Abstract
Plant biomass allocation between below- and above-ground parts can actively adapt to the ambient growth conditions and is a key parameter for estimating terrestrial ecosystem carbon (C) stocks. To investigate how climatic variations affect patterns of plant biomass allocation, we sampled 548 plants belonging to four dominant genera (Stipa spp., Cleistogenes spp., Agropyron spp., and Leymus spp.) along a large-scale (2500 km) climatic gradient across the temperate grasslands from west to east in northern China. Our results showed that Leymus spp. had the lowest root/shoot ratios among the each genus. Root/shoot ratios of each genera were positively correlated with mean annual temperature (MAT), and negatively correlated with mean annual precipitation (MAP) across the transect. Temperature contributed more to the variation of root/shoot ratios than precipitation for Cleistogenes spp. (C4 plants), whereas precipitation exerted a stronger influence than temperature on their variations for the other three genera (C3 plants). From east to west, investment of C into the belowground parts increased as precipitation decreased while temperature increased. Such changes in biomass allocation patterns in response to climatic factors may alter the competition regimes among co-existing plants, resulting in changes in community composition, structure and ecosystem functions. Our results suggested that future climate change would have great impact on C allocation and storage, as well as C turnover in the grassland ecosystems in northern China.
Introduction
Below-ground carbon (C) is the primary component of C stocks in grassland ecosystems, accounting for >80% of plant C stocks [1]–[3]. The below-ground system plays an important role in controlling terrestrial C sequestration and cycling. However, compared to the above-ground system, knowledge of the below-ground system is still limited [4]. Root/shoot ratios have been used to calibrate and estimate C storage from the more easily measurable aboveground biomass [5]. As a critical determinant, root/shoot ratios have also been incorporated into terrestrial ecosystem C modeling [6]–[7]. Quantifying root/shoot ratio and its relationships with climatic factors is not only important for improving our understanding of biomass allocation in terrestrial ecosystems, but also critical for predicting global C sequestration and cycling under climate change. To our knowledge, however, few studies have investigated the influences of variations in climate on root/shoot ratios across geographical scales [8].
From a physiological perspective, root/shoot ratio is usually thought to reflect the differential investment of photosynthates between above- and below-ground organs, induced by abiotic and biotic pressures [4], [9]–[10]. For example, plants would allocate relatively more biomass to roots if their growth is more strongly influenced by below-ground factors (e.g. nutrients, water), whereas they would allocate relatively more biomass to shoots if their growth is more strongly influenced by above-ground factors (e.g. light, CO2) [11]–[12]. Both temperature and water availability are important abiotic factors affecting plant growth [8] and plant biomass allocation [13]–[15]. In previous studies, plant root/shoot ratio in grassland was found to significantly decrease with increasing mean annual temperature and precipitation [4], [16]. However, other studies did not find changes of plant root/shoot ratios with the variation of temperature and precipitation at both individual [17] and community levels [18] in China’s grasslands. The inconsistency may be due to different plant types investigated. Different plant genera, plant functional groups (e.g. C3 and C4 plants), and plant growth groups (e.g. isometric and allometric plants) may have different specialized strategies to regulate their root/shoot ratios in order to maximize the use of external resources [19]–[23]. Therefore, it is important to investigate how different plant genera respond to the changing climatic factors.
Temperate grassland is one of the most widespread biomes on earth and plays a key role in global C cycling [24]. The temperate grassland in northern China, located in the eastern part of the Eurasian grassland biome, supports diverse species of plants and animals and serves the development of socio-economics of the region [25]. Additionally, this grassland, located in temperate continental arid region, has a remarkable transition of climate, soil, vegetation, and biogeochemical cycling from west to east [26]–[28], which offers a unique opportunity for examining the characteristics of grasslands in relation to climatic factors.
Here, we investigated plant biomass allocation between above- and below-ground in four dominant genera (Stipa, Cleistogenes, Leymus and Agropyron) along a large-scale transect of approximately 2500 km across the temperate grasslands of northern China. Plants of these four genera provide the majority of the community primary production, and to a great extent, determine the structure and function of the grassland ecosystem. We aimed to: (1) document the general patterns of root/shoot ratios of these four genera across geographical scale, (2) examine the effects of climatic factors on their root/shoot ratios, and (3) illustrate the relative contribution of different climatic variables to the variation of these root/shoot ratios.
Materials and Methods
Study Area and Sampling Sites
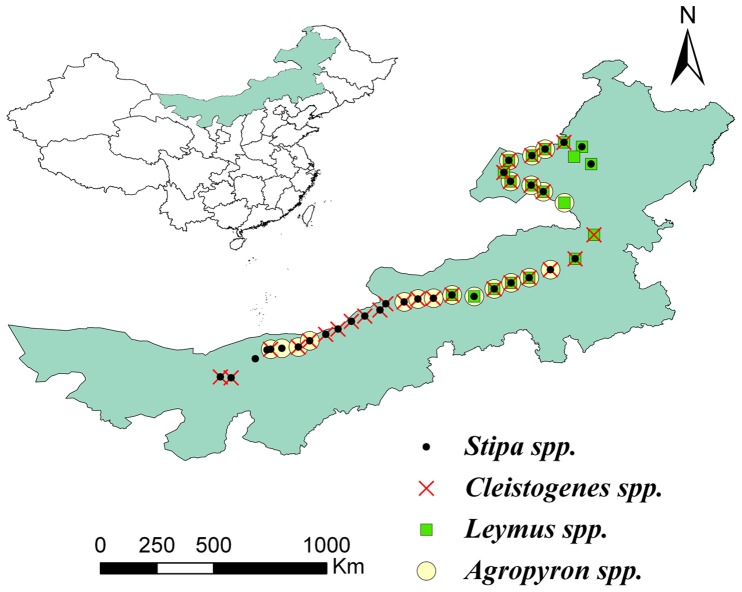
The research was conducted in the semi-arid region of northern China. The selection of sample sites was principally defined by a grassland transect of ∼2500 km ranging from west to east across the temperate grassland of northern China (Figure 1). The longitudinal-latitudinal transect covers latitudes from 40.7°N to 50.1°N and longitudes from 105.6°E to 120.4°E (Figure 1). Across the broad geographical regions and environmental gradients along the west-east transect, there are various vegetation types such as the desert grassland in the western part, the typical grassland in the middle part, and the meadow grassland predominantly in the eastern part.
