SUMMARY
"Extended" (multiple-flow) measurements of exhaled nitric oxide (FeNO) potentially can distinguish proximal and distal airway inflammation, but have not been evaluated previously in large populations. We performed extended NO testing within a longitudinal study of a school-based population, to relate bronchial flux (J'awNO) and peripheral NO concentration (CalvNO) estimates with respiratory health status determined from questionnaires. We measured FeNO at 30, 50, 100, and 300 ml/sec in 1640 subjects aged 12–15 from 8 communities, then estimated J'awNO and CalvNO from linear and nonlinear regressions of NO output vs. flow. J'awNO, as well as FeNO at all flows, showed influences of asthma, allergy, Asian or African ancestry, age, and height (positive), and of weight (negative), generally corroborating past findings. By contrast, CalvNO results were inconsistent across different extended NO regression models, and appeared more sensitive to small measurement artifacts. Conclusions: Extended NO testing is feasible in field surveys of young populations. In interpreting results, size, age, and ethnicity require attention, as well as instrumental and environmental artifacts. J'awNO and conventional FeNO provide similar information, probably reflecting proximal-airway inflammation. CalvNO may give additional information relevant to peripheral-airway, alveolar, or systemic pathology. However, it needs additional research, including testing of populations with independently verifiable peripheral or systemic pathology, to optimize measurement technique and interpretation.
Keywords: exhaled nitric oxide, airway inflammation, airways, asthma, allergy, epidemiology, public health, population survey
INTRODUCTION
The fractional exhaled concentration of nitric oxide (FeNO) is a useful indicator of eosinophilic airway inflammation in some circumstances, and may possibly be useful as a probe of other respiratory pathology. The extensive relevant literature has been reviewed recently elsewhere.1,2 The pattern of change in FeNO with changing expiratory flow – commonly evaluated by “extended NO analysis”, i.e. measurement of FeNO at several standardized controlled flow rates -- may provide information beyond that available from conventional measurements at 50 ml/sec. As a first approximation, "plateau" FeNO varies inversely with expiratory flow over a range from tens to hundreds of ml/sec; and NO output, the product of concentration and flow, increases linearly with flow. This can be explained by a physiologic model that divides the lower respiratory tract into a proximal (airway) compartment with a stable maximal flux of NO laterally from wall into lumen (J'awNO), and a distal (alveolar) compartment with stable (flow-independent) NO concentration (CalvNO) in airspaces.3–8 FeNO is then the sum of the distal concentration CalvNO, plus an increment due to J’awNO which will be larger or smaller in proportion to its dilution by slower or faster expiratory flow in the proximal airways. Diffusion of NO within the respiratory tract can be ignored at sufficiently high expiratory flows (probably 50 ml/sec and higher), allowing estimates of J’awNO and CalvNO by simple linear regression.3,4 Nonlinear behavior of exhaled NO versus flow, observed at slower flows, can be modeled by treating J’awNO as the product of a stable NO concentration within the proximal airway wall and a stable diffusing capacity of the wall-to-lumen interface, such that the effective diffusion rate depends on the NO concentration difference between the proximal wall and the distal airspaces.5,7 Alternative models have focused on axial NO diffusion (from proximal to distal airways) with allowance for distally increasing airway cross-section, and have determined adjustment factors to be applied to results from the simple linear models.9,10 Parameter estimates from either nonlinear or axial-diffusion models are usually increased for J’awNO and decreased for CalvNO, relative to the simple linear models.
Extended NO testing is more demanding than conventional testing, and thus more subject to technical failures. For children especially, maintaining the slowest and/or fastest controlled flows may be difficult. Even with complete and technically satisfactory measurements, problems may arise with nonlinear or axial-diffusion modeling, in that regression estimates may fail to converge, or results may be physiologically implausible – most commonly, with negative estimates of CalvNO. Nevertheless, several studies of children, employing clinic-based populations or convenience samples (250 or fewer subjects) have been reasonably successful in characterizing factors that influence extended NO parameters.11–14 Key findings include the following: a) Elevated J’awNO and elevated CalvNO can occur independently. b) J’awNO tends to increase with growth in later childhood and adolescent years, but CalvNO apparently does not. c) Gender differences are small. d) Although many children with asthma have normal J’awNO and CalvNO, asthma is strongly associated with elevated values, particularly of J’awNO. e) Respiratory allergy without asthma also tends to elevate extended NO parameters as well as conventional FeNO; different studies disagree about the relative importance of allergy compared to asthma. To understand their clinical and public-health implications, these findings need followup in larger, more community-based populations.
The University of Southern California Children's Health Study (CHS) is a large-scale longitudinal community-based epidemiologic study of chronic respiratory health risks, with emphasis on air pollution. We implemented extended NO analysis in the CHS population, with the long-term goal of distinguishing subgroups with different patterns of airway inflammation (proximal, distal, or both), potentially relatable to different patterns of exposure to air pollutants and other risk factors. After a pilot study to verify feasibility,15 we collected extended exhaled NO data in 1,640 middle-school students from a CHS cohort. This paper presents initial cross-sectional data analyses intended to determine how extended NO parameters relate to gender, age, body size, ethnicity, and respiratory health status. These analyses also explore the influences of potential measurement artifacts and confounding factors, as well as alternative choices of physiologic model.
METHODS
During March-June 2010, extended exhaled NO testing was performed as described previously,15 in a current CHS cohort16,17 of middle-school students from 8 diverse Southern California communities. The investigative protocol was reviewed and approved by the Institutional Review Board of the Keck School of Medicine, University of Southern California. After explanation of the testing procedures, assent was obtained from each subject, and informed consent was obtained from a parent or legal guardian. Procedures conformed to published recommendations,18 except that NO concentration was determined from the 3-second plateau interval with minimum coefficient of variation, rather than the first acceptable interval (see below). Testing was conducted at schools in mid-morning through early afternoon, to avoid daily peaks of ambient NO. Height and weight were measured, and a questionnaire concerning recent health status and environmental exposures was administered to each subject, to supplement information from longer questionnaires filled out annually by parents or by subjects. FeNO was measured at flows near 30, 50, 100, and 300ml/s. Usually two blows were recorded at each flow level, but three were recorded at 50 ml/s, to improve comparability with similar tests in prior years. One extra blow could be recorded at each flow level if the technician judged it necessary to replace an unsatisfactory measurement. Testing employed three analyzer systems (Model 1080i, EcoMedics, Ann Arbor, MI/Duernten, Switzerland), with "DeNOx" accessories that removed essentially all NO from inhaled air, rotated among three technicians. Analyzers were calibration-checked monthly against a certified span gas (Scott-Marrin, Inc., Riverside, CA), and zero levels were checked periodically during testing sessions, against the DeNOx output and against air drawn through a separate zero-NO filter (Sievers Division, GE Analytical Instruments, Boulder, CO). Ambient air in the testing area was sampled periodically with the same NO analyzers, and the NO concentration to which each subject was exposed just prior to testing was estimated by linear interpolation between the closest preceding and following ambient measurements.
