Abstract
Objective
Platelets have been implicated in cancer metastasis and prognosis. No population-based study has been reported as to whether preoperative platelet count directly predicts metastatic recurrence of colorectal cancer (CRC) patients.
Design
Using a well-characterized cohort of 1,513 surgically resected CRC patients, we assessed the predictive roles of preoperative platelet count in overall survival, overall recurrence, as well as locoregional and distant metastatic recurrences.
Results
Patients with clinically high platelet count (≥400× 109/L) measured within 1 month before surgery had a significantly unfavorable survival (hazard ratio [HR]=1.66, 95 % confidence interval [CI] 1.34–2.05, P=2.6×10−6, Plog rank= 1.1×10−11) and recurrence (HR=1.90, 1.24–2.93, P=0.003, Plog rank=0.003). The association of platelet count with recurrence was evident only in patients with metastatic (HR=2.81, 1.67–4.74, P=1.1×10−4, Plog rank =2.6×10−6) but not locoregional recurrence (HR=0.59, 95 % CI 0.21–1.68, P= 0.325, Plog rank=0.152). The findings were internally validated through bootstrap resampling (P<0.01 at 98.6 % of resampling). Consistently, platelet count was significantly higher in deceased than living patients (P<0.0001) and in patients with metastatic recurrence than locoregional (P= 0.004) or nonrecurrent patients (P<0.0001). Time-dependent modeling indicated that the increased risks for death and metastasis associated with elevated preoperative platelet counts persisted up to 5 years after surgery.
Conclusion
Our data demonstrated that clinically high level of preoperative platelets was an independent predictor of CRC survival and metastasis. As an important component of the routinely tested complete blood count panel, platelet count may be a cost-effective and noninvasive marker for CRC prognosis and a potential intervention target to prevent metastatic recurrence.
Keywords: Platelet, Thrombocytosis, CRC, Survival, Recurrence, Metastasis
Introduction
Colorectal cancer (CRC) is a leading cause for cancer incidence and mortality in the USA in 2011 [1]. Despite the recent improvements, our understanding of CRC prognosis and metastasis remains limited [2]. Up to 50 % of stage II and III CRC patients develop recurrence after radical surgery [3–6]. Over half of the recurrent patients have distant metastasis, which accounts for 90 % of CRC-related deaths [7, 8]. To further develop predictors of CRC survival and metastasis has important clinical significance.
The mechanisms of cancer metastasis have been extensively studied but still remain elusive. A typical simplification is that metastasis follows an orderly sequence of events, including intravasation of the surrounding tissues, tumor cell dissemination into and extravasation from the circulation system, and metastatic colonization in distant organs [8–12]. During this invasion–metastasis cascade, the dissemination of malignant cells into blood and their transportation play a pivotal role through involving a wide spectrum of blood-related cells such as platelets, lymphocytes, macrophages, and mast cells, as well as other nonblood-related cells [11]. Of the many blood cells linked to metastasis, platelets have been relatively well studied. Platelets facilitate metastasis through mediating disseminated tumor cell survival in the circulation system, extravasation, and angiogenesis in the microenvironment of target sites [13–17]. A recent study reported that the interactions between platelets and tumor cells augment metastasis by promoting epithelial mesenchymal transition (EMT) [18]. Having a clinically high level of platelets is called thrombocytosis. This condition has been associated with advanced stage and death for several malignancies including CRC, although with mixed results [19–26]. Moreover, to the best of our knowledge, no study has been reported on the association between preoperative platelet count and cancer metastasis. In this study, based on a large and well-characterized hospital-based CRC patient cohort, we comprehensively evaluated the association of preoperative platelet count with the prognosis of CRC patients who underwent surgical resection.