Figure 1. Locations of the sampling sites along the transect across the temperate grasslands of northern China.
The investigation was conducted in August 2012 when the phenological difference was relatively negligible and the grasses had matured across the transect. All necessary permits were gained before the beginning of field investigation. Sample sites subjected to minimal grazing and other anthropogenic disturbances were visually selected in the field. Exact sampling locations were GPS-referenced with latitude, longitude and elevation (eTrex Venture, Garmin, ±3 m accuracy). A total of 37 sample sites were investigated along the 2500 km grassland transect and the distance between two adjacent sample sites was about 60 km.
Plant Materials
Stipa, Cleistogenes, Leymus, and Agropyron are four most widely distributed dominant genera in the temperate grasslands in northern China. Dominant plants belonging to these four genera along the transect were sampled. Both Stipa spp. and Agropyron spp. are perennial multi-stems C3 bunchgrasses, Leymus spp. are perennial single-stem C3 rhizome grasses, and Cleistogenes spp. are multi-stems C4 bunchgrasses [29].
Sampling
At each sampling site, ten sampling plots (1-m×1-m each) were established along a 500-m long sampling strip which crosses the transect perpendicularly. The distance between two adjacent plots was 50 m. Five to ten mature and healthy individuals belonging to each of the four genera (Stipa, Cleistogenes, Agropyron, and Leymus) were randomly sampled if existed (Figure 1). The maximum plant height of four dominant genera was measured with a ruler. The majority of root material of these plants were located in the upper 30-cm of soil [29]. The root system was excavated by extracting a soil cylinder of about 15–25 cm in diameter and 20–30 cm in depth with the target plant in the middle, using a spade. The size of the soil cylinder depended on the aboveground morphology of the target plant. Roots of the target plant were carefully collected and separated from soils and other belowground materials. The roots and shoots were then separated from the ground surface [4] and put into paper bags separately. Afterwards, the roots were washed free of soils under running water for 5 minutes before all plant samples were oven-dried at 80°C to constant weight. Root/shoot ratio is defined as the dry root biomass (g) divided by the dry shoot biomass (g) (Appendix S1). Since these data were all collected by a single team of researchers using uniform collection method, this analysis avoids the difficulty of heterogeneous data which previous large-scale analyses of biomass allocation have encountered.
Climate Data
Five climatic variables, i.e. mean annual temperature (MAT, °C), mean annual precipitation (MAP, mm), mean growing season temperature (GST, °C), mean growing season precipitation (GSP, mm) and mean annual potential evapotranspiration (PET, mm year−1), were employed to analyze the climatic controls on the spatial patterns of plant root/shoot ratios. Monthly mean temperature and monthly mean precipitation were extracted from a global climate dataset with a resolution of 0.0083°×0.0083° (approximately 1 km2 at the equator) obtained from http://www.worldclim.org/. Growing season was defined as the months with a mean temperature ≥5°C. PET was extracted from Numerical Terradynamic Simulation Group with a resolution of 0.5°×0.5° obtained from http://www.ntsg.umt.edu/project/mod16. A drought index (DI) based on cumulative values of precipitation and potential evapotranspiration for each site across the transect was calculated, using MAP/PET (Appendix S1).
Statistical Analysis
The Kolmogorov-Smirnov (KS) test was used to test for normality of data before statistical analysis [30]. Differences among means were compared by one-way ANOVA, followed by multiple comparison tests. Semi-variogram analysis was employed to check the spatial autocorrelation of our sampling sites [31]. Results showed that the range of the spatial dependency of MAT and MAP (25 km and 22 km, respectively) were lower than the distance between two adjacent sample sites (60 km), which indicated our sample points had non-significant spatial autocorrelation (Table 1).
Table 1. Semi-variogram test of spatial autocorrelation of mean annual temperature (MAT) and mean annual precipitation (MAP) among sampling sites across a 2500-km long transect in northern China.
| Model | Nugget effect | Sill | Range | R2 | |
| MAP | Gaussian | 1030 | 15280 | 25 | 0.945 |
| MAT | Gaussian | 0.3 | 7.6 | 22 | 0.905 |
Relationships between root/shoot ratios and climatic factors were examined with linear mixed model incorporating random effects, using sample plots as the random factor and climatic variables as the fixed factors [32]. All statistical analyses were performed using the R software package (version 2.15.0). To test the independent contribution of temperature and precipitation to root/shoot ratios, we conducted path analysis using SAS 9.0 (SAS Institute Inc., Cary, NC, USA). Path analysis can be used to partition the relationship between root/shoot ratios and climatic factors into direct and indirect effect [33]. A high path coefficient indicates a strong contribution of a climatic factor (MAP, MAT, GSP, and GST) to root/shoot ratios. Direct path coefficients measure direct effects of a climatic factor (MAP, MAT, GSP, and GST) on root/shoot ratios, while indirect path coefficients specify the effects of a climatic factor passed through other climate factors. Path analysis cannot exclude the random effects of sample plots. Hence, we used the mean root/shoot ratio of each site to demonstrate the independent contribution of temperature and precipitation to root/shoot ratios. This paper only presents the results of MAP and MAT, since the effects of GSP and GST were not different from those of MAP and MAT.