Digitized records of NO concentration and flow as functions of time were processed with a custom-designed computer algorithm that calculated NO concentrations from the initial 3-sec plateau as recommended by professional societies,18 and from subsequent 3-sec plateaus (0.1 sec apart) that met the same criteria (<10% difference between start and end points, <10% difference of any other point from start or end point). Plateaus with lowest coefficients of variation (low-CV) were used for data analysis. Low-CV plateaus were preferred over initial plateaus because they usually occurred near the volume interval previously recommended for extended NO testing19 (with slightly higher concentrations than initial plateaus), with less variance between blows and better linearity of NO output vs. flow. Still, correlations between low-CV and initial plateau data were high (rank correlation > 0.99), so conclusions would have been similar had initial plateaus been analyzed. Exhaled NO plots were reviewed, using visual inspection and statistical outlier tests, by experienced staff members without knowledge of subjects' demographic or health status. Plateaus judged unacceptable were recalculated or excluded from analysis.
Software packages SAS (SAS Institute, Cary, NC) and R (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis. To estimate each subject's extended eNO parameters, we first applied the linear model of Tsoukias et al.3 as in our pilot study.15 By least-squares linear regression we determined the best-fit intercept (J'awNO) and slope (CalvNO) of NO output versus flow, as well as their standard errors and 95% confidence limits. We then performed alternative analyses using nonlinear,5,7 axial-diffusion,9,10 and quadratic15 physiologic models. Data from 30 ml/sec blows were excluded from linear and axial-diffusion model calculations; all data were used otherwise. We found considerable inconsistency in CalvNO results from different models. Results from Tsoukias, Hogman nonlinear,7 and Condorelli axial-diffusion9 models (abbreviated T, H, and C respectively) are presented here, to illustrate the possible range of estimates. For the Condorelli model, fixed adjustment factors (determined from adults)9 were used, rather than adjustments based on airway size (determined from children),15,19 because the fixed factors yielded fewer physiologically implausible CalvNO estimates.
Effects on each extended NO parameter of gender, age, body size, ethnicity, respiratory health status, and external factors thought to be influential were assessed using linear models in SAS. (The latter analyses are referred to here as "population models", as distinct from physiologic models that estimate extended NO parameters for each individual.) FeNO values at each flow level were assessed similarly. Because distributions were skewed, data were log-transformed for analysis; scales were shifted as necessary to deal with negative values. Effects of age, size and ethnicity were expected to differ between boys and girls,15 so two-way interaction terms with gender and these predictor variables were included in preliminary models. Because age, height, and weight were collinear, we modeled their effects in terms of age, residual height for age, and residual weight for height. Residuals were computed from gender-specific linear regressions of height on age and age squared, and weight on height and height squared, using data from our population, which did not closely match published predictions based on nationwide samples. Other effects included in the initial “comprehensive” population models were respiratory health status, community of residence (“town”), recent (within past 12 months) secondhand smoke (SHS) exposure as reported by questionnaire, calendar month of testing, analyzer used for testing, test technician, and ambient NO concentration during testing. Mutually exclusive categories of asthma/allergy status were defined based on questionnaire responses. Subjects were considered to have asthma based on either a previous physician diagnosis or asthma medication use within the past year. Subjects using controller medications, typically inhaled corticosteroids (ICS), were categorized separately from those who only used rescue medications, typically as-needed beta-adrenergic bronchodilators. ICS-using subjects were further divided between those who had and had not used the medication within one week of testing (recently enough to influence FeNO). Subjects with any reports of respiratory symptoms not associated with acute infections were considered to have allergy; they were subdivided according to whether they experienced symptoms within the past year, or only in earlier years. Reported wheeze in the past year was not consistently associated with asthma or allergy, so it was analyzed as a separate categorical variable. No interactions of gender with these other predictors were included; i.e. the population models assumed that effects of disease, environmental factors, or measurement artifacts would be similar in boys and girls. Clock hour of testing was excluded as a predictor after being found nonsignificant in preliminary analyses. (That finding cannot argue against diurnal FeNO variation, however, since each subject was measured only once, and measurements were limited to a small segment of the 24-hour cycle.) All other aforementioned variables were significant or near-significant predictors of one or more extended NO variables. The final “parsimonious” population model applied to each NO variable included predictors that were significant (P < 0.05) or possibly influential (P < 0.15) in preliminary models for that variable. Population modeling was performed first for all subjects with technically acceptable data, and then for only those subjects with no anomalous results (model failures or negative parameter estimates) from T, H, or C models.
RESULTS
Population Characteristics
Of 1,640 subjects tested, acceptable conventional FeNO measurements at 50 ml/sec flow (FeNO(50)) were obtained from 1,631 (99.4%). Technical problems were most common at extreme flows: the percentages of acceptable data for FeNO(30), FeNO(100), and FeNO(300) were 94.3, 98.6, and 96.2 respectively. Subjects unable to provide acceptable FeNO(30) or FeNO(300) showed modestly increased prevalence of asthma (not significant by chi-square tests) compared to other subjects. Subjects lacking FeNO(300) had higher geometric mean FeNO(50) than other subjects (33 vs. 15 ppb, P < 0.001). That was not true for subjects lacking FeNO(30). Extended NO parameter estimates from T, C, and H models were technically acceptable for 98.4%, 96.6%, and 92.0% of subjects respectively. Concerning model H, however, 13.6% of subjects appeared technically acceptable by our criteria but did not meet Hogman’s original validity criteria.7 Population-model results including or excluding that group were not markedly different; results presented here include them. For model C, subjects without acceptable results had asthma prevalence approximately double that for other subjects; otherwise, model failures and health status did not appear to be significantly related.
Table 1 summarizes questionnaire-reported characteristics of subjects who provided any technically acceptable exhaled NO data. More than 20% of subjects had reports of asthma, usually mild or intermittent: fewer than 50% of the subgroup with asthma had used any asthma medication in the past year, fewer than 20% had used ICS, and fewer than 10% had used ICS recently enough to influence FeNO. About half of all subjects reported a history of respiratory allergic symptoms, but the majority of them denied symptoms in the year prior to testing. Fewer than 30% of all subjects were classified as healthy, with no reports of asthma or allergy.
Table 1.
Categorization of Subjects by Factors Potentially Relevant to Exhaled NO, as Determined from Parents' and Subjects' Questionnaire Responses, in Subjects with Technically Acceptable FeNO(50) (N = 1,631)
| CATEGORY | PERCENT |
|---|---|
| GENDER* | |
| Male | 48.9 |
| Female | 51.1 |
| ETHNICITY | |
| Hispanic | 55.9 |
| African-American | 1.8 |
| Asian-American | 3.7 |
| Non-Hispanic White | 30.2 |
| Other/Unknown | 8.4 |
| RESPIRATORY HEALTH GROUPING | |
| Asthma, inhaled corticosteroid use in past week | 1.5 |
| Asthma, inhaled corticosteroid use in past year, not in past week | 1.6 |
| Asthma, other medication use in past year | 5.5 |
| Asthma + allergy, no medication use in past year | 9.8 |
| Asthma, no allergy, no medication use in past year | 1.8 |
| Allergy, symptomatic in past year | 21.3 |
| Allergy, no symptoms in past year | 29.9 |
| Healthy (asthma, allergy reports all negative) | 28.6 |
| WHEEZE IN PAST YEAR | |
| No/not reported | 84.2 |
| Yes | 15.8 |
| SECONDHAND SMOKE EXPOSURE IN PAST YEAR | |
| No/not reported | 91.1 |
| Yes | 8.9 |
Percentage breakdown in other categories did not vary significantly by gender.
