comparison of idarubicin + ara-C-, fludarabine + ara-C-, and topotecan + ara-C-based regimens in treatment of newly diagnosed acute myeloid leukemia, refractory anemia with excess blasts in transformation, or refractory anemia with excess blasts. Blood. 2001;98(13):3575–83. doi: 10.1182/blood.v98.13.3575. [DOI] [PubMed] [Google Scholar]
- 52.Castaigne S, Chevret S, Archimbaud E, et al. Randomized comparison of double induction and timed-sequential induction to a “3 + 7” induction in adults with AML: long-term analysis of the Acute Leukemia French Association (ALFA) 9000 study. Blood. 2004;104(8):2467–74. doi: 10.1182/blood-2003-10-3561. [DOI] [PubMed] [Google Scholar]
- 53.Pautas C, Merabet F, Thomas X, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol. 2010;28(5):808–14. doi: 10.1200/JCO.2009.23.2652. [DOI] [PubMed] [Google Scholar]
- 54.Gibson BE, Webb DK, Howman AJ, De Graaf SS, Harrison CJ, Wheatley K. Results of a randomized trial in children with Acute Myeloid Leukaemia: medical research council AML12 trial. Br J Haematol. 2011;155(3):366–76. doi: 10.1111/j.1365-2141.2011.08851.x. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361(13):1249–59. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lowenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361(13):1235–48. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]
- 57.Peterson BA, Bloomfield CD. Prolonged maintained remissions of adult acute non-lymphocytic leukaemia. Lancet. 1977;2(8030):158–60. doi: 10.1016/s0140-6736(77)90178-7. [DOI] [PubMed] [Google Scholar]
- 58.Gale RP. Advances in the treatment of acute myelogenous leukemia. N Engl J Med. 1979;300(21):1189–99. doi: 10.1056/NEJM197905243002105. [DOI] [PubMed] [Google Scholar]
- 59.Cassileth PA, Harrington DP, Hines JD, et al. Maintenance chemotherapy prolongs remission duration in adult acute nonlymphocytic leukemia. J Clin Oncol. 1988;6(4):583–7. doi: 10.1200/JCO.1988.6.4.583. [DOI] [PubMed] [Google Scholar]
- 60.Cassileth PA, Lynch E, Hines JD, et al. Varying intensity of postremission therapy in acute myeloid leukemia. Blood. 1992;79(8):1924–30. [PubMed] [Google Scholar]
- 61.Rees JK, Gray RG, Swirsky D, Hayhoe FG. Principal results of the Medical Research Council’s 8th acute myeloid leukaemia trial. Lancet. 1986;2(8518):1236–41. doi: 10.1016/s0140-6736(86)92674-7. [DOI] [PubMed] [Google Scholar]
- 62.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994;331(14):896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 63.Bloomfield CD, Lawrence D, Byrd JC, et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998;58(18):4173–9. [PubMed] [Google Scholar]
- 64.Arellano M, Winton E, Pan L, et al. High-dose cytarabine induction is well tolerated and active in patients with de novo acute myeloid leukemia older than 60 years. Cancer. 2012;118(2):428–33. doi: 10.1002/cncr.26290. [DOI] [PubMed] [Google Scholar]
- 65.Cassileth PA, Harrington DP, Appelbaum FR, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med. 1998;339(23):1649–56. doi: 10.1056/NEJM199812033392301. [DOI] [PubMed] [Google Scholar]
- 66.Stelljes M, Beelen DW, Braess J, et al. Allogeneic transplantation as post-remission therapy for cytogenetically high-risk acute myeloid leukemia: landmark analysis from a single prospective multicenter trial. Haematologica. 2011;96(7):972–9. doi: 10.3324/haematol.2011.041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burnett AK, Goldstone AH, Stevens RM, et al. Randomised comparison of addition of autologous bone-marrow transplantation to intensive chemotherapy for acute myeloid leukaemia in first remission: results of MRC AML 10 trial. UK Medical Research Council Adult and Children’s Leukaemia Working Parties. Lancet. 1998;351(9104):700–8. doi: 10.1016/s0140-6736(97)09214-3. [DOI] [PubMed] [Google Scholar]
- 68.Burnett AK, Wheatley K, Goldstone AH, et al. The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: results of the UK MRC AML 10 trial. Br J Haematol. 2002;118(2):385–400. doi: 10.1046/j.1365-2141.2002.03724.x. [DOI] [PubMed] [Google Scholar]
