Abstract
Pericytes play critical roles in the development, maturation and remodeling of blood vessels, and in the central nervous system (CNS), evidence suggests that pericytes also regulate blood flow and form an integral part of the blood-brain barrier. The study of this important cell type has been hampered by the lack of any pericyte-specific marker and by the difficulty of culturing pericytes in adequate numbers to high purity. Here we present a novel yet simple approach to isolate and culture large numbers of pericytes from the mouse CNS that nevertheless leads to very pure pericyte cultures. In our method, vascular cells obtained from adult mice brains are cultured initially under conditions optimized for endothelial cells, but after two passages switched to a medium optimized for pericyte growth. After growing the cells for 1-2 additional passages we obtained a largely homogeneous population of cells that expressed the pericyte markers NG2, PDGF -receptor, and CD146, but were negative for markers of endothelial cells (CD31), microglia (Mac-1) and astrocytes (GFAP). Under these conditions, pericytes could be grown to high passage number, and were maintained highly pure and largely undifferentiated, as determined by antigen expression profile and low levels of -SMA expression, a marker of pericyte differentiation. Furthermore, switching the cells from pericyte medium into DMEM containing 10% FBS promoted -SMA expression, demonstrating that high passage pericytes could still differentiate. Thus, we provide an alternative approach to the culture of CNS pericytes that is easy to establish and provides large numbers of highly pure pericytes for extended periods of time. This system should provide others working in the pericyte field with a useful additional tool to study the behavior of this fascinating cell type.
Keywords: pericyte, brain endothelial cell (BEC), culture, purity, co-culture, matrigel
INTRODUCTION
Pericytes are vascular cells that were originally defined solely by their close vicinity to the endothelium of capillaries (Rouget, 1874). Phenotypically similar to smooth muscle cells, they are found on capillaries and other small diameter vessels (Armulik et al., 2005) but possibly also in the intima, media and adventitia of larger vessels (Andreeva et al., 1998; Canfield et al., 2000). Their function is still not entirely understood but they are important contributors to the development, maturation, stabilization, and remodeling of capillaries and other small vessels. (Hirschi and D’Amore, 1996). Increasing evidence supports the long-held notion that pericytes participate in the regulation of capillary blood flow (Hamilton et al., 2010; Vimtrup, 1922), and play an important instructive role during angiogenesis (Bergers and Song, 2005; Dore-Duffy and LaManna, 2007). Recently the relationship between pericytes and mesenchymal stem cells has come under the spotlight. Some evidence suggests that mesenchymal stem cells are a subgroup of pericytes (Caplan, 2008) and other studies have highlighted pericyte multipotency in a variety of tissues, including brain (Dore-Duffy, 2008; Dore-Duffy et al., 2006), skeletal muscle, smooth muscle, bone, cartilage, and adipose tissue (Brachvogel et al., 2005; Crisan et al., 2008; Dellavalle et al., 2007; Doherty et al., 1998; Farrington-Rock et al., 2004; Schor A, 1998). Within the brain, in addition to being a potential source of pluripotent stem cells (Dore-Duffy, 2008; Dore-Duffy et al., 2006), pericytes are known to be important participants in the establishment and maintenance of the blood-brain-barrier (Balabanov and Dore-Duffy, 1998; Ballabh et al., 2004; Daneman et al., 2010). A good understanding of pericyte biology has clear clinical implications since pericyte dysfunction has been shown to be linked to several pathologies, including hypertension (Herman and Jacobson, 1988; Kutcher and Herman, 2009) and diabetic microangiopathy (Hammes, 2005; Hirschi and D’Amore, 1996). At the same time, the early contribution of pericytes to pathological angiogenesis also makes them an interesting candidate for anti-angiogenesis therapy (Song et al., 2005; Tigges et al., 2008; Wesseling et al., 1995).
The study of pericytes has been greatly facilitated by the isolation and culture of primary pericytes. Most techniques are aimed at the isolation of pericytes from the retina (Gitlin and D’Amore, 1983) or the brain (Dore-Duffy, 2003), two tissues whose capillaries are rich in pericytes. Recently, methods have been devised to isolate pericytes from alternate sources including skeletal muscle, skin, and foetal tissues (Crisan et al., 2008; Mogensen et al., 2011; Sundberg et al., 2002). Pericyte isolation strategies generally start with enzymatic digestion of tissue, usually followed by the isolation of microvessel fragments via successive filtration steps. Fragment outgrowth strategies are relatively easy to do but the cultures generated often contain several different cell types, resulting in impure pericyte populations. Pure pericyte cultures can be obtained through either positive or negative immuno-selection via the use of magnetic beads or flow cytometry; the downside of this approach is that it is expensive, complicated, and often leads to very low cell yields.
