Abstract
Objectives
While the majority of HPV+ oropharyngeal squamous cell carcinomas have a favorable prognosis, we search for markers of poor prognosis by carefully examining a subset of highly-aggressive cases.
Study Design
Seven patients with HPV+ oropharyngeal cancer who presented with non-pulmonary distant metastasis or developed distant metastasis post-treatment were identified. Eight control cases were chosen which responded well to treatment. Pathology and radiological studies were reviewed and compared.
Results
Two cases displayed a small cell carcinoma (SmCC) component upon pathologic review. Biomarker analysis revealed lower expression of NOTCH1 in the aggressive cohort in comparison to controls (p=0.04). Cases showed a predominance of clustering of lymph nodes, extracapsular spread and central tumor necrosis.
Conclusion
While most HPV-related oropharyngeal cancers display a positive prognosis, it is evident that there is a subset, which behave more aggressively. This early investigation identifies pathologic and radiologic features that may help to predict this behavior.
Keywords: Head and neck cancer, Oropharyngeal squamous cell carcinoma, aggressive, HPV, human papillomavirus, neuroendocrine, small cell carcinoma, NOTCH1, p53, radiology
Introduction
Head and neck cancer is the sixth most common cancer worldwide, with an annual burden of over 500,000 cases.1 Recently, molecular and epidemiologic data has established that high-risk human papillomavirus (HPV) is a causative factor for a subset of head and neck squamous cell carcinomas (HNSCC).2–3 HPV, particularly type 16, is most closely associated with HNSCC of the oropharynx (OPSCC), where it is found in 40–60% these tumors.3 Of most concern is the fact that incidence of oropharyngeal HPV-related cancers has been significantly increasing over the last 40 years.3–6 Current evidence points to an increase in sexual promiscuity beginning in the 1960s as the impetus for this trend.1 Interestingly, these HPV-positive cancers have a distinct clinical and biological signature from their HPV-negative counterparts. In contrast to HPV-negative tumors associated with older age and tobacco/alcohol exposure, HPV-positive tumors are associated with a younger age and increased number of sexual partners.5–7 Notably, there is an increased survival associated with HPV-positive OPSCC, partly due to increased sensitivity to chemotherapy and radiation.3,8 This increased chemoradiosensitivity is likely secondary to production of oncogenes E6 and E7 by HPV resulting in preservation of apoptotic pathways.8–9 Currently, there is no standard treatment for dealing with HPV-positive tumors, though clinical trials are examining the possibility of de-escalation therapy for HPV- positive OPSCC. In these trials, the goal is to maximize treatment response, while minimizing deleterious effects of chemoradiotherapy by using milder treatment agents or doses for the HPV-positive patients.1
While the majority of HPV-positive OPSCC may have a favorable prognosis, it is evident that there is a subset within this group which display much more aggressive behavior and lead to poor clinical outcome.10–13 Recently, two papers in the pathology literature have shown that small cell carcinoma (SmCC) of the oropharynx is associated with HPV and that some of these tumors have adjacent squamous cell carcinoma (SCC) components.11–12 Further, SmCC of the oropharynx is known to widely disseminate and follow a very aggressive clinical course.12 This could imply that there may be an aggressive subset of HPV-positive OPSCC cases in which a SmCC component exists.
Based on existing literature, we also chose two biomarkers to investigate. p53 expression has been shown to correlate with disease-specific survival and overall survival in some studies, but its clinical prognostic significance remains controversial.10 The NOTCH1 signaling pathway has been studied in HPV-positive cervical cancers and shown to act as a tumor suppressor, which can repress HPV E6/E7 expression and limit carcinogenesis.14–15 In those with cervical cancer, high NOTCH1 expression has been correlated to less aggressive tumors.15 More recently, two pioneer studies using exome sequencing of tumors from head and neck cancer patients provided strong evidence that NOTCH1 functions as a tumor suppressor in head and neck cancer as well. These studies found that 11–15% of head and neck cancer patients harbor mutations in NOTCH1 and that NOTCH1 deregulation was a major driver of head and neck cancer carcinogenesis.16–18 Radiological studies may also add information as to the aggression of the tumor.19–24 Currently, there is a lack of literature which has examined such cases in a multidisciplinary manner. Accordingly, the purpose of our study is to carefully examine a known subset of patients with HPV-positive OPSCC that had very aggressive clinical features and attempt to elucidate the pathologic, clinical and radiological features that could help explain this unexpected behavior.
Patients and Methods
Cases
Approval for this study was obtained from The Ohio State University Office of Responsible Research Practices Cancer Institutional Review Board. Patients who were treated by the Head and Neck cancer comprehensive team at the James Cancer Hospital from 2005 to 2012 known by the Authors’ to have highly aggressive cancers of the oropharynx were included in this study. Highly aggressive, in this context, was defined as cancers which had distant metastases in unusual sites (non-pulmonary) at presentation or within 1-year after initial treatment during their clinical course. Cases were excluded which did not have biopsy proven results or where metastasis were limited to the lungs. Our rationale for this selection is that patients with advanced HNSCC who have distant metastasis to nonpulmonary sites or multiple sites have worse overall survival (OS) than those with limited pulmonary metastasis. Specifically, in a recent study with 127 patients, those with lung metastases had a median OS of 26 months, compared to 21 months with liver metastases, 14 months in patients with multiple metastatic locations and 13 months with metastases to the skeletal system.25 As our study was designed as a pilot study, our search for cases was not exhaustive, instead authors were asked to identify any patients they had treated which met the above inclusion criteria. HPV-status was checked from electronic medical records. If HPV-status was unknown, it was assayed by p16 immunohistochemistry (IHC) and HPV High-risk chromogenic in-situ hybridization (CISH). Cases were only included if they were deemed HPV-positive, based on a positive result by either of these tests. Representative blocks were chosen for immunohistochemical staining. Medical records were also reviewed to document patient age, sex, tobacco exposure, primary site, clinical course, and clinical outcome.
