Abstract
Background
In a previous study, we demonstrated that swine with metabolic syndrome treated with alcohol had improved insulin signaling. We developed a follow-up study to evaluate the effects of alcohol on ischemic myocardium in animals without metabolic syndrome.
Methods
Fourteen Yorkshire swine underwent placement of an ameroid constrictor to induce chronic myocardial ischemia. Postoperatively, one group was supplemented with ethanol (ETOH), and one group was supplemented with sucrose (SUC) daily to normalize caloric intake.After 7 weeks, all animals underwent dextrose challenge and harvest of non-ischemic and ischemic myocardium. Tissues were analyzed for protein expression and histologic analysis.
Results
There was no difference in BMI, serum glucose or insulin levels. However ethanol supplementation up-regulated PI3K, pAKT, AKT and pFOX01 expression, which may promote insulin signaling and down regulated inhibitors of insulin signaling pIRS1 and pIRS2. There was no difference in intramyocardial glycogen, but there was increased GLUT4 expression in the ETOH group, which may promote glucose utilization.
Conclusions
Despite similar serum glucose and insulin levels, alcohol consumption up-regulates the insulin signaling pathway in the absence of metabolic syndrome in both nonischemic and chronically ischemic myocardium. These results suggest that alcohol selectively up-regulates the insulin signaling pathway despite normoglycemia.
Keywords: chronic myocardial ischemia, insulin signaling, alcohol consumption
Introduction
Insulin resistance and hyperinsulinemia are central components of metabolic syndrome and type 2 diabetes. Over time, insulin resistance promotes the development of cardiovascular disease by increasing vasoconstriction, inflammation and thrombosis. 1 Hyperglycemia and insulin resistance have also been associated with adverse clinical outcomes in patients undergoing coronary artery bypass graft surgery, and aggressive glycemic control has been shown to improve morbidity and mortality in diabetic and non-diabetic patients. 2 Alcohol consumption has emerged as an important modifiable risk factor for the development of diabetes and cardiovascular disease.3, 4 The J-shaped, dose-dependent relationship between alcohol consumption and cardiovascular disease is well established in numerous epidemiologic studies. At low to moderate doses of alcohol consumption, defined as 1-2 drinks or 15-30g ethanol daily reduces the risk of cardiovascular disease by 40% and diabetes by 30% compared to abstainers. 5-7 However at high doses of alcohol consumption, defined as >3-4 drinks or >60g ethanol daily, the cardioprotective effect is reversed and the risk of hypertension, hyperlipidemia, cardiovascular disease and diabetes increases.
One important mechanism for this biphasic effect on cardiovascular disease and diabetes is attributed to the dose dependent alcohol mediated reduction in insulin resistance. Low to moderate doses of daily alcohol improves insulin sensitivity and reduces insulin resistance, whereas high doses of alcohol worsens insulin sensitivity, thereby increasing the risk of diabetes and cardiovascular disease.8, 9 In a previous study conducted in our lab, we found that swine with metabolic syndrome supplemented with vodka and wine had increased insulin signaling in the liver and skeletal muscle compared to the control group despite insulin resistance.10 We developed a clinically relevant follow-up study of ethanol consumption and chronic myocardial ischemia to assess the effect of moderate ethanol consumption on insulin signaling in ischemic and non-ischemic myocardium in the absence of metabolic syndrome.
Methods
ANIMAL MODEL
Fourteen intact male Yorkshire miniswine (Parsons Research, Amherst, MA) were fed 500g/day of regular chow (Sinclair Research, Columbia, MO) daily and underwent ameroid constrictor placement to the left circumflex artery to simulate conditions of chronic myocardial ischemia as described previously11. Postoperatively, one group was supplemented with 90 ml of ethanol daily (50%/V, EtOH, n = 7) and the control group was supplemented with 80g of sucrose of equal caloric value to the alcohol (SUC, n = 7). After 7 weeks of diet supplementation, all animals were anesthetized and the heart was exposed via median sternotomy. The animals were euthanized by exsanguination and samples from the ischemic myocardium (in the distribution of the left circumflex coronary artery) and the non-ischemic normally perfused myocardium (in the distribution of the left anterior descending coronary artery) were collected. Tissue samples were rapidly frozen in liquid nitrogen for histologic and protein expression analysis.
Animals were weighed at the time of harvest. All animals were observed to ensure complete consumption of food and supplement, had unlimited access to water, and were housed in a warm, non-stressful environment for the duration of the experiment.
