Abstract
Following focal cerebral ischemia, blood vessels in the ischemic border, or penumbra, launch an angiogenic response. In light of the critical role for fibronectin in angiogenesis, and the observation that fibronectin and its integrin receptors are strongly upregulated on angiogenic vessels in the hypoxic CNS, the aim of this study was to establish whether angiogenic vessels in the ischemic CNS also show this response. Focal cerebral ischemia was established in C57/Bl6 mice by middle cerebral artery occlusion (MCA:O), and brain tissue analyzed seven days following re-perfusion, a time at which angiogenesis is ongoing. Within the ischemic core, immunofluorescent (IF) studies demonstrated vascular expression of MECA-32, a marker of leaky cerebral vessels, and vascular breakdown, defined by loss of staining for the endothelial marker, CD31, and the vascular adhesion molecules, laminin, dystroglycan and α6 integrin. Within the ischemic penumbra, dual-IF with CD31 and Ki67 revealed the presence of proliferating endothelial cells, indicating ongoing angiogenesis. Significantly, vessels in the ischemic penumbra showed strong upregulation of fibronectin and the fibronectin receptors, α5β1 and αvβ3 integrins. Taken together with our recent finding that the α5β1 integrin plays an important role in promoting cerebral angiogenesis in response to hypoxia, these results suggest that stimulation of the fibronectin-α5β1 integrin signalling pathway may provide a novel approach to amplifying the intrinsic angiogenic response to cerebral ischemia.
Keywords: cerebral ischemia, angiogenesis, endothelial cells, integrin, fibronectin, laminin, astrocyte
INTRODUCTION
Neurons in the central nervous system (CNS) are acutely sensitive to loss of oxygen and glucose, and if starved of these nutrients for even minutes, embark on a pathway of cell death (Dirnagl et al. 1999). The current consensus is that once this destructive pathway is initiated, it continues in an irreversible manner, resulting in wholesale loss of neurons and the well-described clinical hallmarks of stroke: aphasia, paralysis or even death (Sharp et al. 2000). While the clinical defect is ultimately caused by death of neurons and associated glial cells, cerebral ischemia also affects the function and integrity of vascular cells, which in itself, can lead to exacerbation of neuronal death (del Zoppo and Hallenbeck 2000). Shortly following the ischemic insult, cerebral vessels in the ischemic core start to lose their permeability barrier (Hamann et al. 1996), due to loss of endothelial tight junction proteins (Yang et al. 2007). At the same time, extracellular matrix (ECM) proteins in the vascular basal lamina are degraded (Hamann et al. 1995), and endothelial cells and astrocytes lose expression of ECM receptors , resulting in their uncoupling from the vascular basement membrane and cell death (Tagaya et al. 2001; Tagaya et al. 1997; Wagner et al. 1997). This combination of events leads to vessel breakdown, and secondary expansion of the ischemic infarct.
While ischemia has catastrophic consequences for cerebral vessels within the ischemic core, evidence suggests that the milder ischemic stimulus experienced in the ischemic periphery, or penumbra, actually promotes angiogenic remodeling, such that 4-7 days after the ischemic insult, new sprouting capillaries can be seen (Chen et al. 1994; Hayashi et al. 2003; Wei et al. 2001). This has led to the suggestion that stimulation or amplification of this endogenous response could be exploited as a means of encouraging new blood vessel formation in cerebral areas susceptible to ischemia, either before, or immediately after an ischemic event (Greenberg and Jin 2005).
In trying to define the molecular mechanisms that regulate cerebral angiogenesis, studies have revealed a role for specific transcription factors, including hypoxia inducible factor-1α (HIF-1α) (Chavez et al. 2000) and the Hox family (Chen et al. 2004), growth factors, including vascular endothelial growth factor (VEGF) (Kuo et al. 1999), and the angiopoietins (Ang1 and Ang2) (Pichiule and LaManna 2002). In addition, ECM proteins also play important roles in regulating vascular growth and remodeling. Specifically, fibronectin promotes angiogenesis, both during development and in physiological and pathological remodeling events in the adult (Astrof and Hynes 2009). Null mutations in the fibronectin gene or its main cell surface receptors, the α4, α5 or αv integrins, show major defects in developmental angiogenesis, leading to embryonic or postnatal lethality (Bader et al. 1998; George et al. 1993; Yang et al. 1993; Yang et al. 1995). An angiogenic role for fibronectin is further suggested by the observations that fibronectin and its specific integrin receptors are expressed at only low levels in quiescent vessels, but strongly upregulated in developmental or tumor-associated angiogenic vessels (Kim et al. 2000; Risau and Lemmon 1988; Tonnesen et al. 1985).
Studies of cerebral angiogenesis are largely consistent with these findings, with fibronectin and its integrin receptors expressed at high levels by angiogenic vessels in the developing brain, but then downregulated with maturation (Dummula et al. 2010; Milner and Campbell 2002). To determine whether this developmental program is recapitulated during angiogenesis in the adult CNS, we recently examined this process in a mouse model of cerebral hypoxia, in which a strong physiological angiogenic response occurs. This revealed strong induction of fibronectin and the two fibronectin receptors, α5β1 and αvβ3 integrins on angiogenic vessels (Li et al. 2010; Milner et al. 2008a). More recently, we have demonstrated the functional significance of these interactions, by showing that mice deficient in endothelial cell expression of the α5 integrin, show an attenuated angiogenic response to chronic cerebral hypoxia (Li et al, manuscript in preparation). Taken together, this demonstrates that fibronectin promotes cerebral angiogenesis, via the α5β1 integrin. Considering the potential clinical relevance of angiogenic vessels in the ischemic penumbra, one fundamental question yet to be addressed is: do angiogenic vessels in the ischemic penumbra also show upregulation of fibronectin and its associated receptors? In the current study we have addressed this question by establishing cerebral ischemia using the middle cerebral artery occlusion (MCA:O) model in mice, and then examined brains 7 days post-ischemia (the time window at which angiogenesis occurs), to characterize vascular expression of the ECM proteins fibronectin and laminin, and their cell surface receptors.