Controls
For comparison, we identified a set of control patients who had HPV-positive oropharyngeal cancers and favorable outcome. Eight patients were selected who were treated at the James Cancer Hospital from 2005 to 2012. These patients had complete response to primary therapy and had no evidence of distant metastases throughout their treatment course. These cases were found from a tissue microarray, which had been previously assembled as described before.26 Please see Table 1 for features of control cases.
Table 1.
Clinical Features of Non-aggressive HPV+ Oropharyngeal Cancers
| Case # |
Age (y) |
T- stage |
N-stage | Pack- Years |
Site | Sex | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 47 | T3 | N2b | 0 | BOT | M | Surgery and adjuvant CRT |
NED |
| 2 | 65 | T1 | N2c | 0 | BOT | F | Surgery and adjuvant RT |
NED |
| 3 | 74 | T3 | N2b | 0 | Tonsil | M | Neck dissection and CRT |
NED |
| 4 | 68 | T1 | N1 | 0 | Tonsil | M | Neck dissection and CRT |
NED |
| 5 | 71 | T1 | N0 | 0 | BOT | F | Complete resection | NED |
| 6 | 60 | T1 | N0 | 0 | Tonsil | M | Neck dissection and CRT |
NED |
| 7 | 40 | T1 | N0 | 0 | Tonsil | M | Neck Dissection and CRT |
NED |
| 8 | 52 | T2 | N2b | 0 | Tonsil | M | Neck dissection and CRT |
NED |
BOT, base of tongue; CH, chemotherapy; RT, radiotherapy; CRT, chemoradiation; NED, no evidence of disease
Immunohistochemistry
Immunohistochemical studies were performed on 4-micron sections on positively charged slides cut from formalin-fixed and paraffin embedded tissue. Slides with specimens were then placed in a 60 °C oven for 1 hour, cooled, and deparaffinized and rehydrated through xylenes and graded ethanol solutions to water.
For synaptophysin and chromogranin stains, slides were placed on a Dako Autostainer, immunostaining system (Dako North America Incorporated, Carpinteria, CA). The detection system used was a Labeled Streptavidin-Biotin Complex (Dako) applied using standard protocols. The primary antibodies and dilution factors are as follows: Synaptophysin (clone 27G12; Leica Microsystems, Bannockburn, IL; 1:100); chromogranin (clone DAK-A3; Dako; 1:200). For p16 staining, slides were placed on a Ventana Medical Systems Benchmark XT Automatic Staining System (Ventana Medical Systems, Tucson, AZ) and viewed with iView DAB Detection system (Ventana). The primary antibodies used were p16 (clone INK4a; MTM Laboratories, Heidelberg, Germany; prediluted).
Aggressive cases were compared to control cases with favorable treatment response for immunohistochemical staining with p53 and NOTCH1. p53 (1:100 Neomarkers) and NOTCH1 (1:100, Cell Signalling) were stained using the Vectastain ABC kit (Vector Laboratories) as previously described.10 Antibody binding was scored by a pathologist (P.E.W.) who was blinded to the group using a categorical scale. A scale of 1 to 4 was used to assess the proportion of cells staining: 1 was less than 5%; 2, 5% to 20%; 3, 21% to 50%; and 4, 51% to 100% tumor staining. Intensity was scored as 1, equal to no staining; 2, low intensity; 3, moderate; and 4, high intensity. If different areas of a slide revealed varying intensity, the average intensity was used for scoring. Scores for multiple cores from each patient were averaged. To calculate the overall expression score (OES), we multiplied the intensity score (1–4) by the proportion staining score (1–4).
In Situ-Hybridization
A chromogenic in-situ hybridization study for high-risk HPV was performed for unknown cases using the HPV III family 16 probe (Ventana), which detects HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66.
Statistics
For biomarker analysis, aggressive and control groups were compared using an unpaired 2-tailed t-test with unequal variances. Standard errors were also determined. We aimed to achieve a 95% confidence level (α = 0.05) for statistical significance. All calculations were performed using Microsoft Excel.
Radiological Review
For our radiological analysis, all imaging taken at time of diagnosis was reviewed independently by a neuroradiologist and assessed for characteristics of aggression. Specifically, using computed tomography (CT) scans, primary tumor site and largest cervical lymph node dimensions (in anterior-posterior, transverse, and cranial-caudal) were recorded and images were assessed for evidence of extracapsular spread (ECS), central tumor necrosis (CTN) and clustering of lymph nodes. Following prior work, CTN was defined as a central area of low attenuation surrounded by an irregular rim of enhancing tissue.21 ECS was defined as infiltration of adjacent fat or muscle planes or capsular contour irregularity.22 In our study, clustering of lymph nodes was defined as grouping of 3 or more contiguous nodes with loss of intervening soft-tissue planes. Control cases were also assessed for ECS, CTN and clustering of nodes. Volume of primary site and volume of lymph node were extrapolated based on recorded dimensions and approximation of lymph nodes as rectangular prisms. Fluorodeoxyglucose positron emission tomography (FDG-PET) was used to assess breadth of distribution of lymph node spread. The maximal standardized uptake value (SUV) in both the primary site and lymph nodes was noted. Of note, control cases did not have preoperative FDG-PET scans so no data on SUV was available.