The Institutional Animal Care and Use Committee of the Rhode Island Hospital approved all experiments. Animals were cared for in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” (NIH publication no. 5377-3 1996).
SURGICAL INTERVENTIONS
Anesthesia
Anesthesia was induced with an intramuscular injection of telazol (4.4 mg/kg). Animals were endotracheally intubated, mechanically ventilated at 12 – 20 breaths per minute, and general anesthesia was maintained with a gas mixture of oxygen at 1.5 – 2 liters/min and isoflurane at 0.75 – 3.0% concentration.
Ameroid Constrictor Placement
Animals were given a single dose of intravenous enrofloxacin 5mg/kg for antibiotic prophylaxis and general anesthesia was induced and maintained. Animals were prepped and draped in the usual sterile fashion. The heart was exposed through a left mini-thoracotomy and pericardiotomy. The left atrial appendage was retracted, and the left circumflex artery was dissected at the take off of the left main coronary artery. The ameroid constrictor was placed around the left circumflex artery (Research Instruments SW, Escondito, CA). The pericardium was loosely re-approximated followed by a layered closure of the surgical incision. Post-operative pain was controlled with a single dose of intramuscular Buprenorphine (0.03 mg/kg) and a 72 hour Fentanyl patch (4μg/kg). All animals received 325mg of aspirin daily starting 1 day pre-operatively and continuing for a total of 5 days for prophylaxis against thrombo-embolic events. All animals continued perioperative antibiotics: enrofloxacin 68mg orally daily for 5 days.
Cardiac Harvest
Under general anesthesia, the heart was exposed via median sternotomy. Animals were euthanized by exsanguination and chronically ischemic myocardial samples from the left circumflex territory and non-ischemic myocardial samples from the left anterior descending territory were collected for further analysis.
SEROLOGIC STUDIES
Blood samples were drawn from the jugular vein prior to euthanasia and tissue harvest. Blood glucose measurements were taken at baseline (fasting) and 30 and 60 minutes after an intravenous 0.5mg/kg dextrose infusion. Blood samples were also drawn and analyzed for insulin levels. The chemistry laboratory at the Rhode Island Hospital, Providence, RI, analyzed serum samples.
PROTEIN EXPRESSION
Frozen tissue samples were homogenized and in a radio-immunoprecipitation assay solution (Boston BioProducts, Ashland, MA) supplemented with protease and phosphatase inhibitors. Forty micrograms of whole-tissue lysates were fractionated by SDS-PAGE 4-12% Bis-Tris gels (NuPage Novex Mini Gel, Invitrogen, Carlsbad, CA) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA). Membranes were incubated overnight at 4°C with primary antibodies at dilutions recommended by the manufacturer against phosphorylated insulin receptor substrate 1 (p-IRS1) (Ser612), IRS2, PI3K, phosphorylated AKT (Thr 308), AKT, phosphorylated GSK3β (Ser 9), GSK3β, phosphorylated FoxO1 (Ser 256), FoxO1 (all from Cell Signaling, Danvers, MA), and phosphorylated IRS2 (Ser 731) (abcam Cambridge, MA). Membranes were incubated with the appropriate horseradish peroxidase-linked secondary antibody for one hour at room temperature (Jackson ImmunoResearch, West Grove, PA). Immune complexes were visualized with enhanced chemiluminescense and images were captured with a digital camera system (G-Box, Syngene, Cambridge, England). Band densitometry was quantified as arbitrary light units using Image-J software (National Institutes of Health, Bethesda, MD). All membranes were probed with GAPDH to correct for loading error.
IMMUNOHISTOCHEMICAL ANALYSIS
Frozen myocardium was sectioned (12-μ-thickness) and fixed in 10% formalin for 10 minutes. Sections were blocked with 1% bovine serum albumin in phosphate buffered saline for 1 hour at room temperature and incubated with anti-Glut 4 antibody (Epitomics, Burlingame, CA) overnight at 4°C. Sections were then incubated with DyLight 488-conjugated anti-rabbit antibody (Jackson ImmunoResearch) for 45 minutes, then mounted with Vectashield with 4’,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Images were captured at X20 magnification with a Nikon E800 Eclipse microscope (Nikon, Tokyo, Japan) at the same exposure. Brightness was enhanced identically in all images to optimally display immunofluorescence (Adobe Photoshop, San Jose, CA).