It has long been noted that pericytes are a major contaminant of endothelial cell cultures (Gitlin and D’Amore, 1983) and in our laboratory we, too, observed that successive passaging of brain endothelial cells (BECs) is limited, in part by the ability of contaminating pericytes to over-run the EC cultures. Pericyte culture has mainly been performed with Dulbecco’s Modified Eagle Medium (DMEM); however more specialized culture media are now available. Recently introduced media have improved and facilitated the culture of mesenchymal stem cells (Gong et al., 2009), begging the question if the application of optimised media can also improve the culture of pericytes. In this study we wanted to test the notion that repeat passaging of brain vessel cultures and the use of commercially available optimized pericyte medium could lead to satisfactorily pure and undifferentiated pericyte cultures. On the basis of our findings, here we present a novel yet simple approach to pericyte isolation from the mouse CNS that nevertheless leads to very pure pericyte cultures. Using this approach, vascular cells obtained from adult mice brains were cultured initially under conditions optimized for BECs, but after two passages switched to a medium optimized for pericyte growth. After growing the cells for 1-2 additional passages we obtained abundant numbers of pericytes in cultures that were generally free of endothelial cells, microglia and astrocytes. Since it has been well documented that pericytes in culture tend to rapidly differentiate, routine passage of pericyte cultures has been avoided. However, using our modified approach, and using a commercially available optimized medium, we characterized pericytes of low and high passage numbers and present evidence that pericytes cultured under the conditions we describe stay undifferentiated for many passages.
MATERIALS AND METHODS
Animals
All cell cultures were obtained from C57BL/6 mice which were maintained under pathogen-free conditions in the closed breeding colony of The Scripps Research Institute (TSRI).
Primary culture of brain endothelial cells (BECs) and pericytes
Pericyte isolation was initially done as described for the isolation of endothelial cells (ECs) (Milner et al., 2008). Six 6-8-week old mice were euthanized shortly before their brains were extracted and placed in cold Minimum Essential Medium (MEM). The olfactory bulb, cerebellum, and medulla were dissected away and the rest of the brain was minced thoroughly with a sterilized razor blade. The minced brain tissue was washed once with MEM and then washed by centrifuging in an Eppendorf 5810 R centrifuge at 1200 rpm for 5 minutes and then incubated in an enzymatic solution (Worthington, Lakewood, NJ), containing 30 U/ml papain and 40 μg/ml DNase I in Earl’s Balanced Salt Solution (EBSS) for 70 minutes at 37°C. After incubation the digested brain tissue was homogenized by passing it 10 x through an 18 gauge needle and subsequently 10 x through a 21 gauge needle. The homogenized brain cells were then mixed with 1.7 volumes of 22% bovine serum albumin (BSA) in phosphate buffered saline (PBS) and centrifuged at 4000 rpm for 10 minutes. The lipid layer on top of the vial was carefully removed, and the cell pellet was re-suspended in 5 ml of endothelial cell growth medium (ECGM) consisting of Hams F12, supplemented with 10% FBS, Heparin, ascorbic acid, L-glutamine, penicillin/streptomycin (all from Sigma, St. Louis, MO) and endothelial cell growth supplement (ECGS) (Upstate Cell Signaling Solutions, Lake Placid, NY), and centrifuged for 5 minutes at 1200 rpm. Subsequently, the cells were re-suspended in ECGM and plated on two wells of a 6-well plate (Nunc) that had been coated with collagen I (Sigma, 0.02% solution) for 2 hours at 37°C. After 20 hours, cells were washed 3 times with PBS and fresh ECGM added. At this time point, in order to select for BECs, cultures were incubated with 4 μg/ml puromycin (Alexis GmbH, Grunberg, Germany) for two days to remove contaminating cell types, and then kept in ECGM until required. The medium was changed every 3 days. Endothelial cell purity was > 98% as determined by CD31 in flow cytometry. For all experiments, BEC were used only for the first passage.
When the aim was to culture pericytes the puromycin step was omitted. Cells were grown in ECGM, with the medium changed every 3 days. After 7-9 days, the cells reach confluency, and the cultures were harvested with trypsin and passaged 1:4 onto fresh collagen-coated 6-well plates. Cells were grown to confluency and then passaged again as described above. During the first two passages, the cells were kept in ECGM; following the third passage, cells were maintained in pericyte medium (ScienCell Research Laboratories, Carlsbad, CA) containing 2% FBS. Cells cultured for immunocytochemical analysis were treated under the same conditions as described above but were passaged onto collagen-coated Lab-Tek II chamber slides (Nunc) and grown for 4 days unless indicated differently.
Antibodies
Polyclonal rabbit anti-NG2 and anti PDGFβ-receptor and polyclonal guinea pig anti-NG2 antibodies were generated and provided by Dr. William Stallcup (Sanford-Burmham Institute, La Jolla, CA). Polyclonal rabbit anti-CD146 and monoclonal hamster anti-CD31 antibodies were obtained from AbCam (Cambridge, MA). Monoclonal rat anti-CD11b (Mac-1) antibody was purchased from BD Pharmingen (La Jolla, CA). Monoclonal mouse anti-α-smooth muscle actin ( -SMA) and anti-glial fibrillary acidic protein (GFAP) antibodies were obtained from Sigma-Aldrich (St. Louis, MO). The Cy3, and Cy5-conjugated secondary antibodies against rabbit IgG and guinea pig IgG were obtained from Jackson Immunoresearch (West Grove, PA, USA). Alexa Fluor 488 and Alexa Fluor 568-conjugated secondary antibodies against rabbit IgG and rat IgG were obtained from Invitrogen (Carlsbad, CA). The DyLight488-conjugated antibody against hamster IgG was obtained from Biolegend (San Diego, CA).