Results
Clinical Features
Seven highly aggressive HPV-positive cancers of the oropharynx were identified. The clinical features of these cancers are displayed in Table 2. Cases were diagnosed between 2005 and 2011. Age of patients at diagnosis ranged from 38 to 78, with a mean of 59. Patients were predominantly male (86%). Four patients (57%) had a smoking history, with a mean of 48 pack-years. Two patients (28%) had a small cell component to their tumor. Both these patients were smokers (mean 65 pack-years). In one of these patients, there was an as intermixed squamous cell component to the tumor. For the other, the patient had a prior oral cavity SCC. Three patients presented with neck masses; two patients presented with an oropharyngeal mass; one patient presented with dysphagia; and one patient had a persistent sore throat. Four patients presented with distant metastases, while the other three developed distant metastases at 3, 6, and 12 months post-treatment. Bony metastases developed in five patients (71%); Liver metastases were detected in three patients (43%); one patient developed lung metastasis (along with bony metastasis); and one patient developed thyroid and mediastinal lymph node metastasis. In patients with distant metastases at presentation, treatment had a palliative intent in three (75%) of the patients. Palliative treatment consisted of either combination chemotherapy with radiation (CRT) or chemotherapy alone (CH). In one patient who initially presented with distant metastases (thyroid and mediastinal LNs), definitive CRT was attempted and the patient had complete remission. For the three cases that developed metastases post-treatment, initial treatment was either definitive CRT or surgery. Once distant metastases developed, either palliative CH or radiation therapy was attempted.
Table 2.
Clinical Features of Highly aggressive HPV+ Oropharyngeal Cancers
| Case # |
Age (y) |
T- stage |
Pack- Years |
Site | Sex | Presentation | Treatment | Clinical Course | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | T1 | 23 | GTS | M | Neck metastasis |
Palliative CRT |
Liver metastasis at presentation |
DWD |
| 2 | 62 | T3 | 0 | Tonsil | M | Oropharyngeal mass |
Palliative CRT |
Lung and spine metastasis at presentation |
DWD |
| 3 | 38 | T4a | 0 | BOT | M | Neck metastasis |
Definitive CRT then salvage CH |
Thyroid and mediastinal LN metastases at presentation; recurrence in mediastnal LNs × 2 |
AWD |
| 4 | 48 | T2 | 0 | Tonsil | M | Persistent sore throat |
Palliative CH | Spine metastasis at presentation |
DWD |
| 5 | 65 | T4a | 40 | BOT | M | Dysphagia | Definitive CRT followed by palliative RT |
Iliac bone metastasis found 3 months after CRT |
DWD |
| 6 | 78 | T1 | 116 | BOT/ FOM | F | Oropharyngeal mass |
Surgery with positive margins followed by palliative CH |
Liver and bony metastases found 1 year post-operatively |
DWD |
| 7 | 58 | T1 | 13 | Tonsil | M | Neck metastasis |
Definitive CRT followed by palliative CH |
Liver and bony metastases found 6 months after CRT |
DWD |
GTS, glossotonsillar sulcus; BOT, base of tongue; CH, Chemotherapy; RT, Radiotherapy; CRT, Chemoradiation; DWD, dead with disease; AWD, alive with disease
Pathologic Features
Under pathologic review, there were two cases in our cohort that manifested a small cell phenotype. Evidence of a small cell phenotype was seen through hematoxylin and eosin (H&E) stains showing small cells with a high nuclear:cytoplasm ratio and hyperchromatic nuclei. Immunohistochemical evidence of neuroendocrine differentiation was also seen as both cases expressed chromogranin and synaptophysin and lost expression of p63. See Table 3. One of these cases (Case # 7) also contained a SCC component to their tumor, which was seen contiguous to the small cell component. Further, there were several areas where the cell types were intermixed. Both the SCC component and SmCC component were positive for p16 staining, implying a dual-association with HPV. See Figure 1.
Table 3.