HEMATOXYLIN-EOSIN AND PERIODIC ACID-SCHIFF
Hematoxylin-Eosin and Periodic Acid-Schiff staining was performed on frozen tissue sections by the Pathology and Histology Core Facility at Rhode Island Hospital. Images were obtained using Aperio ScanScope technology (Vista, CA) and captured at 20X magnification. Hematoxylin-Eosin stained tissue was analyzed for necrosis, inflammatory cell infiltrate, and morphologic differences between groups.
DATA ANALYSIS
All results are reported as mean ± standard error of the mean. Dextrose challenge serum glucose levels were analyzed with a two-way ANOVA followed by a post-hoc Bonferroni test. A Student's T-test was used to compare the means of all other studies using GraphPad Prism 5.0 Software (GraphPad Software Inc., San Diego, CA).
Results
ANIMAL MODEL
All animals included in the analysis survived the entire experiment. One animal in the SUC group died intraoperatively during ameroid constrictor placement of intractable ventricular arrhythmia and another animal in the ETOH group died on post-operative day 3 of a perforated gastric ulcer. The animals that did not survive were excluded from analysis. Prior to euthanasia and tissue collection, the SUC and ETOH groups had similar body mass index (BMI) (34.05kg/m2 and 34.58kg/m2 respectively p=0.68) (Table).
Table. BMI, Serum Glucose and Insulin Levels.
There was no difference in baseline BMI, blood glucose or insulin levels. After 30 and 60 minutes of dextrose infusion, there was no difference in serum glucose.
| SUC | ETOH | p Value | |
|---|---|---|---|
| BMI kg/m2 | 34.05±0.97 | 34.58±0.83 | 0.68 |
| Dextrose Challenge | ANOVA 0.30 | ||
| Blood Glucose 0 min | 44.71±7.69 | 63.29±8.73 | |
| Blood Glucose 30 min | 203.71±18.44 | 203.00±14.63 | |
| Blood Glucose 60 min | 124.86±14.12 | 141.86±14.34 | |
| Insulin (mU/L) | 3.41±0.35 | 3.37±0.28 | 0.93 |
Fold Change ± Standard Error of the Mean
SEROLOGIC STUDIES
At baseline, the SUC and ETOH groups had similar fasting serum glucose levels (44.7mg/dL and 63.3mg/dL respectively). Also, at thirty and sixty minutes after dextrose infusion, the SUC and ETOH groups had similar serum glucose (203.7mg/dL, 124.9mg/dL vs 203.0mg/dL, 141.86mg/dL respectively p=0.30). Fasting serum insulin levels were not statistically different in the SUC and ETOH groups (3.41mU/L and 3.37mU/L respectively p=0.93) (Table).
NON-ISCHEMIC TERRITORY PROTEIN EXPRESSION
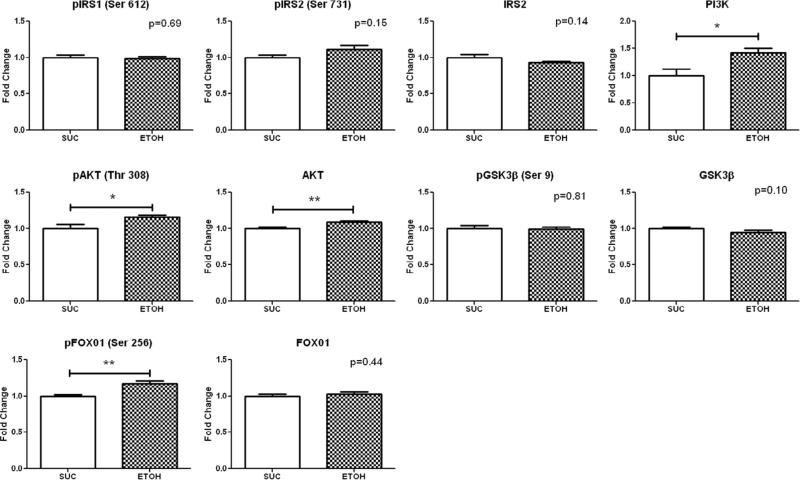
Western blot analysis in the non-ischemic territory demonstrated increased expression of PI3K (1.42±0.08 p =0.01), AKT (1.09±0.01 p=0.001), pAKT (Thr 308) (1.16±0.03 p=0.03) and pFOX01 (Ser 256) (1.17±0.04 p=0.003) in the ETOH compared to SUC (Figure 1).
Figure 1. Insulin Signaling Protein Expression in the Non-Ischemic Territory.
