Abstract
Technological innovations have driven the advancement of the surgical treatment of movement disorders, from the invention of the stereotactic frame to the adaptation of deep brain stimulation (DBS). Along these lines, this review will describe recent advances in getting neuromodulation modalities, including DBS, to the target; and in the delivery of therapy at the target. Recent radiological advances are altering the way that DBS leads are targeted and inserted, by refining the ability to visualize the subcortical targets using high-field strength MRI and other innovations such as diffusion tensor imaging, and the development of novel targeting devices enabling purely anatomical implantations without the need for neurophysiological monitoring. New portable CT scanners also are facilitating lead implantation without monitoring as well as improving radiological verification of DBS lead location. Advances in neurophysiological mapping include efforts to develop automatic target verification algorithms, and probabilistic maps to guide target selection. The delivery of therapy at the target is being improved by the development of the next generation of internal pulse generators (IPGs). These include constant current devices that mitigate the variability introduced by impedance changes of the stimulated tissue, and in the near future, devices that deliver novel stimulation patterns with improved efficiency. Closed-loop adaptive IPGs are being tested, which may tailor stimulation to ongoing changes in the nervous system reflected in Œbiomarkers1 continuously recorded by the devices. Finer grained DBS leads, in conjunction with new IPGs and advanced programming tools, may offer improved outcomes via Œcurrent steering1 algorithms. Finally, even thermocoagulation - essentially replaced by DBS - is being advanced by new Œminimally-invasive1 approaches that may improve this therapy for selected patients in whom it may be preferred. Functional neurosurgery has a history of being driven by technological innovation, a tradition that continues into its future.
Keywords: Deep brain stimulation, closed-loop neuromodulation, MRI, constant current
INTRODUCTION
The surgical treatment of movement disorders has always relied on technological advances, the most enabling of which was the stereotactic frame [1, 2]. Other particularly notable innovations included devices for thermo- and cryoablation; refinements in stereotactic frames by Leksell, and Brown, Cosman, Roberts, and Wells amongst others; Leksell’s Gamma Knife (developed specifically for functional neurosurgery); the CT and MRI scans; cellular recording devices and of course deep brain stimulation (DBS). All of these innovations, and those discussed below, reflect the symbiosis of advances in technology to visualize a target in the brain that cannot be directly seen, and then to ‘touch’ it (i.e. stereotaxis) therapeutically. Such will be the organization of this review: we will discuss advances in image-guided targeting of subcortical regions, followed by advances in the technology of therapeutics for movement disorders. Most of the discussion will relate to DBS, which remains the mainstay of the treatment of movement disorders as it has for nearly two decades, although new technology for thermocoagulation will be discussed as well. Although other approaches to neuromodulation continue to vie for a role (e.g. gene/cell therapy), none have advanced sufficiently in humans to be considered an established treatment and will not be covered.
ADVANCES IN DBS LEAD TARGETING AND IMPLANTATION
Advances in MRI definition of the target
While intraoperative mapping and clinical responses have been the ‘standard’ for targeting, advances in imaging inexorably have led and will further lead to better preoperative identification of the targets and shortened mapping procedures and – I believe – will ultimately largely replace mapping in the awake patient in the next decade. Not only have sequences been better refined for the 1.5 Tesla (T) MRI scanner over the years, but now 3.0 T scanners are ubiquitous, in the developed world at least, and new advances have been made in defining targets on these scanners. For example, Kerl et al. (2012) compared several popular sequences, using standard installations, showing better definition of the subthalamic nucleus (STN) on the T2*-FLASH2D sequence (coronal) [3], and also of the globus pallidus interna (GPi) with the same sequence (axial) [4]. A more recent advance is quantitative susceptibility mapping, based on the susceptibility induced by iron in the basal ganglia, which very clearly displays both GPi and STN as compared to standard images (Figure 1) [5]. These advances will aid direct targeting of these structures, especially important in purely anatomically-based procedures. Even the thalamus, the subdivisions of which are indistinguishable on standard MRI (even 3.0 T), has been parcellated by new techniques to reveal the ventralis intermedius (Vim) nucleus, but visualization in the individual patient has been much less robust [6].
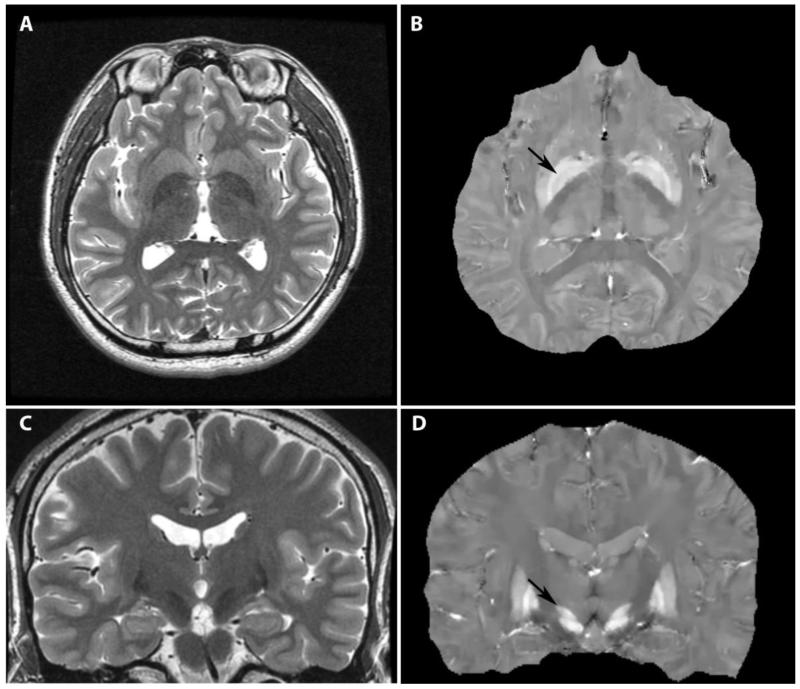
Fig. 1.
Quantitative Susceptibility Mapping clearly demonstrating the globus pallidus internus (GPi) on axial images (B, arrow) and the subthalamic nucleus (STN) on coronal images (D, arrow), as compared to traditional T2-weighted imaging (A, C). (Images courtesy of Brian Kopell, Mt. Sinai School of Medicine, and Tian Liu, Cornell University)
Abosch et al. demonstrated improved definition of the STN and GPi on high field strength (7T) MR imaging in human subjects, due to increases in signal-to-noise ratio amongst other advantages [7, 8]. Their work also suggests potential for visualization of some of the thalamic parcellations, including the Vim, in individual subjects. A major concern with 7T MRI, however, is the greater tissue distortion and susceptibility artifacts at high field strength. This issue was addressed by Duchin et al., who demonstrated that it is feasible to obtain precise (1 voxel) co-registration between 1.5T and 7T image sets in the diencephalon/midbrain region (but not more peripherally), where DBS leads are implanted [9].
Diffusion Tensor Imaging and DBS
It is now almost universally accepted that electrical stimulation preferentially activates efferent axonal fibers and that, in many instances, axon fiber tracts comprise the neural elements that mediate the effects of DBS (reviewed in [10, 11]). Diffusion tensor imaging (DTI) has been used to demonstrate the proximity of effectively implanted DBS leads to these white matter tracts. In DBS of the STN, contacts in the dorsolateral region are most effective. In this location, the electric field established around the contact (volume of tissue activated, VTA) is in position to activate both STN projection axons and the fibers of the lenticular fasciculus (a portion of the internal pallidal fibers projecting to the ventral thalamus), as shown in macaque monkeys by overlaying realistic computational models that predict VTA upon a brain atlas and DTI [12]. This approach has recently been used to create a ‘probabilistic stimulation atlas’ that may in the future help to guide optimal DBS implantations [13].
Similarly, effective Vim DBS was shown to correlate with proximity of the effective contact to the dentatorubrothalamic (DRT) tract entering the ventral lateral thalamus [14], and ineffective stimulation resulted from an electrode that missed this DTI-determined tract [11]. These findings are consistent with growing appreciation that better tremor results occur with implantation in regions that contain the cerebello-thalamic afferents, such as the caudal zona incerta, aka posterior subthalamic area [15]. In fact, DTI has advanced to the point that deterministic tract tracing may be utilized in individual patients to target fiber tracts, such as the DTR, in the absence of intraoperative mapping or testing [16]. Another useful approach is to identify the fiber pathways surrounding target nuclear structures and thus constrain and thereby identify the target itself, as was retrospectively done in 4 patients who had undergone STN or GPi DBS [17].