Immunocytochemistry and microscopy
Cell cultures growing on collagen I-coated chamber slides were fixed in methanol (-20°C) for 3 minutes and subsequently washed 2 x for 5 minutes in room temperature PBS, and then briefly rinsed with a small amount of Dako dilution buffer (Dako, Carpinteria, CA). Cultures were then incubated with antibodies (diluted in Dako dilution buffer (1:100)) for 30 minutes at 37°C in a moisturised chamber, washed for 5 minutes in PBS, and again briefly rinsed in the dilution buffer. Cultures were then incubated with fluorescent-conjugated secondary antibodies diluted 1:300 in Dako dilution buffer for 30 minutes at 37 °C, then washed briefly in PBS and incubated for 5 minutes with Hoechst nuclear stain (Sigma). The cultures were finally washed 3 times for 10 minutes in PBS with 0.1% tween-20, briefly rinsed in tap water, and mounted in Aquamount (Polysciences, Warrington, PA). All IF microscopy work was done with a Zeiss Axio Imager M1m microscope and Zeiss Axiocam MRC digital camera. Phase contrast pictures were taken on a Zeiss Axio Observer A1 microscope. Software used for cell image analysis was Image J 1.44p. For the quantification of cellular composition of cell cultures, four experiments were performed, in duplicate within each experiment at each passage number. Pericyte expression of -SMA was examined by measuring the -SMA+ fluorescent area per field of view using Image J software (version 1.43U). All data were presented as the mean ± SEM, and statistical significance assessed by Student’s t test, in which p < 0.05 was defined as statistically significant.
Pericyte cell proliferation assay
Lab-Tek II chamber slides were coated with collagen I for 2 hours, then washed, and pericytes plated and cultured in pericyte medium until cells reached ~50% confluence. Pericytes were then cultured for 6 hours in the presence of BrdU (Invitrogen, Carlsbad, CA), then fixed in acid/alcohol and processed for BrdU immunocytochemistry according to the manufacturer’s instructions. Pericyte proliferation was assessed by quantifying the number of BrdU-positive cells as a percentage of the total number of cells (Hoechst staining), and the results expressed as the mean ± SEM of three experiments. Statistical significance was assessed by Student’s t test, in which p < 0.05 was defined as statistically significant.
Pericyte and endothelial cell co-culture in matrigel
The co-culture method was adapted from a method used by Masanobu Komatsu (personal communication). One day before the cells were co-cultured they were labelled with red or green cell tracker (1:1000 dilution) for 30 minutes in serum-free medium, according to the manufacturer’s instructions (Molecular Probes, Eugene, Oregon). In order to co-culture pericytes and BECs, 50 μL of ice cold matrigel (BD Bioscience, La Jolla, CA) was pipetted into the wells of an 8-well chamber slide and allowed to polymerize. Both pericytes and BECs were then harvested by trypsinization and counted. Approximately 100,000 BECs and 10,000 pericytes were added to the polymerized matrigel in each well, and cultured in ECGM for 24 hours. After that time the cultures were analyzed by fluorescent microscopy.
RESULTS
Multiple passage of brain vascular cultures yields pure cultures of pericytes
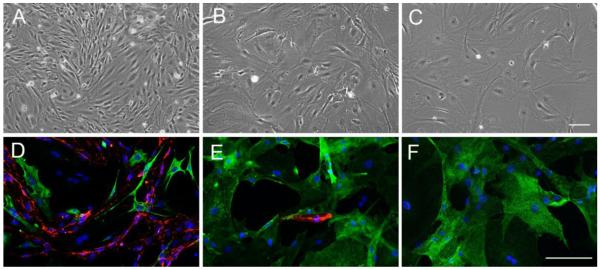
We routinely isolate brain endothelial cells (BEC) from the brain of 6-8 week old mice by enzymatic disruption, and culturing on collagen I in endothelial cell growth medium (ECGM) containing puromycin for days 1-3, to kill all non-endothelial cells. This approach yields homogeneous cultures, displaying the elongated spindle-shaped morphology, typical of endothelial cells that are > 98% endothelial, as assessed by CD31 immunoflourescence (IF). By contrast, in cultures not treated with puromycin, a different type of cell starts to emerge after 5-7 days, displaying the rhomboid morphology typical of pericytes. At confluence, primary cultures not exposed to puromycin consist predominantly of BECs with a small minority of pericytes. To favor the growth of pericytes, we switch these cultures from ECGM to pericyte medium from the second passage onwards. Interestingly, with subsequent passages the relative proportions of BECs and pericytes shifts dramatically, with pericytes predominating at the expense of BECs. As shown in Figure 1, while the first passage cultures contain more BECs than pericytes (A), by the third passage these cultures contain only a small number of BECs (B), and by later passages (>5) BECs have entirely disappeared, leaving a pure population of pericytes, showing the typical rhomboid morphology of pericytes (C).
Figure 1.
Characterization of pericyte cultures. Pericyte cultures were established as described in Materials and Methods. Phase contrast pictures of the cultures at passage 1 (A), passage 3 (B) and passage 5 (C). Scale bar = 100μm. Note that passaging shifts the composition of the cultures from the typical spindle-shaped endothelial morphology at passage 1 to the rhomboid morphology typical of pericytes at passage 5. D-F shows dual-IF with the pericyte marker NG2 (green) and the endothelial cell marker CD31 (red). Scale bar = 100μm. Note that passage 1 cultures contained a majority of endothelial cells with some pericytes. By passage 3, NG2-positive pericytes dominated, and by passage 5 the culture contained only pericytes, with no endothelial cells present.