Levels represent fold change ± standard error of the mean compared to SUC. Western blot analysis demonstrated that alcohol consumption significantly up-regulated PI3K, pAKT (Thr 308), AKT, and pFOX01 (Ser 256) expression. *p<0.05 **p<0.01
ISCHEMIC TERRITORY PROTEIN EXPRESSION
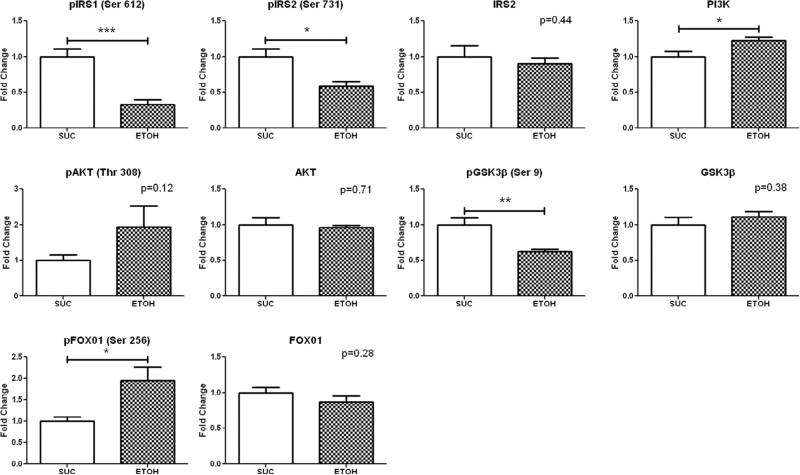
Western blot analysis in the ischemic territory demonstrated increased expression of PI3K (1.22±0.06 p=0.04) and pFOX01 (Ser 256) (1.95±0.31 p=0.01), and decreased expression of pIRS1 (Ser 612) (0.33±0.07 p=0.0003), pIRS2 (Ser 731) (0.59±0.06 p=0.01) and pGSK3β (Ser 9) (0.62±0.04 p=0.004) in the ETOH group compared to SUC. (Figure 2).
Figure 2. Insulin Signaling Protein Expression in the Ischemic Territory.
Levels represent fold change ± standard error of the mean compared to SUC. Western blot analysis demonstrated that alcohol consumption significantly up-regulated expression of PI3K and pFOX01 (Ser 256), and down-regulated expression of pIRS1 (Ser 612), pIRS2 (Ser 731) and pGSK3β (Ser 9) compared to SUC. *p<0.05 **p<0.01 ***p<0.001
GLUT4 IMMUNOHISTOCHEMISTRY IN NON-ISCHEMIC AND ISCHEMIC MYOCARDIUM
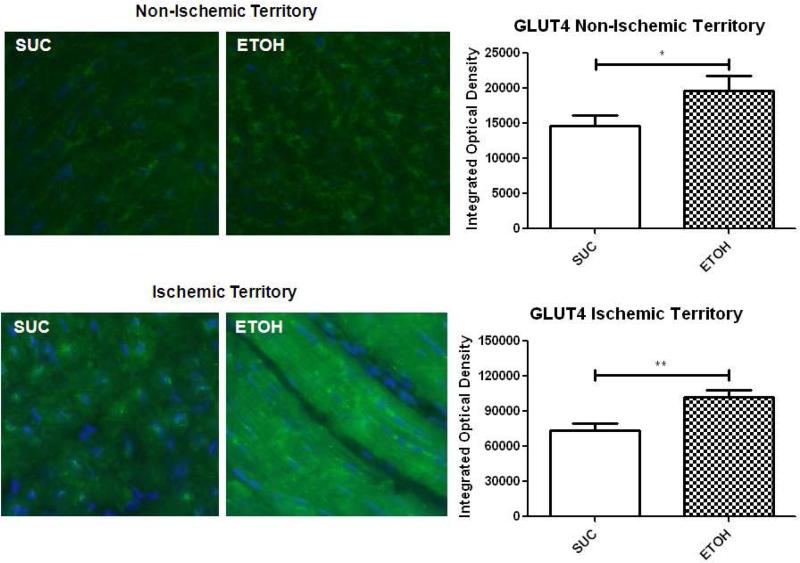
There was a significant increase in membrane-bound GLUT4 staining in the nonischemic territory in the ETOH group compared to SUC (19,652OD vs 14,590OD respectively p=0.03). Also, there was a marked increase in membrane-bound GLUT4 staining in the ischemic myocardium in the ETOH group compared to SUC (102,200OD vs 73,880OD respectively p=0.005). In comparison to the normal ventricle, there was more intense GLUT4 expression in the ischemic territory of the ETOH group (Figure 3).