Another way in which DTI will allow better targeting is by using projection regions to define anatomical subregions of potential DBS targets, i.e. by defining the ‘connectome’ of the subcortical target [11], as has been done with the human thalamus to define Vim, for example [6]. A note of caution with this technique is raised, however, by disparities in the parcellation thus derived compared to that from T1/T2 maps, which is based on tissue characteristics (in the same subject group) [6]. Fiber connectivity should indeed not be expected to precisely mirror anatomical boundaries in all cases. The connectome of the most effective Vim DBS electrode contact has also been analyzed. Whereas one report of 12 patients showed, as expected, higher connectivity to the primary motor cortex, another report of 6 patients surprisingly showed higher connectivity to the premotor cortex [18].
The three subregions of the STN (limbic, associative, motor) were also identified by probabilistic DTI originating from the associated projection regions [19]. This approach may allow better MRI-based targeting of the STN and potentially obviate microelectrode recording, but remains to be accomplished on an individual patient. However, high field strength MRI does allow the delineation of functional and anatomical connections, including of the basal ganglia and thalamus, in individual human subjects [8], but is only available as a research tool in few centers, and has yet to be used prospectively for a DBS implantation.
We have used a probabilistic approach to map the frontal and subcortical projection regions in our effective DBS contacts in the subgenual cingulate region for depression [20], but more recently have been using deterministic DTI of those projections for targeting in individual patients (Figure 2) [20, 21].
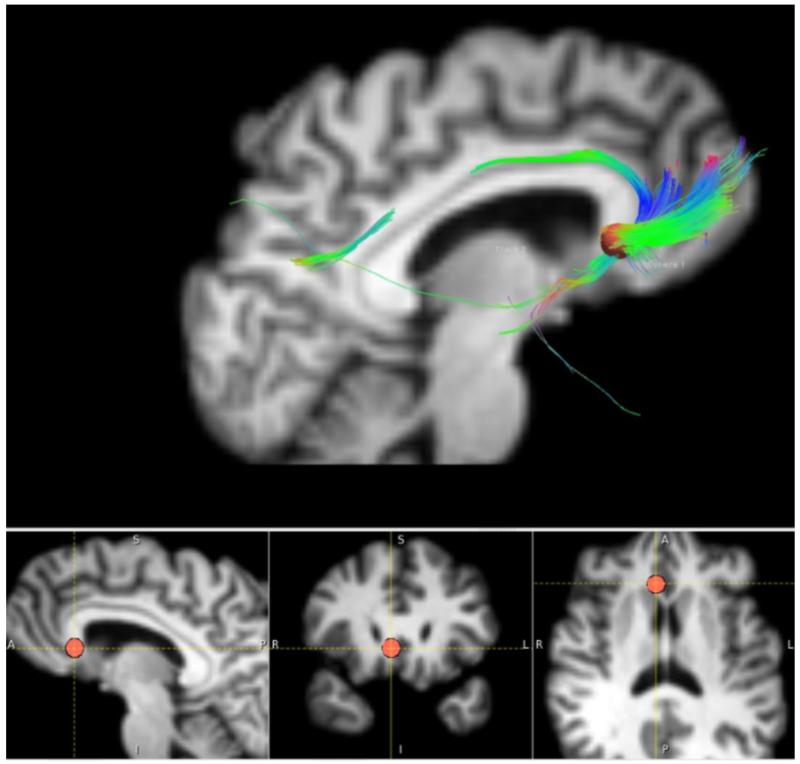
Fig. 2.
Deterministic diffusion tensor imaging (DTI) used in an individual patient undergoing deep brain stimulation of the subgenual cingulate cortex (SCC) for treatment-resistant major depressive disorder. The DBS site chosen is depicted by the red dot (bottom images), at the nexus of fibers projecting to the ventromedial orbitofrontal cortex, the cingulate bundle, and the nucleus accumbens. (Images courtesy of Helen Mayberg and Patrico Riva Posse, Emory University).
DBS implantation in the interventional/intraoperative MRI scanner
The ability to directly and reliably identify DBS targets in the MRI has been inexorably leading to direct targeting, without neurophysiological or clinical feedback, and in turn, to completing the entire implantation within the MRI environment. This approach offers the advantages of: 1) targeting following the making of the burr hole and durotomy, after most or all of the brain shift has occurred; 2) elimination of microelectrode recording (MER) and therefore, in some instances, requiring fewer brain penetrations; 3) 3D anatomical radiologic control of DBS position prior to closure, when the electrode can be repositioned if necessary; 4) performing the procedure under general anesthesia which is better tolerated by patients; 5) potentially shorter procedure times, and 6) real-time monitoring for hemorrhagic complications [22]. The initial approach involved adaption of a skull-mounted platform originally designed for frameless implantation with optical tracking, for the MRI environment (NexFrame® MR, Medtronic) [23]. In 29 patients, 87% of 53 electrodes were implanted in STN in a single pass, with greater accuracy as compared to frame-based techniques, with no hematomas and comparable clinical outcomes. In 17 PD patients bilaterally implanted in STN, the results (49.4% decrease in the UPDRS motor scale at 6 months) were comparable to patients implanted with standard techniques. Recently, this group led the development of a targeting platform specifically designed for MRI use, including the necessary dedicated software (ClearPoint®, MRI Interventions) [24]. Targeting experiments in cadavers showed improved accuracy compared to the NexFrame MR. In addition, MRI-based targeting is easily and advantageously adapted for use in future applications such as gene- [25] and cell-based [26] approaches.
We have found the ClearPoint MRI approach to be very effective, particularly in GPi DBS implantations (Figure 3). GPi is larger than STN and more difficult to map in the operating room, requiring, in our experience, a greater number of microelectrode passes. Moreover, for dystonia patients in particular, frame-based approaches can be problematic, general anesthesia is often required, and it is not possible to observe clinical benefits from stimulation intraoperatively. In contrast, GPi is relatively easier to see in the MRI than is STN, with our particular MRI scanner. Using the ClearPoint system, we have implanted 37 electrodes in 28 patients: 29 in GPi, 6 in STN and 2 in centromedian/parafasiciular (CMPF). Bilateral DBS leads were implanted in one session in 9 patients and the remaining 19 patients underwent unilateral implantation, in whom 7 leads were contralateral to a previously implanted lead. Five DBS leads were repositioned in the MRI scanner, and 3 were reimplantations of previously removed DBS leads. Sixteen patients had PD (15 GPi leads; 6 STN leads), 11 patients had dystonia (all GPi leads) and 1 patient had Tourette Syndrome (bilateral CMPf leads). In our early experience, post-operative lead locations and outcomes appear to be satisfactory.
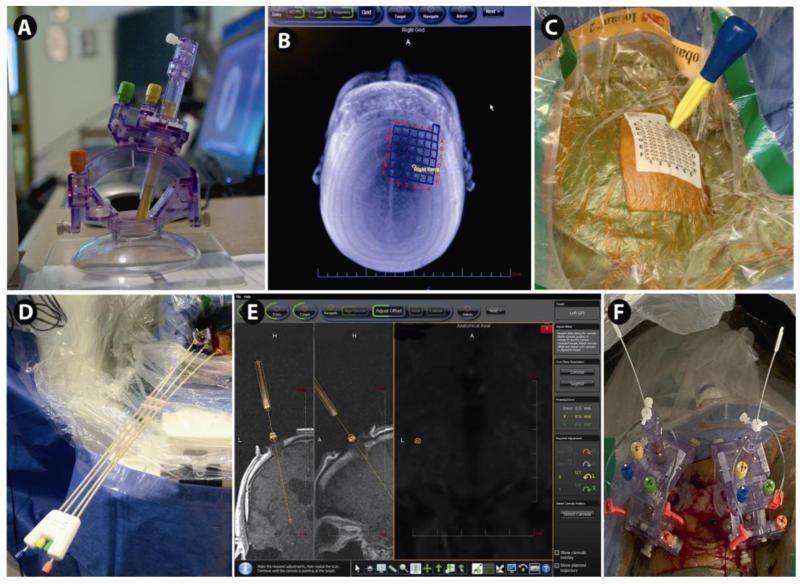
Fig. 3.