Immunocytochemical characterization of the pericyte cultures
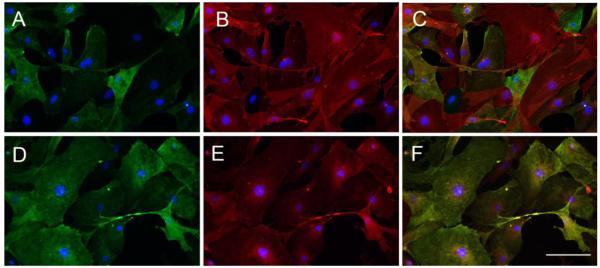
To evaluate the purity of our pericyte cultures we stained the cells with the pericyte markers NG2, platelet-derived growth factor-beta-receptor (PDGFβ-receptor), CD146, and α-smooth muscle actin (α-SMA). As shown in Figures 1D-F, passage 1 cultures contained a mixture of CD31-positive BECs, and NG2-positive pericytes. By passage 3, most CD31-positive cells had disappeared from the cultures, and by passage 5, the cultures consisted of NG2-positive cells with no CD31-positive cells present. Passage 5 pericytes were further evaluated for expression of other pericyte markers (Figure 2). In these cultures, > 98% of cells were positive for the PDGFβ-receptor while > 80% of cells were positive for the markers NG2 or CD146. Our finding that all cells expressed at least one pericyte marker demonstrates the high purity of our pericyte cultures, and the relative heterogeneity of expression of NG2 and CD146 is consistent with the previously described heterogeneity of pericytes (Bandopadhyay et al., 2001).
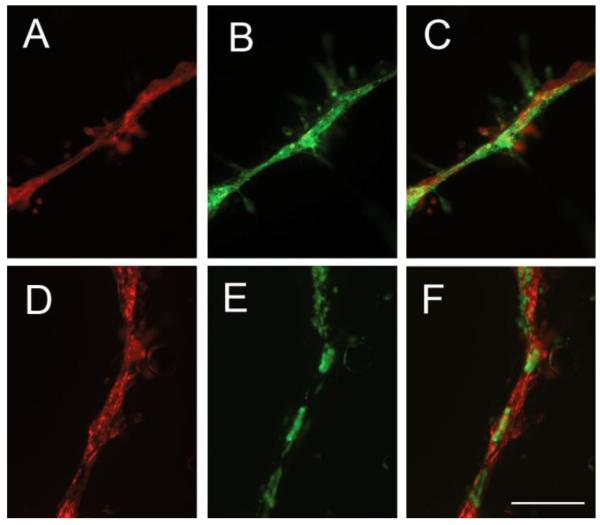
Figure 2.
Further characterization of pericyte cultures. Pericyte cultures were established as described in Materials and Methods, and passage 5 pericytes analyzed by dual-IF for the pericyte markers NG2 (A), PDGF -receptor (B), with merge (C), and NG2 (D), CD146 (E), and merge (F). Scale bar = 100μm. Note that while virtually all cultured pericytes expressed the PDGFβ-receptor (B), a slightly smaller subset of pericytes expressed NG2 (A), and a similar subset of pericytes also expressed CD146 (E), which was virtually co-expressed with NG2 (D,F).
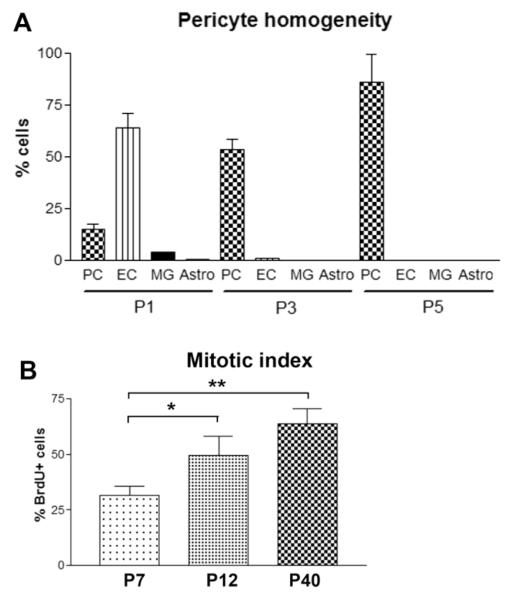
Cell culture of brain pericytes is often frustrated by the presence of high numbers of astrocytes and microglia. In order to determine how many astrocytes and microglia were present in our pericyte cultures we immunostained the cultures with antibodies against the astrocyte marker GFAP and the microglial marker Mac-1 (CD11b). At early passages (P1) we occasionally saw low to moderate numbers of microglia and low numbers of astrocytes. At passage 3 the cultures consistently contained less than 2% CD11b-positive microglia and less than 0.2% GFAP-positive astrocytes (Figure 3A). Thus, serial passaging tremendously increased the purity of the pericyte cultures, and all contaminating endothelial cells, astrocytes and microglia had virtually disappeared by the fifth passage.
Figure 3.
A. Quantification of pericyte culture purity. Cell cultures of passage numbers 1, 3 and 5 were analyzed immunocytochemically with antibodies against pericytes (PC; NG2), endothelial cells (EC; CD31), microglia (MG; CD11b), and astrocytes (Astro; GFAP). All points represent the mean SEM of 4 experiments. Note that passage 1 (P1) cultures contained predominantly endothelial cells, passage 3 (P3) cultures predominantly pericytes with only a few endothelial cells and no microglia or astrocytes present, and passage 5 (P5) cultures contained only pericytes. At P5 the percentage of NG2-expressing cells increased further, suggesting that higher passage pericytes are more mature. B. Examination of the effect of passage number on proliferation rate. Pericyte cultures were established as described in Materials and Methods, and cultures of different passage number (P7, P12 and P40) examined for mitotic index using the BrdU incorporation technique. All points represent the mean SEM of 3 experiments. Note that the pericyte cultures displayed a progressive increase in proliferation rate with increase in passage number. ** p < 0.005, * NS (p = 0.086).