MATERIALS AND METHODS
Animals
The present study was conducted in accordance with NIH guidelines for the care and use of animals in research and under protocols approved by the Center for Laboratory Animal Care at The University of Connecticut Health Center. Focal transient cerebral ischemia was induced in male C57Bl6 mice (20-25g) by right middle cerebral artery occlusion (MCAO) followed by reperfusion as described previously (Li et al. 2007). Mice were anesthetized with 4% isoflurane mixed with room air and oxygen for induction and then maintained with 1-2% isoflurane/room air and oxygen mixture for all surgical procedures. At the end of ischemia (90 minutes MCAO, the animal was briefly re-anesthetized, and reperfusion was initiated by filament withdrawal. Cortical perfusion (Laser Doppler Flowmetry; LDF) were evaluated throughout MCAO and early reperfusion as described previously (Li et al. 2007).
Behavioral measurements
Neurological deficits (NDS) were scored immediately after MCA:O and at daily intervals up to day 7 post-stroke. The scoring system was as follows: 0, no deficit; 1, forelimb weakness and torso turning to the ipsilateral side when held by tail; 2, circling to affected side; 3, unable to bear weight on affected side; and 4, no spontaneous locomotor activity or barrel rolling (McCullough et al. 2005). Median NDS scores immediately after MCA:O, and after 3 days and 7 days post-ischemia were: 3 (range 3-4), 1 (range 1-2) and 1 (range 0-1) respectively (n = 7). Weights of the mice at the same time-points were: 23.9 ± 0.87 g, 19.5 ± 1.54 g, and 21.8 ± 2.27 g, respectively.
Immunofluorescent studies and antibodies
Seven days following MCA:O, mice were euthanized by perfusion with ice-cold saline, and the brains rapidly dissected, equilibrated in 30 % sucrose for 3 days, and then stored at −80°C. In parallel, untreated fresh brain slices, staining with TTC was performed, as previously described (Liu et al. 2009), to demarcate the cerebral infarct. Immunofluorescent (IF) studies were performed as previously described (Milner et al. 2008a) on 10 μm thick frozen coronal sections. The following monoclonal antibodies were obtained from BD Pharmingen (La Jolla, CA): rat monoclonal antibodies reactive for the α5 integrin subunit (clone 5H10-27), α6 integrin (clone GoH3), Mac-1 (αMβ2 integrin, clone M1/70), CD31 (PECAM-1) (clone MEC13.3), and clone MECA-32, and the hamster monoclonal antibodies reactive for the α5 (clone HMα5-1) and β3 integrin subunits (clone 2C9.G2). The mouse monoclonal antibody reactive for β-dystroglycan (clone 43DAG/8D5) was obtained from Novocastra (Newcastle, United Kingdom). The Cy-3 conjugated mouse monoclonal against GFAP (clone G-A-5) was obtained from Sigma. Rabbit polyclonal antibodies against the following proteins were used in this study: fibronectin (Sigma), laminin (Sigma), Ki67 (Vector Laboratories, Burlingame, CA). The Cy3-conjugated anti-rabbit and anti-hamster secondary antibodies were obtained from Jackson Immunoresearch (West Grove, PA), and the AlexaFluor 488-conjugated anti-rat, anti-mouse, and anti-rabbit secondary antibodies were obtained from Invitrogen (Carlsbad, CA). TUNEL staining was performed using the TUNEL kit (Invitrogen) according to the manufacturer’s instructions. Briefly, frozen brain sections were processed for TUNEL-positive cells, and then counter-stained with the nuclear marker Hoechst (Sigma).
Quantification and statistical analysis
Quantification of the number of blood vessels positive for the different antigens was performed by capturing images of the three regions of interest (contralateral hemisphere, ischemic core and penumbra) with a X 20 objective on a Zeiss Axio Observer A1 microscope and Zeiss Axiocam MRC digital camera. Multiple images were taken of each section, the number of positive events per field of view quantified in the three different regions in each brain (n = 5 mice), and the results expressed as the mean ± SEM of the number of antigen-positive events per field of view. Statistical significance was assessed by using Student’s t test, in which p < 0.05 was defined as statistically significant.
RESULTS
Characterization of cell death and glial activation responses in the ischemic CNS
C57BL/6 mice were subject to 90 minutes of ischemia by temporary occlusion of the middle cerebral artery (MCA:O), followed by 7 days reperfusion. The size of the ischemic lesion in these mice varied between 50-75% of the affected hemisphere (Figure 1A). The ischemic hemisphere showed typical hallmark signs of severe focal cerebral ischemia, including widespread areas of TUNEL-positive staining, indicating massive cell death (Figure 1B). Within the ischemic hemisphere, astrocytes and microglia showed a marked glial activation response (Figure 2A). GFAP immunofluoresence (IF) revealed an almost total absence of astrocytes within the ischemic core, suggesting that most astrocytes within this area had undergone cell death. In contrast, at the boundary between the ischemic core and penumbra, a very intense GFAP reaction was apparent, with highly elevated levels of GFAP expression within all astrocytes. Mac-1 IF demonstrated that within the ischemic penumbra and core, microglia and/or macrophages showed increased cell density and increased levels of Mac-1 expression, typical of the activated phenotype (Hanisch and Kettenmann 2007).