DBS implantation by direct targeting technique in the intraoperative or interventional MRI scanner: Implantation of globus pallidus internus (GPi) DBS lead(s) using the ClearPoint® SMARTframe (MRI Interventions, Irvine, CA). The skull-mounted targeting cannula is shown in A. Adjustments of pitch/roll and X/Y movements are driven by gears (colored knobs). The entry is determined with respect to a fiducial grid (B, C), and the software determines the point on the grid through which to mark the bone (C). Iterative adjustments are made to the pitch/roll and X/Y using the controller (D) attached to the SMARTFrame, as determined by the software, to align the cannula with the target (E). After final alignment to submillimeter accuracy, an MRI-compatible (i.e. minimal artifact and approved for use in MRI) ceramic stylet is inserted to target through a peel-away catheter. After MR-confirmation of accurate targeting, the stylet is replaced with the DBS lead(s) (F), followed in some centers by a final MRI demonstrating accurate DBS lead position, and finally removal of the peel-away catheter.
In my view, the major advantage of performing the DBS procedure in the MRI scanner is immediate and definitive intraoperative radiological control for the lead implantation. ClearPoint can be used in the interventional MRI (i.e. the diagnostic scanner suite turned into an operating room) or in the operating room using the intraoperative MRI (e.g. IMRIS®). Another means for immediate intraoperative radiological control is the intraoperative CT (iCT) scanner, requiring the additional step of co-registration of the post-implantation iCT to the pre-operative MRI scan [27]. The O-arm® (Medtronic, Minneapolis, MN), which became available several years ago [28], is a flat-panel cone-beam CT unit that can perform both standard 2D-fluoroscopy as well as 3D-imaging. Although the image quality is less than traditional fan-beam CT, the bore is large enough to image the whole head and thus allow co-registration to other imaging sets such as preoperative MRI and/or traditional CT for 3D radiological intraoperative control (Figure 4). The most quantitative assessment of the accuracy of the O-arm was performed by Holloway and Docef (2013) who used it in conjunction with the NexFrame [29]. This modality is particularly useful in surgery with skull-mounted miniframes, where lack of a frame-based fiducial system precludes standard stereotactic 2D fluoroscopy. They found that the measurement accuracy for implanted objects was 0.72 ± 0.38 mm comparing the O-arm to post-op CT, demonstrating the adequacy of this tool for verification of DBS lead localization. Shahlaie et al. [30] found a 1.65 mm radial discrepancy between O-arm iCT and postop MRI location of the DBS leads, whereas Smith and Bakay [31] found a 1.5 mm discrepancy between the post-implant iCT and postop MRI. The latter two findings, being somewhat greater than that of Holloway and Docef [29], were perhaps due in part to the decreased accuracy of measuring DBS location on post-op MRI as compared to CT, resulting from larger DBS lead artifacts.
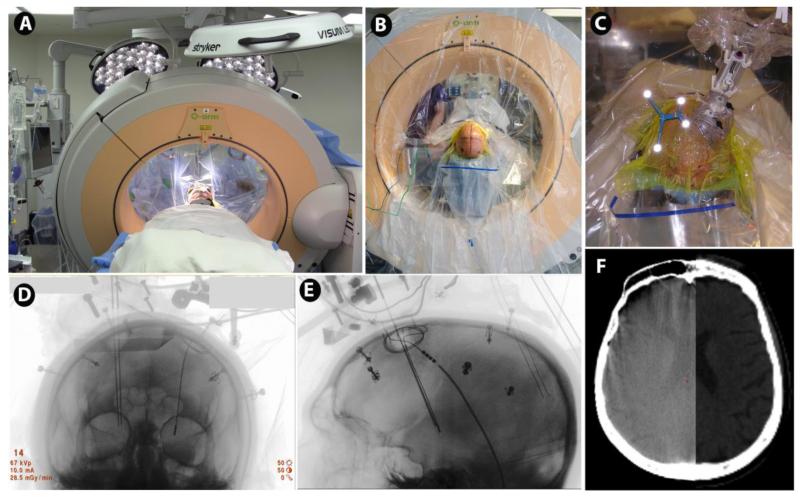
Fig. 4.
Stereotactic intraoperative CT scanning for 3D radiological control of DBS implantation. Patient positioned within the O-arm® (Medtronic) (A, B). The reference frame for ‘frameless’ navigation is shown (light reflecting off fiducials) to the left of the NexFrame®, a skull-mounted targeting frame with microelectrode inserted (C). The O-arm allows both anterior-posterior (D) and lateral (E) radiography that can be co-registered to the preoperative MRI or CT, using the Stealth® Framelink® navigation workstation (Medtronic). This may be used to track the accuracy of microelectrode insertions, as shown, contributing to the interpretation of neurophysiological recordings. The O-arm also allows 3D CT imaging (F, left), which can be co-registered to the pre-operative imaging (F, right), that can be used for both post-implantation DBS location verification, as well as for bone fiducial registration, allowing the entire procedure (beyond the preoperative MRI scan) to be completed in the operating room. (Images courtesy of Kathryn Holloway, Medical College of Virginia).
Holloway and Docef [29] further demonstrated the usefulness of ‘real-time’ 3D radiological feedback during microelectrode recording. They found that 1) the mean stereotactic error of the first microelectrode tracks was 2.12 ± 1.04 mm, and that 2) the error of subsequent, ostensibly parallel, tracks was 1.12 ± 0.74, ranging from 0.21 to 3.04 mm, with 22% of tracks having an error of >1.8 mm. The authors felt that incorporating the 3D information on actual microelectrode track location aided in their interpretation. Similarly, Smith and Bakay [31], using O-arm after each microelectrode repositioning with the NexFrame, noted several instances where the microelectrode did not move appropriately, possibly resulting from deflection or from the microelectrode going down the previous track.
It is interesting, as in these reports, to combine microelectrode recording with 3D radiological control. Smith and Bakay [31] found a 3mm discrepancy between their initial radiological target (which presumably would be the final target in solely MRI-based procedures) and their final DBS location based on MER (they accepted the latter location even if it was discrepant with the “direct” target selected on iCT/pre-op MRI co-registration). Which was the right target? The authors had good clinical results, but without a large comparison study the answer is difficult to know. Others have instead relocated leads when they did not appear in good position on O-arm iCT. Patil relocated 2 out of 13 leads, which were laterally misplaced, in 8 patients [32], and Shahlaie et al. repositioned 1 lead more laterally in 15 patients during frame-based DBS implantation [30].
A final advantage of the O-arm was shown by Holloway and Docef [29], who demonstrated that it could be used for bone fiducial registration used with the NexFrame as accurately as could preoperative CT scan, but with the advantage of being able to implant the fiducials in the operating room and obviating the need to go back to the CT- or MRI-scanner.
Recently, a true (fan-beam) portable CT (Ceretom®) has been used for 3D lead verification, and will likely increase in popularity due to its portability, size and lower price than the O-arm. Raslan et al. reported in abstract form the results of using iCT with frameless (NexFrame) implantation, without MER, in 51 patients (102 electrodes) with a radial error of 1.5 mm from preoperative MRI [33].
Some form of 3D radiological control coupled with improved preoperative imaging promises to lead to a proliferation of cases performed solely with direct targeting, under anesthesia, replacing microelectrode mapping. Is one radiological technique better than another? The iMRI offers the advantage of ‘one image set for one patient on one day’ (P. Larson, UCSF, personal communication). However, the availability of iMRI for many centers will be challenging, especially given the opportunity cost of occupying the MRI scanner for surgical cases in lieu of diagnostic cases. In contrast, while the iCT requires image fusion to the pre-operative MRI, it is an economical modality (<$600,000 depending on the particular instrument) and, moreover, can be combined for use both in the OR and as a portable CT scan for diagnostic cases. I suspect that iCT will proliferate faster than will the iMRI.
Advances in physiological characterization of the DBS target
Left unresolved, for the time being, is the question of which means of DBS targeting leads to better clinical outcomes – neurophysiology/clinical monitoring or direct targeting with radiological control and no monitoring – an issue that is especially important given the apparent discrepancy between the two [31]. Recent technological advances have been made in physiological identification of targets as well. Efforts have been going forward to automatically identify target regions during microelectrode mapping to remove ‘human error’, potentially improving outcomes and improving efficiency. One approach has been the use of Hidden Markov Models [34-36], which infers unknown (hidden) structures (e.g. STN) – so-called ‘states’ - from some known quantities referable to those states (e.g. MER signals). Retrospectively using patient MER data, detection sensitivities have been in the 86-95% range for detecting thalamus, ZI, STN and SNr, with recent refinements (semi-HMM) yielding >98% accuracy, 99.5% sensitivity and 96.5% specificity [34]. This approach has been coupled to intraoperative neuronavigation as well [35].