Table 2 shows summary statistics for measured variables, and for extended NO parameter estimates from all 3 models, based on all acceptable data for each variable. Boys averaged taller and heavier than girls, and typically were higher in FeNO and J'awNO, but not clearly different in CalvNO. In population models, main effects of gender were nonsignificant, but interactions of gender with age or size were often significant (see later). Distributions of parameter estimates for J’awNO and CalvNO from the 3 models were noticeably different: as observed previously by others, the more complex models tended to find higher J’awNO and lower CalvNO than the simple linear model. Negative point estimates of CalvNO were found for 1.1% of subjects with model T, 5.7% with H, and 34.8% with C. In most of those cases, 95% confidence limits of CalvNO estimates extended above zero. For the C and H models, proportions of negative CalvNO estimates were modestly but significantly increased in subjects with asthma or allergy. Only 765 subjects (46.6%) had no anomalous data, i.e. no missing or negative estimates of T, C, or H model parameters. Negative estimates of CalvC were the most common anomalies (N = 554), followed by failures to meet the H model’s original acceptability criteria7 (N = 224).
Table 2.
Field Testing Data: Summary Statistics
| Girls | |||||
|---|---|---|---|---|---|
| Arithmetic Mean |
Standard Deviation |
Median | Interquartile Range |
Range | |
| Physical Characteristics | |||||
| Age, yr | 13.4 | 0.6 | 13.3 | 12.9 -- 13.9 | 12.2 — 15.1 |
| Height, cm | 158.0 | 6.7 | 158 | 154 -- 163 | 134 -- 175 |
| Weight, kg | 54.1 | 13.2 | 52.6 | 44.9 — 59.4 | 28.6 — 115.6 |
| BMI, kg/m2 | 21.6 | 4.7 | 20.8 | 18.1 -- 23.8 | 13.1 — 43.5 |
| Measured Variables | |||||
| FeNO(30), ppb | 29.2 | 29.3 | 18.6 | 13.6 — 32.7 | 4.2 — 249 |
| FeNO(50), ppb | 19.1 | 19.0 | 12.1 | 8.9 — 21.5 | 2.7 — 139 |
| FeNO(100), ppb | 11.1 | 10.6 | 7.4 | 5.4 — 12.3 | 1.7 — 83.4 |
| FeNO(300), ppb | 4.7 | 3.8 | 3.4 | 2.6 — 5.2 | 0.3 — 30.6 |
| Ambient NO, ppb | 3.7 | 6.5 | 1.9 | 0.7—4.2 | 0 — 59 |
| Parameter Estimates from Tsoukias (T), Condorelli (C), and Hogman (H) Models | |||||
| J’awT, pl/sec | 839 | 877 | 525 | 363 -- 986 | 77 -- 6530 |
| CalvT, ppb | 1.88 | 1.31 | 1.63 | 1.14 – 2.27 | -1.87 – 11.68 |
| J’awC, pl/sec | 1402 | 1478 | 882 | 615 -- 1595 | 132 -- 11101 |
| CalvC, ppb | 0.34 | 1.23 | 0.44 | −0.13 – 1.01 | −7.17 – 3.22 |
| J’awH, pl/sec | 978 | 1047 | 618 | 410 -- 1139 | 101 -- 7816 |
| CalvH, ppb | 1.32 | 0.96 | 1.23 | 0.78 – 1.82 | -2.68 – 7.40 |
| Boys | |||||
| Physical Characteristics | |||||
| Age, yr | 13.5 | 0.6 | 13.5 | 13.0 – 14.0 | 12.3 — 15.2 |
| Height, cm | 162.2 | 9.1 | 162 | 156 -- 169 | 137 -- 190 |
| Weight, kg | 56.9 | 15.8 | 54.0 | 45.4 — 65.8 | 29.0 — 139.2 |
| BMI, kg/m2 | 21.5 | 5.0 | 20.1 | 17.8 – 24.1 | 13.4 — 46.6 |
| Measured Variables | |||||
| FeNO(30), ppb | 36.9 | 39.1 | 22.3 | 15.1 — 42.2 | 3.9 — 314 |
| FeNO(50), ppb | 24.7 | 26.5 | 14.7 | 10.0 — 27.3 | 2.4 — 219 |
| FeNO(100), ppb | 14.3 | 15.0 | 8.8 | 6.2 — 15.9 | 1.8 — 125 |
| FeNO(300), ppb | 5.8 | 5.3 | 4.0 | 2.9 — 6.3 | 0.7 — 47.8 |
| Ambient NO, ppb | 3.8 | 6.0 | 2.0 | 0.7—4.4 | 0 — 56 |
| Parameter Estimates from Tsoukias (T), Condorelli (C), and Hogman (H) Models | |||||
| J’awT, pl/sec | 1106 | 1234 | 657 | 417 – 1224 | 43 – 9526 |
| CalvT, ppb | 2.05 | 1.59 | 1.67 | 1.16 – 2.51 | -0.88 – 14.40 |
| J’awC, pl/sec | 1859 | 2089 | 1109 | 706 – 2051 | 74 -- 16194 |
| CalvC, ppb | −0.01 | 1.71 | 0.29 | −0.38 – 0.83 | −12.98 – 4.40 |
| J’awH, pl/sec | 1335 | 1563 | 791 | 492 – 1469 | 30 -- 12569 |
| CalvH, ppb | 1.25 | 1.19 | 1.15 | 0.62 – 1.77 | −3.53 – 7.68 |
Relationships among FeNO measures and extended eNO parameter estimates
FeNO variation within individuals between different flows was small in comparison to variation between individuals, as evidenced by high correlations of FeNO measurements at different flows. Pairwise Spearman rank correlation coefficients (r) ranged from 0.91 for FeNO(30) vs. FeNO(300), to 0.98 for FeNO(50) vs. FeNO(100). Furthermore, J'awNO estimates from T, C, and H models correlated highly with FeNO at all flows: Spearman r was 0.86 to 0.88 for J’awNO vs. FeNO at 300 ml/sec, and 0.93 or higher for J’awNO vs. FeNO at slower flows. However, different models disagreed concerning the relationship of CalvNO to FeNO: Spearman r was positive for the T model (0.54 to 0.76), negative for the C model (−0.49 to −0.24), and weakly positive for the H model (0.16 to 0.39). In all 3 instances the most positive relationship was between CalvNO and FeNO(300), consistent with the expectation that the influence of distal NO on FeNO is greatest at fastest flows.