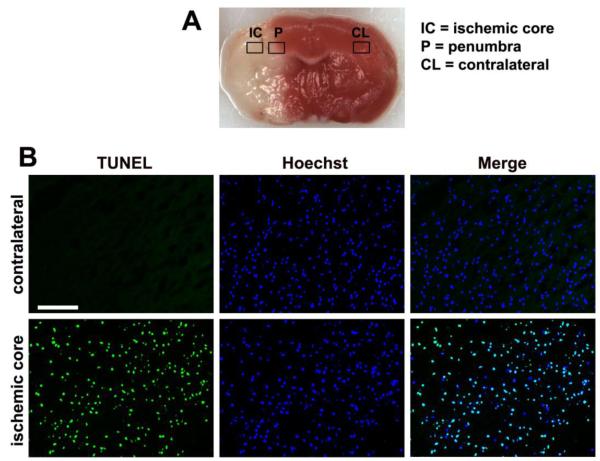
Figure 1.
Examination of cell death within the cerebral infarct seven days after middle cerebral artery occlusion (MCA:O). A. TTC staining differentiates the infarct (white) from viable tissue (red). Boxes outline the regions of interest, including the ischemic core (IC), ischemic penumbra (P), and the contralateral hemisphere (CL). B. TUNEL staining reveals massive cell death within the ischemic core. Scale bar = 50 μm.
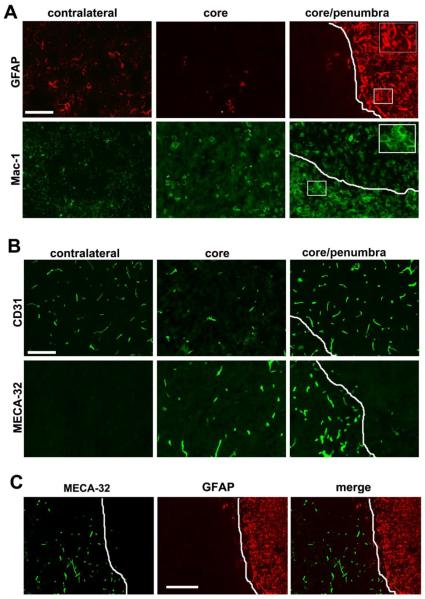
Figure 2.
Glial and vascular activation responses seven days post-focal cerebral ischemia. White lines demarcate the ischemic core/penumbra boundary. A. GFAP immunofluorescence (IF) revealed a paucity of astrocytes within the ischemic core, but a marked astrocytic activation within the ischemic penumbra. Mac-1 IF demonstrated a microglia/macrophage activation response in the ischemic core and penumbra. Scale bar = 50 μm (inserts, scale bar = 15 μm). B. CD31 IF showed a reduction in vessel density within the ischemic core, but an increased density in the ischemic penumbra. MECA-32 IF revealed no staining in the contralateral hemisphere, but strong induction on vessels in the ischemic core and penumbra. Scale bar = 50 μm. C. Dual-IF with MECA-32 and GFAP revealed an exclusive relationship between the two markers; astrocyte-ensheathed vessels never expressed the MECA-32 antigen, but vessels denuded of astrocyte contact showed strong MECA-32 induction. Scale bar = 100 μm.
Cerebral ischemia leads to alterations in blood vessel morphology and expression of vascular markers
Blood vessels were identified with the endothelial-specific marker CD31 (PECAM-1). Compared with the contralateral hemisphere, the density of CD31-positive vessels within the ischemic core was markedly reduced (20.5 ± 3.5 vessels/field compared with 47.5 ± 5.4 vessels/field in the contralateral hemisphere, p < 0.01), demonstrating that ischemia leads to degeneration of blood vessels within the ischemic core. In contrast, vessel density in the ischemic penumbra was significantly elevated (67.0 ± 8.1 vessels/field compared with 47.5 ± 5.4 vessels/field in the contralateral hemisphere, p < 0.01), suggesting the growth of new blood vessels in the ischemic penumbra (Figure 2B). Next, we examined vessel expression of mouse endothelial cell antigen-32 (MECA-32), as this gives an indication of the immaturity or leakiness of cerebral vessels. MECA-32 is not expressed by vessels in the mature CNS, but is expressed by immature developing cerebral blood vessels (up to embryonic day 15) before being switched off (Hallman et al. 1995), and is also induced on adult cerebral vessels following endothelial cell disruption during infection (Engelhardt et al. 1994). We have previously demonstrated that MECA-32 is transiently induced on angiogenic vessels in the hypoxic adult CNS (Milner et al. 2008a). Consistent with previous findings, blood vessels in the contralateral hemisphere were negative for MECA-32, whereas in contrast, vessels in the ischemic core expressed high levels of this antigen (Figure 2B). In addition, vascular expression of MECA-32 appeared to be inversely related to the presence of GFAP expression, with the on-off border of both markers occurring at the ischemic core/penumbra boundary. To more closely examine this relationship, we performed dual-IF for GFAP and MECA-32. This revealed that vessels covered by GFAP-positive astrocyte processes were negative for MECA-32, while in contrast, vessels not contacted by GFAP processes expressed high levels of MECA-32 (Figure 2C).