Figure 3. GLUT4 Immunohistochemistry in the Non-Ischemic and Ischemic Myocardium.
Green staining indicates GLUT4 and blue staining indicates nuclei. Alcohol supplementation significantly up-regulated membrane-bound GLUT4 expression in both the non-ischemic and ischemic territories compared to SUC. There was also more intense GLUT4 staining in the ischemic territory of SUC and ETOH compared to the non-ischemic territory. *p<0.05 **p<0.01
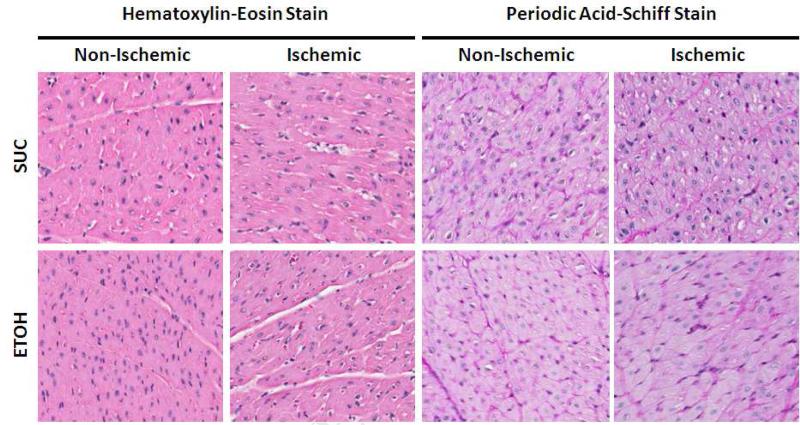
HISTOLOGIC ANALYSIS OF NON-ISCHEMIC AND ISCHEMIC MYOCARDIUM
There were no morphologic differences in the non-ischemic and ischemic myocardium when stained with Hematoxylin-Eosin. Periodic acid-Schiff staining for glycogen was homogenous in distribution and similar in both the non-ischemic and ischemic territory of the ETOH and SUC groups. (Figure 4).
Figure 4. Histologic Analysis of the Non-Ischemic and Ischemic Territory.
The Hematoxylin-Eosin stain did not demonstrate any morphologic differences between the SUC and ETOH groups. The Periodic Acid-Schiff stain did not demonstrate any difference in intramyocardial glycogen deposition in the non-ischemic or ischemic territories.
Discussion
This study demonstrated that Yorkshire swine supplemented with a moderate daily dose of ethanol had biochemical evidence of increased insulin signaling in the ischemic and non-ischemic myocardium. Both groups had similar serum insulin levels and serum glucose levels at baseline and thirty and sixty minutes after dextrose infusion suggesting that the animals did not have glucose intolerance or insulin resistance. This result was expected since both the SUC and ETOH groups were fed regular chow and this was not an animal model of diabetes or metabolic syndrome. As expected, there was no difference in BMI between the SUC and ETOH groups because both groups consumed the same number of calories daily.
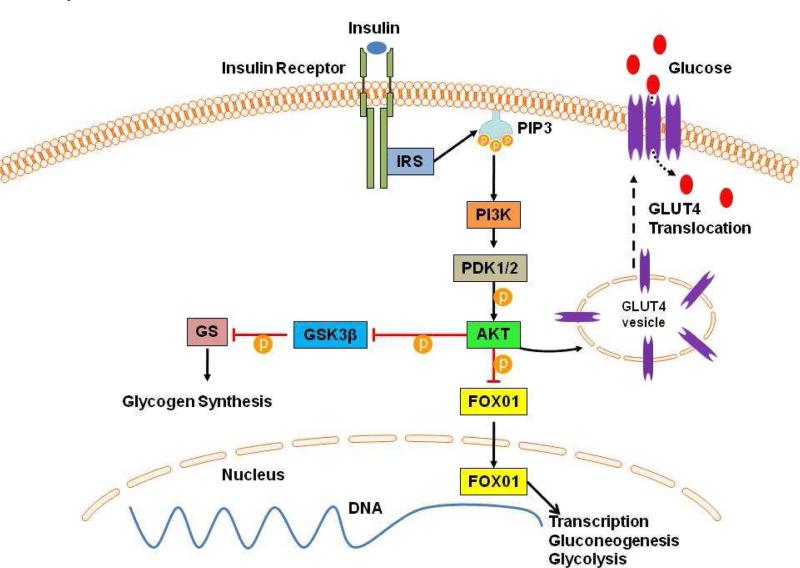
Despite the lack of insulin resistance or glucose intolerance, the ethanol treated animals had augmented insulin signaling in the ischemic and non-ischemic myocardium based on expression of proteins involved in insulin signaling. The insulin-signaling pathway starts with insulin binding the insulin receptor, resulting in tyrosine phosphorylation of IRS, which activates phosphoinostitide 3-kinase (PI3K) (Figure 5).12 In turn, PI3K activates PDK1/2, which activates AKT, a serine kinase. AKT is a central component of the insulin-signaling cascade, which triggers the translocation of transmembrane GLUT4 from intracellular vesicles to the plasma membrane. GLUT4 facilitates the movement of glucose into the cell from the extracellular space, thereby improving glucose utilization and reducing serum glucose levels. AKT also phosphorylates and inhibits GSK3β, which is an inhibitor of glycogen synthase. By inhibiting GSK3β, AKT effectively activates glycogen synthase, and allows for improved intracellular glucose utilization and glycogen synthesis. In a well-fed state, when insulin levels are high, AKT phosphorylates FOX01, a member of the forkhead box transcription factor family, which excludes it from the nucleus and inhibits its transcription activity of gluconeogenic and glycolysis genes.13