Table 3 shows pairwise Spearman rank correlations of extended NO parameters from the 3 models, for all subjects with technically acceptable data (below diagonal) and for the subgroup with no model failures or negative estimates (above diagonal). Different models agreed well in ranking subjects’ J’awNO; but disagreed concerning which subjects had elevated CalvNO, and concerning the relationship of CalvNO to J’awNO. CalvT and CalvC showed the most marked disagreement. CalvH agreed well with CalvT in subjects with no anomalous data, but not as well for all subjects. High J’awNO was associated with high CalvNO in the T model, but low (or negative) CalvNO in the C model. In the H model, J’awNO and CalvNO were essentially uncorrelated for all subjects, but somewhat positively correlated in those with no anomalous data.
Table 3.
Pairwise Spearman Rank Correlations of Extended NO Parameter Estimates from Tsoukias (T), Condorelli (C) and Hogman (H) Models. Below diagonal: all subjects with technically acceptable data for both variables (1496 ≤ N ≤ 1597). Above diagonal: subjects with no model failures or physiologically implausible parameter estimates (N = 765). Correlations of J’awNO with CalvNO from the same model are underlined.
| CalvT | CalvC | CalvH | J’awT | J’awC | J’awH | |
|---|---|---|---|---|---|---|
| CalvT | 0.49 | 0.90 | 0.59 | 0.59 | 0.63 | |
| CalvC | 0.27 | 0.69 | −0.17 | −0.17 | −0.21 | |
| CalvH | 0.74 | 0.67 | 0.48 | 0.48 | 0.43 | |
| J’awT | 0.47 | −0.54 | 0.07 | 1.00 | 0.96 | |
| J’awC | 0.47 | −0.54 | 0.07 | 1.00 | 0.96 | |
| J’awH | 0.46 | −0.62 | −0.01 | 0.97 | 0.97 |
The above comparisons address the entire range of extended NO model parameters, but unusually high values are of most interest. Table 4 compares models with respect to high values – those above 95th percentiles for healthy subjects – for the entire population with technically acceptable data. (Non-health-related influences on high NO, not directly relevant to this comparison, are ignored.) For each pairing of alternative J’awNO or CalvNO estimates, Table 4 shows their percentage of agreement in identifying “high” subjects. Agreement was good (75%–89%) between J’awNO estimates, but poor (13%–41%) between CalvNO estimates. By the same approach, agreement was 79% between FeNO(50) and FeNO(300), consistent with the finding that FeNO values at different flows correlate highly with each other and with J’awNO.
Table 4.
Agreement Between Different Models in Identifying Subjects with High J’awNO or CalvNO*
| Variables Compared (Respective Normal Limits) |
% of Subjects High, Either |
% of Subjects High, Both |
% Agreement |
|---|---|---|---|
| J’awT, J’awC (2118, 3803 pl/sec) | 10.1 | 9.0 | 89 |
| J’awT, J’awH (2118, 2316 pl/sec) | 11.7 | 9.8 | 84 |
| J’awC, J’awH (3803, 2316 pl/sec) | 11.7 | 8.8 | 75 |
| CalvT, CalvC (3.36, 2.11 ppb) | 11.4 | 1.5 | 13 |
| CalvT, CalvH (3.36, 2.80 ppb) | 10.8 | 4.4 | 41 |
| CalvC, CalvH (2.11, 2.80 ppb) | 8.0 | 2.5 | 31 |
| FeNO(50), FeNO(300) (46.5, 9.7 ppb) | 11.2 | 8.9 | 79 |
High is defined as above normal limit (95th percentile) for healthy subjects.
Table 5 compares different models’ performance in identifying patterns of high NO output, by adapting a phenotypic classification developed by Puckett et al.14 It shows percentages of subjects identified as high in J’awNO only, CalvNO only, or both, by each model. All models showed an overall increase in high-NO findings among subjects with asthma; but otherwise they disagreed. By the T model, many subjects with high J’awNO also had high CalvNO; whereas by the C model, very few were high in both. This is consistent with aforementioned overall correlations – positive for T, negative for C. The pattern for the H model fell between those extremes.
Table 5.
Comparison of Extended NO Models in Identifying Different High-NO Phenotypes*
| Model | Percentage of all subjects high in: | |||
|---|---|---|---|---|
| neither | J’awNO only | CalvNO only | both | |
| Tsoukias (T) | 85.6 | 4.2 | 3.9 | 6.3 |
| Condorelli (C) | 87.5 | 8.8 | 3.4 | 0.3 |
| Hogman (H) | 84.8 | 9.0 | 3.7 | 2.5 |
| Percentage of subjects with asthma high in: | ||||
| neither | J’awNO only | CalvNO only | both | |
| Tsoukias (T) | 72.3 | 7.1 | 6.5 | 14.1 |
| Condorelli (C) | 79.2 | 17.9 | 2.2 | 0.7 |
| Hogman (H) | 73.0 | 17.5 | 3.6 | 5.9 |
High is defined as above normal limit (95th percentile) for healthy subjects.
Influences of health status and other personal characteristics on FeNO and extended NO parameter estimates
The online supplement presents detailed results from parsimonious population models applied to FeNO(50) and FeNO(300) (Table E-1 for entire population, Table E-2 for subgroup with no anomalous results), J’awNO from the 3 alternative extended NO models (Tables E-3, E-4), and CalvNO likewise (Tables E-5, E-6). General patterns appeared similar for the entire population and the subgroup with no anomalous results. Effect sizes and significance levels tended to be less for the subgroup, consistent with its smaller size and possibly better overall respiratory health.
Figure 1 shows estimated geometric mean J'awNO and its upper 95% confidence limit for each health-status subgroup, adjusted for other influential factors, according to T, C, and H extended NO models. All 3 models showed highly significant (P < 0.0001) progressive increases of J’awNO with increasing degrees of chronic respiratory disturbance, ranging from 10–15% for subjects with allergy history but no symptoms in the past year, to 70–80% for subjects with medication-dependent asthma who had used ICS in the week before testing. Variations of FeNO by health grouping (not graphed) were proportionately similar. For the 765 subjects with no anomalous data, patterns were similar but estimated effect sizes tended to be smaller (online supplement Tables E-1 – E-4). After adjustment for asthma/allergy status, reported wheeze had a slight positive influence on J’awNO (around 10%) that fell short of statistical significance. Ethnicity also influenced J’awNO significantly (P < 0.05 for overall group differences). Compared to non-Hispanic whites, increases in geometric mean were < 10% in Hispanic and unclassified groups, 20–30% in African-Americans, and 30–40% in Asian-Americans. Considering only the 765 subjects with no anomalous data, the Asian difference persisted, while differences among other groups tended to disappear (Table E-4). Increasing age and height-for-age had independent positive influences on J’awNO, while increasing weight-for-height had a negative influence in boys only (Table 6).
Figure 1.