- 69.Sievers EL, Larson RA, Stadtmauer EA, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19(13):3244–54. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 70.Bross PF, Beitz J, Chen G, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7(6):1490–6. [PubMed] [Google Scholar]
- 71.Petersdorf S, Kopecky K, Stuart R, et al. Preliminary results of Southwest Oncology Group Study S0106: An international intergroup phase 3 randomized trial comparing the addition of gemtuzumab ozogamicin to standard induction therapy followed by a second randomization to post-consolidation gemtuzumab ozogamicin versus no additional therapy for previously untreated acute myeloid leukemia. Blood. 2009;114:790. [Google Scholar]
- 72.Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29(4):369–77. doi: 10.1200/JCO.2010.31.4310. [DOI] [PubMed] [Google Scholar]
- 73.Castaigne S, Pautas C, Terre C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379(9825):1508–16. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 74.Burnett AK, Hills RK, Hunter AE, et al. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia. 2013;27(1):75–81. doi: 10.1038/leu.2012.229. [DOI] [PubMed] [Google Scholar]
- 75.Burnett AK, Russell NH, Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol. 2012;30(32):3924–31. doi: 10.1200/JCO.2012.42.2964. [DOI] [PubMed] [Google Scholar]
- 76.Bauduer F, Ducout L, Dastugue N, Capdupuy C, Renoux M. De novo and secondary acute myeloid leukemia in patients over the age of 65: a review of fifty-six successive and unselected cases from a general hospital. Leuk Lymphoma. 1999;35(3–4):289–96. doi: 10.3109/10428199909145732. [DOI] [PubMed] [Google Scholar]
- 77.Leith CP, Kopecky KJ, Godwin J, et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89(9):3323–9. [PubMed] [Google Scholar]
- 78.Menzin J, Lang K, Earle CC, Kerney D, Mallick R. The outcomes and costs of acute myeloid leukemia among the elderly. Arch Intern Med. 2002;162(14):1597–603. doi: 10.1001/archinte.162.14.1597. [DOI] [PubMed] [Google Scholar]
- 79.Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28(4):556–61. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 80.Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci U S A. 2010;107(16):7473–8. doi: 10.1073/pnas.1002650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boumber Y, Kantarjian H, Jorgensen J, et al. A randomized study of decitabine versus conventional care for maintenance therapy in patients with acute myeloid leukemia in complete remission. Leukemia. 2012;26:2428–31. doi: 10.1038/leu.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562–9. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 83.Keating GM. Azacitidine: a review of its use in the management of myelodysplastic syndromes/acute myeloid leukaemia. Drugs. 2012;72(8):1111–36. doi: 10.2165/11209430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 84.Kantarjian HM, Erba HP, Claxton D, et al. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol. 2010;28(4):549–55. doi: 10.1200/JCO.2009.23.3130. [DOI] [PubMed] [Google Scholar]
- 85.Claxton D, Erba HP, Faderl S, et al. Outpatient consolidation treatment with clofarabine in a phase 2 study of older adult patients with previously untreated acute myelogenous leukemia. Leuk Lymphoma. 2012;53(3):435–40. doi: 10.3109/10428194.2011.616960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109(6):1114–24. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 87.Lo-Coco F, Ammatuna E. The biology of acute promyelocytic leukemia and its impact on diagnosis and treatment. Hematology Am Soc Hematol Educ Program. 2006:156–61. 514. doi: 10.1182/asheducation-2006.1.156. [DOI] [PubMed] [Google Scholar]
- 88.Sanz MA, Tallman MS, Lo-Coco F. Tricks of the trade for the appropriate management of newly diagnosed acute promyelocytic leukemia. Blood. 2005;105(8):3019–25. doi: 10.1182/blood-2004-09-3475. [DOI] [PubMed] [Google Scholar]
- 89.Barbui T, Finazzi G, Falanga A. The impact of all-trans-retinoic acid on the coagulopathy of acute promyelocytic leukemia. Blood. 1998;91(9):3093–102. [PubMed] [Google Scholar]