Allometric relationships between traits have been generally understood as exponential relationships described by Log y = a (Log x)+b, where “x” and “y” are the two variables, “a” represents the scaling slope and “b” the intercept [34]. The relationship between the log-transformed root biomass and shoot biomass was tested with standardized major axis (SMA) regression. SMA regression protocols are appropriate when both regression variables are interdependent and subject to measurement errors as in the case of shoot biomass and root biomass [35]. SMA slopes and intercepts were obtained using the SMART software package developed by Falster et al. [36]. SMA slopes were tested against 1.0. Non-significant difference from 1.0 in the slopes indicates an allometric relationship between root and shoot biomass [35].
Results
Root/Shoot Ratios of the Four Dominant Genera
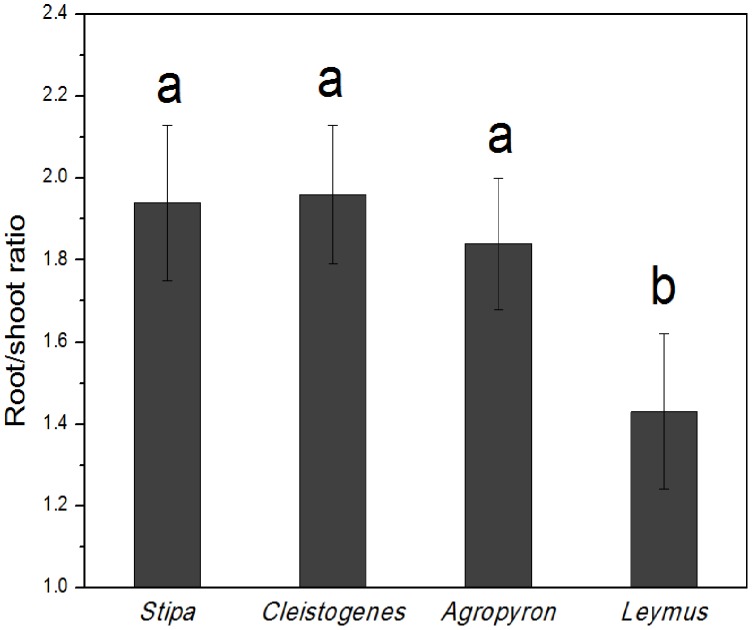
Plant root/shoot ratios were significantly drawn from a normally distributed population for each genus (Table 2). Mean root/shoot ratio was 1.94 (ranging from 1.12 to 2.72) for Stipa spp., 1.97 (1.15–2.98) for Cleistogenes spp., 1.84 (1.22–2.50) for Agropyron spp., and 1.43 (0.75–2.4) for Leymus spp. (Table 2; Figure 2), whereas the mean maximum plant height was 19 cm (ranging from 5 to 49 cm) for Stipa spp., 10 cm (3–23 cm) for Cleistogenes spp.,19 (10–25 cm) for Agropyron spp. and 32 cm (13–54 cm) for Leymus spp. (Table 2). CV of root/shoot ratios ranged from 13% to 21% (Table 2).
Table 2. Statistics of plant root/shoot ratios of four dominant genera (Stipa, Cleistogenes, Agropyron, and Leymus) across a 2500-km long transect in northern China’s temperature grassland.
| Genus | n | Range | Mean ± SD | CV (%) | K-S test | MPH(cm) |
| Stipa | 196 | 1.12–2.72 | 1.94±0.29 | 14.95 | Normal | 19(5–49) |
| Cleistogenes | 151 | 1.15–2.98 | 1.97±0.27 | 13.71 | Normal | 10(3–23) |
| Agropyron | 96 | 1.22–2.50 | 1.84±0.26 | 14.13 | Normal | 19(10–25) |
| Leymus | 105 | 0.75–2.40 | 1.43±0.29 | 20.28 | Normal | 32(13–45) |
Number of replicates (n), mean, range, standard deviation (SD), coefficient of variation (CV: defined as SD/mean) and maximum plant height (MPH) and Kolmogorov-Smirnov (K-S) test results are all reported.
Figure 2. Root/shoot ratios (mean ±1 SE) of four grasses genera (Stipa, Cleistogenes, Agropyron, and Leymus).
Bars with dissimilar letters denote signiffcant difference (p<0.01).
Relationships between Root/Shoot Ratios and Climatic Factors
Linear mixed model analysis showed that root/shoot ratios were positively correlated with MAT (all p<0.05) and negatively correlated with MAP (all p<0.001) excluding the random effects of sample plots at both intra- and inter-genus levels (Table 3). Path analysis showed that direct dependence of root/shoot ratios on MAP were greater than on MAT for Stipa spp., Agropyron spp. and Leymus spp. (Table 4). For Cleistogenes spp., however, MAT had stronger direct effects on root/shoot ratios than MAP (Table 4). The direct path coefficient of MAP on the root/shoot ratios was −0.577 for Stipa spp., −0.231 for Agropyron spp., −0.417 for Leymus spp. and −0.413 for Cleistogenes spp., with the indirect path coefficient via MAT of −0.252, −0.399, −0.053 and −0.064, respectively. The direct path coefficient of MAT were 0.285 for Stiap spp., 0.468 for Cleistogenes spp., 0.064 for Agropyron spp., and 0.21 for Leymus spp. with the indirect path coefficient via MAP of 0.509, 0.21, 0.342 and 0.126, respectively.