Pericytes remain undifferentiated over many passages when kept in pericyte medium
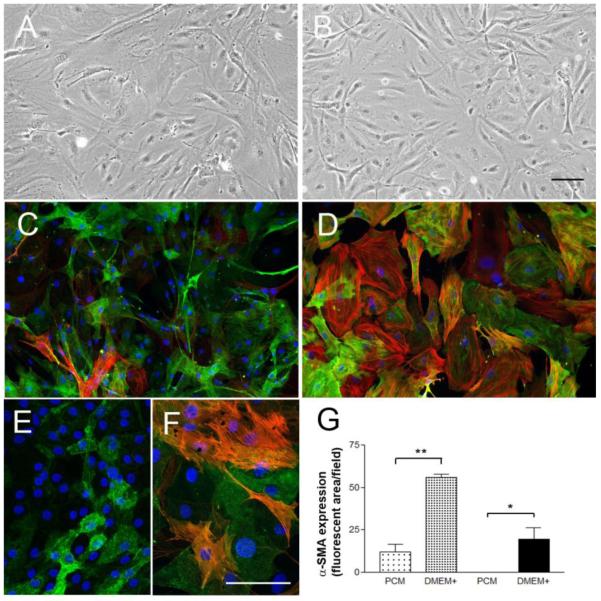
Smooth muscle cells (SMC) are known to differentiate and change their fundamental properties when passaged repeatedly in culture (Orlidge and D’Amore, 1986). Since pericytes originate from a related lineage, many groups in the pericyte field have adopted a low-passage policy to avoid this problem. To investigate whether pericytes in our system alter their properties with time in culture, we passaged our cultures to high passage number (>40 times), and examined the cells for changes in morphology, proliferation rate and expression of phenotypic markers. Significantly, pericytes obtained by our method and cultured in pericyte medium remained undifferentiated. Pericyte cultures passaged up to 40 times appeared highly homogeneous over time. Pericytes passaged up to 10 times showed no visible change in morphology (Figure 4A-B), while cells in higher passage cultures (>10) decreased somewhat in size (Figure 4, compare C with E) but otherwise still exhibited typical pericyte morphology. Interestingly, rather than showing a decline in mitotic index which would be predicted if higher passage cells were becoming more differentiated, the pericyte cultures actually displayed a progressive increase in proliferation rate with increase in passage number (Figure 3B). As pericyte differentiation is associated with increased α-SMA expression, we next examined levels of this marker to determine if high passage cells showed enhanced expression. This revealed that low passage (P3) pericyte cultures contained only a small number of α-SMA-positive cells (Figure 4C), and that higher passage (P40) pericytes contained even fewer (Figure 4E), but after being switched into DMEM containing 10% FBS (DMEM+) for 4 days, α-SMA was strongly up-regulated in the vast majority of pericytes, both in early (Figure 4D) and late (Figure 4F) passage pericytes (Figure 4G). This demonstrates that high passage pericyte cultures still retain the ability to differentiate in response to serum.
Figure 4.
Switching from pericyte medium to DMEM containing 10% FBS promotes pericyte differentiation. Pericyte cultures were established as described in Materials and Methods. Phase contrast pictures of passage 3 (A) and passage 40 (B) maintained in pericyte medium. Scale bar = 100μm. Note that pericytes cultured in pericyte medium for many passages do not visibly change their morphology. Passage 3 (C and D) and passage 40 (E and F) pericytes were grown in pericyte medium (C and E) or DMEM + 10% FBS (D and F) for 4 days, then analyzed for NG2-green/ -SMA-red by dual-IF. Scale bar = 100μm. G. Quantification of α-SMA expression by fluorescent imaging using Image J. Note that when cultured in pericyte medium, early passage pericytes expressed very low levels of α-SMA (C), and higher passage pericytes expressed even less (E), but when switched into DMEM containing 10% FBS (DMEM+), α-SMA was strongly up-regulated in the vast majority of pericytes, both in early (D) and late (F) passage pericytes. * p < 0.05, ** p < 0.001.
Pericytes associate with endothelial cells to create vascular cords in matrigel co-culture experiments
It is well established that when pericytes are co-cultured with endothelial cells in the biological matrix matrigel, pericytes line up along the length of the endothelial vascular cords, as they do in blood vessels in vivo (Bryan and D’Amore, 2008). To examine whether the pericytes derived in our system also show this ability, we co-cultured pure populations of pericytes (labeled with red cell tracker) with pure BEC cultures (labeled with green cell tracker) in matrigel. As shown in Figure 5, in this system, pericytes aligned with endothelial cords and extended processes along the length of the cords. This property was shown both by early (P3) and late passage (P17) pericytes. Quantification revealed that after 24 hours of co-culture, more than 95% of both early and late passage pericytes were closely associated with endothelial cords. This demonstrates first, that pericytes derived in our culture system behave like pericytes derived from other approaches, and second that high passage pericytes derived from this system retain the ability to closely associate with endothelial cords.
Figure 5.