Cerebral vessels in the ischemic core show loss of the cell surface laminin receptors, dystrogycan and α6β1 integrin, but a slower breakdown of vascular basement membrane laminin
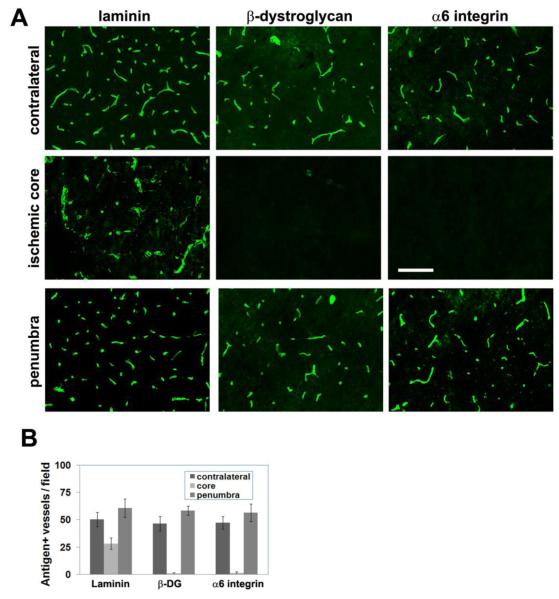
Previous studies of MCA:O in non-human primate and rat have shown that cerebral ischemia leads to loss of basement membrane laminin and the laminin receptors, α6β1 and dystroglycan from cerebral vessels (Hamann et al. 1995; Milner et al. 2008b; Tagaya et al. 2001). To investigate whether this also occurs in mice, we examined expression of these cell adhesion molecules in this study. Interestingly, in our 7 day post-ischemic samples, dystroglycan and the α6β1 integrin, which are expressed by all vessels in the non-ischemic brain, were totally absent from vessels in the ischemic core (Figure 3A). This loss of dystroglycan is entirely consistent with our previous observation that astrocyte foot processes are the major source of dystroglycan on cerebral vessels (Milner et al. 2008b); thus it follows that absence of astrocytes in the ischemic core would lead to disappearance of dystroglycan from cerebral vessels. In contrast to the total loss of dystroglycan and α6β1 integrin, vessels in the ischemic core still expressed residual levels of laminin, though staining revealed dilated dysmorphic vascular structures within the ischemic core. By comparison, vessels in the ischemic penumbra showed no loss of expression of laminin, α6β1 integrin or dystroglycan.
Figure 3.
The influence of focal cerebral ischemia on vascular expression of laminin and laminin receptors. A. Seven days following cerebral ischemia, expression of the laminin receptors dystroglycan and α6 integrin was totally eliminated from vessels in the ischemic core. Laminin immunoreactivity was still present on vessels in the core region, but the staining revealed dilated degenerating vessels. Scale bar = 50 μm. B. Quantification of vascular expression of laminin and its receptors. Note the total loss of β-dystroglycan and α6 integrin expression from vessels in the ischemic core, but the delay in loss of laminin.
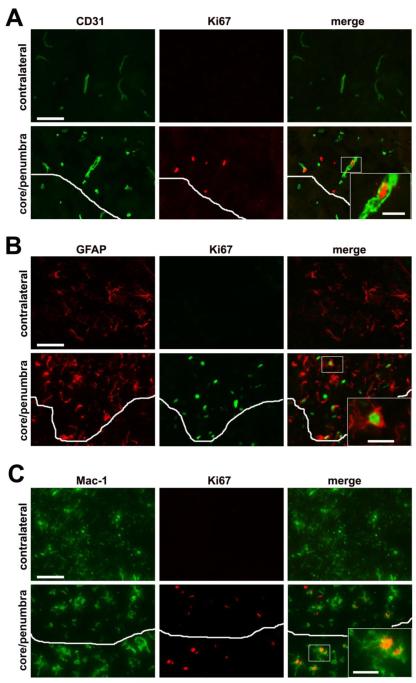
Vascular remodeling in the ischemic penumbra is associated with proliferation of endothelial cells, as well as proliferation of astrocytes and microglia
It is well established that an angiogenic response occurs within the ischemic penumbra (Chen et al. 1994; Hayashi et al. 2003; Wei et al. 2001). To examine this response in the mouse MCA:O model, we performed dual-IF for the endothelial marker CD31, and the cell proliferation marker, Ki67. As shown in Figure 4A, whereas no Ki67-positive cells were observed in the contralateral hemisphere, numerous CD31/Ki67 dual-positive cells were seen in the ischemic penumbra, demonstrating brain endothelial cell proliferation and angiogenic remodeling. This analysis also revealed many Ki67-positive cells that were CD31-negative, suggesting the presence of other proliferating cell types, both in the ischemic penumbra and core. To investigate whether astrocytes or microglia also proliferate in the ischemic penumbra, we performed dual IF for GFAP/Ki67 and Mac-1/Ki67, which confirmed the presence of proliferating astrocytes and microglia respectively (Figures 4B and C).
Figure 4.
Identification of proliferating cells in the ischemic penumbra seven days following focal cerebral ischemia. Dual-IF with cell-specific markers and the proliferation marker Ki67 was performed on frozen sections of ischemic penumbra or contralateral hemisphere to identify: (A) endothelial cells (CD31), (B) astrocytes (GFAP), or (C) microglia/macrophages (Mac-1). White lines demarcate the ischemic core/penumbra boundary. Scale bar = 25μm (inserts = 7.5μm). Note that no cell proliferation was detected in the contralateral hemisphere. In contrast, the ischemic penumbra contained proliferating endothelial cells, astrocytes and microglia/macrophages.