Table 3. Effects of MAT and MAP on root/shoot ratios of four dominant genera (Stipa, Cleistogenes, Agropyron, and Leymus) across a 2500-km long transect in northern China’s temperature grassland.
| Root/shoot ratiosfor Stipa | ||||
| Fixed effect | ||||
| Variables | Slope | Intercept | R | p-value |
| MAT | 0.071 | 1.77 | 0.629 | p<0.001 |
| MAP | −0.002 | 2.33 | 0.938 | p<0.001 |
| Root/shoot ratios for Cleistogenes | ||||
| Fixed effect | ||||
| MAT | 0.054 | 1.85 | 0.646 | p<0.001 |
| MAP | −0.001 | 2.30 | 0.936 | p<0.001 |
| Root/shoot ratiosfor Agropyron | ||||
| Fixed effect | ||||
| MAT | 0.040 | 1.80 | 0.468 | p<0.05 |
| MAP | −0.001 | 2.24 | 0.972 | p<0.001 |
| Root/shoot ratiosfor Leymus | ||||
| Fixed effect | ||||
| MAT | 0.059 | 1.46 | 0.269 | p<0.05 |
| MAP | −0.002 | 2.06 | 0.986 | p<0.001 |
| Root/shoot ratios forall four genera | ||||
| Fixed effect | ||||
| MAT | 0.076 | 1.72 | 0.266 | p<0.05 |
| MAP | −0.001 | 2.14 | 0.824 | p<0.001 |
MAT, mean annual temperature; MAP, mean annual precipitation.
A linear mixed model was employed, using sample plots as the random factor and climate variables as the fixed factors. R is the correlation coefficients between climate factors and root/shoot ratios. P-values are estimated using restricted maximum likelihood (REML) estimates and are reported for significant (p<0.05) model terms.
Table 4. Path coefficients between climate factors (MAT and MAP) and plant root/shoot ratios of four dominant genera (Stipa, Cleistogenes, Agropyron, and Leymus) across a 2500-km long transect in northern China’s temperature grassland.
| Genus | R | Direct path | Indirect path coefficient | ||
| coefficient | Via MAP | Via MAT | |||
| Stipa | MAP–R/S | −0.829 | −0.577 | −0.252 | |
| MAT–R/S | 0.794 | 0.285 | 0.509 | ||
| Cleistogenes | MAP–R/S | −0.630 | −0.231 | −0.399 | |
| MAT–R/S | 0.678 | 0.468 | 0.210 | ||
| Agropyron | MAP–R/S | −0.470 | −0.417 | −0.053 | |
| MAT–R/S | 0.406 | 0.064 | 0.342 | ||
| Leymus | MAP–R/S | −0.477 | −0.413 | −0.064 | |
| MAT–R/S | 0.336 | 0.210 | 0.126 | ||
MAT, mean annual temperature; MAP, mean annual precipitation; R/S, root/shoot ratio.
Indirect path coefficient means the indirect dependency of root/shoot ratios on certain climate factor and R is the correlation coefficient between the climate factors and the root/shoot ratios.
Allometric Relationships between Root and Shoot Biomass
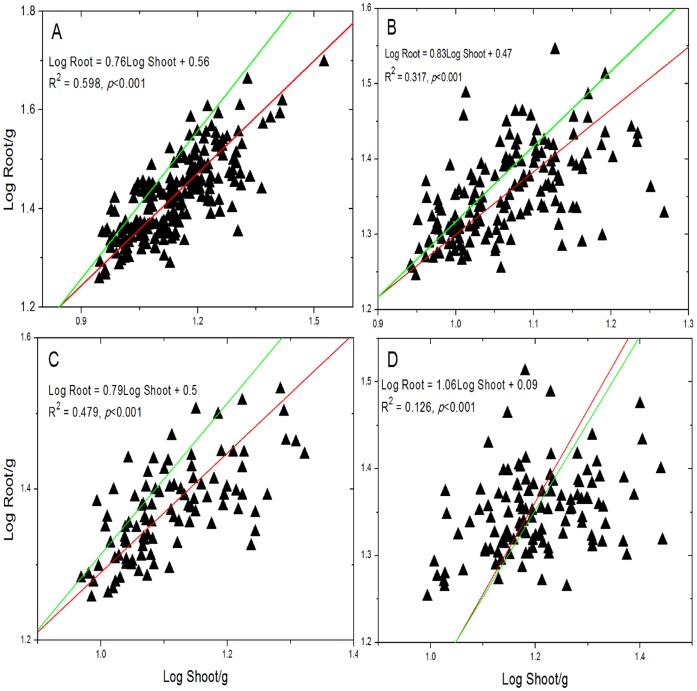
Shoot biomass was positively correlated with root biomass (p<0.001) for each genus we sampled (Figure 3; Table 5). The allometric slope of the relationship between log-root and log-shoot biomass obtained by the reduced major axis analysis for Stipa spp., Cleistogenes spp., Agropyron spp. and Leymus spp. was 0.76 (with a 95% confidence interval of 0.69–0.83), 0.83 (0.72–0.94), 0.79 (0.68–0.91), and 0.93 (0.78–1.09), respectively (Table 5). These values differed significantly from 1.0, except for the last value (p = 0.360), indicating isometric growth for Leymus spp. and allometric growth for the rest three genera (Table 5).