Figure 5. Insulin Signaling Pathway.
The effects of insulin on glucose uptake and insulin sensitivity presented here are discussed in detail in the text. PIP3 Phosphatidylinostitol Triphospate; PDK1/2 3-Phosphoinostitide-Dependent Kinase 1; GS Glycogen Synthase.
Although the histologic analysis did not reveal any differences in morphology or intramyocardial glycogen stores between the ETOH and SUC groups, there were significant differences in insulin signaling protein expression. In the normally perfused, non-ischemic myocardium, alcohol supplementation increased the expression of PI3K, pAKT, AKT and pFOX01, which all promote insulin sensitivity. Alcohol supplementation also significantly up-regulated membrane-bound GLUT4 immunofluorescence staining in the non-ischemic myocardium, confirming the overall trend observed in the insulin signaling protein expression.
In the collateral dependent chronically ischemic myocardium, there was down-regulation of serine phosphorylated IRS1 and IRS2. Serine phosphorylation of IRS inactivates it, and arrests the insulin signal. Insulin receptor substrate 1 is predominantly expressed in the liver, and IRS2 is expressed in the liver, muscle and adipose tissue.14 The net result of down-regulation of serine phosphorylated IRS1 and IRS2 promotes insulin signal transduction. There was also up-regulation of the down-stream target of IRS1 and IRS2, PI3K, and up-regulation of pFOX01. Interestingly, pGSK3β was down-regulated in the ETOH group, which inhibits glycogen synthase and worsens insulin sensitivity. Nonetheless, overall alcohol supplementation promoted insulin signaling in the ischemic myocardium, and this trend was further confirmed with significant ethanol-mediated up-regulation of membrane-bound GLUT4 immunofluorescence staining.
In comparing the intensity of GLUT4 staining in the ischemic territory to the non-ischemic territory, there was a marked increase in GLUT4 expression in the ischemic territory. This observation is consistent with known myocardial energy metabolism. Under normal conditions, non-ischemic myocardium primarily utilizes free fatty acids for energy. In the setting of ischemia, the myocardium can no longer metabolize free fatty acids, and glucose is increasingly utilized as the primary energy substrate.15 Therefore, in the ischemic myocardium, there is an increased demand for glucose to generate ATP, which may be the reason why there was a substantial increase in GLUT4 expression in the ischemic myocardium compared to the non-ischemic myocardium.