Vessels in the ischemic penumbra show increased expression of fibronectin, and enhanced endothelial expression of α5β1 and αvβ3 integrins
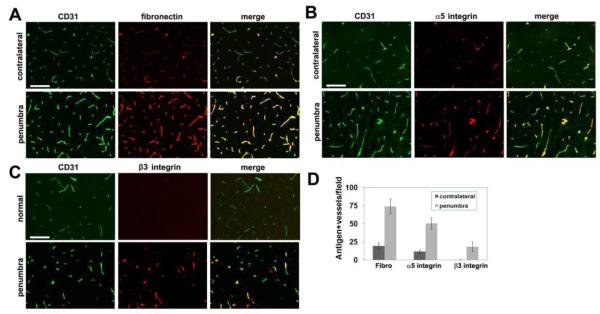
We have shown that angiogenic vessels in the hypoxic CNS strongly upregulate expression of fibronectin and its receptors, α5β1 and αvβ3 (Li et al. 2010; Milner et al. 2008a). Now, we addressed whether a similar induction also occurs on angiogenic vessels in the ischemic CNS. Consistent with previous findings, vessels in the contralateral hemisphere expressed low levels of fibronectin. By comparison, vessels within the ischemic penumbra showed markedly elevated levels of fibronectin expression (Figure 5A). Quantification revealed that the number of fibronectin-positive vessels in the ischemic penumbra was significantly greater than in the contralateral hemisphere (74 ± 10.3 fibronectin-positive vessels/field compared with 19.5 ± 4.2 in the contralateral hemisphere, p < 0.001). In a similar manner, while endothelial cells in the contralateral hemisphere expressed only low levels of α5β1 and no αvβ3 integrin, both fibronectin receptors were strongly upregulated on vessels in the ischemic penumbra (50.5 ± 7.7 α5 integrin-positive vessels/field in the penumbra compared with 11.8 ± 2.5 in the contralateral hemisphere, p < 0.001, and 18.5 ± 6.5 β3 integrin-positive vessels/field compared with 0.3 ± 0.5 in the contralateral hemisphere, p < 0.002), (Figures 5B-D). This confirms that angiogenic vessels in the ischemic penumbra show clear upregulation of fibronectin and its cognate receptors, α5β1 and αvβ3 integrins. We also investigated the expression of fibronectin and the α5 and β3 integrins on vessels in the ischemic core (Figure 6). This analysis was complicated by the fact that the ischemic core was heavily infiltrated with inflammatory leukocytes expressing high levels of the α5 integrin that were surrounded by a fibronectin-rich ECM. In addition, while the β3 integrin was not expressed at high levels by the inflammatory infiltrates, it was apparent that within the ischemic core, the pattern of β3 staining was often more extensive than the CD31 staining, suggesting that some inflammatory cells transmigrating across the leaky damaged vessels may also express the β3 integrin. Despite these complications, dual-IF with CD31/fibronectin, CD31/α5 integrin, and CD31/β3 integrin suggested that vessels in the ischemic core also express elevated levels of fibronectin and the α5 and β3 integrins, as shown in the merged images in Figure 6.
Figure 5.
Upregulation of fibronectin and its integrin receptors on cerebral vessels in the ischemic penumbra seven days after focal cerebral ischemia. Frozen sections of ischemic penumbra or contralateral hemisphere were examined for vascular expression of: (A) fibronectin, (B) α5 integrin, or (C) β3 integrin. Scale bar = 50 μm. Note that while few vessels in the contralateral hemisphere expressed fibronectin or its receptors, vessels in the ischemic penumbra strongly upregulated fibronectin and the α5 and β3 integrins (D).
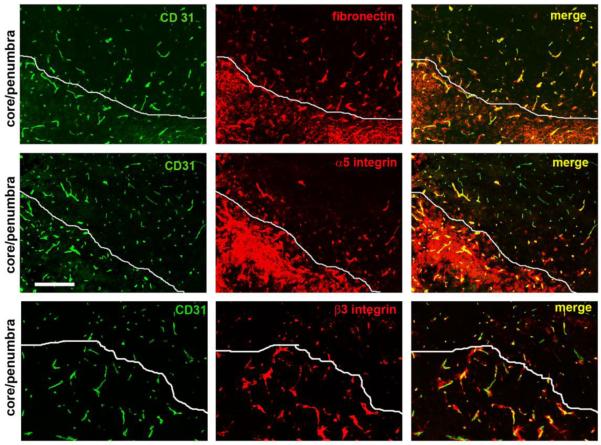
Figure 6.
Examination of vascular expression of fibronectin and its receptors in the ischemic core seven days after focal cerebral ischemia. Frozen sections encompassing the ischemic core and penumbra were examined for vascular expression of fibronectin, α5 integrin, or β3 integrin. White lines demarcate the ischemic core/penumbra boundary. Scale bar = 100 μm. Note that inflammatory leukocytes in the ischemic core expressed high levels of the α5 integrin and were surrounded by a fibronectin-rich ECM. In addition, vessels in the ischemic core expressed elevated levels of fibronectin and the α5 and β3 integrins, as demonstrated in the merged images.
DISCUSSION
Focal cerebral ischemia results in a central ischemic core and a surrounding ischemic periphery, or penumbra (Sharp et al. 2000). Ischemia within the core is so severe that most of the cells in this area, including neurons and glia, die within days of the initial insult (Dirnagl et al. 1999; Sharp et al. 2000). In the days following the ischemic insult, the ischemic core expands in size as more cells undergo cell death, likely as a result of secondary pathological mechanisms. While much effort has gone into understanding what happens to neurons and glia following ischemia, less is known about the fate and pathogenic mechanisms affecting vascular cells. This is fundamentally important for two reasons. First, expansion of the ischemic territory that occurs in the days following the primary ischemic insult (Liu et al. 2009) is likely a result of vascular breakdown and resulting edema. If the vascular damage could be limited, then the size of ischemic infarct could be minimized. Second, blood vessels in the ischemic penumbra launch an adaptive regenerative response, sprouting new blood vessels as part of an intrinsic attempt to deliver blood to the oxygen-starved region (Chen et al. 1994; Hayashi et al. 2003; Wei et al. 2001). Thus it follows that if this endogenous response could be enhanced, this would also minimize the size of ischemic infarct.