Pericytes align with endothelial vascular cords in co-cultures. Pericytes and BECs were established as described in Materials and Methods, then labeled with red or green cell tracker respectively and co-cultured in a 1:10 ratio in Matrigel in ECGM for 24 hours. Top panels (A-C) show P3 pericytes while lower panels (D-F) show P17 pericytes. Scale bar = 100μm. Note that the green-labeled BECs (B and E) were closely invested with the red-labeled pericytes (A and D), and that virtually all pericytes strongly co-localized with BECs, to form vessel-like structures.
DISCUSSION
In this study we have described a technique of isolating and purifying brain pericytes that is simple and easy to perform, but still leads to a highly pure pericyte culture. The straightforward approach we applied consistently allowed us to obtain pericyte cultures that had significantly less endothelial cells, astrocytes and microglia present than other published techniques that we tried in our laboratory. Since our method relies on the repeated passage of pericytes, one important question is how differentiated are the pericytes produced by this culture system? That pericytes can rapidly differentiate in vitro is a well known problem, and the stability of pericytes in culture remains a controversial question. While some have reported pericytes to be stable over several passages (Capetandes and Gerritsen, 1990; Gitlin and D’Amore, 1983; Helmbold et al., 2001; von Beckerath et al., 2000) others have claimed that pericytes rapidly differentiate in culture (Armulik et al., 2005; Dellavalle et al., 2007; Sundberg et al., 2002). If passaging of pericytes is to be avoided, then one either has to work with relatively impure primary cultures, when large numbers of cells are required, or with small numbers of cells when more demanding pericyte purification protocols are applied. This predicament has led some groups to develop immortalized pericytes derived from rat or mouse tissues (Dore-Duffy et al., 2011; Kondo et al., 2003).
The approach described here relies on the use of endothelial cell growth medium for the first two passages, followed by a switch to specialized pericyte medium that keeps pericytes undifferentiated. When we cultured from the outset in pericyte medium, we noticed that the number of contaminating astrocytes and microglia was significantly higher; thus we always started cultures in ECGM and switched to pericyte medium after 2 passages. Interestingly, in most published isolation approaches, pericytes are cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10-20% fetal bovine serum (FBS). However, our results demonstrate that culturing pericytes in DMEM with FBS leads to a significant upregulation of α-SMA, a decrease in growth rate, and cell enlargement, all clear signs of pericyte differentiation. That the use of DMEM containing 10% FBS leads to pericyte differentiation has been noted before (Dellavalle et al., 2007). In contrast, when cultured in pericyte medium, most pericytes express only very low levels of α-SMA, and under these conditions the pericytes could be passaged over 40 times without showing any of the above mentioned signs of differentiation. Also, when high passage cells were co-cultured with BECs, they still behaved like pericytes by closely aligning along the endothelial vascular cords. Importantly, a high proportion of both low passage (> 90%) and high passage (> 60%) pericytes could be induced to differentiate when culture medium was changed to DMEM containing 10% FBS. While we did not investigate every possible differentiation “signifier” we showed clear evidence that pericytes cultured under the maintenance conditions described had not undergone differentiation.
During the first 10 passages pericytes seem to remain very stable; however, at passages above 10 we encountered some changes. The proliferation rate increased, the overall α-SMA expression and the cell size were reduced. These observations could be interpreted such that at higher passage number the pericytes in our cultures underwent de-differentiation. De-differentiation has been described for several different cell types of the mesenchymal lineage. Adipocytes can de-differentiate into a fibroblast-like cell (Shen et al., 2011) and smooth muscle cells have long been known to undergo de-differentiation (Gabbiani, 1986). De-differentiation has also been described for cultured pericytes isolated from the bovine retina (Bryan and D’Amore, 2008). Interestingly, as our data shows, any de-differentiation was generally reversed when cells were shifted back into DMEM containing FBS. That high passage pericytes (> P10) retain the ability to differentiate is supported by our observation that when passage 17 pericytes were co-cultured with BECs in matrigel in endothelial cell medium, the pericytes nearly perfectly co-localized with the endothelial cells, just as we had seen with passage 3 pericytes. In this regard we noticed that any passage > P10 had characteristic features including smaller cell size and increased proliferation rate, relative to low passage pericytes.
Pericytes are often identified in vivo by their close apposition to endothelial cells and their basement membrane. When pericytes are not in this location, they are difficult to identify because, to date, no single pericyte-specific cell marker has been identified. In pericyte cultures the usual approach for their identification is to check for the presence and absence of multiple markers. Based on the work of others, in this study we used NG2 (Ozerdem et al., 2001), PDGFβ-receptor (Sundberg et al., 1993) and CD146 (Covas et al., 2008; Li et al., 2003) to positively identify pericytes. CD146 expression has also been described on endothelial cells (Yan et al., 2003). In fact, we found that BECs expressed markedly higher levels of CD146 than did pericytes, making it easy to differentiate between BECs and pericytes. In addition to using positive markers to identify pericytes, we also used CD11b (Mac-1), GFAP and CD31 for negative identification. While -SMA has been used to identify pericytes, it is not a very specific marker, especially in vivo as smooth muscle cells also express α-SMA, and indeed many pericytes within blood vessels do not express this marker. In vitro, it has been reported that α-SMA expression can be used as a marker for pericyte differentiation, as few freshly isolated pericytes express this antigen, but with time in culture, almost 100% of cells express α-SMA (Balabanov and Dore-Duffy, 1998; Dore-Duffy, 2008). We also used a functional assay, the matrigel pericyte-endothelial cell co-culture, as a means to verify that the cultured cells behaved like pericytes, closely co-aligning along the endothelial vascular cords. The combined data we obtained from both the phenotypic analysis and the functional assay strongly support the claim that our method yields highly pure cultures of pericytes. However, we did encounter some differences in the expression pattern of pericyte markers in our cultures. While all cells expressed PDGFβ-receptor, the other markers including NG2, CD146 and α-SMA were expressed in a way that hinted that the pericytes we isolated were not entirely homogenous. This observation is in agreement with the assumption that pericytes in vivo are a heterogeneous group of cells and that within a given tissue the pericytes from different types of vessels differ (Bandopadhyay et al., 2001; Murfee et al., 2005). Murfee and co-workers for example showed that while NG2 is present on pericytes associated with capillaries and arterioles, it is not expressed in pericytes on venules (Murfee et al., 2005), though interestingly, pericytes on venules induce NG2 expression as part of the activation response during vascular remodeling (Murfee et al., 2006).