Patients and Methods
Study Population
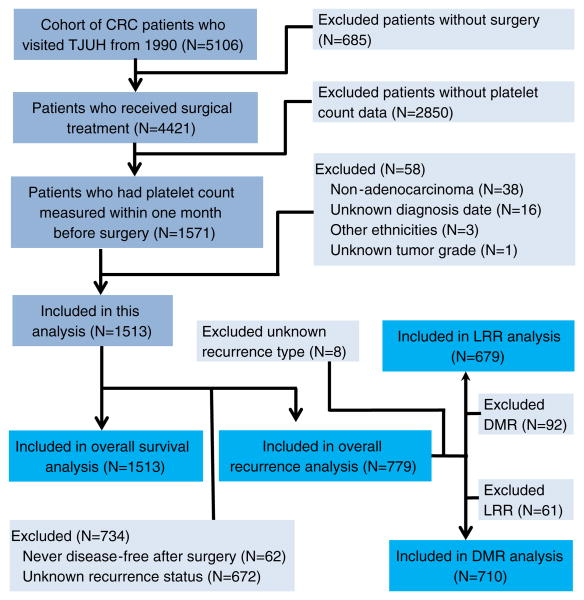
Subjects in this study were identified from a hospital-based cohort of histologically confirmed CRC patients who visited the Kimmel Cancer Center at the Thomas Jefferson University Hospital from 1990 to 2010. There are a total of 5,106 patients in this cohort. For this study, we selected CRC adenocarcinoma patients who were surgically treated and had at least one platelet count measurement taken within 1 month before surgery. Finally, 1,513 patients with an average follow-up time of 54.0 (standard deviation [SD], 39.9) months were selected based on these criteria and after excluding three Hispanic patients and one patient with unknown tumor grade (Fig. 1). All the 1,513 patients had complete data on survival status and were included in the analysis for overall survival. Next, after excluding 672 patients with unknown recurrence status and 62 patients who were never disease-free after surgery, we retained 779 patients with an average follow-up time of 40.8 (SD, 37.0) months for the analysis of overall recurrence. Among the 779 patients, 679 patients were included in the analysis of locoregional recurrence (LRR), and 710 were included in the analysis of distant metastatic recurrence (DMR) (Fig. 1). This study was approved by the Institutional Review Board of Thomas Jefferson University.
Fig. 1.
CONSORT diagram of study population. CRC colorectal cancer, LRR locoregional recurrence, DMR distant metastatic recurrence, TJUH Thomas Jefferson University Hospital
Collection of Demographic and Clinical Data
Demographic and clinical data were obtained from medical chart review. Demographic variables analyzed in this study included age, gender, ethnicity, smoking status, and drinking status. Clinical variables included primary tumor site, tumor stage, tumor grade, and treatment (surgery, chemotherapy, and radiation therapy). The values of platelet count, which was measured through routine clinical laboratory tests, were obtained from chart review.
Statistical Analysis
Four major clinical endpoints were analyzed in this study: overall survival, overall recurrence, LRR, and DMR. Overall survival time was defined as the time from initial surgery to death from any cause. Patients were followed in the Thomas Jefferson University Hospital by a multidisciplinary team through standard of care treatment of colorectal cancer, including a combined evaluation of physical examinations, imaging findings (ultrasound, computed tomography, positron emission tomography, and magnetic resonance imaging), and laboratory results (mainly carcinoembryonic antigen test). Time to recurrence was defined as the time from initial surgery to LRR or DMR. Patients who were living or recurrence-free at the last follow-up were censored for survival or recurrence analysis, respectively. Recurrence was defined as, after the surgical removal of the primary tumor, the regrowth of tumor in the original or near organ or regional lymph nodes (LRR), or distant organs (DMR). The analysis of LRR excluded patients who developed DMR or had unknown recurrence type, whereas the analysis of DMR excluded patients who developed LRR or had unknown recurrence type (Fig. 1). Comparisons of demographic and clinical variables were performed using the chi-square test for categorical variables and Student's t test or one-way analysis of variance for continuous variables. Determination of thrombocytosis was based on a clinical cut-off of 400× 109/L as defined previously [30, 31]. The associations between platelet count and patient outcomes were estimated using hazard ratio (HR) and 95 % confidence interval (95 % CI) calculated by multivariate Cox proportional hazards model adjusting for age, gender, ethnicity, smoking status, drinking status, primary tumor site, tumor stage, tumor grade, chemotherapy, and radiation therapy, where appropriate. The results of the main effects analyses were internally validated using the bootstrap resampling method [27]. A total of 500 bootstrap samples were generated for each analysis. Each time a bootstrap sample was drawn from the original dataset and the P value for the analysis was calculated. The number of times with a P value<0.01 was counted. Survival curves were constructed using the Kaplan–Meier method and compared using the log rank test. Interaction analysis was performed by adding an interaction term in the Cox regression model. Dose-dependent effects of platelet count on CRC outcomes were analyzed using fractional polynomial regression model adjusting for all the host variables. Time-dependent analyses of platelet count and long-term outcomes after surgery were conducted using flexible parametric modeling framework that analyzes the interaction between platelet count and time and thus confers a time-dependent effect to platelet count. Under this framework, platelet count was modeled as a time-dependent variable and HRs were estimated as functions of time and platelet count while adjusting other non-time-dependent host variables. SAS (Version 9.2, SAS Institute, Cary, NC, USA) and STATA (Version 11.0, STATA Corp., College Station, TX, USA) software packages were used for these analyses. All P values were two sided. P≤0.05 was considered the threshold of statistical significance.