Figure 3. Allometric relationship between root biomass and shoot biomass.
Stipa spp. (A), Cleistogenes spp. (B), Agropyron spp. (C), and Leymus spp. (D). Red lines are the standardized major axis regression curves (for a summary of regression statistics, see Table 5). Green lines are isometric lines with slope equal to 1 and y-intercept equal to that of the corresponding red lines.
Table 5. Linear regression between above- and below-ground biomass for Stipa spp., Cleistogenes spp., Agropyron spp., and Leymus spp. using a standardized major axis (SMA) method.
| Genus | R2 | Slope(95% CI) | Intercept | p (H0:slope = 1.0) |
| Stipa | 0.60*** | 0.76 (0.69−0.83) | 0.56 (0.48−0.63) | <0.001 |
| Cleistogenes | 0.32*** | 0.83 (0.72−0.94) | 0.47 (0.36−0.59) | <0.001 |
| Agropyron | 0.48*** | 0.79 (0.68−0.91) | 0.50 (0.37−0.63) | <0.01 |
| Leymus | 0.13*** | 1.06 (0.88−1.16) | 0.09 (−0.21-0.06) | = 0.360 |
95% CI, 95% confidence interval; ***, p<0.001.
Both above- and below-ground biomass were log10–transformed. p is the significance level at which the estimated slopes are different from 1.0.
Discussion
Our results showed that Leymus spp. had significantly lower root/shoot ratios than Stipa spp., Cleistogenes spp. and Agropyron spp. (Figure 2). This could be partly explained by the relatively high plant height of Leymus spp., since root/shoot ratios generally deceased with increasing plant height [37]. Li et al. [37] found that root/shoot ratios of grasses decreased logarithmically with increasing plant height in the temperate grasslands of northern China. Chapin and Chapin [38], Snowdon et al. [39], and Henry and Thomas [40] also reported that shorter plants usually had higher root/shoot ratios in natural grasslands, shrubs and forests ecosystems, respectively.
Root/shoot ratio is considered to be a strong indicator for the capacity of plants to take up light, water and nutrients. Plants would demonstrate optimal distribution patterns to adapt to particular environmental conditions, such as shade or drought [48]–[49]. Grasses grown in drought environment are expected to allocate more biomass to root systems for water uptake [41], [50]. Our data within each genus and across the four genera supported this hypothesis. From west to east of our sampling transect, MAP increased from 100 mm to 450 mm and MAT decreased from 8.1°C to −3.1°C. Along the transect, plants allocated, correspondingly, less biomass to root system for taking up soil water and more biomass to shoot system for light, leading to a decreasing trend in root/shoot ratios from west to east. Warming may stimulate evaportranspiration and reduce soil water content, which explains the observed higher root/shoot ratios with increasing MAT. Moreover, lower temperature in the eastern part of the transect may have more negatively influenced the root growth rather than the shoot growth. This is consistent with what Kummerow [42] found in arctic sedges, where lower root/shoot ratios were observed in areas with lower temperature, indicating that a relatively large fraction of the photosynthate was allocated to shoot production under cold conditions. Mokany et al. [4] found that root/shoot ratios in grassland decreased significantly with both increasing MAP and increasing MAT (p<0.001). Yang et al. [18] found that root/shoot ratios did not vary markedly with MAP and MAT for grassland plants at the community level in China. However, our results indicated positive relationships between root/shoot ratios and MAT but negative relationships between root/shoot ratios and MAP for the four dominant genera across the west-east transect (Table 3), suggesting positive effects of temperature or radiation but negative effects of water availability on root/shoot ratios in arid and semi-arid regions. Our results agreed with findings of Xu et al. [43], which indicated that high temperature in combination with soil drought increased the root/shoot ratios of Leymus chinensis. Temperature has been well known for stimulating soil enzyme activities and increasing decomposition and mineralization of organic matter [44]. Additionally, temperature can also stimulate nutrient transportation in soils and nutrient uptake by roots [45]. Hence, plant root/shoot ratios are expected to decrease with increasing temperature under favorable below-ground conditions.
However, in arid and semi-arid regions, water stress becomes the most limiting factor for plant growth. Therefore, the influence of temperature on C allocation is mainly reflected in its interaction with the limited precipitation [22], [35], [46], explaining the positive correlation between temperature and root/shoot ratios we found. Our results may also be partly explained further by the significantly positive correlation between root/shoot ratios and PET (all p<0.05) and the significantly negative correlation between root/shoot ratios and DI (all p<0.05) (Table 6). Our results suggested that future climate change characterized by increases in both temperature and precipitation in the semiarid grasslands of northern China may result in changes in C allocation between the above- and belowground part of plants in this ecosystem.