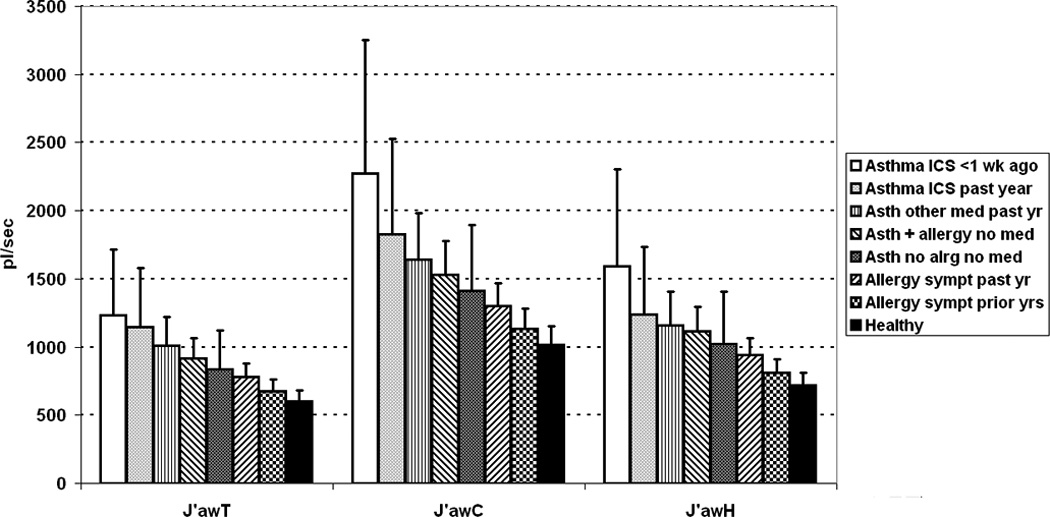
J’awNO differences by respiratory health status, as determined by extended NO models of Tsoukias (J’awT), Condorelli (J’awC), and Hogman (J’awH), for entire population with technically acceptable data. Bar indicates geometric mean; flag indicates upper 95% confidence limit of geometric mean. Differences are significant (P < 0.0001) by all extended NO models.
Table 6.
Significant Influences on Alternative Extended NO Parameter Estimates, Other Than Respiratory Health and Ethnicity [a]
| J’awT | J’awC | J’awH | CalvT[d] | CalvC [d] | CalvH[d] | |
|---|---|---|---|---|---|---|
| Age/Size: | ||||||
| Age (per year) | +13** | +13** | +16** | NS | −0.36** | −13** |
| Height for age (per cm): Girls | +1.0** | +1.1** | +1.3** | +0.64* | −0.019* | +0.6 |
| Height for age (per cm): Boys [b] | +1.0** | +1.1** | +1.3** | +0.64* | −0.019* | −1.3* |
| Weight for height (per kg): Girls | +0.09 | +0.02 | NS | −0.78** | −0.007† | −1.0** |
| Weight for height (per kg): Boys [b] | −0.43* | −0.45* | NS | −0.78** | −0.007† | −1.0** |
| Environmental/Instrumental: [c] | ||||||
| Secondhand smoke exposure | −10† | −11† | NS | NS | NS | NS |
| Town (highest vs. lowest) | NS | NS | NS | +30** | +0.7* | +54** |
| Month of test (highest vs. lowest) | NS | NS | NS | +16* | +0.3† | +33** |
| Analyzer (highest vs. lowest) | +14* | +14* | +14* | +8† | NS | NS |
| Technician (highest vs. lowest) | NS | NS | NS | +16* | +0.5* | +43** |
| Ambient NO | NS | NS | NS | +28* | NS | +36† |
Estimated percentage change per unit or between categories, from parsimonious population models, adjusted for ethnic and respiratory-health influences. Significance tests for slope different from zero or for overall variation between categories: NS = not significant in preliminary model, excluded from parsimonious model;
P < 0.15;
P < 0.05;
P < 0.005.
Where same value is reported for boys and girls, gender interaction term is nonsignificant; significance test relates to main effect of height or weight.
In general, highest and lowest categories were the same for J’awT, J’awC, and J’awH; but otherwise were different between parameters. Significance tests address overall difference among categories.
Because of scale shifting to deal with negative values, percentage estimates for CalvNO are not constant across the range, and not comparable between models. For best overall consistency, percentage changes are calculated for values near the median CalvNO for the given model (shown in Table 2). For CalvC, predicted ranges may approach or cross zero, so estimates are given in ppb NO rather than percent.
Figure 2 shows estimated geometric mean CalvNO and its upper 95% confidence limit for each health-status subgroup and each extended NO model. The 3 models disagreed substantially concerning effects of respiratory health on CalvNO. CalvT showed a highly significant progressive rise from the healthy to the recently-ICS-using asthmatic subgroup, proportionately smaller but otherwise similar to findings for J’awNO. CalvC also varied significantly according to health status, but less consistently, and generally in the opposite direction: more negative geometric means were associated with asthma. CalvH was modestly increased in ICS-using subgroups, but differences by health status did not reach statistical significance. Ethnic differences were significant for CalvT in the entire population, and for CalvT and CalvH in the subgroup with no anomalous data, with Asian-American subjects showing high values in each instance (Tables E-5, E-6).
Figure 2.
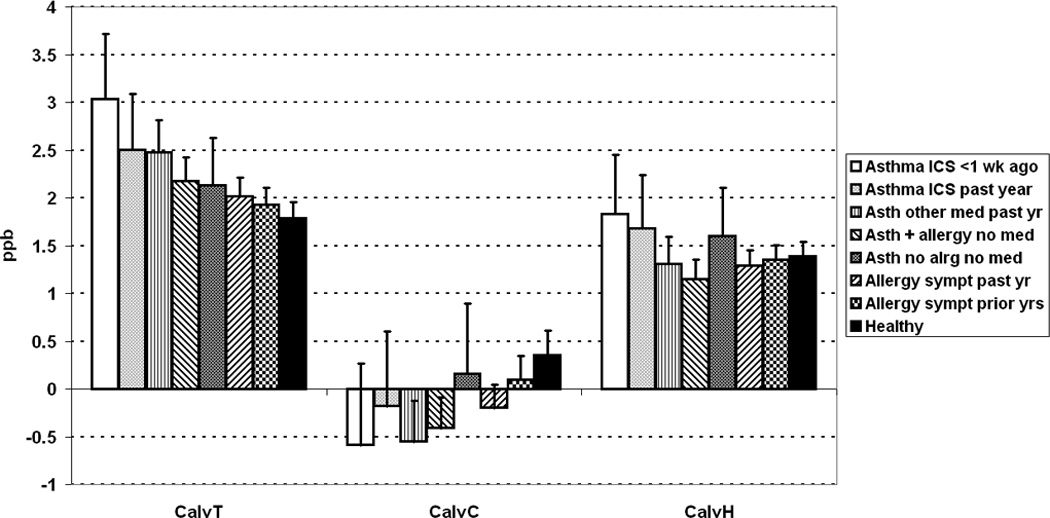
CalvNO differences by respiratory health status, as determined by extended NO models of Tsoukias (CalvT), Condorelli (CalvC), and Hogman (CalvH), for entire population with technically acceptable data. Bar indicates geometric mean; flag indicates upper 95% confidence limit of geometric mean. Significance of differences: P < 0.0001 for CalvT and CalvC, P = 0.12 for CalvH.