- 90.Sanz MA, Tallman MS, Lo-Coco F. Practice points, consensus, and controversial issues in the management of patients with newly diagnosed acute promyelocytic leukemia. Oncologist. 2005;10(10):806–14. doi: 10.1634/theoncologist.10-10-806. [DOI] [PubMed] [Google Scholar]
- 91.Ohno R, Asou N, Ohnishi K. Treatment of acute promyelocytic leukemia: strategy toward further increase of cure rate. Leukemia. 2003;17(8):1454–63. doi: 10.1038/sj.leu.2403031. [DOI] [PubMed] [Google Scholar]
- 92.Huang ME, Ye YC, Chen SR, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72(2):567–72. [Google Scholar]
- 93.Sanz MA, Martin G, Gonzalez M, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood. 2004;103(4):1237–43. doi: 10.1182/blood-2003-07-2462. [DOI] [PubMed] [Google Scholar]
- 94.Fenaux P, Le Deley MC, Castaigne S, et al. Effect of all transretinoic acid in newly diagnosed acute promyelocytic leukemia. Results of a multicenter randomized trial. European APL 91 Group. Blood. 1993;82(11):3241–9. [PubMed] [Google Scholar]
- 95.Lo-Coco F, Avvisati G, Vignetti M, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA Group. Blood. 2010;116(17):3171–9. doi: 10.1182/blood-2010-03-276196. [DOI] [PubMed] [Google Scholar]
- 96.Ades L, Chevret S, Raffoux E, et al. Is cytarabine useful in the treatment of acute promyelocytic leukemia? Results of a randomized trial from the European Acute Promyelocytic Leukemia Group. J Clin Oncol. 2006;24(36):5703–10. doi: 10.1200/JCO.2006.08.1596. [DOI] [PubMed] [Google Scholar]
- 97.Sanz MA, Lo Coco F, Martin G, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96(4):1247–53. [PubMed] [Google Scholar]
- 98.Sanz MA, Montesinos P, Rayon C, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115(25):5137–46. doi: 10.1182/blood-2010-01-266007. [DOI] [PubMed] [Google Scholar]
- 99.Kelaidi C, Chevret S, De Botton S, et al. Improved outcome of acute promyelocytic leukemia with high WBC counts over the last 15 years: the European APL Group experience. J Clin Oncol. 2009;27(16):2668–76. doi: 10.1200/JCO.2008.18.4119. [DOI] [PubMed] [Google Scholar]
- 100.Soignet SL, Maslak P, Wang ZG, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339(19):1341–8. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 101.Soignet SL, Frankel SR, Douer D, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19(18):3852–60. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- 102.Mathews V, George B, Lakshmi KM, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood. 2006;107(7):2627–32. doi: 10.1182/blood-2005-08-3532. [DOI] [PubMed] [Google Scholar]
- 103.Mathews V, George B, Chendamarai E, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: long-term follow-up data. J Clin Oncol. 2010;28(24):3866–71. doi: 10.1200/JCO.2010.28.5031. [DOI] [PubMed] [Google Scholar]
- 104.Ghavamzadeh A, Alimoghaddam K, Rostami S, et al. Phase II study of single-agent arsenic trioxide for the front-line therapy of acute promyelocytic leukemia. J Clin Oncol. 2011;29(20):2753–7. doi: 10.1200/JCO.2010.32.2107. [DOI] [PubMed] [Google Scholar]
- 105.Lo-Coco FM, Avvisati G, Ferrara F, et al. ATRA and arsenic trioxide (ATO) versus ATRA and idarubicin (AIDA) for newly diagnosed, non high-risk acute promyelocytic leukemia (APL): results of the phase III, prospective, randomized, intergroup APL0406 study by the Italian-German cooperative groups Gimema-SAL-AMLSG. Blood. 2012;120:6. [Google Scholar]
- 106.Ravandi F, Estey E, Jones D, et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009;27(4):504–10. doi: 10.1200/JCO.2008.18.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Powell BL, Moser B, Stock W, et al. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood. 2010;116(19):3751–7. doi: 10.1182/blood-2010-02-269621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23(9):1969–78. doi: 10.1200/JCO.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 109.Uhlman DL, Bloomfield CD, Hurd DD, Peterson BA. Prognostic factors at relapse for adults with acute myeloid leukemia. Am J Hematol. 1990;33(2):110–6. doi: 10.1002/ajh.2830330207. [DOI] [PubMed] [Google Scholar]