Table 6. Effects of PEI and DI on root/shoot ratios of four dominant genera (Stipa, Cleistogenes, Agropyron, and Leymus) across a 2500-km long transect in northern China’s temperature grassland.
| Root/shoot ratiosfor Stipa | ||||
| Fixed effect | ||||
| Variables | Slope | Intercept | R | p-value |
| PET | 0.001 | 0.47 | 0.992 | p<0.001 |
| DI | −0.099 | 2.26 | 0.903 | p<0.001 |
| Root/shoot ratiosfor Cleistogenes | ||||
| Fixed effect | ||||
| PET | 0.001 | 0.97 | 0.992 | p<0.001 |
| DI | −1.747 | 2.25 | 0.970 | p<0.001 |
| Root/shoot ratiosfor Agropyron | ||||
| Fixed effect | ||||
| PET | 0.001 | 1.32 | 0.993 | p<0.05 |
| DI | −1.530 | 2.12 | 0.952 | p<0.05 |
| Root/shoot ratiosfor Leymus | ||||
| Fixed effect | ||||
| PET | 0.001 | 0.91 | 0.996 | p<0.05 |
| DI | −2.201 | 1.97 | 0.980 | p<0.05 |
| Root/shoot ratios forfour genera | ||||
| Fixed effect | ||||
| PET | 0.001 | 0.33 | 0.991 | p<0.001 |
| DI | −2.687 | 2.32 | 0.911 | p<0.001 |
PEI, mean annual potential evapotranspiration; DI, drought index.
A linear mixed model was employed, using sample plots as the random factor and climate variables as the fixed factors. R is the correlation coefficients between climate factors and root/shoot ratios. P-values are estimated using restricted maximum likelihood (REML) estimates and are reported for significant (p<0.05) model terms.
According to theoretical predictions and prior observations, plant growth strategies should be mainly regulated by precipitation in the arid and semi-arid grassland of northern China because water availability is the dominant limiting factor for primary productivity in this region [46]. However, our results revealed that the relative contribution of MAT and MAP to the variations of root/shoot ratios was genus-dependent. Root/shoot ratios of C3 plants (Stipa spp., Agropyron spp. and Leymus spp.) were more influenced by precipitation, whereas root/shoot ratios of C4 plants (Cleistogenes spp.) were more controlled by temperature. James et al. [47] and Wang [48] also found similar phenomena. This can be explained by the more effective quantum yield of CO2 fixation [49] and the higher maximum photosynthetic rate of C4 plants at higher temperature [50]. Therefore, more C can be invested to roots for nutrients uptake, resulting in higher root/shoot ratios at higher temperature. In addition, C4 pathway could overcome the deleterious effects of drought on photosynthesis and enables C4 plants to photosynthesize more efficiently at lower water content and higher temperature. C4 plants usually have higher photosynthetic water-use efficiency than C3 plants [51]. Similarly, Pau et al. [52] demonstrated that C4 grasses showed a preference for regions with higher temperatures and lower precipitation compared with C3 grasses at the global scale. Sage and Kubien [50] suggested that C4 species may be more readily respond to warming in a manner that promotes rapid root proliferation and colonization in disturbed habitats. This is consistent with the widely held but unproven hypotheses that C4 plants should be favored by global warming [49].
The isometric hypothesis suggests that above-ground biomass scales one-to-one with respect to the below-ground biomass at both individual and community levels [53], [54]. We only found that the scaling of root biomass vs. shoot biomass of Leymus spp. followed 1∶1 line (isometric plants) (Figure 3; Table 5). The other three genera (Stipa, Cleistogenes and Agropyron) were allometric plants, which showed asymmetrical change between root and shoot biomass with changing environments (Table 5; Figure 3). This difference among the four genera may be caused by the differences in plant morphology and may also be influenced by natural selection. It makes larger ecological sense that plants allocate more of their biomass to belowground in response to water limitation regardless of an isometric or allometric relationship between shoot and root biomass (Table 3; Table 6). Such an allometic or isometric relationship is controlled by both the slope (relative growth rate of shoot and root biomass) and the y-intercept (the absolute value of root/shoot ratio). All the y-intercepts for the four genera were significantly different from zero, indicating that the absolute values of root/shoot ratios for the four genera were not consistent. Therefore, our findings indicated that the relationship between root and shoot biomass in relation to environment conditions may be genus-specific.
Conclusion
Our results suggested that plants in the temperate grassland would alter their carbon allocation pattern between above- and belowground parts in response to climate changes. Under warming condition, plants, especially C4 plants, may allocate relatively less biomass to aboveground, resulting in higher competitive capacity of C4 plants compared to C3 plants, which may finally change community structure and ecosystem function. Given the sensitivity of root/shoot ratios of the examined dominant genera to climatic factors, future climate change will have great impacts on C storage and turnover in the grasslands of northern China.
Supporting Information
Dataset on the root/ratios of four dominant genera ( Stipa , Cleistogenes , Agropyron , and Leymus ), and information on the geographic location and climate of the study area.
(XLSX)
Acknowledgments
We thank all members of Shenyang sampling campaign teams from Institute of Applied Ecology, Chinese Academy of Sciences for their assistance with field sampling. We gratefully acknowledge Quansheng Chen and Chunming Jiang for their comments on the earlier version of this manuscript.
Funding Statement
This work was financially supported by the National Basic Research Program of China (2011CB403204) and by the National Natural Science Foundation of China (31000200). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jackson RB, Canadell J, Ehleringer JR, Mooney HA, Sala OE, et al. (1996) A global analysis of root distributions for terrestrial biomes. Oecologia 108: 389–411. [DOI] [PubMed] [Google Scholar]
- 2.Saugier B, Roy J, Mooney HA (2001) Academic Press, San Diego. In: Roy J, Saugier B, Mooney HA, editors. Estimations of global terrestrial productivity: converging toward a single number? In: Terrestrial Global Productivity. 543–556.