Immunohistochemical and In Situ Hybridization Findings of Highly aggressive HPV+ Oropharyngeal Cancers
| Case # |
HPV High-Risk ISH |
p16 IHC | Synaptophysin | Chromogranin | p63 | Impression |
|---|---|---|---|---|---|---|
| 1 | + | + | − | − | + | SCC |
| 2 | − | + | − | − | + | SCC |
| 3 | + | + | − | − | + | SCC |
| 4 | − | + | − | − | + | SCC |
| 5 | + | + | − | − | + | SCC |
| 6 | − | + | + | + | − | SmCC and prior oral cavity SCC |
| 7 | + (SCC) + (SmCC) |
+ (SCC) + (SmCC) |
− (SCC) + (SmCC) |
− (SCC) + (SmCC) |
+ (SCC) − (SmCC) |
SmCC with contiguous SCC |
SmCC, small cell carcinoma; SCC, squamous cell carcinoma; HPV, human papillomavirus; IHC, immunohistochemistry; ISH, in-situ hybridization
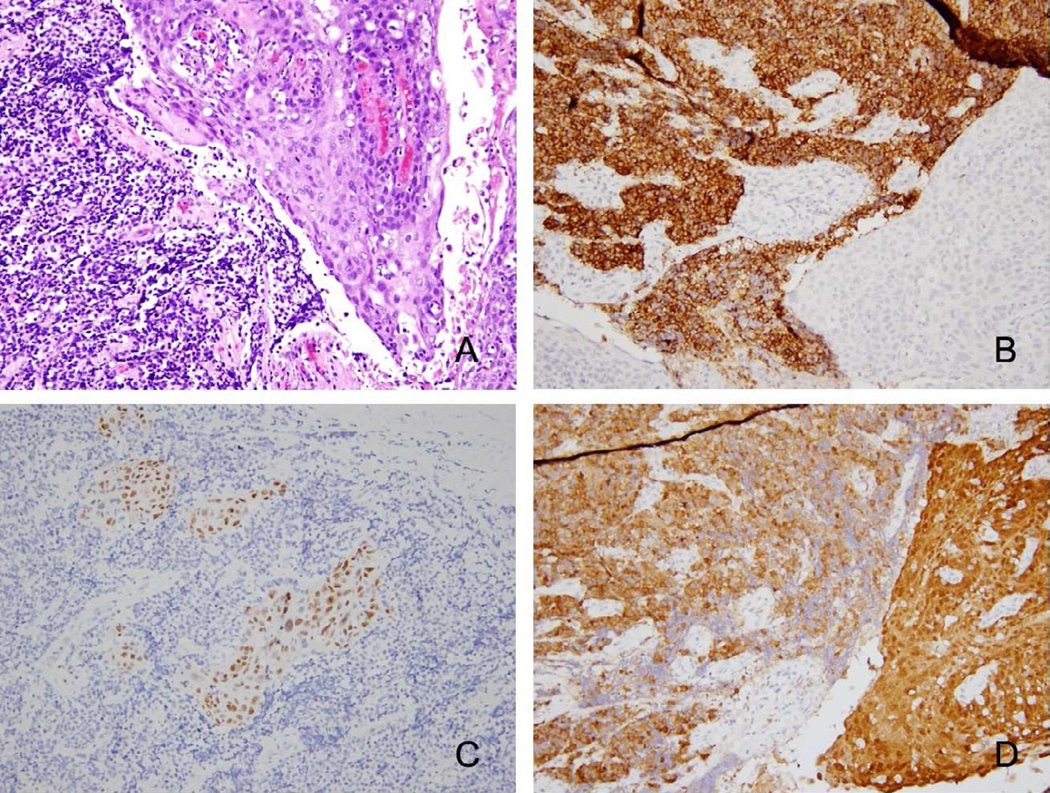
Figure 1.
HPV+ oropharyngeal carcinoma shows both small cell and squamous cell components in Case #7. A) Hematoxylin and eosin staining shows SmCC (left) and SCC (right). B) SmCC component (left) shows expression of synaptophysin. C) SCC component expresses p63; SmCC exhibits loss of p63 expression. D) HPV exists in both components as seen by p16 expression. Images are 200× magnification
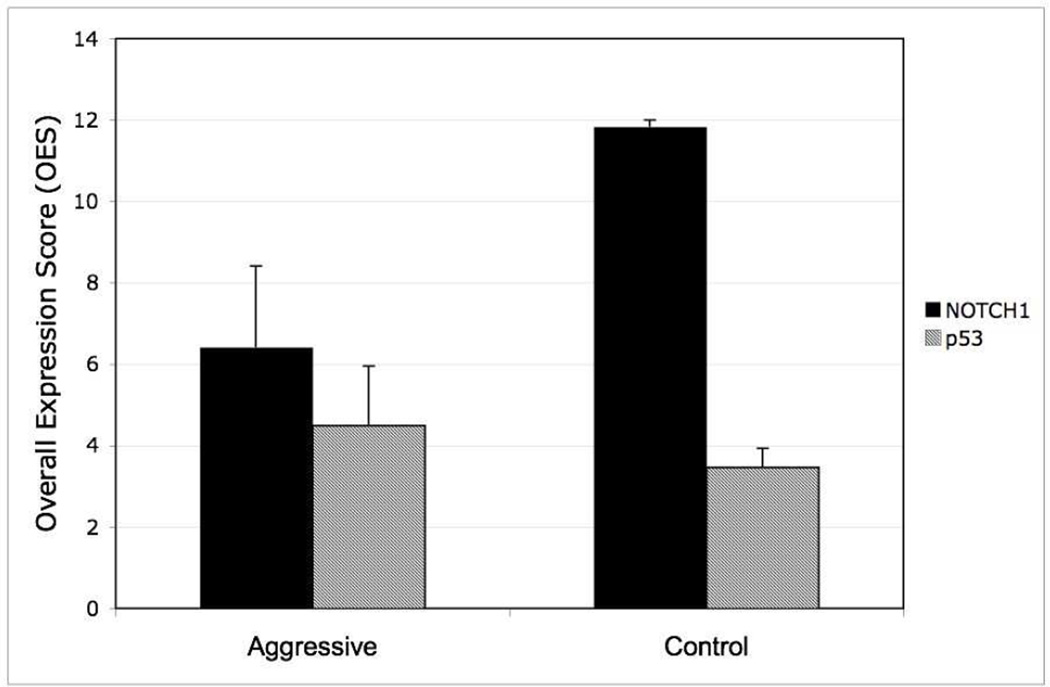
We also determined the expression of two biomarkers in our aggressive cohort and compared these expression levels to control cases with favorable outcome. For our case with mixed pathology, only the squamous cell portion showed expression of NOTCH1, while the small cell component was negative. NOTCH1 expression was absent in our case with entirely SmCC pathology. NOTCH1 expression was generally robust in control cases. See Figure 2. Quantitatively, our aggressive group had an average OES of 6.4 ± 2.0 (n=6), while our control group had an average OES of 11.8 ± 0.1 (n=8). Staining levels of NOTCH1 were shown to be significantly lower in the aggressive cohort (p=0.04). Of particular interest, our case with entirely small cell pathology showed >50% staining for mutant-p53, a finding that was not seen for any of the other HPV-positive tumors. See Figure 3. For p53 expression, our aggressive group had an average OES of 4.5 ± 1.5 (n=6), while our control group had an OES of 3.5 ± 0.5 (n=8). The differential staining of p53 did not reach statistical significance (p=0.5). See Figure 4.