- 110.Sander A, Zimmermann M, Dworzak M, et al. Consequent and intensified relapse therapy improved survival in pediatric AML: results of relapse treatment in 379 patients of three consecutive AML-BFM trials. Leukemia. 2010;24(8):1422–8. doi: 10.1038/leu.2010.127. [DOI] [PubMed] [Google Scholar]
- 111.Kurosawa S, Yamaguchi T, Miyawaki S, et al. Prognostic factors and outcomes of adult patients with acute myeloid leukemia after first relapse. Haematologica. 2010;95(11):1857–64. doi: 10.3324/haematol.2010.027516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chevallier P, Labopin M, Turlure P, et al. A new Leukemia Prognostic Scoring System for refractory/relapsed adult acute myelogeneous leukaemia patients: a GOELAMS study. Leukemia. 2011;25(6):939–44. doi: 10.1038/leu.2011.25. [DOI] [PubMed] [Google Scholar]
- 113.Duval M, Klein JP, He W, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28(23):3730–8. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Becker PS, Kantarjian HM, Appelbaum FR, et al. Clofarabine with high dose cytarabine and granulocyte colony-stimulating factor (G-CSF) priming for relapsed and refractory acute myeloid leukaemia. Br J Haematol. 2011;155(2):182–9. doi: 10.1111/j.1365-2141.2011.08831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Faderl S, Ferrajoli A, Wierda W, et al. Clofarabine combinations as acute myeloid leukemia salvage therapy. Cancer. 2008;113(8):2090–6. doi: 10.1002/cncr.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Faderl S, Wetzler M, Rizzieri D, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. J Clin Oncol. 2012;30(20):2492–9. doi: 10.1200/JCO.2011.37.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Amadori S, Stasi R, Martelli AM, et al. Temsirolimus, an mTOR inhibitor, in combination with lower-dose clofarabine as salvage therapy for older patients with acute myeloid leukaemia: results of a phase II GIMEMA study (AML-1107) Br J Haematol. 2012;156(2):205–12. doi: 10.1111/j.1365-2141.2011.08940.x. [DOI] [PubMed] [Google Scholar]
- 118.Ho AD, Lipp T, Ehninger G, et al. Combination of mitoxantrone and etoposide in refractory acute myelogenous leukemia—an active and well-tolerated regimen. J Clin Oncol. 1988;6(2):213–7. doi: 10.1200/JCO.1988.6.2.213. [DOI] [PubMed] [Google Scholar]
- 119.Amadori S, Arcese W, Isacchi G, et al. Mitoxantrone, etoposide, and intermediate-dose cytarabine: an effective and tolerable regimen for the treatment of refractory acute myeloid leukemia. J Clin Oncol. 1991;9(7):1210–4. doi: 10.1200/JCO.1991.9.7.1210. [DOI] [PubMed] [Google Scholar]
- 120.Trifilio SM, Rademaker AW, Newman D, et al. Mitoxantrone and etoposide with or without intermediate dose cytarabine for the treatment of primary induction failure or relapsed acute myeloid leukemia. Leuk Res. 2012;36(4):394–6. doi: 10.1016/j.leukres.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 121.Jackson GH. Use of fludarabine in the treatment of acute myeloid leukemia. Hematol J. 2004;5(Suppl 1):S62–7. doi: 10.1038/sj.thj.6200392. [DOI] [PubMed] [Google Scholar]
- 122.Estey E, Thall P, Andreeff M, et al. Use of granulocyte colony-stimulating factor before, during, and after fludarabine plus cytarabine induction therapy of newly diagnosed acute myelogenous leukemia or myelodysplastic syndromes: comparison with fludarabine plus cytarabine without granulocyte colony-stimulating factor. J Clin Oncol. 1994;12(4):671–8. doi: 10.1200/JCO.1994.12.4.671. [DOI] [PubMed] [Google Scholar]
- 123.Jackson G, Taylor P, Smith GM, et al. A multicentre, open, non-comparative phase II study of a combination of fludarabine phosphate, cytarabine and granulocyte colony-stimulating factor in relapsed and refractory acute myeloid leukaemia and de novo refractory anaemia with excess of blasts in transformation. Br J Haematol. 2001;112(1):127–37. doi: 10.1046/j.1365-2141.2001.02551.x. [DOI] [PubMed] [Google Scholar]
- 124.Virchis A, Koh M, Rankin P, et al. Fludarabine, cytosine arabinoside, granulocyte-colony stimulating factor with or without idarubicin in the treatment of high risk acute leukaemia or myelodysplastic syndromes. Br J Haematol. 2004;124(1):26–32. doi: 10.1046/j.1365-2141.2003.04728.x. [DOI] [PubMed] [Google Scholar]