Insulin signaling is critically important in the setting of myocardial ischemia. Insulin promotes glucose uptake and optimizes glucose metabolism by stimulating pyruvate dehydrogenase and aerobic metabolism.16 Insulin also inhibits the release of free fatty acids, which prevents free fatty acid mediated depressed contractility, oxidative stress, endothelial dysfunction, and inhibition of insulin signaling.16-18 Insulin also has anti-inflammatory properties by inhibiting pro-inflammatory transcription factor NFKB and decreasing inflammatory mediators including IL-6 and TNFα.19 Taken together, the beneficial effects of insulin in ischemic myocardium on the cellular level translate to improved outcomes in patients undergoing coronary artery bypass surgery supplemented with insulin. Lazar and coworkers conducted a clinical trial in which non-diabetic patients receiving a solution of glucose, insulin and potassium early in the post-operative period had improved cardiac function, spent less time on the ventilator and had shorter ICU and hospital length of stays.20 Furnary and colleagues demonstrated that diabetic patients undergoing coronary artery bypass who had strict serum glucose control with continuous insulin infusion using the Portland Protocol resulted in a 50% reduction in operative mortality.21
Although alcohol's cardioprotective mechanism is not completely understood, it appears that insulin signaling is key in optimizing myocardial function in the setting of myocardial ischemia. This study demonstrates that a moderate dose of alcohol supplementation daily augmented insulin signaling in both ischemic and non-ischemic myocardium in normoglycemic, non-diabetic swine. These results are consistent with previously reported rodent studies demonstrating that low-moderate doses of alcohol supplementation improve insulin sensitivity22, 23 and augments insulin signaling.24-26 Given the known beneficial effects of alcohol on insulin sensitivity, perhaps the insulin signaling pathway one mechanism by which ethanol is cardioprotective. Further investigation on the dose dependent effect of ethanol on insulin signaling in ischemic myocardium is warranted in future studies.
Acknowledgments
Funding
Funding for this research was provided by the National Heart, Lung, and Blood Institute (R01HL46716, R01HL69024, and R01HL85647, Dr. Sellke), NIH Training grant 5T32-HL076134 (Dr. Lassaletta), NIH Training grant 5T32-HL094300-03, Drs. Elmadhun and Sabe).
ABBREVIATIONS
- SUC
Sucrose Supplemented Swine (control)
- ETOH
Ethanol Supplemented Swine
- IRS1
Insulin Receptor Substrate 1
- pIRS1
Phosphorylated Insulin Receptor Substrate 1
- IRS2
Insulin Receptor Substrate 2
- PI3K
Phosphoinostitide 3 kinase
- PIP3
Phosphatidylinostitol Triphospate
- PDK1/2
3-Phosphoinostitide-Dependent Kinase 1
- AKT
Protein Kinase B
- pAKT
Phosphorylated AKT
- FOX01
Forkhead Box 01
- pFOX01
Phosphorylated FOX01
- GSK3β
Glycogen Synthase Kinase 3β
- GS
Glycogen Synthase
- GLUT4
Glucose Transporter Type 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: Molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 2.Smiley DD, Umpierrez GE. Perioperative glucose control in the diabetic or nondiabetic patient. Southern Medical Journal. 2006;99:580–589. doi: 10.1097/01.smj.0000209366.91803.99. [DOI] [PubMed] [Google Scholar]
- 3.Tanasescu M, Hu FB, Willett WC, Stampfer MJ, Rimm EB. Alcohol consumption and risk of coronary heart disease among men with type 2 diabetes mellitus. Journal of the American College of Cardiology. 2001;38:1836–1842. doi: 10.1016/s0735-1097(01)01655-2. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L, Investigators IS. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the interheart study): Case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 5.Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: A meta-analysis of prospective observational studies. Diabetes Care. 2005;28:719–725. doi: 10.2337/diacare.28.3.719. [DOI] [PubMed] [Google Scholar]
- 6.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the french paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 7.Marmot MG. Alcohol and coronary heart disease. International Journal of Epidemiology. 2001;30:724–729. doi: 10.1093/ije/30.4.724. [DOI] [PubMed] [Google Scholar]
- 8.Kiechl S, Willeit J, Poewe W, Egger G, Oberhollenzer F, Muggeo M, Bonora E. Insulin sensitivity and regular alcohol consumption: Large, prospective, cross sectional population study (bruneck study). BMJ. 1996;313:1040–1044. doi: 10.1136/bmj.313.7064.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: A randomized controlled trial. JAMA. 2002;287:2559–2562. doi: 10.1001/jama.287.19.2559. [DOI] [PubMed] [Google Scholar]