Another approach that has been used is ‘support vector machines’, a supervised machine learning approach, to identify the target based on 6 mathematical features characterizing the MER signal [37]. During MER for PD, the support vector machine was able to correctly identify 99.4% of neural recordings from the thalamus, zona incerta, STN and SNr. It is nevertheless unclear whether these and other new approaches to physiological monitoring will improve effectiveness and/or safety of what is already a very well-established methodology [38].
ADVANCES IN IMPLANTABLE PULSE GENERATORS AND PROGRAMMING
New implantable pulse generators: Constant current stimulation
The approved pulse generators for DBS had, until recently, utilized only constant voltage. However, capacitative current is the determinant of downstream neuronal effects, whereby the current density acting across resistive elements in the tissue establishes an extracellular voltage gradient that alters neuronal excitability. In a constant voltage device, current is determined by impedance of the tissue and electrode-tissue interface, which theoretically can be temporally variable. Thus, a constant current device automatically accommodates for impedance changes and might thereby improve effectiveness or tolerability. However, exactly how tissue impedance varies temporally in patients is unknown. Lempka et al. [39] showed impedance increases over days after implantation of an appropriately scaled DBS electrode in rhesus macaques, which may accord with clinicians’ preference to delay programming for up to a month following implantation when using constant voltage devices. Conversely, Sillay et al [40], examining impedances of the Responsive Neurostimulation System (RNS®, NeuroPace), saw little intra-but large inter-patient variability in a patient cohort over longer periods of time. Interestingly, Lempka et al. also observed rapid (over ~30 minutes) decreases in impedance upon activation of stimulation, possibly via alteration of the electrode-tissue interface [41]. Thus, it seems reasonable to posit that a constant current device would be useful in the short term for earlier programming initiation, and that it may decrease the time needed for programming by eliminating the effects of progressive decrease in impedance (and resulting increased VTA) over the beginning of an initial programming session. Okun et al. [42] reported the results of a randomized sham-stimulation controlled (but not blinded) trial of STN DBS using a constant current device in PD, with outcomes on the UPDRS motor score (39% improvement) comparable to previous randomized controlled trials (25 – 46% improvements) [43-46]. Programming factors were not reported, nor was there a comparison to a voltage-controlled device. Constant current devices are now widely available.
DBS programming tools
Present pulse generators offer the possibility of stimulation via one or more contacts (as cathodes) in so-called monopolar mode (IPG case as return electrode), or bipolar with one (or more electrodes) as cathode or anode. Recent studies have characterized the fields of activation associated with different electrode configurations. Realistic computational modeling studies, examining VTA with realistic anatomical configurations of nuclei and fiber pathways based on DTI (see above), have advanced the understanding of how different electrode activation configurations can influence benefits and adverse effects [12]. These studies show that a priori knowledge of the exact anatomical location and orientation of DBS electrodes can inform a more effective and efficient programming, and have led to software that can aid the clinician in utilizing this knowledge (www.CiceroneDBS.org) [47, 48]. This type of computer analysis might also be used to plan the electrode location and orientation [49].
Current Steering
Constant current stimulation offers the possibility of ‘current steering’ by independently setting each electrode to different currents, or ‘multi-source stimulation’. Computer modeling studies have shown that this approach can achieve greater activation of target neuron fibers with less activation of off-target fibers such as the internal capsule [50]. The Vercise® DBS system (Boston Scientific), which is the first system to offer this capability, has received CE Mark approval in Europe, but no studies of the benefits of this approach in clinical practice are yet available. Carried still further, testing was performed in the non-human primate with a DBS electrode array comprising 16 rows of 4 disc-shaped contacts facing each cardinal direction, where each contact is individually programmable (Sapiens Steering Brain Stimulation, BV, Netherlands), to achieve even greater increases in the therapeutic window [51]. ‘Directionally-segmented’ electrodes, a similar approach, were computationally modeled, based on actual DBS electrodes implanted in 3 patients for ET. The modeling suggested that current-steering would improve benefits and minimize paresthesias in these patients, especially with a misplaced electrode[52].
Novel stimulation waveforms
At present, available implanted pulse generators produce charge-balanced bipolar square-waves pulses with a regular, non-adjustable pattern, with only amplitude, frequency, and pulse width being adjustable. Recent research suggests, however, that alternative patterns might improve effectiveness and/or efficiency. With our collaborators, we recently compared the effects of four novel, irregular patterns to the standard regular pattern on bradykinesia measured by finger-tapping in awake patients during replacement of the IPG [53]. All patterns were 185 Hz overall, but the irregular patterns had unique temporal characteristics. Three of the 4 irregular patterns suppressed bradykinesia more effectively than did regular stimulation, and the effectiveness of each pattern correlated with its ability to suppress beta-band pathological oscillatory activity in a biophysical computational model of STN DBS actions on GPi activity. Another study showed that lower frequency stimulation (80 Hz) in a burst-pattern was as effective on bradykinesia as regular 135 Hz stimulation in the non-human MPTP parkinson model [54]. It remains to be determined if the irregular stimulation patterns are more effective than regular trains on other parkinsonian features, suggested by the magnitude of change on the motor UPDRS predicted by our results (12-15 points) [53]. Notably, previous attempts to use irregular patterns on tremor (in ET as well as PD) [55, 56] failed to show benefits, likely due to pauses that allowed pathological activity to propagate through the network [57]. The effects of varying stimulation patterns will need to be evaluated on each of the features of PD, as well as other movement disorders.
Closed-loop or adaptive stimulation approaches
Movement disorder symptoms are dynamic with time scales varying from the sub-second range as in tremor to hours or days as in motor fluctuations in PD. Standard DBS stimulation parameters, however, are adjusted over time scales of weeks (initially) to months, with more frequent parameter adjustments correlating with improved outcomes [47]. Bi-directional brain-machine interfaces, which are capable of ‘sensing’ in addition to stimulation, allow for adaptive, or closed-loop – feedback control of stimulation parameters in response to ongoing changes in brain state or other physiological or behavioral parameters [58, 59]. This adaptive approach, at least theoretically, may be more effective and more efficient than the standard unidirectional open-loop approach, leading to improved outcomes and less frequent generator changes or recharging. Moreover, adaptive systems may mitigate demands on clinical resources presently required during manual programming.
Rosin et al. [60] utilized a closed-loop adaptive algorithm in the African green monkey MPTP model. They recorded single-unit and local field potentials from M1 cortex, and used the detection of an action potential in M1 (the control signal or biomarker) to provoke a train of 7 pulses at 130Hz delivered into GPi. Strikingly, this algorithm was more effective than continuous open-loop GPi DBS in improving movement and correspondingly pathological oscillatory activity, despite an overall frequency of stimulation that was only 30 Hz and highly irregular. A similarly irregular, low frequency pattern in a non-adaptive design was ineffective. The African green monkey model used manifests a great degree of tremor, and the benefits on ‘kinesis’ derived to a large degree from amelioration of tremor, and correspondingly the oscillatory activity mitigated was in the tremor (4 – 7 Hz) and ‘double-tremor’ (9 – 15 Hz) bands, not in the beta range known to be associated with bradykinesia. The utility of closed-loop vs. open-loop stimulation on other PD features, including kinesis unrelated to tremor, the appropriate control signal(s) for those symptoms, and the relationship to changes in beta oscillatory activity remain to be determined [59].
The only pulse generators presently approved in the U.S. or Europe for the treatment of movement disorders are open-loop devices. Recently, the Responsive Neurostimulation System (briefly discussed above), a closed-loop device designed for the treatment of partial onset epilepsy, has been shown to decrease seizure frequency in a controlled clinical trial [61]. This cranially-implanted device is capable of acquiring a continuous electrocorticogram (ECoG), which is analyzed in a customizable way in real-time for evidence of seizure activity (e.g. increase in ‘line-length’ of the ECoG tracing), which in turn triggers customizable stimulation for the abrogation of seizure activity. Okun et al. [62] were the first to use this device for a movement disorder, in this case bilateral centromedian (CM) stimulation in 5 patients with Tourette Syndrome. The pilot clinical trial did not use a closed-loop approach, as the proper control signal has not yet been identified. Rather, a scheduled stimulation regime (2 seconds on/2 seconds off in 2 subjects; 16 seconds on/120 seconds off in 2; and 10 seconds on/10 seconds off in 1) was used, to conserve battery life in the context of a paroxysmal movement disorder and with a device with a limited duty cycle. Statistically significant improvements (although <50%) were observed in the Yale Global Tic Severity Score (YGTSS) from this regime. Perhaps more importantly, the bi-directional capability of the RNS device was capitalized on to collect LFPs from the CM target before and after stimulation, acutely and – for the first time – chronically, in patients with a movement disorder [63]. LFP recordings showed increased power in the gamma range months after the onset of stimulation, the magnitude of which correlated with improvements in the YGTSS; decreased theta power was also seen in the two best responders. This paradigm will serve as a model for similar studies in other movement disorders, in particular PD, where pathological beta oscillations inversely correlate with motor performance.