- 3. Yang YH, Fang JY, Ji CJ, Ma WH, Mohammat A, et al. (2012) Widespread decreases in topsoil inorganic carbon stocks across China’s grasslands during 1980s–2000s. Global Change Biology 18: 3672–3680. [Google Scholar]
- 4. Mokany K, Raison RJ, Prokushkin AS (2006) Critical analysis of root: shoot ratios in terrestrial biomes. Global Change Biology 12: 84–96. [Google Scholar]
- 5. Milchunas DG (2009) Estimating root production: comparison of 11 methods in shortgrass steppe and review of biases. Ecosystems 12: 1381–1402. [Google Scholar]
- 6. Scurlock JMO, Johnson K, Olson RJ (2002) Estimating net primary productivity from grassland biomass dynamics measurements. Global Change Biology 8: 736–753. [Google Scholar]
- 7. Sack L, Maranon T, Grubb PJ (2002) Global allocation rules for patterns of biomass partitioning. Science 296: A1923–A1923. [DOI] [PubMed] [Google Scholar]
- 8. Wang RZ, Gao Q (2003) Climate-driven changes in shoot density and shoot biomass in Leymus chinensis (Poaceae) on the North-east China Transect (NECT). Global Ecology and Biogeography 12: 249–259. [Google Scholar]
- 9. Barboni D, Harrison SP, Bartlein PJ, Jalut G, New M, et al. (2009) Relationships between plant traits and climate in the Mediterranean region: a pollen data analysis. Journal of Vegetation Science 15: 635–646. [Google Scholar]
- 10. Szabo ND, Algar AC, Kerr JT (2009) Reconciling topographic and climatic effects on widespread and range-restricted species richness. Global Ecology and Biogeography 18: 735–744. [Google Scholar]
- 11. Shipley B, Meziane D (2002) The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Functional Ecology 16: 326–331. [Google Scholar]
- 12. Xu X, Niu SL, Sherry RA, Zhou XH, Zhou JZ, et al. (2012) Interannual variability in responses of belowground net primary productivity (NPP) and NPP partitioning to long-term warming and clipping in a tallgrass sprairie. Global Change Biology 18: 1648–1656. [Google Scholar]
- 13. Swemmer AM, Knapp AK, Snyman HA (2007) Intra-seasonal precipitation patterns and above-ground productivity in three perennial grasslands. Journal of Ecology 95: 780–788. [Google Scholar]
- 14. Yang HJ, Li Y, Wu MY, Zhang Z, Li LH, et al. (2011) Plant community responses to nitrogen addition and increased precipitation: the importance of water availability and species traits. Global Change Biology 17: 2936–2944. [Google Scholar]
- 15. Luo YJ, Wang XK, Zhang XQ, Booth TH, Lu F (2012) Root: shoot ratios across China’s forests: Forest type and climatic effects. Forest Ecology and Management 269: 19–25. [Google Scholar]
- 16.Bhattachan A, Tatlhego M, Dintwe K, O’Donnell F, Caylor KK, et al.. (2012) Evaluating Ecohydrological Theories of Woody Root Distribution in the Kalahari. PLoS ONE 7, e33996. [DOI] [PMC free article] [PubMed]
- 17. Wang LA, Niu KC, Yang YH, Zhou P (2010) Patterns of above- and belowground biomass allocation in China’s grasslands: evidence from individual-level observations. Science China-Life Science 53: 851–857. [DOI] [PubMed] [Google Scholar]
- 18. Yang YH, Fang JY, Ma WH, Guo DL, Mohammat A (2010) Large-scale pattern of biomass partitioning across China’s grasslands. Global Ecology and Biogeography 19: 268–277. [Google Scholar]
- 19. Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, et al. (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytologist 193: 30–50. [DOI] [PubMed] [Google Scholar]
- 20. Müller I, Schmid B, Weiner J (2000) The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspectives in Plant Ecology, Evolution and Systematics 3: 115–127. [Google Scholar]
- 21. Auerswald K, Wittmer MHOM, Bai YF, Yang H, Taube F, et al. (2012) C4 abundance in an Inner Mongolia grassland system is driven by temperature–moisture interaction, not grazing pressure. Basic and Applied Ecology 13: 67–75. [Google Scholar]
- 22. Vogan PJ, Sage RF (2012) Effects of low atmospheric CO2 and elevated temperature during growth on the gas exchange responses of C-3, C-3-C-4 intermediate, and C-4 species from three evolutionary lineages of C-4 photosynthesis. Oecologia 169: 341–352. [DOI] [PubMed] [Google Scholar]
- 23. Liu YS, Pan QM, Zheng SH, Bai YF, Han XG (2012) Intra-seasonal precipitation amount and pattern differentially affect primary production of two dominant species of Inner Mongolia grassland. Acta Oecologica 44: 2–10. [Google Scholar]
- 24. Fan JW, Zhong HP, Harris W, Yu GR, Wang SQ, et al. (2008) Carbon storage in the grasslands of China based on field measurements of above- and below-ground biomass. Climate Change 86: 375–396. [Google Scholar]
- 25. Kang L, Han XG, Zhang ZB, Sun QJ (2007) Grassland ecosystems in China: review of current knowledge and research advancement. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 362: 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xia L (1995) Modelling the response of vegetation in North-East China transect to global change. Journal of Biogeography 22: 515–522. [Google Scholar]
- 27. Liu YS, Pan QM, Zheng SH, Bai YF, Han XG (2012) Intra-seasonal precipitation amount and pattern differentially affect primary production of two dominant species of Inner Mongolia grassland. Acta Oecologica 44: 2–10. [Google Scholar]
- 28. Ni J, Wang GH, Bai YF, Li XZ (2007) Scale-dependent relationships between plant diversity and above-ground biomass in temperate grasslands south-eastern Mongolia. Journal of Arid Environments 68: 132–142. [Google Scholar]
- 29. Zhao W, Chen SP, Lin GH (2007) Compensatory growth responses to clipping defoliation in Leymus chinensis (Poaceae) under nutrient addition and water deficiency conditions. Plant Ecology 196: 85–99. [Google Scholar]
- 30. Hubert WL (1967) On the Kolmogorov-Smirnov Test for Normality with Mean and Variance Unknown. Journal of the American Statistical Association 62: 399–402. [Google Scholar]
- 31. Griffith DA, Csillag F (1993) Exploring relationships between semi-variogram and spatial autoregressive models. Papers in Regional Science 72: 283–295. [Google Scholar]
- 32.Pinheiro JC, Bates M (2000) Mixed-Effects Models in S and S-PLUS. Springer-Verlag, New York.