Table 6 compares J’awNO and CalvNO across the T, C, and H physiologic models, with respect to effects of age, size, and environmental/instrumental factors, for the entire population. Because they are determined from log-transformed data, effect sizes are given as percentages. (For CalvC, percentage estimates are difficult to interpret because reference levels may be near zero or negative, so results are expressed in ppb NO.) The T, C, and H models disagreed on the significance and direction of age and height effects on CalvNO, but were more consistent concerning the effect of weight-for-height. They indicated a decrease of roughly 0.007 to 0.013 ppb for each added kg of weight in subjects with CalvNO near the median. The range of residual weight-for-height, excluding extreme percentiles, was −19 to +41 kg, implying a possible variation of CalvNO by 0.4 to 0.8 ppb just from this source. For comparison, the interquartile range of CalvNO from each model was between 1.0 and 1.2 ppb (Table 2). Figure 3 illustrates the relationship of CalvH with weight-for-height.
Figure 3.
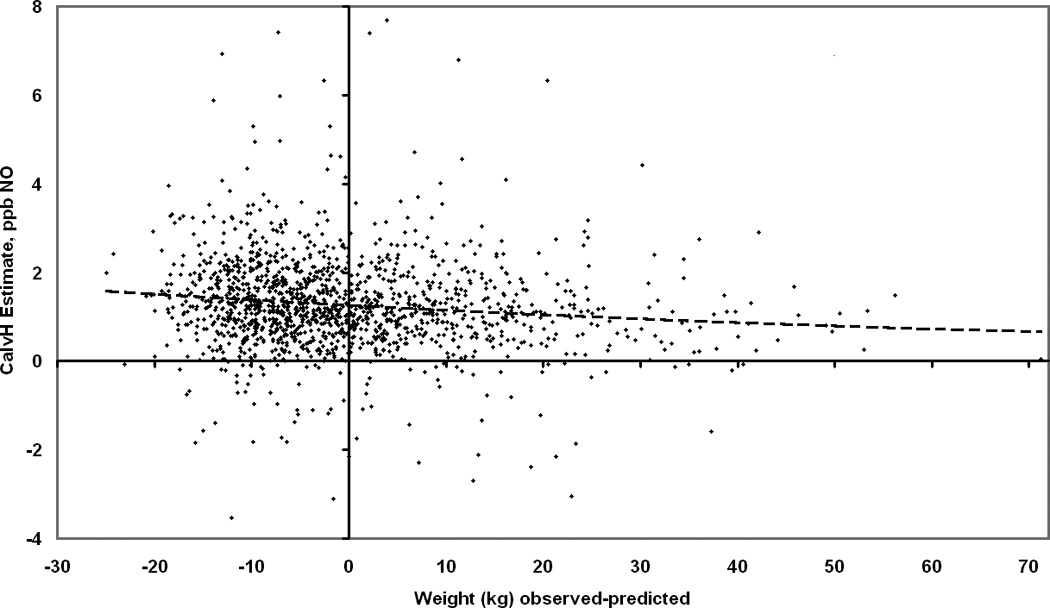
Individual CalvNO estimated by Hogman (H) physiologic model, as a function of residual weight-for-height (observed weight, minus weight predicted by gender-specific regression analyses of this population), with best-fit quadratic regression line (broken line).
Influences of environmental or instrumental factors on FeNO and extended NO parameter estimates
Exact counterbalancing of analyzer and technician assignments by time and location was not logistically feasible, so statistical confounding among the predictor categorical variables NO analyzer, technician, town, and calendar month cannot be ruled out. Analyzer differences were statistically significant for FeNO and J'awNO (Table 6, online supplement Tables E-1 – E-4), suggesting consistent differences in analyzer response, not prevented by routine calibration checks. The effect was small with respect to clinical testing, but appreciable with respect to population modeling. Subjects who reported exposure to SHS averaged about 10% lower in geometric mean FeNO(50), J’awT, or J’awC than other subjects, although differences did not reach significance. Otherwise, environmental or measurement-artifact effects on J’awNO were nonsignificant. By contrast, CalvNO estimates from all 3 models showed significant or near-significant variation related to technician, town, and calendar month (Tables 6, E-5, E-6). CalvT, CalvH, and FeNO(300) also showed significant or nearly significant increases in subjects whose ambient NO exposures at the time of testing were among the highest 5% -- between 12 and 59 ppb (Tables 6, E-1, E-5).
DISCUSSION
We found that extended NO parameters can be measured in large-scale field studies of young people, and potentially can provide refined phenotypic data on airway physiology. These initial results, from an unprecedentedly large population, generally reinforce others’ prior findings in smaller groups, summarized in the Introduction. Multiple extrinsic factors, as well as host factors independent of respiratory health, may influence conventional or extended exhaled NO measurements, and are important to consider in etiologic research in populations. Importantly, these results point out the difficulty of measuring peripheral-airway or alveolar NO in a consistent and interpretable manner.
The results reinforce prior findings that conventional FeNO(50) is strongly correlated with maximum proximal airway wall NO flux (J'awNO),1 and show that the correlation is still strong for FeNO at flows up to 300 ml/sec. Thus, for most subjects the proximal NO influence appears dominant even for FeNO(300), emphasizing the difficulty of measuring peripheral NO precisely. Different physiologic models agree well in measuring proportionate J’awNO differences among groups. However, numerical estimates depend strongly on the model used (possibly also on the specific flows at which FeNO is measured), and so are not usually comparable across studies.
Concerning peripheral NO concentration (CalvNO), disagreement between models is more serious. In this large-scale field survey, relatively few data points were obtainable from each subject, and the range of flows was not necessarily optimal for each model, limiting the precision of parameter estimates. However, some “model failures” are usually found even in more intensive, well controlled laboratory studies. Thus, even the more advanced physiologic models, applied under optimum conditions, do not describe respiratory-tract NO dynamics accurately in all cases. Despite shortcomings of current models, comparison of CalvNO across different models may offer useful insight into peripheral airway status. We suggest the following interpretation of the present data: CalvT, not corrected for axial back-diffusion, measures peripheral NO plus back-diffused proximal NO. The back-diffused component increases with J’awNO, giving rise to the positive correlation of J’awT with CalvT. Increased CalvT in subgroups with asthma is thus attributable to back-diffused proximal-airway NO, and not necessarily to elevated peripheral NO. CalvC explicitly corrects for back-diffusion, but its adjustment factors derive from a very different population. They apparently over-correct in our population, in light of the many negative CalvC values. Furthermore, negative values were concentrated in subgroups with more marked asthma and high J’awNO, consistent with others’ prior observations that airway obstruction in asthma may impair back-diffusion and thus exacerbate any over-correction error.20 CalvH does not address back-diffusion as such, but does allow for intra-airway diffusion dependent on the relative intensity of proximal and peripheral NO sources, and so may provide a reasonable estimate of peripheral NO. Moreover, the present extended NO testing equipment and protocol were designed for use with the H model (which, however, ideally employs more flow levels in a wider range). Increased geometric mean CalvH in ICS-using asthmatic subjects (Figure 2), as well as increased prevalence of high CalvH accompanying high J’awH in asthmatics (Table 5), suggests modest increases in peripheral NO in some of the present subjects with asthma. The apparently small overall effects of respiratory health disturbances on CalvNO may reflect a narrow clinical range – between good health and relatively mild asthma – in our population. Further studies including subjects with moderate-severe asthma, or with independently verifiable peripheral respiratory or systemic inflammation, may provide more insight into disease-related differences in CalvNO.