Such studies will be markedly facilitated by the availability for clinical research studies of another bi-directional implantable pulse generator, constructed by modification of the presently approved/marketed ActivaPC™ DBS system (Medtronic) with a novel neural recording and processing subsystem [64]. The new neural interface device offers the capability to record 4 channels of ECoG/LFP from the implanted leads; contains a three-axis accelerometer for non-neural feedback using motor activity; and provides algorithmic processing and telemetry for data uploading. Various criteria were used in the design, including the ability to record LFPs over a wide range of frequencies and voltages (e.g. 1μV signals such as beta oscillations in PD to >100 μV signals during epileptic seizures), as well as not increasing power usage over that required for standard stimulation by more than 10%. The accelerometer allows quantification of posture, activity, and axial tremor. The system, being made available for research applications in patients, will enable identification of biomarker states that can guide neuromodulation, both as a clinical outcome parameter to guide programming (e.g. decrease in beta power in PD), and conceivably in an automated, closed-loop application. The bi-directional neural interface system has been used to record μV signals from the non-human primate motor cortex to direct cursor movement on a computer screen in an established brain-machine interface application [64]. Proof-of-principle of an automated closed-loop application was shown in a sheep model where sensing of seizure-related activity in a hippocampal electrode was used to shut down stimulation of an anterior thalamic electrode [65, 66].
Electrochemical detection for closed-loop neuromodulation
Technology developed over the last few years may enable the sensing of neurotransmitter concentrations for adaptive control of DBS. The Wireless Instantaneous Neurochemical Concentration Sensor (WINCS) uses fast scan cyclic voltammetry (FSCV) and amperometry for nearly real-time (millisecond) detection of dopamine, serotonin and adenosine, as well as glutamate and other neurotransmitters [67]. Its use has been demonstrated in animal models, and recently in 8 patients undergoing Vim DBS to demonstrate increased adenosine concentrations after DBS insertion [68]. Carbon fiber electrodes (~5μm diameter) are presently used, but carbon nanofiber nanoelectrode arrays may allow larger regions to be sampled [69]. Clinical applications are still some time away, however.
ADVANCES IN NEUROABLATION
DBS has all but replaced ablative surgery. The epitome of an open-loop approach, neuroablation has nevertheless remained a legitimate and effective treatment for PD, ET and dystonia [70]. Radiofrequency thermocoagulation is the predominant means for creating lesions at present, but radiosurgical (gamma ray) induced necrosis – e.g. gamma thalamotomy – remains a viable, minimally invasive option for tremor, especially in older patients [71, 72]. However, new technology is becoming available for thermocoagulative surgery. Recently, transcranial MRI-guided focused ultrasound (tMRgFUS, or FUS), using a hemispheric, 1024-element phased array 650 Hz transducer (ExAblate®, Insightec, LtD), has been pioneered for the creation of thermal sonication lesions, using MRI thermometry to provide ‘closed-loop’ control of local temperatures [73, 74]. The feasibility of this technology for functional procedures was demonstrated in 9 patients who underwent FUS – induced medial thalamotomy (central lateral nucleus) for chronic pain [75]. Safety and effectiveness is insured by the ability, as with RF lesions, to produce sub-threshold lower temperature ‘temporary’ lesions in awake patients to assess for effects (therapeutic and adverse). A permanent 3 – 5 mm sonication lesion is produced by iterative 10-20 second lesions up to a peak temperature of 51 - 60°C, with temperature control provided by real-time MRI thermometry. As with gamma thalamotomy, the procedure is non-invasive, but in contrast the effects are immediate, allowing for more controlled evaluation for benefits and adverse effects (although at present the entire head must be shaved for this procedure). A proof-of-concept report of 4 patients was recently published, demonstrating 81% improvement in tremor at 3 months in the treated hand, with one patient experiencing persistent parasthesia in the hand [76]. An FDA-approved Phase 1 study of FUS for essential tremor is underway, with other trials for PD anticipated (W. J. Elias, personal communication)[74]. As with RF or gamma thalamotomy or pallidotomy, only unilateral lesions will be considered safe with this technique.
CONCLUSION: What does the future look like?
MRI technology is only going to improve, as will our understanding of the anatomical appearance of the appropriate targets for DBS. Hence, the need for physiologically characterization of the DBS target – which is essentially a surrogate for the anatomical site – is expected to wane over the next decade. Anatomical targeting will take into account patient-specific variance, including the shape as well as connectivity of their DBS target, which will likely be white matter in nature to maximize network effects. The orientation of the implantation will consider the VTA desired, planned ahead of time. Patients will be able to be implanted while asleep, knowing that they will wake up with a confirmed, well-placed DBS lead, with an even smaller chance for a complication than they face today. Within the next few years, implanted electrodes will offer finer-grained options, from a greater number of smaller contacts in the context of implantable pulse generators that offer flexibility with respect to current steering, to any array of contacts, as well as customizable stimulation patterns. The programming of such a complex system will naturally need to be managed by new software tools available to clinicians to aid in the programming. Indeed, much of this will occur automatically by bi-directional closed-loop generators, which can determine the appropriate electrodes based on the recording of established biomarkers. Clinician burden will be mitigated by downloading this information from the internet in a telemedicine paradigm, as in the RNS device. Many of these changes will improve individual and group outcomes, while decreasing energy demand from the devices, which in the context of improved batteries and recharging capability, will virtually eliminate the need for frequent pulse generator replacements. The first generation of closed-loop devices are already available for research, including in movement disorders (Medtronic), and will likely be approved by the FDA in the near future for epilepsy (RNS, NeuroPace). Will all of this be moot, as new medicines, gene-based therapy, cell-based therapy or optogenetic therapy replace the need for electrical neuromodulation? The time horizon for those advances keeps receding like a mirage. DBS neuromodulation, in our opinion, has a comfortable period remaining to have the stage essentially to itself.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Robert E. Gross declares board membership for NeuroPace, consultancy for Medtronic, Boston Scientific, St. Judes Medical Corp., Deep Brain Innovations, and Visualase, as well as speakers’ bureaus for Visualase and NeuroPace.
Margaret E. McDougal declares no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Clarke RH, Horsley V. On a method of investigating the deep ganglia and tracts of the central nervous system (cerebellum) Br Med J. 1906;2:1799–800. doi: 10.1097/BLO.0b013e31814d4d99. [DOI] [PubMed] [Google Scholar]
- 2.Spiegel EA, Wycis HT, Marks M, Lee AJ. Stereotaxic Apparatus for Operations on the Human Brain. Science. 1947;106(2754):349–50. doi: 10.1126/science.106.2754.349. doi:10.1126/science.106.2754.349. [DOI] [PubMed] [Google Scholar]
- 3.Kerl HU, Gerigk L, Pechlivanis I, Al-Zghloul M, Groden C, Nolte I. The subthalamic nucleus at 3.0 Tesla: choice of optimal sequence and orientation for deep brain stimulation using a standard installation protocol: clinical article. Journal of neurosurgery. 2012;117(6):1155–65. doi: 10.3171/2012.8.JNS111930. doi:10.3171/2012.8.jns111930. [DOI] [PubMed] [Google Scholar]
- 4.Nolte IS, Gerigk L, Al-Zghloul M, Groden C, Kerl HU. Visualization of the internal globus pallidus: sequence and orientation for deep brain stimulation using a standard installation protocol at 3.0 Tesla. Acta Neurochir (Wien) 2012;154(3):481–94. doi: 10.1007/s00701-011-1242-8. doi:10.1007/s00701-011-1242-8. [DOI] [PubMed] [Google Scholar]
- 5•.Liu T, Eskreis-Winkler S, Schweitzer A, Chen W, Kaplitt M, Tsiouris A, et al. Improved Subthalamic Nucleus Depiction with Quantitative Susceptibility Mapping Radiology. 2013 doi: 10.1148/radiol.13121991. Forthcoming. This report (and others by this group) show remarkable depictions of both STN and GPi targets using quantitative susceptibility mapping, a technique which will gain more widespread availability in the coming year or two.