Results
Characteristics of Study Population
A total of 1,513 CRC patients were included in this study, with an average age of 64.9 (SD, ±13.6) years. The distributions of demographic and clinical variables are summarized in Table 1. Most patients were Caucasians (76.7 %) with a primary tumor site in the colon (70.5 %) and a moderately differentiated tumor grade (66.3 %). There was a relatively even distribution of males and females (48.6 vs. 51.4 %), never smokers and current or previous smokers (48.8 vs. 46.0 %), as well as never drinkers and current or previous drinkers (44.1 vs. 48.5 %). Over half of the patients had early stage disease (stage 0, I, or II, 57.2 %) or did not receive chemotherapy (56.3 %) or radiation therapy (82.4 %). During a median follow-up duration of 46.7 (range, 19.6–84.7) months, 624 (41.2 %) patients died. Among the 1,513 patients, 672 (44.4 %) patients had unknown recurrence status and 62 (4.1 %) patients were never disease-free after surgery and were excluded from the recurrence analysis. The remaining 779 patients with recurrence status were included for the analysis of overall recurrence. Among them, 20.7 % developed recurrence, including 61 patients with LRR, 92 patients with DMR, and 8 patients with unknown recurrence type (Table 1). The distributions of the average values of platelet count measured within 1 month before surgery by host characteristics were also summarized in Table 1. Significantly different distributions of platelet count were observed for almost all the variables (P values ranged from <0.0001 to 0.053). We observed a significant trend of increasing average platelet count along with the increase in tumor stage (238.8, 249.0, 275.3, 281.9, and 316.8×10−9/L for stage 0, I, II, III, and IV, respectively) and tumor grade (262.2, 272.7, and 289.4×10−9/L for well, moderately, and poorly differentiated tumors, respectively) (Table 1). We also noticed a significantly increased level of average platelet count in deceased compared to living patients (281.4 vs. 265.4×10−9/L, P=0.001) and in patients with overall (288.3×10−9/L, P=0.004) or distant recurrence (311.3×10−9/L, P<0.0001) compared to nonrecurrent (NR) patients (264.5×10−9/L). No significant difference was noted between patients with locoregional recurrence and nonrecurrent patients (254.4 vs. 264.5×10−9/L, P=0.387) (Table 1). Excluding the 28 patients with unknown ethnicity from the main effect analysis yielded very similar results. Results of all the analyses were similar when the 55 patients with stage 0 diseases were excluded (data not shown).
Table 1. Host characteristics of the study population and distributions of platelet count by average values.
| Variables | No. of patients (%), n=1,513 | Platelet counta (mean ± SEM) | P value |
|---|---|---|---|
| Age, mean (±SD) | 64.9 (±13.6) | ||
| Gender | |||
| Male | 736 (48.6) | 251.0±3.3 | |
| Female | 777 (51.4) | 219.8±43.6 | <0.0001 |
| Race | |||
| Asian | 50 (3.3) | 295.1 ±14.0 | |
| Black | 275 (18.2) | 299.3±6.2 | |
| Caucasian | 1,160 (76.7) | 265.3±2.7 | <0.0001 |
| Unknown | 28 (1.8) | ||
| Smoking status | |||
| Never smoking | 739 (48.8) | 276.2±3.4 | |
| Current smoking | 222 (14.7) | 283.5±6.8 | |
| Previous smoking | 473 (31.3) | 262.1 ±4.4 | 0.008 |
| Unknown | 79 (5.2) | ||
| Drinking status | |||
| Never drinking | 667 (44.1) | 279.6±3.9 | |
| Current drinking | 679 (44.9) | 268.2±3.5 | |
| Previous drinking | 55 (3.6) | 252.1 ±15.3 | 0.024 |
| Unknown | 112 (7.4) | ||
| Primary tumor sites | |||
| Colon | 1,066 (70.5) | 285.5±3.0 | |
| Rectosigmoid junction | 138 (9.1) | 257.3±7.6 | |
| Rectum | 309 (20.4) | 231.8±3.9 | <0.0001 |
| Tumor stages | |||
| Stage 0 | 55 (3.6) | 238.8±8.5 | |
| Stage I | 399 (26.4) | 249.0±4.0 | |
| Stage II | 412 (27.2) | 275.3±4.6 | |
| Stage III | 362 (23.9) | 281.9±4.9 | |
| Stage IV | 199 (13.2) | 316.8±8.4 | < 0.0001 |
| Unknown | 86 (5.7) | ||
| Tumor grades | |||
| Well differentiated | 155 (10.2) | 262.2±7.0 | |
| Moderately differentiated | 1,003 (66.3) | 272.7±3.0 | |
| Poorly differentiated | 201 (13.3) | 289.4±7.6 | |
| Cell type not determined | 154 (10.2) | 254.6±7.0 | 0.004 |
| Chemotherapy | |||
| No | 851 (56.3) | 267.1 ±3.1 | |
| Yes | 601 (39.7) | 276.1 ±4.1 | 0.053 |
| Unknown | 61 (4) | ||
| Radiation therapy | |||
| No | 1,246 (82.4) | 279.1 ±2.8 | |
| Yes | 259 (17.1) | 237.1 ±4.6 | <0.0001 |
| Unknown | 8 (0.5) | ||
| Vital status | |||
| Alive | 889 (58.8) | 265.4±2.9 | |
| Dead | 624 (41.2) | 281.4±4.2 | 0.001 |
| Recurrence status | |||
| No recurrence (NR) | 618 (40.9) | 264.5±3.5 | |