- 125.Estey EH, Thall PF, Pierce S, et al. Randomized phase II study of fludarabine + cytosine arabinoside + idarubicin +/− all-trans retinoic acid +/− granulocyte colony-stimulating factor in poor prognosis newly diagnosed acute myeloid leukemia and myelodysplastic syndrome. Blood. 1999;93(8):2478–84. [PubMed] [Google Scholar]
- 126.Pastore D, Specchia G, Carluccio P, et al. FLAG-IDA in the treatment of refractory/relapsed acute myeloid leukemia: single-center experience. Ann Hematol. 2003;82(4):231–5. doi: 10.1007/s00277-003-0624-2. [DOI] [PubMed] [Google Scholar]
- 127.Martin MG, Augustin KM, Uy GL, et al. Salvage therapy for acute myeloid leukemia with fludarabine, cytarabine, and idarubicin with or without gemtuzumab ozogamicin and with concurrent or sequential G-CSF. Am J Hematol. 2009;84(11):733–7. doi: 10.1002/ajh.21545. [DOI] [PubMed] [Google Scholar]
- 128.Milligan DW, Wheatley K, Littlewood T, Craig JI, Burnett AK. Fludarabine and cytosine are less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: results of the MRC AML-HR randomized trial. Blood. 2006;107(12):4614–22. doi: 10.1182/blood-2005-10-4202. [DOI] [PubMed] [Google Scholar]
- 129.Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105(1):54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 130.Langdon WY. FLT3 signaling and the development of inhibitors that target FLT3 kinase activity. Crit Rev Oncog. 2012;17(2):199–209. doi: 10.1615/critrevoncog.v17.i2.50. [DOI] [PubMed] [Google Scholar]
- 131.Levis M, Ravandi F, Wang ES, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse. Blood. 2011;117(12):3294–301. doi: 10.1182/blood-2010-08-301796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mark J, Levis M, Perl Alexander E, et al. Final results of a phase 2 open-label, monotherapy efficacy and safety study of quizartinib (ac220) in patients with flt3-itd positive or negative relapsed/refractory acute myeloid leukemia after second-line chemotherapy or hematopoietic stem cell transplantation. Blood. 2012;120:673. [Google Scholar]
- 133.Marcucci G, Haferlach T, Dohner H. Molecular genetics of adult acute myeloid leukemia: prognostic and therapeutic implications. J Clin Oncol. 2011;29(5):475–86. doi: 10.1200/JCO.2010.30.2554. [DOI] [PubMed] [Google Scholar]
- 134.Gregory TK, Wald D, Chen Y, Vermaat JM, Xiong Y, Tse W. Molecular prognostic markers for adult acute myeloid leukemia with normal cytogenetics. J Hematol Oncol. 2009;2:23. doi: 10.1186/1756-8722-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Preisler H, Davis RB, Kirshner J, et al. Comparison of three remission induction regimens and two postinduction strategies for the treatment of acute nonlymphocytic leukemia: a cancer and leukemia group B study. Blood. 1987 May;69(5):1441–49. [PubMed] [Google Scholar]
- 136.Wiernik PH, Banks PL, Case DC, Jr, et al. Cytarabine plus idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemia. Blood. 1992 Jan 15;79(2):313–19. [PubMed] [Google Scholar]
- 137.Weick JK, Kopecky KJ, Appelbaum FR, et al. A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia: a Southwest Oncology Group study. Blood. 1996 Oct 15;88(8):2841–51. [PubMed] [Google Scholar]
- 138.Lowenberg B, Pabst T, Vellenga E, et al. Cytarabine dose for acute myeloid leukemia. The New England journal of medicine. 2011 Mar 17;364(11):1027–36. doi: 10.1056/NEJMoa1010222. [DOI] [PubMed] [Google Scholar]
- 139.Cassileth PA, Harrington DP, Appelbaum FR, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. The New England journal of medicine. 1998 Dec 3;339(23):1649–56. doi: 10.1056/NEJM199812033392301. [DOI] [PubMed] [Google Scholar]
- 140.Moore JO, George SL, Dodge RK, et al. Sequential multiagent chemotherapy is not superior to high-dose cytarabine alone as postremission intensification therapy for acute myeloid leukemia in adults under 60 years of age: Cancer and Leukemia Group B Study 9222. Blood. 2005 May 1;105(9):3420–27. doi: 10.1182/blood-2004-08-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]