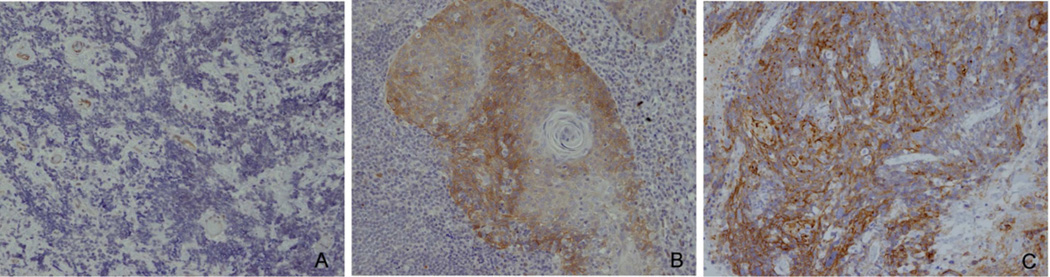
Figure 2.
HPV+ oropharyngeal cancers display differential staining of NOTCH1. A) Aggressive Case #7 shows lack of NOTCH1 expression in more aggressive small cell histology. B) Moderate expression of NOTCH1 is seen in less aggressive squamous cell histology. C) Control Case #3 shows robust staining of NOTCH1. Images are 200× magnification.
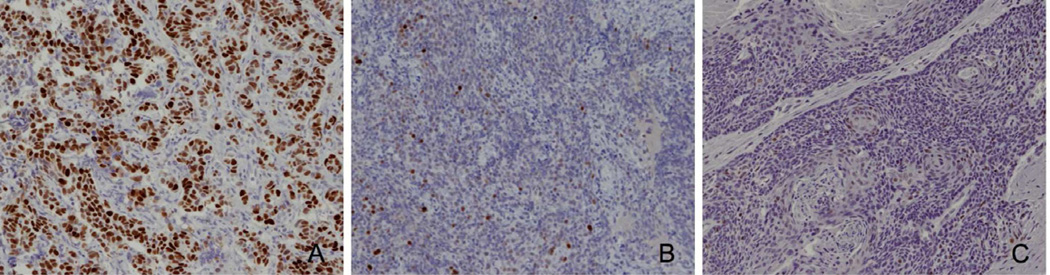
Figure 3.
HPV+ oropharyngeal cancers display differential staining of p53. A) Aggressive Case # 6 shows small cell phenotype and robust staining for p53. B) Aggressive Case #1 with SCC shows minimal staining with p53. C) Control Case #3 shows minimal staining with p53. Images are 200× magnification.
Figure 4.
Immunohistochemistry results for NOTCH1 and p53 staining in aggressive and control groups. Overall expression score (OES) for NOTCH1 is significantly lower in aggressive group than control (p=0.04). OES difference for p53 staining is not significant. Error bars signify standard error.