Conclusion
In this paper, we describe a technique of isolating and purifying brain pericytes that is simple and easy to perform, but still leads to a highly pure pericyte culture. Using this approach, and combined with the use of specialized pericyte medium, we were able to routinely passage pericytes more than 40 times, to produce highly pure pericyte cultures that show the rhomboid morphology, expression pattern of phenotypic markers, and alignment with endothelial vascular cords, typical of pericytes. This novel system should provide others working in the pericyte field with a useful additional tool to study the behavior of this fascinating cell.
Highlights.
We describe a novel simple method of culturing brain pericytes
Cultures are highly homogeneous, expressing NG2, PDGF -receptor, and CD146
Closely align with endothelial vascular cords in matrigel co-culture
Can be passaged to high number without premature differentiation
Both low and high passage cells upregulate α-SMA when cultured in DMEM + 10% FBS
ACKNOWLEDGEMENTS
The authors greatly appreciate the gift of the NG2 and PDGFβ-receptor antibodies from Dr. William Stallcup (Sanford-Burmham Institute, La Jolla, CA), and Dr. Masanobu Komatsu (Sanford-Burnham Institute, Florida) for his advice on establishing pericyte-endothelial co-cultures. This work was supported by the National Multiple Sclerosis Society: by a Harry Weaver Neuroscience Scholar Award to RM (JF 2125A1/1), and a Post-Doctoral Fellowship to JVW (FG 1879-A-1), and by the NIH RO1 grant NS060770. This is manuscript number 21587 from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andreeva ER, et al. Continuous subendothelial network formed by pericyte-like cells in human vascular bed. Tissue Cell. 1998;30:127–35. doi: 10.1016/s0040-8166(98)80014-1. [DOI] [PubMed] [Google Scholar]
- Armulik A, et al. Endothelial/pericyte interactions. Circ Res. 2005;97:512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998;53:637–44. doi: 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Ballabh P, et al. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Bandopadhyay R, et al. Contractile proteins in pericytes at the blood-brain and blood-retinal barriers. J Neurocytol. 2001;30:35–44. doi: 10.1023/a:1011965307612. [DOI] [PubMed] [Google Scholar]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–64. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachvogel B, et al. Perivascular cells expressing annexin A5 define a novel mesenchymal stem cell-like population with the capacity to differentiate into multiple mesenchymal lineages. Development. 2005;132:2657–68. doi: 10.1242/dev.01846. [DOI] [PubMed] [Google Scholar]
- Bryan BA, D’Amore PA. Pericyte isolation and use in endothelial/pericyte coculture models. Methods Enzymol. 2008;443:315–31. doi: 10.1016/S0076-6879(08)02016-8. [DOI] [PubMed] [Google Scholar]
- Canfield AE, et al. Role of pericytes in vascular calcification: a review. Z Kardiol. 2000;89(Suppl 2):20–7. doi: 10.1007/s003920070096. [DOI] [PubMed] [Google Scholar]
- Capetandes A, Gerritsen ME. Simplified methods for consistent and selective culture of bovine retinal endothelial cells and pericytes. Invest Ophthalmol Vis Sci. 1990;31:1738–44. [PubMed] [Google Scholar]
- Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–30. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Covas DT, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–54. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Crisan M, et al. Purification and culture of human blood vessel-associated progenitor cells. Curr Protoc Stem Cell Biol. 2008 doi: 10.1002/9780470151808.sc02b02s4. Chapter 2, Unit 2B 2 1-2B 2 13. [DOI] [PubMed] [Google Scholar]
- Daneman R, et al. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–6. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–67. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Doherty MJ, et al. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res. 1998;13:828–38. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P. Isolation and characterization of cerebral microvascular pericytes. Methods Mol Med. 2003;89:375–82. doi: 10.1385/1-59259-419-0:375. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P. Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des. 2008;14:1581–93. doi: 10.2174/138161208784705469. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, et al. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–24. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, LaManna JC. Physiologic Angiodynamics in the Brain. Antioxidants and Redox Signaling. 2007;9:1363–1371. doi: 10.1089/ars.2007.1713. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, et al. Immortalized CNS pericytes are quiescent smooth muscle actin-negative and pluripotent. Microvasc Res. 2011;82:18–27. doi: 10.1016/j.mvr.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington-Rock C, et al. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–32. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- Gabbiani G. The cytoskeleton of rat aortic smooth muscle cells. Normal conditions, experimental intimal thickening, and tissue culture. Ann N Y Acad Sci. 1986;488:196–8. [PubMed] [Google Scholar]