The observed highly significant negative relationship of CalvNO to weight (Table 6, online supplement Tables E-5, E-6) supports the possibility that CalvNO may provide useful evidence of systemic inflammatory processes. Negative relationships of J'awNO and FeNO with weight were also significant in some instances (Table 6, Tables E-1 – E-4), particularly for FeNO(300), most influenced by peripheral NO. Negative relationships of body mass index or weight-for-height with conventional FeNO were found in this population at younger ages,21 and in a young Chinese population,22 but were not found in some other studies.23,24 The present findings suggest that the relationship is governed at least in part by peripheral NO. Mechanistic explanation will require more research, but present data allow some tentative inferences. The CalvNO-weight relationship did not appear to be driven by markedly overweight subjects (Figure 3). That argues against a strictly pulmonary-mechanical mechanism involving lung restriction due to obesity, and thus favors the possibility that the effect relates to a systemic metabolic process that involves NO and is influenced by body weight. However, the direction of effect is counter-intuitive, assuming that excess weight promotes inflammatory processes tending to raise NO concentrations.
These results help to clarify effects of growth on conventional FeNO and J’awNO in early teenage years: being older, and being taller for one's age, both tend to increase NO output. One plausible explanation is that both increasing age and increasing height contribute to growth in proximal airway surface area. If NO flux per unit surface area is relatively stable, then aggregate proximal flux (J'awNO) will increase in proportion to this growth, as will FeNO at any fixed expiratory flow. Further investigation of extended NO tests in conjunction with spirometry should help to clarify these relationships.
The relatively slight effects of respiratory allergy on FeNO or J’awNO observed in this population may reflect limitations of clinical characterization, based only questionnaire responses from subjects and their parents. Had immunologic testing been available, possibly fewer subjects would have been classified as allergic, and larger effects of allergy on exhaled NO would have been found. Asthma seemed to have stronger effects than allergy, although again the limitations of questionnaire-based categorization must be recognized. Even though ICS is expected to reduce exhaled NO, among our subjects with asthma ICS was associated with increased FeNO(50) and J'awNO, more so in those who reported more recent usage. This presumably reflects more severe asthma in those using ICS. A similar association of ICS usage with increased FeNO(50) has been found in an adult population.25
The finding of elevated FeNO and J’awNO in subjects of Asian descent agrees with previous observations in this population21 and others.23,24 A biological explanation is lacking, to the best of our knowledge. Our present evidence does not clearly answer the question whether Asian ancestry also predicts elevated CalvNO (Tables E-5, E-6). New investigations comparing larger numbers of Asians with non-Asians are indicated.
These results highlight the need to address technical issues of measurement, particularly for CalvNO, to minimize imprecision or bias due to the measurement system itself or the testing environment, in order to reliably detect true biological effects of environmental or host factors. Concerning the measurement system, analyzer effects were most consistently significant. Statistical detection of analyzer differences in a large dataset is not surprising: instrument response depends on interactive aerodynamic, chemical, and electronic processes likely to differ slightly from one unit to another, even if all are carefully maintained, frequently calibration-checked, and consistently meet applicable standards of precision. Technician effects were also often significant, raising the possibility that test results are sensitive to subtle differences in coaching of subjects. Differences between communities and between calendar months might reflect real biological effects of season or of local environmental quality, or chance differences in factors that introduce bias. At present the latter explanation seems more plausible, in that significant differences did not show obvious patterns related to time or location.
Two environmental factors showed at least weak statistical evidence consistent with real biological effects on exhaled NO – secondhand smoke exposure as reported on questionnaires, and ambient NO concentration during the testing session. The SHS effects on FeNO or J’awNO, though marginally statistically significant at best, are noteworthy in that changes were in the direction observed in active smokers, opposite to changes expected from increased proximal airway inflammation in nonsmokers. The ambient NO effect on CalvNO – significant for CalvT, close to significance for CalvH – is consistent with a short-term effect of air pollution. If the association is causal, the active agent might be NO itself or other pollutant(s) that commonly accompany NO, e.g. particulate matter from motor vehicle exhaust. Associations between short-term urban air pollution exposure and acutely increased FeNO have been observed previously in panel studies of children.26,27
In conclusion, this large-scale study of a young population makes clear that extended exhaled NO measurements, as well as conventional FeNO measurements, may be influenced by body size, age, ethnicity, test instruments, technicians, and the local environment. J'awNO is the major contributor to conventional FeNO levels, probably reflecting the inflammatory state of proximal airways. CalvNO, as currently measured, may not accurately reflect true peripheral respiratory tract NO concentration, but nevertheless enhances the information available from FeNO or J’awNO, and may facilitate assessment of peripheral-airway, alveolar, or systemic dysfunction. To improve interpretability of CalvNO, extended NO studies in populations with inflammatory conditions involving the deep lung and/or the circulatory system are indicated. Concurrent evaluation of airway obstruction, by spirometry or more complex tests, is also indicated, because airway obstruction may influence intra-airway NO behavior, and thus alter extended NO measurements.20,28
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge the contributions of the field team: Steven Howland (leader), Reyna Diaz Leyva, Blanca Garcia, Martha Perez, Ned Realiza, and Lisa Valencia. This work was supported by the National Heart Lung and Blood Institute (grant 5R01HL076647); the Southern California Environmental Health Sciences Center (grant # 5P30ES007048) funded by the National Institute of Environmental Health Sciences; the Children’s Environmental Health Center (grant #s 5P01ES009581, R826708-01 and RD831861-01) funded by the National Institute of Environmental Health Sciences and the Environmental Protection Agency; the National Institute of Environmental Health Sciences (grant # 5P01ES011627); and the Hastings Foundation.