- 10.Elmadhun NY, Lassaletta AD, Chu LM, Bianchi C, Sellke FW. Vodka and wine consumption in a swine model of metabolic syndrome alters insulin signaling pathways in the liver and skeletal muscle. Surgery. 2012;152:414–422. doi: 10.1016/j.surg.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lassaletta AD, Chu LM, Robich MP, Elmadhun NY, Feng J, Burgess TA, Laham RJ, Sturek M, Sellke FW. Overfed ossabaw swine with early stage metabolic syndrome have normal coronary collateral development in response to chronic ischemia. Basic Research in Cardiology. 2012;107:243. doi: 10.1007/s00395-012-0243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertrand L, Horman S, Beauloye C, Vanoverschelde JL. Insulin signalling in the heart. Cardiovascular Research. 2008;79:238–248. doi: 10.1093/cvr/cvn093. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, Heidenreich KA, Sajan MP, Farese RV, Stolz DB, Tso P, Koo SH, Montminy M, Unterman TG. Foxo1 regulates multiple metabolic pathways in the liver: Effects on gluconeogenic, glycolytic, and lipogenic gene expression. The Journal of Biological Chemistry. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- 14.Sykiotis GP, Papavassiliou AG. Serine phosphorylation of insulin receptor substrate-1: A novel target for the reversal of insulin resistance. Molecular Endocrinology. 2001;15:1864–1869. doi: 10.1210/mend.15.11.0725. [DOI] [PubMed] [Google Scholar]
- 15.Opie LH. Effects of regional ischemia on metabolism of glucose and fatty acids. Relative rates of aerobic and anaerobic energy production during myocardial infarction and comparison with effects of anoxia. Circulation Research. 1976;38:I52–74. [PubMed] [Google Scholar]
- 16.Lazar HL. Glycemic control during coronary artery bypass graft surgery. ISRN Cardiology. 2012;2012:292490. doi: 10.5402/2012/292490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Q, Docherty JC, Rendell JC, Clanachan AS, Lopaschuk GD. High levels of fatty acids delay the recovery of intracellular ph and cardiac efficiency in post-ischemic hearts by inhibiting glucose oxidation. Journal of the American College of Cardiology. 2002;39:718–725. doi: 10.1016/s0735-1097(01)01803-4. [DOI] [PubMed] [Google Scholar]
- 18.Langouche L, Vanhorebeek I, Vlasselaers D, Vander Perre S, Wouters PJ, Skogstrand K, Hansen TK, Van den Berghe G. Intensive insulin therapy protects the endothelium of critically ill patients. The Journal of Clinical Investigation. 2005;115:2277–2286. doi: 10.1172/JCI25385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeschke MG, Klein D, Bolder U, Einspanier R. Insulin attenuates the systemic inflammatory response in endotoxemic rats. Endocrinology. 2004;145:4084–4093. doi: 10.1210/en.2004-0592. [DOI] [PubMed] [Google Scholar]
- 20.Lazar HL, Philippides G, Fitzgerald C, Lancaster D, Shemin RJ, Apstein C. Glucose-insulin-potassium solutions enhance recovery after urgent coronary artery bypass grafting. The Journal of Thoracic and Cardiovascular Surgery. 1997;113:354–360. doi: 10.1016/S0022-5223(97)70333-7. discussion 360-352. [DOI] [PubMed] [Google Scholar]
- 21.Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. The Journal of Thoracic and Cardiovascular Surgery. 2003;125:1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 22.Hong J, Smith RR, Harvey AE, Nunez NP. Alcohol consumption promotes insulin sensitivity without affecting body fat levels. International Journal of Obesity. 2009;33:197–203. doi: 10.1038/ijo.2008.266. [DOI] [PubMed] [Google Scholar]
- 23.Paulson QX, Hong J, Holcomb VB, Nunez NP. Effects of body weight and alcohol consumption on insulin sensitivity. Nutrition Journal. 2010;9:14. doi: 10.1186/1475-2891-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He L, Marecki JC, Serrero G, Simmen FA, Ronis MJ, Badger TM. Dose-dependent effects of alcohol on insulin signaling: Partial explanation for biphasic alcohol impact on human health. Molecular Endocrinology. 2007;21:2541–2550. doi: 10.1210/me.2007-0036. [DOI] [PubMed] [Google Scholar]
- 25.Qu W, Zhao L, Peng X, Yang X, Ying C, Hao L, Sun X. Biphasic effects of chronic ethanol exposure on insulin-stimulated glucose uptake in primary cultured rat skeletal muscle cells: Role of the akt pathway and glut4. Diabetes/Metabolism Research and Reviews. 2011;27:47–53. doi: 10.1002/dmrr.1152. [DOI] [PubMed] [Google Scholar]
- 26.Tomie Furuya D, Binsack R, Onishi ME, Monteiro Seraphim P, Fabres Machado U. Low ethanol consumption induces enhancement of insulin sensitivity in liver of normal rats. Life Sciences. 2005;77:1813–1824. doi: 10.1016/j.lfs.2004.12.046. [DOI] [PubMed] [Google Scholar]