- 6.Traynor CR, Barker GJ, Crum WR, Williams SC, Richardson MP. Segmentation of the thalamus in MRI based on T1 and T2. NeuroImage. 2011;56(3):939–50. doi: 10.1016/j.neuroimage.2011.01.083. doi:10.1016/j.neuroimage.2011.01.083. [DOI] [PubMed] [Google Scholar]
- 7•.Abosch A, Yacoub E, Ugurbil K, Harel N. An assessment of current brain targets for deep brain stimulation surgery with susceptibility-weighted imaging at 7 tesla. Neurosurgery. 2010;67(6):1745–56. doi: 10.1227/NEU.0b013e3181f74105. discussion 56. doi:10.1227/NEU.0b013e3181f74105. Demonstration of the power of high field strength MRI: remarkable definition of basal ganglia including STN and GPi including internal architecture, and demonstration of individual nuclei (e.g. Vim) within the thalamus in individual human subjects.
- 8.Lenglet C, Abosch A, Yacoub E, De Martino F, Sapiro G, Harel N. Comprehensive in vivo mapping of the human basal ganglia and thalamic connectome in individuals using 7T MRI. PloS one. 2012;7(1):e29153. doi: 10.1371/journal.pone.0029153. doi:10.1371/journal.pone.0029153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duchin Y, Abosch A, Yacoub E, Sapiro G, Harel N. Feasibility of using ultra-high field (7 T) MRI for clinical surgical targeting. PloS one. 2012;7(5):e37328. doi: 10.1371/journal.pone.0037328. doi:10.1371/journal.pone.0037328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deniau JM, Degos B, Bosch C, Maurice N. Deep brain stimulation mechanisms: beyond the concept of local functional inhibition. The European journal of neuroscience. 2010;32(7):1080–91. doi: 10.1111/j.1460-9568.2010.07413.x. doi:10.1111/j.1460-9568.2010.07413.x. [DOI] [PubMed] [Google Scholar]
- 11.Henderson JM. “Connectomic surgery”: Diffusion tensor imaging (DTI) tractography as a targeting modality for surgical modulation of neural networks. Frontiers in Integrative Neuroscience. 2012;6 doi: 10.3389/fnint.2012.00015. doi:10.3389/fnint.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miocinovic S, Parent M, Butson CR, Hahn PJ, Russo GS, Vitek JL, et al. Computational analysis of subthalamic nucleus and lenticular fasciculus activation during therapeutic deep brain stimulation. Journal of neurophysiology. 2006;96(3):1569–80. doi: 10.1152/jn.00305.2006. doi:10.1152/jn.00305.2006. [DOI] [PubMed] [Google Scholar]
- 13.Butson CR, Cooper SE, Henderson JM, Wolgamuth B, McIntyre CC. Probabilistic analysis of activation volumes generated during deep brain stimulation. NeuroImage. 2011;54(3):2096–104. doi: 10.1016/j.neuroimage.2010.10.059. doi:10.1016/j.neuroimage.2010.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coenen VA, Madler B, Schiffbauer H, Urbach H, Allert N. Individual fiber anatomy of the subthalamic region revealed with diffusion tensor imaging: a concept to identify the deep brain stimulation target for tremor suppression. Neurosurgery. 2011;68(4):1069–75. doi: 10.1227/NEU.0b013e31820a1a20. discussion 75-6. doi:10.1227/NEU.0b013e31820a1a20. [DOI] [PubMed] [Google Scholar]
- 15.Sandvik U, Koskinen LO, Lundquist A, Blomstedt P. Thalamic and subthalamic deep brain stimulation for essential tremor: where is the optimal target? Neurosurgery. 2012;70(4):840–5. doi: 10.1227/NEU.0b013e318236a809. discussion 5-6. doi:10.1227/NEU.0b013e318236a809. [DOI] [PubMed] [Google Scholar]
- 16.Coenen V, Allert N, Mädler B. A role of diffusion tensor imaging fiber tracking in deep brain stimulation surgery: DBS of the dentato-rubro-thalamic tract (drt) for the treatment of therapy-refractory tremor. Acta Neurochirurgica. 2011;153(8):1579–85. doi: 10.1007/s00701-011-1036-z. doi:10.1007/s00701-011-1036-z. [DOI] [PubMed] [Google Scholar]
- 17.Sedrak M, Gorgulho A, Bari A, Behnke E, Frew A, Gevorkyan I, et al. Diffusion tensor imaging (DTI) and colored fractional anisotropy (FA) mapping of the subthalamic nucleus (STN) and the globus pallidus interna (GPi) Acta Neurochir (Wien) 2010;152(12):2079–84. doi: 10.1007/s00701-010-0813-4. doi:10.1007/s00701-010-0813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pouratian N, Zheng Z, Bari AA, Behnke E, Elias WJ, Desalles AA. Multi-institutional evaluation of deep brain stimulation targeting using probabilistic connectivity-based thalamic segmentation. Journal of neurosurgery. 2011;115(5):995–1004. doi: 10.3171/2011.7.JNS11250. doi:10.3171/2011.7.jns11250. [DOI] [PubMed] [Google Scholar]
- 19.Lambert C, Zrinzo L, Nagy Z, Lutti A, Hariz M, Foltynie T, et al. Confirmation of functional zones within the human subthalamic nucleus: patterns of connectivity and sub-parcellation using diffusion weighted imaging. NeuroImage. 2012;60(1):83–94. doi: 10.1016/j.neuroimage.2011.11.082. doi:10.1016/j.neuroimage.2011.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutman DA, Holtzheimer PE, Behrens TE, Johansen-Berg H, Mayberg HS. A tractography analysis of two deep brain stimulation white matter targets for depression. Biological psychiatry. 2009;65(4):276–82. doi: 10.1016/j.biopsych.2008.09.021. doi:10.1016/j.biopsych.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cerebral cortex. 2008;18(6):1374–83. doi: 10.1093/cercor/bhm167. doi:10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin AJ, Larson PS, Ostrem JL, Keith Sootsman W, Talke P, Weber OM, et al. Placement of deep brain stimulator electrodes using real-time high-field interventional magnetic resonance imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2005;54(5):1107–14. doi: 10.1002/mrm.20675. doi:10.1002/mrm.20675. [DOI] [PubMed] [Google Scholar]
- 23••.Starr PA, Martin AJ, Ostrem JL, Talke P, Levesque N, Larson PS. Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: technique and application accuracy. Journal of neurosurgery. 2010;112(3):479–90. doi: 10.3171/2009.6.JNS081161. doi:10.3171/2009.6.jns081161. Series of 53 STN DBS implanted in 29 patients using an adaptation for MRI-guidance of a skull mounted miniframe. 87% implanted in single pass with greater accuracy than frame-based procedures, no hematomas and comparable clinical outcomes. Set the stage for next reference describing development of a novel system for use in MRI-based DBS implantation (Larson et al., 2012).
- 24.Larson PS, Starr PA, Bates G, Tansey L, Richardson RM, Martin AJ. An optimized system for interventional magnetic resonance imaging-guided stereotactic surgery: preliminary evaluation of targeting accuracy. Neurosurgery. 2012;70(1 Suppl Operative):95–103. doi: 10.1227/NEU.0b013e31822f4a91. discussion doi:10.1227/NEU.0b013e31822f4a91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson RM, Varenika V, Forsayeth JR, Bankiewicz KS. Future applications: gene therapy. Neurosurg Clin N Am. 2009;20(2):205–10. doi: 10.1016/j.nec.2009.04.004. doi:10.1016/j.nec.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roskom J, Swistowski A, Zeng X, Lim DA. Future directions: use of interventional MRI for cell-based therapy of Parkinson disease. Neurosurg Clin N Am. 2009;20(2):211–8. doi: 10.1016/j.nec.2009.04.005. doi:10.1016/j.nec.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Fiegele T, Feuchtner G, Sohm F, Bauer R, Anton JV, Gotwald T, et al. Accuracy of stereotactic electrode placement in deep brain stimulation by intraoperative computed tomography. Parkinsonism & related disorders. 2008;14(8):595–9. doi: 10.1016/j.parkreldis.2008.01.008. doi:10.1016/j.parkreldis.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Caire F, Gantois C, Torny F, Ranoux D, Maubon A, Moreau JJ. Intraoperative use of the Medtronic O-arm for deep brain stimulation procedures. Stereotactic and functional neurosurgery. 2010;88(2):109–14. doi: 10.1159/000280823. doi:10.1159/000280823. [DOI] [PubMed] [Google Scholar]
- 29•.Holloway K, Docef A. A quantitative assessment of the accuracy and reliability of O-arm images for deep brain stimulation surgery. Neurosurgery. 2013;72(1 Suppl Operative):47–57. doi: 10.1227/NEU.0b013e318273a090. doi:10.1227/NEU.0b013e318273a090. Quantitative analysis of the accuracy of the O-arm®, a cone-beamed portable CT scanner for intraoperative 3D imaging, in DBS location verification, tracking of serial microelectrode trajectories, and fiducial registration.