When contemplating the use of angiogenic therapy for stroke, two important issues should be addressed. The first is to define the optimal time of treatment: is it best to treat before, or immediately after ischemic stroke? Logic suggests that it would be optimal to promote angiogenesis before the damage has been done, but in order to do that, it is necessary to identify those patients who are at high risk of stroke. Such a group of patients might include those who have recently suffered a transient ischemic attack (TIA), as this group show high risk of subsequent stroke (Johnston et al. 2007; Rothwell et al. 2005). An alternative strategy would be to apply angiogenic therapy immediately after stroke. The down-side of this approach is that most ischemic neuronal cells die within days of ischemia, well before the growth of new blood vessels occurs, thus limiting the protective impact of post-stroke angiogenic therapy. However, post-ischemic therapy may have a role to play in promoting repair and neurogenesis following ischemic damage, which by itself may still promote clinical improvement. In addition to optimizing the timing of delivery, it is also imperative that angiogenic therapy creates functional cerebral vessels without causing deleterious side-effects, such as breakdown of blood-brain barrier (BBB) or hemorrhagic transformation, such as that observed in angiogenic studies using VEGF (Zhang et al. 2000). Clearly, if angiogenic therapy is to have any place in the clinic, these two issues need to be resolved first.
Fibronectin is an important regulator of vascular remodeling, and recent data from our lab suggests that fibronectin and its receptors play an important pro-angiogenic role in the CNS, both during development and in the angiogenic response to hypoxia in the adult (Li et al. 2010; Milner and Campbell 2002; Milner et al. 2008a). In light of this, the main aim of this study was to determine whether angiogenic vessels in the ischemic penumbra show upregulation of fibronectin and the α5β1 and αvβ3 integrins. Our main findings were as follows: (i) blood vessels in the ischemic core show strong induction of MECA-32, a marker of immature/leaky cerebral endothelium, and this induction correlates with loss of coverage from GFAP-positive astrocyte endfeet, (ii) vessels within the ischemic core degenerate and lose expression of the adhesion molecules laminin, dystroglycan, and α6β1 integrin, (iii) the appearance of CD31/Ki67 dual-positive cells in the ischemic penumbra demonstrates angiogenic vessels in this region, and (iv) vessels in the ischemic penumbra show marked upregulation of fibronectin and the fibronectin receptors, α5β1 and αvβ3 integrins.
Blood vessels in the ischemic core express MECA-32, a marker of immature cerebral endothelium, and this correlates with loss of vessel coverage by astrocyte endfeet
MECA-32 is a marker of immature or leaky brain endothelium. During development, MECA-32 labels brain endothelial cells only up to embryonic day 15 (E15) (Hallman et al. 1995). It also labels angiogenic endothelial cells in the adult CNS following cerebral hypoxia (Milner et al. 2008a) or after bacterial infection (Engelhardt et al. 1994). In this study, we determined that a large number of vessels within the ischemic core expressed MECA-32 at high levels, and that MECA-32-positive vessels extended up to the ischemic border. Interestingly, MECA-32 and GFAP expression displayed a mutually exclusive relationship, suggesting that cerebral vessels denuded of astrocyte contact lose their mature blood-brain barrier (BBB) properties, and re-express MECA-32. This would be consistent with the seminal work of Janzer and Raff, who demonstrated that astrocyte contact is essential for cerebral vessels to acquire BBB-specific properties of mature brain vessels (Janzer and Raff 1987). Our work suggests that MECA-32 is a good marker of brain vessels that have lost astrocyte contact, and would also explain why MECA-32 is expressed only transiently during the physiological adaptive angiogenic response in the hypoxic CNS (Milner et al. 2008a). During this response, remodeling cerebral vessels only momentarily lose their astrocyte contact, before re-adopting mature BBB characteristics. This is in contrast to vessels in the ischemic core, which lose astrocyte contact permanently, resulting in strong and sustained expression of MECA-32. Taken together, this evidence suggests that astrocytes play a central role in maintaining vascular integrity in the CNS, raising the notion that protecting astrocytes might be key in the treatment of ischemic stroke.
Differential loss of laminin and laminin receptors from cerebral vessels in the ischemic core
Our finding that blood vessels in the ischemic core show reduced expression of laminin and its cognate receptors, α6β1 integrin and dystroglycan are largely consistent with previous studies of MCA:O in other species (Hamann et al. 1995; Milner et al. 2008b; Tagaya et al. 2001). However, it should be noted that in our study we observed a differential loss of adhesion molecules in the ischemic core, with a total absence of dystroglycan and α6β1integrin, but only a partial reduction in laminin, 7 days post-ischemia. Several explanations may account for this. First, the most likely reason is that as blood vessels degenerate, endothelial cells undergo cell death, with concomitant loss of cell surface receptors, including dystroglycan and integrins. In contrast, ECM components of the vascular basal lamina including laminin are not destroyed immediately upon cell death, but remain for extended time in the form of a dysfunctional vascular scaffold. Second, dystroglycan and α6β1 integrin may be more sensitive to the effect of destructive proteases, than laminin. This would be consistent with our recent findings that dystroglycan expression is rapidly lost in the ischemic core of the non-human primate, in an MMP-dependent manner (Milner et al. 2008b). Third, taken with our recent observation that the vast majority of dystroglycan is expressed by astrocyte end-feet (Milner et al. 2008b), and our current finding, that by 7 days post-ischemia, the ischemic core is almost entirely devoid of astrocytes, it seems entirely feasible that loss of dystroglycan immunoreactivity directly reflects the absence of astrocytes in this region.