REFERENCES
- 1.Barnes PJ, Dweik RA, Gelb AF, Gibson PG, George SC, Grasemann H, et al. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest. 2010;138:682–692. doi: 10.1378/chest.09-2090. [DOI] [PubMed] [Google Scholar]
- 2.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am J Respirat Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsoukias NM, George SC. A two-compartment model of pulmonary nitric oxide exchange dynamics. J Appl Physiol. 1998;85:653–666. doi: 10.1152/jappl.1998.85.2.653. [DOI] [PubMed] [Google Scholar]
- 4.Pietropaoli AP, Perillo IB, Torres A, Perkins PT, Frasier LM, Utell MJ, et al. Simultaneous measurement of nitric oxide production by conducting and alveolar airways of humans. J Appl Physiol. 1999;87:1532–1542. doi: 10.1152/jappl.1999.87.4.1532. [DOI] [PubMed] [Google Scholar]
- 5.George SC, Hogman M, Permutt S, Silkoff PE. Modeling pulmonary nitric oxide exchange. J Appl Physiol. 2004;96:831–839. doi: 10.1152/japplphysiol.00950.2003. [DOI] [PubMed] [Google Scholar]
- 6.Puckett JL, George SC. Partitioned exhaled nitric oxide to non-invasively assess asthma. Respir Physiol Neurobiol. 2008 Nov 30;163(1–3):166–177. doi: 10.1016/j.resp.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogman M, Merilainen P. Extended NO analysis in asthma. J Breath Res. 2007;1 doi: 10.1088/1752-7155/1/2/024001. 024001 (8pp) [DOI] [PubMed] [Google Scholar]
- 8.García-Río F, Casitas R, Romero D. Utility of two-compartment models of exhaled nitric oxide in patients with asthma. J Asthma. 2011;48:329–334. doi: 10.3109/02770903.2011.565847. [DOI] [PubMed] [Google Scholar]
- 9.Condorelli P, Shin HW, Aledia AS, Silkoff PE, George SC. A simple technique to characterize proximal and peripheral nitric oxide using constant flow exhalations and an axial diffusion model. J Appl Physiol. 2007;102:417–425. doi: 10.1152/japplphysiol.00533.2006. [DOI] [PubMed] [Google Scholar]
- 10.Kerckx Y, Michils A, Van Muylem A. Airway contribution to alveolar nitric oxide in healthy subjects and stable asthma patients. J Appl Physiol. 2008;104:918–924. doi: 10.1152/japplphysiol.01032.2007. [DOI] [PubMed] [Google Scholar]
- 11.Paraskakis E, Brindicci C, Fleming L, Krol R, Kharitonov SA, Wilson NM, Barnes PJ, Bush A. Measurement of bronchial and alveolar nitric oxide production in normal children and children with asthma. Am J Respir Crit Care Med. 2006;174:260–267. doi: 10.1164/rccm.200506-962OC. [DOI] [PubMed] [Google Scholar]
- 12.Latzin P, Beck J, Griese M. Exhaled nitric oxide in healthy children: Variability and a lack of correlation with atopy. Pediatr Allergy Immunol. 2002;13:37–46. doi: 10.1034/j.1399-3038.2002.00066.x. [DOI] [PubMed] [Google Scholar]
- 13.Sepponen A, Lehtimaki L, Huhtala H, Kaila M, Kankaanranta H, Moilanen E. Alveolar and bronchial nitric oxide output in healthy children. Pediatr Pulmonol. 2008;43:1242–1248. doi: 10.1002/ppul.20953. [DOI] [PubMed] [Google Scholar]
- 14.Puckett JL, Taylor RWE, Leu SY, Guijon OL, Aledia AS, Galant SP, George SC. Clinical patterns in asthma based on proximal and distal airway nitric oxide categories. Respir Res. 2010;11 doi: 10.1186/1465-9921-11-47. 47 (9pp) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linn WS, Rappaport EB, Berhane KT, Bastain TM, Salam MT, Gilliland FD. Extended exhaled nitric oxide analysis in field surveys of schoolchildren: A pilot study. Pediatr Pulmonol. 2009;44:1033–1042. doi: 10.1002/ppul.21101. [DOI] [PubMed] [Google Scholar]
- 16.Eckel SP, Berhane K, Salam MT, Rappaport EB, Linn WS, Bastain TM, et al. Residential traffic-related pollution exposures and exhaled nitric oxide in the Children's Health Study. Environ Health Perspect. 2011;119:1472–1477. doi: 10.1289/ehp.1103516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salam MT, Byun HM, Lurmann F, Breton CV, Wang X, Eckel SP, Gilliland FD. Genetic and epigenetic variations in inducible nitric oxide synthase promoter, particulate pollution, and exhaled nitric oxide levels in children. J Allergy Clin Immunol. 2012;129:232–239. doi: 10.1016/j.jaci.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Thoracic Society/European Respiratory Society. ATS/ERS Recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 19.Puckett JL, Taylor RWE, Galant SP, George SC. Impact of analysis interval of the multiple exhalation flow technique to partition exhaled nitric oxide. Pediatr Pulmonol. 2010;45:182–191. doi: 10.1002/ppul.21182. [DOI] [PubMed] [Google Scholar]
- 20.Verbanck S, Malinovschi A, George S, Gelb AF, Vincken W, Van Muylem A. Bronchial and alveolar components of exhaled nitric oxide and their relationship. Eur Respir J. 2012;39:1258–1261. doi: 10.1183/09031936.00105611. [DOI] [PubMed] [Google Scholar]
- 21.Linn WS, Rappaport EB, Berhane KT, Bastain TM, Avol EL, Gilliland FD. Exhaled nitric oxide in a population-based study of Southern California schoolchildren. Respir Res. 2009;10:28. doi: 10.1186/1465-9921-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao TC, Ou LS, Lee WI, Yeh KW, Chen LC, Huang JL. Exhaled nitric oxide discriminates children with and without allergic sensitization in a population-based study. Clin Exp Allergy. 2011;41:556–564. doi: 10.1111/j.1365-2222.2010.03687.x. [DOI] [PubMed] [Google Scholar]
- 23.Wong GWK, Liu EKH, Leung TF, Yung E, Ko FWS, Hui DSC, Fok TF, Lai CKW. High levels and gender difference of exhaled nitric oxide in Chinese schoolchildren. Clin Exp Allergy. 2005;35:889–893. doi: 10.1111/j.1365-2222.2005.02263.x. [DOI] [PubMed] [Google Scholar]
- 24.Kovesi T, Dales R. Exhaled nitric oxide and respiratory symptoms in a community sample of school aged children. Pediatr Pulmonol. 2008;43:1198–1205. doi: 10.1002/ppul.20927. [DOI] [PubMed] [Google Scholar]
- 25.Olin AC, Rosengren A, Thelle DS, Lissner L, Bake B, Toren K. Height, age, atopy are associated with fraction of exhaled nitric oxide in a large adult general population sample. Chest. 2006;130:1319–1325. doi: 10.1378/chest.130.5.1319. [DOI] [PubMed] [Google Scholar]
- 26.Lin W, Huang W, Zhu T, Hu M, Brunekreef B, Zhang Y, Liu X, Cheng H, Gehring U, Li C, Tang X. Acute respiratory inflammation in children and black carbon in ambient air before and during the 2008 Beijing Olympics. Environ Health Perspect. 2011;119:1507–1512. doi: 10.1289/ehp.1103461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarnat SE, Raysoni AU, Li WW, Holguin F, Johnson BA, Flores Luevano S, Garcia JH, Sarnat JA. Air pollution and acute respiratory response in a panel of asthmatic children along the U.S.-Mexico border. Environ Health Perspect. 2012;120:437–444. doi: 10.1289/ehp.1003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Muylem A, Kerckx Y, Michils A. Acinar effect of inhaled steroids evidenced by exhaled nitric oxide. J Allergy Clin Immunol. 2010;126:730–735. doi: 10.1016/j.jaci.2010.06.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