- Gitlin JD, D’Amore PA. Culture of retinal capillary cells using selective growth media. Microvasc Res. 1983;26:74–80. doi: 10.1016/0026-2862(83)90056-0. [DOI] [PubMed] [Google Scholar]
- Gong Z, et al. Influence of culture medium on smooth muscle cell differentiation from human bone marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2009;15:319–30. doi: 10.1089/ten.tea.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, et al. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010:2. doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes HP. Pericytes and the pathogenesis of diabetic retinopathy. Horm. Metab. Res. 2005;37:39–43. doi: 10.1055/s-2005-861361. [DOI] [PubMed] [Google Scholar]
- Helmbold P, et al. Isolation and in vitro characterization of human dermal microvascular pericytes. Microvasc Res. 2001;61:160–5. doi: 10.1006/mvre.2000.2292. [DOI] [PubMed] [Google Scholar]
- Herman IM, Jacobson S. In situ analysis of microvascular pericytes in hypertensive rat brains. Tissue Cell. 1988;20:1–12. doi: 10.1016/0040-8166(88)90002-x. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–98. [PubMed] [Google Scholar]
- Kondo T, et al. Establishment of conditionally immortalized rat retinal pericyte cell lines (TR-rPCT) and their application in a co-culture system using retinal capillary endothelial cell line (TR-iBRB2) Cell Struct Funct. 2003;28:145–53. doi: 10.1247/csf.28.145. [DOI] [PubMed] [Google Scholar]
- Kutcher ME, Herman IM. The pericyte: cellular regulator of microvascular blood flow. Microvasc. Res. 2009;77:235–46. doi: 10.1016/j.mvr.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, et al. Differential expression of CD146 in tissues and endothelial cells derived from infantile haemangioma and normal human skin. J. Pathol. 2003;201:296–302. doi: 10.1002/path.1443. [DOI] [PubMed] [Google Scholar]
- Milner R, et al. The rapid decrease in astrocyte-associated dystroglycan expression by focal cerebral ischemia is protease-dependent. J Cereb Blood Flow Metab. 2008;28:812–823. doi: 10.1038/sj.jcbfm.9600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen C, et al. Isolation and functional characterization of pericytes derived from hamster skeletal muscle. Acta Physiol (Oxf) 2011;201:413–26. doi: 10.1111/j.1748-1716.2010.02206.x. [DOI] [PubMed] [Google Scholar]
- Murfee WL, et al. Perivascular cells along venules upregulate NG2 expression during microvascular remodeling. Microcirculation. 2006;13:261–73. doi: 10.1080/10739680600559153. [DOI] [PubMed] [Google Scholar]
- Murfee WL, et al. Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: identifying a venule-specific phenotype. Microcirculation. 2005;12:151–60. doi: 10.1080/10739680590904955. [DOI] [PubMed] [Google Scholar]
- Orlidge A, D’Amore PA. Cell specific effects of glycosaminoglycans on the attachment and proliferation of vascular wall components. Microvasc Res. 1986;31:41–53. doi: 10.1016/0026-2862(86)90005-1. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, et al. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–27. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Rouget C. Note sur le development de la tunique contractile des vaisseaux. Compt. Rend. Acad. Sci. 1874;59:559–562. [Google Scholar]
- Schor A, C. D. Osteogenic potential of vascular pericytes in marrow stromal cell cultures. In: O. M., Beresford J, editors. Marrow stromal cell culture. University of Cambridge Press; Cambridge: 1998. pp. 128–148. [Google Scholar]
- Shen JF, et al. Dedifferentiated fat cells: an alternative source of adult multipotent cells from the adipose tissues. Int J Oral Sci. 2011;3:117–24. doi: 10.4248/IJOS11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, et al. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–9. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg C, et al. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab Invest. 2002;82:387–401. doi: 10.1038/labinvest.3780433. [DOI] [PubMed] [Google Scholar]
- Sundberg C, et al. Microvascular pericytes express platelet-derived growth factor-beta receptors in human healing wounds and colorectal adenocarcinoma. Am J Pathol. 1993;143:1377–88. [PMC free article] [PubMed] [Google Scholar]
- Tigges U, et al. FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development. 2008;135:523–32. doi: 10.1242/dev.002071. [DOI] [PubMed] [Google Scholar]
- Vimtrup B. Beitraege zur Anatomic der Kapillaren: ueber kontraktile Elemente in der Gefasswand der Blutkapillaren. Z. Anat. Entwicklungsgesch. 1922;65:150–182. [Google Scholar]
- von Beckerath N, et al. An inward rectifier and a voltage-dependent K+ current in single, cultured pericytes from bovine heart. Cardiovasc Res. 2000;46:569–78. doi: 10.1016/s0008-6363(00)00055-9. [DOI] [PubMed] [Google Scholar]
- Wesseling P, et al. Early and extensive contribution of pericytes/vascular smooth muscle cells to microvascular proliferation in glioblastoma multiforme: an immuno-light and immuno-electron microscopic study. J Neuropathol Exp Neurol. 1995;54:304–10. doi: 10.1097/00005072-199505000-00003. [DOI] [PubMed] [Google Scholar]
- Yan X, et al. A novel anti-CD146 monoclonal antibody, AA98, inhibits angiogenesis and tumor growth. Blood. 2003;102:184–191. doi: 10.1182/blood-2002-04-1004. [DOI] [PubMed] [Google Scholar]