- 30.Shahlaie K, Larson PS, Starr PA. Intraoperative computed tomography for deep brain stimulation surgery: technique and accuracy assessment. Neurosurgery. 2011;68(1 Suppl Operative):114–24. doi: 10.1227/NEU.0b013e31820781bc. discussion 24. doi:10.1227/NEU.0b013e31820781bc. [DOI] [PubMed] [Google Scholar]
- 31.Smith AP, Bakay RA. Frameless deep brain stimulation using intraoperative O-arm technology. Clinical article. Journal of neurosurgery. 2011;115(2):301–9. doi: 10.3171/2011.3.JNS101642. doi:10.3171/2011.3.jns101642. [DOI] [PubMed] [Google Scholar]
- 32.Patil AA. Intraoperative image fusion to ascertain adequate lead placement. Stereotactic and functional neurosurgery. 2011;89(4):197–200. doi: 10.1159/000327030. doi:10.1159/000327030. [DOI] [PubMed] [Google Scholar]
- 33.Raslan AM, Burchiel KJ, Griffith SE, Anderson VC, editors. Accuracy of DBS electrode placement using NexFrame and the Ceretom intraoperative CT Scanner; CNS 2012 Annual Meeting; Chicago, IL. 2012. [Google Scholar]
- 34.Taghva A. Hidden semi-Markov models in the computerized decoding of microelectrode recording data for deep brain stimulator placement. World neurosurgery. 2011;75(5-6):758–63 e4. doi: 10.1016/j.wneu.2010.11.008. doi:10.1016/j.wneu.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Taghva A. An automated navigation system for deep brain stimulator placement using hidden Markov models. Neurosurgery. 2010;66(3 Suppl Operative):108–17. doi: 10.1227/01.NEU.0000365369.48392.E8. discussion 17. doi:10.1227/01.NEU.0000365369.48392.E8. [DOI] [PubMed] [Google Scholar]
- 36.Zaidel A, Spivak A, Shpigelman L, Bergman H, Israel Z. Delimiting subterritories of the human subthalamic nucleus by means of microelectrode recordings and a Hidden Markov Model. Movement disorders : official journal of the Movement Disorder Society. 2009;24(12):1785–93. doi: 10.1002/mds.22674. doi:10.1002/mds.22674. [DOI] [PubMed] [Google Scholar]
- 37.Guillen P, Martinez-de-Pison F, Sanchez R, Argaez M, Velazquez L. Characterization of subcortical structures during deep brain stimulation utilizing support vector machines; Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference; 2011; 2011. pp. 7949–52. doi:10.1109/iembs.2011.6091960. [DOI] [PubMed] [Google Scholar]
- 38.Alegre M, Hallett M, Olanow CW, Obeso JA. Technical advances in deep brain stimulation: how far is enough? Movement disorders: official journal of the Movement Disorder Society. 2012;27(3):341–2. doi: 10.1002/mds.24965. doi:10.1002/mds.24965. [DOI] [PubMed] [Google Scholar]
- 39.Lempka SF, Miocinovic S, Johnson MD, Vitek JL, McIntyre CC. In vivo impedance spectroscopy of deep brain stimulation electrodes. Journal of neural engineering. 2009;6(4):046001. doi: 10.1088/1741-2560/6/4/046001. doi:10.1088/1741-2560/6/4/046001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sillay KA, Chen JC, Montgomery EB. Long-term measurement of therapeutic electrode impedance in deep brain stimulation. Neuromodulation. 2010;13(3):195–200. doi: 10.1111/j.1525-1403.2010.00275.x. doi:10.1111/j.1525-1403.2010.00275.x. [DOI] [PubMed] [Google Scholar]
- 41.Lempka SF, Johnson MD, Miocinovic S, Vitek JL, McIntyre CC. Current-controlled deep brain stimulation reduces in vivo voltage fluctuations observed during voltage-controlled stimulation. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2010;121(12):2128–33. doi: 10.1016/j.clinph.2010.04.026. doi:10.1016/j.clinph.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okun MS, Gallo BV, Mandybur G, Jagid J, Foote KD, Revilla FJ, et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: an open-label randomised controlled trial. Lancet neurology. 2012;11(2):140–9. doi: 10.1016/S1474-4422(11)70308-8. doi:10.1016/s1474-4422(11)70308-8. [DOI] [PubMed] [Google Scholar]
- 43.Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet neurology. 2010;9(6):581–91. doi: 10.1016/S1474-4422(10)70093-4. doi:10.1016/S1474-4422(10)70093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odekerken VJ, van Laar T, Staal MJ, Mosch A, Hoffmann CF, Nijssen PC, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet neurology. 2013;12(1):37–44. doi:10.1016/S1474-4422(12)70264-8. [Google Scholar]
- 45.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. The New England journal of medicine. 2006;355(9):896–908. doi: 10.1056/NEJMoa060281. doi:10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 46.Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. The New England journal of medicine. 2010;362(22):2077–91. doi: 10.1056/NEJMoa0907083. doi:10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- 47.Frankemolle AM, Wu J, Noecker AM, Voelcker-Rehage C, Ho JC, Vitek JL, et al. Reversing cognitive-motor impairments in Parkinson’s disease patients using a computational modelling approach to deep brain stimulation programming. Brain : a journal of neurology. 2010;133(Pt 3):746–61. doi: 10.1093/brain/awp315. doi:10.1093/brain/awp315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wårdell K, Diczfalusy E, Åström M. Patient-Specific Modeling and Simulation of Deep Brain Stimulation. In: Gefen A, editor. Patient-Specific Modeling in Tomorrow’s Medicine. Studies in Mechanobiology, Tissue Engineering and Biomaterials: Springer Berlin Heidelberg. 2012. pp. 357–75. [Google Scholar]
- 49.Beriault S, Xiao Y, Bailey L, Collins DL, Sadikot AF, Pike GB. Towards computer-assisted deep brain stimulation targeting with multiple active contacts. Med Image Comput Comput Assist Interv. 2012;15(Pt 1):487–94. doi: 10.1007/978-3-642-33415-3_60. [DOI] [PubMed] [Google Scholar]
- 50.Chaturvedi A, Foutz TJ, McIntyre CC. Current steering to activate targeted neural pathways during deep brain stimulation of the subthalamic region. Brain Stimul. 2012;5(3):369–77. doi: 10.1016/j.brs.2011.05.002. doi:10.1016/j.brs.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martens HC, Toader E, Decre MM, Anderson DJ, Vetter R, Kipke DR, et al. Spatial steering of deep brain stimulation volumes using a novel lead design. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2011;122(3):558–66. doi: 10.1016/j.clinph.2010.07.026. doi:10.1016/j.clinph.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 52.Keane M, Deyo S, Abosch A, Bajwa JA, Johnson MD. Improved spatial targeting with directionally segmented deep brain stimulation leads for treating essential tremor. Journal of neural engineering. 2012;9(4):046005. doi: 10.1088/1741-2560/9/4/046005. doi:10.1088/1741-2560/9/4/046005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Brocker DT, Swan BD, Turner DA, Gross RE, Tatter SB, Miller Koop M, et al. Improved efficacy of temporally non-regular deep brain stimulation in Parkinson’s disease. Experimental neurology. 2013;239:60–7. doi: 10.1016/j.expneurol.2012.09.008. doi:10.1016/j.expneurol.2012.09.008. Showed that irregular patterns of stimulation are more efficacious than regular patterns on bradykinesia in Parkinson’s disease, and these patterns also more effectively mitigated pathological beta band oscillations in computer models. Perhaps sets the stage for more effective and/or efficient stimulation parameters.