- 33. Wei XR, Hao MD, Shao MG, Gale WJ (2006) Changes in soil properties and the availability of soil micronutrients after 18 years of cropping and fertilization. Soil & Tillage Research 91: 120–130. [Google Scholar]
- 34. Niklas KJ (2006) A phyletic perspective on the allometry of plant biomass-partitioning patterns and functionally equivalent organ-categories. New Phytologist 71: 27–40. [DOI] [PubMed] [Google Scholar]
- 35.Niklas KJ (1994) Plant Allometry: The scaling of form and process. Chicago: University of Chicago Press.
- 36.Falster DS, Warton DI, Wright IJ. Smatr: Standardized major axis tests and routines Version 2.0. Available: http://www.bio.mq.edu.au/ecology/smatr. Accessed 2007 Jun01.
- 37. Li YH, Luo TX, Lu Q (2008) Plant height as a simple predictor of the root to shoot ratio: Evidence from alpine grasslands on the Tibetan Plateau. Journal of Vegetation Science 19: 245–252. [Google Scholar]
- 38. Chapin FS III, Chapin MC (1981) Ecotypic differentiation of growth processes in Carex aquatilis along latitudinal and local gradients. Ecology 62: 1000–1009. [Google Scholar]
- 39.Snowdon P, Eamus D, Gibbons P (2000) Synthesis of allometrics: review of root biomass and design of future woody biomass sampling strategies. NCAS Technical Report 17, Australian Greenhouse Office, Canberra, AU.
- 40. Henry HAL, Thomas SC (2002) Interactive effects of lateral shade and wind on stem allometry, biomass allocation, and mechanical stability in Abutilon theophrasti (Mal-vaceae). American Journal of Botany 89: 1609–1615. [DOI] [PubMed] [Google Scholar]
- 41.Chapin FSIII, Autumn K, Pugnaire F (1993) Evolution of suites of traits in response to environmental stress. American Naturalist 142 (Suppl.): S78–S92.
- 42. Kummerow J, Ellis BA (1984) Temperature effect on biomass production and root/shoot biomass ratios in two arctic sedges under controlled environmental conditions. Canadian Journal of Botany 62: 2150–2153. [Google Scholar]
- 43. Xu ZZ, Zhou GS (2006) Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis . Planta 224: 1080–1090. [DOI] [PubMed] [Google Scholar]
- 44. Reich PB, Oleksyn J (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. Proceedings of the National Academy of Sciences United States of America 101: 11001–11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hobbie SE, Nadelhoffer KJ, Högberg P (2002) A synthesis: The role of nutrients as constraints on carbon balances in boreal and arctic regions. Plant and Soil 242: 163–170. [Google Scholar]
- 46. Lü XT, Kong DL, Pan QM, Simmons ME, Han XG (2012) Nitrogen and water availability interact to affect leaf stoichiometry in a semi-arid grassland. Oecologia 168: 301–310. [DOI] [PubMed] [Google Scholar]
- 47. James RE, Thure EC, Brent RH (1997) C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112: 285–299. [DOI] [PubMed] [Google Scholar]
- 48. Wang RZ (2004) C4 species and their response to large-scale longitudinal climate variables along the Northeast China Transect (NECT). Photosynthetica 42: 71–79. [Google Scholar]
- 49. Ehleringer JR, Cerling TE, Helliker BR (1997) C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112: 285–299. [DOI] [PubMed] [Google Scholar]
- 50. Sage RF, Kubien DS (2003) Quo vadis C4? An ecophysiological perspective on global change and the future of C4 plants. Photosynthesis Research 77: 209–225. [DOI] [PubMed] [Google Scholar]
- 51. Sage RF, Kubien DS (2007) The temperature response of C-3 and C-4 photosynthesis. Plant Cell and Environment 30: 1086–1106. [DOI] [PubMed] [Google Scholar]
- 52. Pau S, Edwards EJ, Still CJ (2013) Improving our understanding of environmental controls on the distribution of C3 and C4 grasses. Global Change Biology 19: 184–196. [DOI] [PubMed] [Google Scholar]
- 53. Enquist BJ, Niklas KJ (2002) Global allocation rules for patterns of biomass partitioning in seed plants. Science 295: 1517–1520. [DOI] [PubMed] [Google Scholar]
- 54. Kerkhoff AJ, Enquist BJ, Elser JJ, Fagan WF (2005) Plant allometry, stoichiometry and the temperature-dependence of primary productivity. Global Ecology and Biogeography 14: 585–598. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dataset on the root/ratios of four dominant genera ( Stipa , Cleistogenes , Agropyron , and Leymus ), and information on the geographic location and climate of the study area.
(XLSX)