| Recurrence (total) | (161) | 288.3±8.6 | 0.004* |
| Locoregional recurrence (LRR) | 61 (4) | 254.4±10.6 | 0.387** |
| Distant metastasis recurrence (DMR) | 92 (6.1) | 311.3± 12.6 | <0.0001*** |
| Unknown recurrence type | 8 (0.5) | 0.002**** | |
| Recurrence rate (%) | (20.7) | ||
| Never disease free during follow-up | 62 (4.1) | ||
| Unknown | 672 (44.4) | ||
| Median survival time (95 % CI) | 93.7 (82.6–103.3) | ||
| Follow-up time (month), median (interquartile range) | 46.7 (19.6–84.7) | ||
| Time to death (month), median (interquartile range) | 29.7 (12.6–57.8) | ||
| Time to recurrence (month), median (interquartile range) | 20.7 (12.1–39.0) | ||
| Average platelet count (109/L), median (range)b | 257 (52.8–727) | ||
| Maximum platelet count (109/L), median (range)b | 270 (64–1,246) | ||
| Minimum platelet count (109/L), median (range)b | 241 (32–694) | ||
| Platelet count measured frequency, median (range) | 2 (1–31) | ||
SEM standard error
P value for comparison between NR and total recurrence;
P value for LRR and no recurrence;
P value for DMR and no recurrence;
P value for LRR and DMR
Platelet counts were based on the average value measured within 1 month before surgery
Platelet counts were based on the average, maximum, or minimum value measured within 1 month before surgery
The Associations of Preoperative Platelet Count and CRC Outcomes
The associations between preoperative platelet count and CRC clinical outcomes (overall survival, overall recurrence, LRR, and DMR) were estimated using multivariate Cox proportional hazard model adjusting all host variables (Table 2). Since platelet has a high turnover (∼1011 platelets are produced by an adult each day) and fluctuates under different pathological conditions [28, 29], to minimize chance findings, we conducted the analyses by testing the average, maximum, and minimum values of multiple measurements of platelet count within 1 month before surgery. The median platelet count was 257.0×10−9/L (range, 52.8– 727.0) by the average, 270 (range, 64–1246) by the maximum, and 241 (range, 32–694) by the minimum value (Table 1). The results of the three tests were similar. However, the results based on the maximum value exhibited the highest significance for all four endpoints. For the overall survival analysis, compared to the normal range of platelet count (<400×109/L), thrombocytosis (platelet count ≥400× 109/L) conferred a significantly shortened overall survival with an HR of 1.66 (95 % CI 1.34–2.05, P=2.6×10−6) based on the use of the maximum value. The HR was 1.54 (1.19–1.99, P=9.1×10−4) and 1.32 (0.98–1.79, P=0.071) when using the average and minimum values, respectively. For the overall recurrence analysis, patients with thrombocytosis by the maximum value had an increased HR of 1.90 (1.24–2.93, P=0.003). This association was only evident in patients with DMR (HR=2.81, 1.67–4.74, P= 1.1×10−4). In comparison, thrombocytosis tended to have a protective effect in relation to LRR, although the association was not statistically significant (HR=0.59, 0.21–1.68, P= 0.325). Analyses by the average and minimum values exhibited similar associations with attenuated effects (Table 2). We also conducted an internal validation by conducting 500 bootstrap resampling and calculated the number of times that the bootstrap P value was <0.01 (Table 2). Consistent with the main effects analyses, bootstrap analysis using thrombocytosis determined by the maximum platelet count resulted in more than 90 % of times with a P<0.01 for both overall survival and DMR analyses, whereas 74 % of times for the overall recurrence analysis and zero time for the LRR analysis (Table 2). Kaplan–Meier curves and log rank tests also indicated that the maximum value of platelet count had the best discriminative capacity of patient outcomes. As shown in Fig. 2, all three tests indicated a significant difference between the thrombocytosis and the normal groups: thrombocytosis determined using the maximum value resulted in the highest discrimination in the analyses of overall survival (log rank P=1.1×10−11), overall recurrence (log rank P=0.003), and DMR (log rank P=2.6×10−6) (Fig. 2e, f, and h, respectively). Similar but attenuated effects were observed in the analyses by the average (Fig. 2a, b, and d, respectively) or the minimum value (Fig. 2i, j, and l, respectively). Also in line with the Cox analysis results, thrombocytosis had a nonsignificant protective effect on LRR when determined by the average (log rank P=0.131), maximum (log rank P=0.152), or minimum (log rank P=0.112) platelet count value (Fig. 2c, g, and k, respectively).