- 54.Baker KB, Zhang J, Vitek JL. Pallidal stimulation: effect of pattern and rate on bradykinesia in the non-human primate model of Parkinson’s disease. Experimental neurology. 2011;231(2):309–13. doi: 10.1016/j.expneurol.2011.06.012. doi:10.1016/j.expneurol.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuncel AM, Cooper SE, Wolgamuth BR, Clyde MA, Snyder SA, Montgomery EB, Jr., et al. Clinical response to varying the stimulus parameters in deep brain stimulation for essential tremor. Movement disorders : official journal of the Movement Disorder Society. 2006;21(11):1920–8. doi: 10.1002/mds.21087. doi:10.1002/mds.21087. [DOI] [PubMed] [Google Scholar]
- 56.Birdno MJ, Kuncel AM, Dorval AD, Turner DA, Grill WM. Tremor varies as a function of the temporal regularity of deep brain stimulation. Neuroreport. 2008;19(5):599–602. doi: 10.1097/WNR.0b013e3282f9e45e. doi:10.1097/WNR.0b013e3282f9e45e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Birdno MJ, Kuncel AM, Dorval AD, Turner DA, Gross RE, Grill WM. Stimulus features underlying reduced tremor suppression with temporally patterned deep brain stimulation. Journal of neurophysiology. 2012;107(1):364–83. doi: 10.1152/jn.00906.2010. doi:10.1152/jn.00906.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newman JP, Zeller-Townson R, Fong MF, Arcot Desai S, Gross RE, Potter SM. Closed-Loop, Multichannel Experimentation Using the Open-Source NeuroRighter Electrophysiology Platform. Frontiers in neural circuits. 2012;6:98. doi: 10.3389/fncir.2012.00098. doi:10.3389/fncir.2012.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Little S, Brown P. What brain signals are suitable for feedback control of deep brain stimulation in Parkinson’s disease? Ann N Y Acad Sci. 2012;1265:9–24. doi: 10.1111/j.1749-6632.2012.06650.x. doi:10.1111/j.1749-6632.2012.06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60••.Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z, et al. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron. 2011;72(2):370–84. doi: 10.1016/j.neuron.2011.08.023. doi:10.1016/j.neuron.2011.08.023. First evidence that a closed-loop ‘adaptive’ stimulation strategy may be more effective in Parkinson’s disease: this study found improved ‘kinesis’ in vervet MPTP model using closed loop paradigm at 30Hz irregular stimulation, whereas open-loop using similar parameters was ineffective.
- 61.Morrell MJ, Group RNSSiES Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295–304. doi: 10.1212/WNL.0b013e3182302056. doi:10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 62•••.Okun MS, Foote KD, Wu SS, Ward HE, Bowers D, Rodriguez RL, et al. A Trial of Scheduled Deep Brain Stimulation for Tourette Syndrome: Moving Away From Continuous Deep Brain Stimulation Paradigms. Arch Neurol. 2012:1–10. doi: 10.1001/jamaneurol.2013.580. doi:10.1001/jamaneurol.2013.580. First use of a closed-loop stimulation system (RNS, Neuropace) in patients with movement disorders, in this case Tourette Syndrome. While not used in closed loop mode, nevertheless ‘sensing’ was used to collect data on the response of the brain to stimulation (Maling et al., 2012), showing gamma oscillations in the centromedian thalamus increase with time in proportion to clinical response. May define a ‘biomarker’ that can in the future be used to tune stimulation in a true closed-loop adaptive algorithm.
- 63.Maling N, Hashemiyoon R, Foote KD, Okun MS, Sanchez JC. Increased thalamic gamma band activity correlates with symptom relief following deep brain stimulation in humans with Tourette’s syndrome. PloS one. 2012;7(9):e44215. doi: 10.1371/journal.pone.0044215. doi:10.1371/journal.pone.0044215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rouse AG, Stanslaski SR, Cong P, Jensen RM, Afshar P, Ullestad D, et al. A chronic generalized bi-directional brain-machine interface. Journal of neural engineering. 2011;8(3):036018. doi: 10.1088/1741-2560/8/3/036018. doi:10.1088/1741-2560/8/3/036018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stanslaski S, Afshar P, Cong P, Giftakis J, Stypulkowski P, Carlson D, et al. Design and validation of a fully implantable, chronic, closed-loop neuromodulation device with concurrent sensing and stimulation. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2012;20(4):410–21. doi: 10.1109/TNSRE.2012.2183617. doi:10.1109/TNSRE.2012.2183617. [DOI] [PubMed] [Google Scholar]
- 66••.Afshar P, Khambhati A, Stanslaski S, Carlson D, Jensen R, Linde D, et al. A translational platform for prototyping closed-loop neuromodulation systems. Frontiers in neural circuits. 2012;6:117. doi: 10.3389/fncir.2012.00117. doi:10.3389/fncir.2012.00117. Details design parameters of a new bi-directional closed-loop neurostimulator available for research applications in human subject, constructed by adding ‘sensing’ from 4 channels of local field potentials and an integrated accelerometer to a standard approved implantable pulse generator.
- 67.Van Gompel JJ, Chang SY, Goerss SJ, Kim IY, Kimble C, Bennet KE, et al. Development of intraoperative electrochemical detection: wireless instantaneous neurochemical concentration sensor for deep brain stimulation feedback. Neurosurgical focus. 2010;29(2):E6. doi: 10.3171/2010.5.FOCUS10110. doi:10.3171/2010.5.FOCUS10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang SY, Kim I, Marsh MP, Jang DP, Hwang SC, Van Gompel JJ, et al. Wireless fast-scan cyclic voltammetry to monitor adenosine in patients with essential tremor during deep brain stimulation. Mayo Clinic proceedings Mayo Clinic. 2012;87(8):760–5. doi: 10.1016/j.mayocp.2012.05.006. doi:10.1016/j.mayocp.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koehne JE, Marsh M, Boakye A, Douglas B, Kim IY, Chang SY, et al. Carbon nanofiber electrode array for electrochemical detection of dopamine using fast scan cyclic voltammetry. The Analyst. 2011;136(9):1802–5. doi: 10.1039/c1an15025a. doi:10.1039/c1an15025a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gross RE. What happened to posteroventral pallidotomy for Parkinson’s disease and dystonia? Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2008;5(2):281–93. doi: 10.1016/j.nurt.2008.02.001. doi:10.1016/j.nurt.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elias WJ. Editorial: Tremor. Journal of neurosurgery. 2013 doi: 10.3171/2012.10.JNS121654. doi:10.3171/2012.10.JNS121654. [DOI] [PubMed] [Google Scholar]
- 72.Kooshkabadi A, Lunsford LD, Tonetti D, Flickinger JC, Kondziolka D. Gamma Knife thalamotomy for tremor in the magnetic resonance imaging era. Journal of neurosurgery. 2013 doi: 10.3171/2013.1.JNS121111. doi:10.3171/2013.1.JNS121111. [DOI] [PubMed] [Google Scholar]
- 73.Medel R, Monteith SJ, Elias WJ, Eames M, Snell J, Sheehan JP, et al. Magnetic resonance-guided focused ultrasound surgery: Part 2: A review of current and future applications. Neurosurgery. 2012;71(4):755–63. doi: 10.1227/NEU.0b013e3182672ac9. doi:10.1227/NEU.0b013e3182672ac9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Monteith S, Sheehan J, Medel R, Wintermark M, Eames M, Snell J, et al. Potential intracranial applications of magnetic resonance-guided focused ultrasound surgery. Journal of neurosurgery. 2013;118(2):215–21. doi: 10.3171/2012.10.JNS12449. doi:10.3171/2012.10.JNS12449. [DOI] [PubMed] [Google Scholar]
- 75.Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Annals of neurology. 2009;66(6):858–61. doi: 10.1002/ana.21801. doi:10.1002/ana.21801. [DOI] [PubMed] [Google Scholar]
- 76••.Lipsman N, Schwartz ML, Huang Y, Lee L, Sankar T, Chapman M, et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet neurology. 2013;12(5):462–8. doi: 10.1016/S1474-4422(13)70048-6. doi:10.1016/S1474-4422(13)70048-6. First series of 4 patients with “non-invasive” focused ultrasound for Vim thalamotomy. A prospective clinical trial of FUS for thalamotomy is underway with larger number of patients directed by W. Elias, University of Virginia.