Induction of fibronectin and the α5β1 and αvβ3 integrins on angiogenic vessels in the ischemic penumbra
An angiogenic response in the ischemic penumbra has been well described, both following stroke in humans (Krupinski et al. 1994) or experimental focal cerebral ischemia in animal models (Chen et al. 1994; Hayashi et al. 2003; Wei et al. 2001). One of the primary aims of this study was to determine whether the angiogenic vessels show induction of fibronectin and associated cell surface receptors, as has been demonstrated on angiogenic vessels in the hypoxic CNS. We found that this was indeed the case, with a large number of vessels in the ischemic penumbra showing markedly increased levels of expression of fibronectin and the α5β1 and αvβ3 integrins. These findings are consistent with previous studies showing that the αvβ3 integrin is induced on vessels in the post-ischemic CNS, both in non-human primate (Okada et al. 1996) and rat (Wei et al. 2001). This supports the growing concept that fibronectin provides an important angiogenic drive in the CNS, via the endothelial cell surface receptors α5β1 and αvβ3. Recent studies have demonstrated an important instructive role for β1 integrin signalling in post-ischemic angiogenesis (Lathia et al. 2010). Taken with our recent demonstration that the α5β1 integrin is a key mediator of cerebral angiogenesis (Li et al, manuscript in preparation), this strengthens the concept that manipulation of the α5 integrin signaling pathway may provide a novel approach to promote angiogenesis in the ischemic CNS. Interestingly, vessels in the ischemic core also expressed high levels of fibronectin and its integrin receptors. This most likely reflects expansion of the ischemic territory with time, i.e.: yesterday’s ischemic penumbra is today’s ischemic core. This is consistent with the idea that a hypoxic insult promotes endothelial expression of fibronectin and its integrin receptors, and that whether this angiogenic switch results in successful production of new vessels depends on how far the ischemic core expands. If the vessels become swallowed up by the expanding ischemic core, the vessels will breakdown and degenerate. However, if the vessels remain outside the core, they have the potential to form new functional vessels.
Previous studies have highlighted an important protective role for fibronectin following cerebral ischemia and trauma. Deletion of plasma fibronectin in transgenic mice led to a worse clinical outcome following cerebral ischemia, and this correlated with increased levels of neuronal death (Sakai et al. 2001). In another study, brain trauma induced greater neuronal death and lesion size in mice deficient in plasma fibronectin (Tate et al. 2007). The authors of both these studies suggested that plasma-derived fibronectin leaks into cerebral tissue following injury and BBB breakdown, providing a survival cue for neurons. Our studies provide an alternative explanation for these experiments by suggesting that fibronectin plays an important role in stimulating post-ischemic angiogenesis, which would provide an indirect protective effect on neuronal survival. In summary, we have demonstrated that fibronectin and the associated endothelial fibronectin receptors, α5β1 and αvβ3 integrins, are strongly upregulated on angiogenic vessels in the ischemic penumbra. In the next set of experiments, we will test the functional significance of this induction by determining whether cerebral angiogenesis is attenuated in transgenic mice lacking endothelial expression of the α5β1 or αvβ3 integrins.
Highlights.
Cerebral ischemia leads to angiogenesis in the ischemic penumbra.
We study the molecular mechanisms that underlie this process.
Angiogenic vessels upregulate fibronectin and integrin receptors, α5β1 and αvβ3.
This suggests that fibronectin may drive cerebral angiogenesis.
ACKNOWLEDGMENTS
RM was supported by a Harry Weaver Neuroscience Scholar Award (JF 2125A1/1) from the National Multiple Sclerosis Society (RM), and by the NIH RO1 grant NS060770. LDM was supported by NIH RO1 NS050505. This is manuscript number 21368 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Astrof S, Hynes RO. Fibronectins in vascular development. Angiogenesis. 2009;12:165–175. doi: 10.1007/s10456-009-9136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader B, Rayburn H, Crowley D, Hynes R. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- Chavez JC, Agani F, Pichiule P, LaManna JC. Expression of hypoxic inducible factor 1α in the brain of rats during chronic hypoxia. J Appl Physiol. 2000;89:1937–1942. doi: 10.1152/jappl.2000.89.5.1937. [DOI] [PubMed] [Google Scholar]
- Chen HH, Chien CH, Liu HM. Correlation between angiogenesis and basic fibroblast growth factor expression in experimental brain infarct. Stroke. 1994;25:1651–7. doi: 10.1161/01.str.25.8.1651. [DOI] [PubMed] [Google Scholar]
- Chen Y, Xu B, Arderiu G, Hashimoto T, Young WL, Boudreau NJ, Yang GY. Retroviral delivery of homeobox D3 gene induces cerebral angiogenesis in mice. J Cereb Blood Flow Metab. 2004;24:1280–1287. doi: 10.1097/01.WCB.0000141770.09022.AB. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thrombosis Research. 2000;98:V73–V81. doi: 10.1016/s0049-3848(00)00218-8. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathophysiology of ischemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Dummula K, Vinukonda G, Xu H, Hu F, Zia MT, Braun A, Shi Q, Wolk J, Ballabh P. Development of integrins in the vasculature of germinal matrix, cerebral cortex, and white matter of fetuses and premature infants. J Neurosci Res. 2010;88:1193–1204. doi: 10.1002/jnr.22301. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Conley FK, Butcher EC. Cell adhesion molecules on vessels during neuroinflammation in the mouse central nervous system. J Neuroimmunol. 1994;51:199–208. doi: 10.1016/0165-5728(94)90082-5. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- Hallman R, Mayer DN, Berg EL, Broermann R, Butcher EC. Novel mouse endothelial cell surface marker is suppressed during differentiation of the blood brain barrier. Dev Dyn. 1995;202:325–332. doi: 10.1002/aja.1002020402. [DOI] [PubMed] [Google Scholar]