Radiologic Features
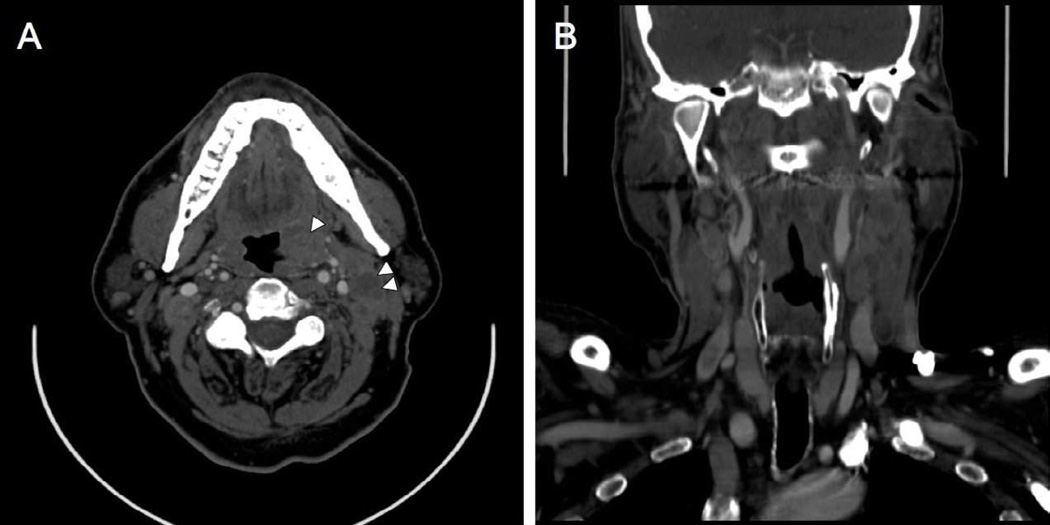
Analysis of computed tomography scans of patients in the aggressive cohort showed a range of primary tumor volume from 4.1 to 73.0 cm3, with an average volume of 24.0 cm3. Volume of largest metastatic cervical lymph node ranged from 8.0 to 51.4 cm3, with an average of 28.1 cm3. CT scans also showed aggressive features of lymph nodes: ECS of tumor and clustering of nodes was seen in all cases that demonstrated lymphadenopathy. One case did not demonstrate lymphadenopathy as the patient had prior surgery with radiation for a previous oral cavity cancer. Five cases (83%) also demonstrated significant CTN in the lymph nodes. See Figure 5. For individual case specifics of these findings, see Table 4. Control cases with good outcome showed CTN in two cases (25%) and ECS in one case (13%). No clustering of nodes was seen in control cases.
Figure 5.
Computed tomography scan post-iodinated contrast of Case #2. A) Axial image shows left tonsil primary (single arrowhead) and lymph nodes (double arrowhead) with CTN. B) Coronal reconstruction shows clustering of nodes and ECS.
Table 4.
Radiologic Features of Highly-aggressive HPV+ Oropharyngeal Cancers
| Primary Tumor | Cervical Lymph Nodes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case # | ∼ Volume (mL) |
SUV |
Level of largest LN |
Largest dimension (cm) |
∼Volume (mL) |
Ipsilateral LNs |
SUV | ECS | CTN | Clustering |
| 1 | 6.1 | NR | IIa/IIb | 4.7 | 42.8 | 20 | II, V | + | + | + |
| 2 | 49.2 | 14 | IIb | 2.9 | 12.2 | 14 | I−III | + | + | + |
| 3 | 24.0 | 15 | IIa/IIb | 3.5 | 30.2 | 13 | I−VI | + | + | + |
| 4 | 4.1 | NR | IIa | 3.3 | 24.1 | 14 | II−III | + | − | + |
| 5 | 73.0 | 26 | IIa | 5.2 | 51.4 | 12 | II, IV | + | + | + |
| 6 | 6.1 | NR | DTA | DTA | DTA | DTA | DTA | DTA | DTA | DTA |
| 7 | 5.2 | 9 | IIa | 2.5 | 8.0 | 9 | II | + | + | + |
ECS, Extracapsular spread; CTN, central tumor necrosis; SUV, standard uptake value; NR, not recorded; LN, lymph node; DTA, difficult to assess as patient had prior radiation therapy to necks
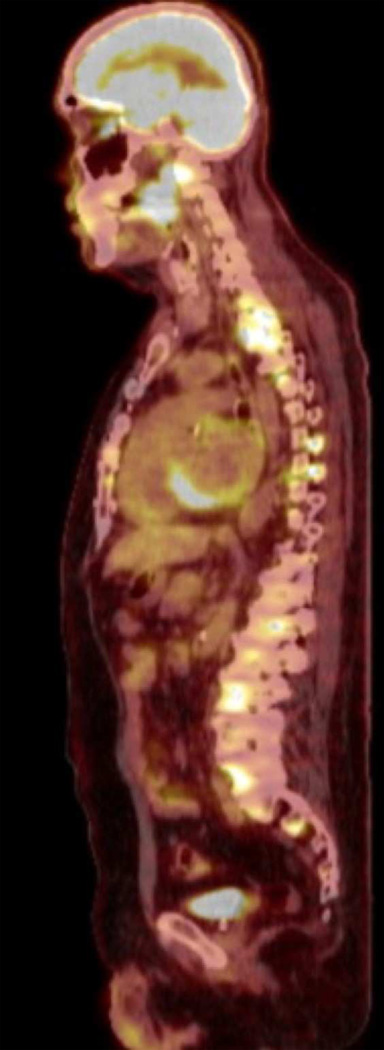
FDG-PET imaging showed metabolically active lymph nodes in all cases with lymphadenopathy. Contralateral active lymph nodes were seen in five cases (83%). Lymph node distribution was seen primarily in the upper-mid jugular chain (Level II-III), though active submandibular (Level Ib), paratracheal (Level VI), supraclavicular (Level IV) and posterior triangle (Level V) lymph nodes were also seen. SUV range for most-active lymph node ranged from 9 to 20, with an average of 14. SUV range for primary lesions was from 9 to 26, with an average of 16. See Figure 6.
Figure 6.
PET-FDG imaging of Case #2 shows intensely hypermetabolic focus in palatine tonsil as well as diffuse hypermetabolic foci in the spine.