- Hamann GF, Okada Y, del Zoppo GJ. Hemorrhagic transformation and microvascular integrity during focal cerebral ischemia. J Cereb Blood Flow Metab. 1996;16:1373–1378. doi: 10.1097/00004647-199611000-00036. [DOI] [PubMed] [Google Scholar]
- Hamann GF, Okada Y, Fitridge R, del Zoppo GJ. Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke. 1995;26:2121–21266. doi: 10.1161/01.str.26.11.2120. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–80. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, Sidney S. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369:283–92. doi: 10.1016/S0140-6736(07)60150-0. [DOI] [PubMed] [Google Scholar]
- Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin a5b1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–8. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- Kuo N-T, Benhayon D, Przybylski RJ, Martin RJ, LaManna JC. Prolonged hypoxia increases vascular endothelial growth factor mRNA and protein in adult mouse brain. J Appl Physiol. 1999;86:260–264. doi: 10.1152/jappl.1999.86.1.260. [DOI] [PubMed] [Google Scholar]
- Lathia JD, Chigurupati S, Thundyil J, Selvaraj PK, Mughal MR, Woodruff TM, Chan SL, Karamyan VT, Mattson MP, Arumugam TV. Pivotal role for beta-1 integrin in neurovascular remodelling after ischemic stroke. Exp Neurol. 2010;221:107–114. doi: 10.1016/j.expneurol.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007;38(11):2992–9. doi: 10.1161/STROKEAHA.107.490904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Welser JV, Milner R. Absence of the αvβ3 integrin dictates the time-course of angiogenesis in the hypoxic central nervous system: accelerated endothelial proliferation correlates with compensatory increases in α5β1 integrin expression. J Cereb Blood Flow Metab. 2010;30:1031–1043. doi: 10.1038/jcbfm.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Schafer DP, McCullough LD. TTC, Fluro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods. 2009;179:1–8. doi: 10.1016/j.jneumeth.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280(21):20493–502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL. Developmental regulation of β1 integrins during angiogenesis in the central nervous system. Mol Cell Neurosci. 2002;20:616–626. doi: 10.1006/mcne.2002.1151. [DOI] [PubMed] [Google Scholar]
- Milner R, Hung S, Erokwu B, Dore-Duffy P, LaManna JC, del Zoppo GJ. Increased expression of fibronectin and the α5β1 integrin in angiogenic cerebral blood vessels of mice subject to hypobaric hypoxia. Mol Cell Neurosci. 2008a;38:43–52. doi: 10.1016/j.mcn.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, Hung S, Wang X, Spatz M, del Zoppo G. The rapid decrease in astrocyte-associated dystroglycan expression by focal cerebral ischemia is protease-dependent. J Cereb Blood Flow Metab. 2008b;28:812–823. doi: 10.1038/sj.jcbfm.9600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Copeland BR, Hamann GF, Koziol JA, Cheresh DA, del Zoppo GJ. Integrin αvβ3 is expressed in selective microvessels following focal cerebral ischemia. Am J Pathol. 1996;149:37–44. [PMC free article] [PubMed] [Google Scholar]
- Pichiule P, LaManna JC. Angiopoietin-2 and rat brain capillary remodeling during adaptation and de-adaptation to prolonged mild hypoxia. J Appl Physiol. 2002;93:1131–1139. doi: 10.1152/japplphysiol.00318.2002. [DOI] [PubMed] [Google Scholar]
- Risau W, Lemmon V. Changes in the Vascular Extracellular Matrix during Embryonic Vasculogenesis and Angiogenesis. Dev Biol. 1988;125:441–450. doi: 10.1016/0012-1606(88)90225-4. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Giles MF, Flossmann E, Lovelock CE, Redgrave JN, Warlow CP, Mehta Z. A simple score (ABCD) to identify individuals at high risk of early stroke after transient ischaemic attack. Lancet. 2005;366:29–36. doi: 10.1016/S0140-6736(05)66702-5. [DOI] [PubMed] [Google Scholar]
- Sakai T, Johnson KJ, Murozono M, Sakai K, Magnuson MA, Wieloch T, Cronberg T, Isshiki A, Erickson HP, Fassler R. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia bit is not essential for skin-wound healing and hemostasis. Nature Med. 2001;7:324–330. doi: 10.1038/85471. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Lu A, Tang Y, Millhorn DE. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:1011–1032. doi: 10.1097/00004647-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Tagaya M, Haring H-P, Stuiver J, Wagner S, Abumiya T, Lucero J, Lee P, Copeland B, Seiffert D, del Zoppo G. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab. 2001;21:835–46. doi: 10.1097/00004647-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Tagaya M, Liu KF, Copeland BR, Seiffert D, Engler R, Garcia JH, del Zoppo GJ. DNA scission after focal brain ischemia. Temporal differences in two species. Stroke. 1997;28:1245–54. doi: 10.1161/01.str.28.6.1245. [DOI] [PubMed] [Google Scholar]
- Tate CA, Garcia AJ, LaPlaca MC. Plasma fibronectin is neuroprotective following traumatic brain injury. Exp Neurol. 2007;207:13–22. doi: 10.1016/j.expneurol.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Tonnesen MG, Jenkins D, Siegal SL, Lee LA, Huff JC, Clark RA. Expression of fibronectin, laminin, and factor VIII-related antigen during development of the human cutaneous microvasculature. J Invest Dermatol. 1985;85:564–568. doi: 10.1111/1523-1747.ep12277410. [DOI] [PubMed] [Google Scholar]
- Wagner S, Tagaya M, Koziol J, Quaranta V, del Zoppo GJ. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin α6β4 during focal cerebral ischemia/reperfusion. Stroke. 1997;28:858–865. doi: 10.1161/01.str.28.4.858. [DOI] [PubMed] [Google Scholar]
- Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–2184. doi: 10.1161/hs0901.094282. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in α5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by α4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen NV, Chopp M. VEGF enhances angiogenesis and promoted blood-brain barrier leakage in the ischemic brain. Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]