Discussion
This cohort of patients with widely metastatic cancer represents a distinct subset of HPV-positive oropharyngeal carcinoma. Pathologically, two of our cases show a small cell component to their tumor pathology. This is of particular interest given the recent reports describing an aggressive HPV-related small cell variant.11–12 We also showed that this small cell component can closely incorporate itself among the more typical squamous cell component. This implies that there may be other HPV-positive oropharyngeal tumors with a contiguous neuroendocrine element that were missed on pathological exam.
Our biomarker analysis revealed a number of interesting findings. First, the NOTCH1 expression of our aggressive cohort was significantly lower than our control group. This finding of NOTCH1 as a predictive biomarker in HPV-related oropharyngeal cancer represents a novel observation. Further support from our data is seen in our case with mixed phenotype, where the more aggressive component (small cell component) lacked NOTCH1 expression, while the squamous cell component expressed it. See Figure 2. Our small cell only tumor had no NOTCH1 staining. This correlates well with recent data showing NOTCH1 expression is absent in small cell lung cancer and that re-expression can lead to growth inhibition.18 A significant limitation of our study is our small sample size. With such few data points, we cannot vouch for the normal distribution of data in our cohorts, and therefore, cannot confirm the fundamental assumption of the t-test. In this sense, our data should be seen as pilot data that sheds light on a potential of novel biomarker.
One case in our cohort showed a particularly interesting finding regarding p53 expression. The case of small cell carcinoma had a >50% expression level for p53. Generally, HPV inactivates and destroys p53, via its early protein E6, so only wild type p53 is present and stains at low levels.10 Therefore, the pathogenesis of the small cell variant may use an alternate pathway that does not involve p53 inactivation. This is less supported by our mixed phenotype case, where p53 expression was not seen in the small cell component. Further study is needed to elucidate the role of p53 in HPV-related small cell carcinoma.
The radiological images of our cases at the time of initial diagnosis showed a predominance (>80%) of cases with CTN, ECS and clustering of nodes. Our control cases showed relatively less CTN (40%) and ECS (20%) than our aggressive cohort. Most striking was the complete lack of node clustering in our control cases compared to all our aggressive cases showing clustering of nodes. As a generalized reference for these values, a recent study examining lymph node volume with computed tomography in HNSCC patients showed only 31% of cases with CTN and 21% of cases with clustering of lymph nodes.19 In a recent publication which looked at clustered nodes as a predictor of aggressiveness, it was found that in an HPV-positive cohort, 6 of 52 patients died of distant metastasis and 5 of the 6 patients that died of distant metastasis had matted nodes.13 This data correlates well with our aggressive cohort, which showed a predominance of clustered nodes. In regard to lymph node volume, one study showed that patients with histological confirmation of lymph node metastasis had a median volume of 2.7 cm3 versus our patient population with median of 6.1 cm3 (average 28.1 cm3).19
Recently, it was shown that SUVs for lymph nodes in HNSCC from FDG-PET scanning correlates to prognosis.24 Specifically, the average nodal SUV value was 10.4 for patients with distant recurrence at one year versus 7.0 for those without. Our cohort supports this evidence and showed an average nodal SUV of 14. The same study showed that overall survival was significantly worse for a primary tumor SUV > 8.0.24 Again, our cohort showed an elevated mean primary tumor SUV at 16.
This cohort of highly aggressive patients represents an important subset of HPV-related oropharyngeal cancer. Such patients who develop non-pulmonary metastasis are certainly a rare deviation among the usual HPV-related cases. Patients like these would not be amenable to de-escalation therapy and would best be treated with aggressive multimodality treatments. First, and foremost, this paper hopes to bring awareness to the head and neck oncology community that HPV association does not necessarily correlate to positive prognosis and that certain HPV-related cases have high metastatic potential. In order to tailor treatment appropriately, we must gain an understanding of which diagnostic modalities have value in predicting prognosis. In our aggressive cohort, we were able to highlight some radiological and pathological markers that could be used to stratify such HPV-positive oropharyngeal cancers in the future. Most striking were the HPV-associated small cell variants, which we showed may co-exist with an intermixed squamous cell tumor and portend a poor prognosis. Given these results, it is of vital importance that clinicians are aware of this variant and that all pathological specimens are carefully screened for a small cell component, as it may exist adjacent to the usual SCC component. Further, we showed for the first time that NOTCH1 has potential for a future biomarker, as its expression was significantly lower in our aggressive cohort. Given this exciting preliminary data, our lab is currently working to characterize NOTCH1 expression on a larger cohort of patients. Future research needs to be directed at the utility of screening for NOTCH1 in pre-operative fine needle aspirates (FNA) as well as post-operative specimens, to help guide initial treatment or adjuvant therapy. Of note, our future studies will have less stringent inclusion criteria for aggressive cases and allow us to expand our study size. While prior studies and established staging algorithms predict aggressive cases to show a higher lymph node volume, larger maximal lymph node diameter and higher SUVs for primary site and lymph nodes, we show here that there are additional, more subtle, radiological features of value. Particularly, our cases showed a predominance of clustering of nodes, CTN, and ECS at rates much higher than reported in the literature and in our control group. As seen in our cohort, these locoregional markers of tumor spread correlate with distant metastasis. These radiological features should be thoroughly assessed in all pre-operative scans by a qualified neuroradiologist as they may predict an aggressive phenotype. While this study is limited by small sample size due to its narrow inclusion criteria, it brings awareness to the community, highlights the importance of a multimodality approach, and serves as a platform for future research with larger cohorts to validate these markers for clinical use.
Statement of Clinical Relevance.
This paper brings awareness to all practitioners involved with HPV+ oropharyngeal cancers that there is a subset that behaves very aggressively. It shows the benefit of multimodality research and paves the way for future research to characterize markers of aggression.
Acknowledgments
Grants: Joan’s Fund (Kumar B, Kumar P); NIH/NCI-CA133250 (Kumar P); The Ohio State University Comprehensive Cancer Center Start-up Funds (Teknos TN, Kumar P)
Role of the Funding Source: Acquiring FFPE tissue samples; applying immunohistochemical stains; biomarker analysis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No authors have a conflict of interest with the work submitted here.
References
- 1.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begum S, Cao D, Gillison M, Zahurak M, Westra WH. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res. 2005;11:5694–5699. doi: 10.1158/1078-0432.CCR-05-0587. [DOI] [PubMed] [Google Scholar]
- 5.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 6.Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31(6):744–754. doi: 10.1053/j.seminoncol.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Ramqvist T, Dalianis T. Oropharyngeal cancer epidemic and human papillomavirus. Emerg Infect Dis. 2010;16:1671–1677. doi: 10.3201/eid1611.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto Hd, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 9.D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 10.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishop JA, Westra WH. Human papillomavirus-related small cell carcinoma of the oropharynx. Am J of Surg Pathol. 2011;35:1679–1684. doi: 10.1097/PAS.0b013e3182299cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraft S, Faquin WC, Krane JF. HPV-associated Neuroendocrine Carcinoma of the Oropharynx: A Rare New Entity With Potentially Aggressive Clinical Behavior. Am J Surg Pathol. 2012;36:321–330. doi: 10.1097/PAS.0b013e31823f2f17. [DOI] [PubMed] [Google Scholar]
- 13.Spector ME, Gallagher KK, Light E, Ibrahim M, Chanowski EJ, Moyer JS, et al. Matted Nodes: Poor prognostic marker in oropharyngeal squamous cell carcinoma independent of HPV and EGFR status. Head Neck. 2012;34:1727–1733. doi: 10.1002/hed.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talora C, Cialfi S, Segatto O, Morrone S, Kim Choi J, Frati L, et al. Constitutively active Notch1 induces growth arrest of HPV-positive cervical cancer cells via separate signaling pathways. Exp Cell Res. 2005;305:343–354. doi: 10.1016/j.yexcr.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Sun L, Song Y, Tong T, Lingying W, Wenhua Z, Qimin Z. Down-modulation of Notch1 expression in cervical cancer is associated with HPV-induced carcinogenesis. Clin Onc and Cancer Res. 2009;6:401–405. [Google Scholar]
- 16.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333(6046):1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist. 2007;12(5):535–542. doi: 10.1634/theoncologist.12-5-535. [DOI] [PubMed] [Google Scholar]
- 19.Liang MT, Chen CC, Wang CP, Wang CC, Lin WD, Liu SA. The association of lymph node volume with cervical metastatic lesions in head and neck cancer patients. Eur Arch Otorhinolaryngol. 2010;266:883–888. doi: 10.1007/s00405-008-0818-2. [DOI] [PubMed] [Google Scholar]
- 20.Alvi A, Johnson JT. Extracapsular spread in the clinically negative neck (N0): implications and outcome. Otolaryngol Head Neck Surg. 1996;114:65–70. doi: 10.1016/S0194-59989670285-1. [DOI] [PubMed] [Google Scholar]
- 21.Som PM. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am J Roentgenol. 1992;158:961–969. doi: 10.2214/ajr.158.5.1566697. [DOI] [PubMed] [Google Scholar]
- 22.Yousem DM, Som PM, Hackney DB, Schwaibold F, Hendrix RA. Central nodal necrosis and extracapsular neoplastic spread in cervical lymph nodes: MR imaging versus CT. Radiology. 1992;182:753–759. doi: 10.1148/radiology.182.3.1535890. [DOI] [PubMed] [Google Scholar]
- 23.Zoumalan RA, Kleinberger AJ, Morris LGT, Ranade A, Yee H, DeLacure MD, et al. Lymph node central necrosis on computed tomography as predictor of extracapsular spread in metastatic head and neck squamous cell carcinoma: pilot study. J Laryngol Otol. 2010;124(12):1284–1288. doi: 10.1017/S0022215110001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubicek GJ, Champ C, Fogh S, Wang F, Reddy E, Intenzo C, et al. FDG-PET staging and importance of lymph node SUV in head and neck cancer. Head and Neck Oncology. 2010;2:19. doi: 10.1186/1758-3284-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauswald H, Simon C, Hecht S, Debus J, Lindel C. Long-term outcome and patterns of failure in patients with advanced head and neck cancer. Radiation Oncology. 2011;6:70. doi: 10.1186/1748-717X-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar B, Yadav A, Lang JC, Cipolla M, Schmitt AC, Arradaza N, et al. YM155 reverses cisplatin resistance in head and neck cancer by decreasing cytoplasmic survivin levels. Mol. Cancer Therapy. 2012;11:1998. doi: 10.1158/1535-7163.MCT-12-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]