Table 2. Associations between thrombocytosis and CRC outcomes by average, maximum, and minimum values of platelet count measured 1 month before surgery.
| Platelet countsa | Overall survival (n = 1,513) | Overall recurrence (n=779) | LRR (n =679) | DMR (n =710) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||||||
| Alive | Dead | HR (95 % CI)b | P | No | Yes | HR (95 % CI)b | P | No | Yes | HR (95 % CI)b | P | No | Yes | HR (95 % CI)b | P | |
| By average value | ||||||||||||||||
| Normal range | 818 | 542 | 1 (reference) | 563 | 140 | 1 (reference) | 563 | 59 | 1 (reference) | 563 | 74 | 1 (reference) | ||||
| Thrombocytosis | 71 | 82 | 1.54 (1.19 –1.99) | 9.1×10−4 | 55 | 21 | 1.54(0.91–2.60) | 0.109 | 55 | 2 | 0.45 (0.11–1.89) | 0.274 | 55 | 18 | 2.14 (1.17– 3.94) | 0.014 |
| Log rank P | 5.4×10−5 | 0.061 | 0.131 | 3.6×10−4 | ||||||||||||
| No. of times with a bootstrap P<0.01 | 418 | 77 | 1 | 184 | ||||||||||||
| By maximum value | ||||||||||||||||
| Normal range | 790 | 492 | 1 (reference) | 537 | 128 | 1 (reference) | 537 | 57 | 1 (reference) | 537 | 65 | 1 (reference) | ||||
| Thrombocytosis | 99 | 132 | 1.66 (1.34–2.05) | 2.6 ×10−6 | 81 | 33 | 1.90 (1.24–2.93) | 0.003 | 81 | 4 | 0.59 (0.21–1.68) | 0.325 | 81 | 27 | 2.81 (1.67–4.74) | 1.1 × 10−4 |
| Log rank P | 1.1×10−11 | 0.003 | 0.152 | 2.6×l0−6 | ||||||||||||
| No. of times with a bootstrap P<0.01 | 493 | 370 | 0 | 460 | ||||||||||||
| By minimum value | ||||||||||||||||
| Normal range | 839 | 570 | 1 (reference) | 580 | 144 | 1 (reference) | 580 | 60 | 1 (reference) | 580 | 76 | 1 (reference) | ||||
| Thrombocytosis | 50 | 54 | 1.32(0.98–1.79) | 0.071 | 38 | 17 | 1.80 (1.03–3.16) | 0.040 | 38 | 1 | 0.30 (0.04–2.20) | 0.235 | 38 | 16 | 2.93 (1.57–5.45) | 7.1×10−4 |
| Log rank P | 0.009 | 0.063 | 0.112 | 7.9×10−5 | ||||||||||||
| No. of times with a bootstrap P<0.01 | 249 | 120 | 0 | 321 | ||||||||||||
Average, maximum, and minimum values of platelet count were calculated within 1 month before surgery. Normal range<400 × 109 /L; thrombocytosis≥400 × 109 /L
HRs adjusting for age, gender, ethnicity, smoking, drinking, primary tumor site, tumor stage, tumor grade, chemotherapy, and radiation therapy p = 0.05 were considered as statistical significance and are bold highlighted in the table
Fig. 2.
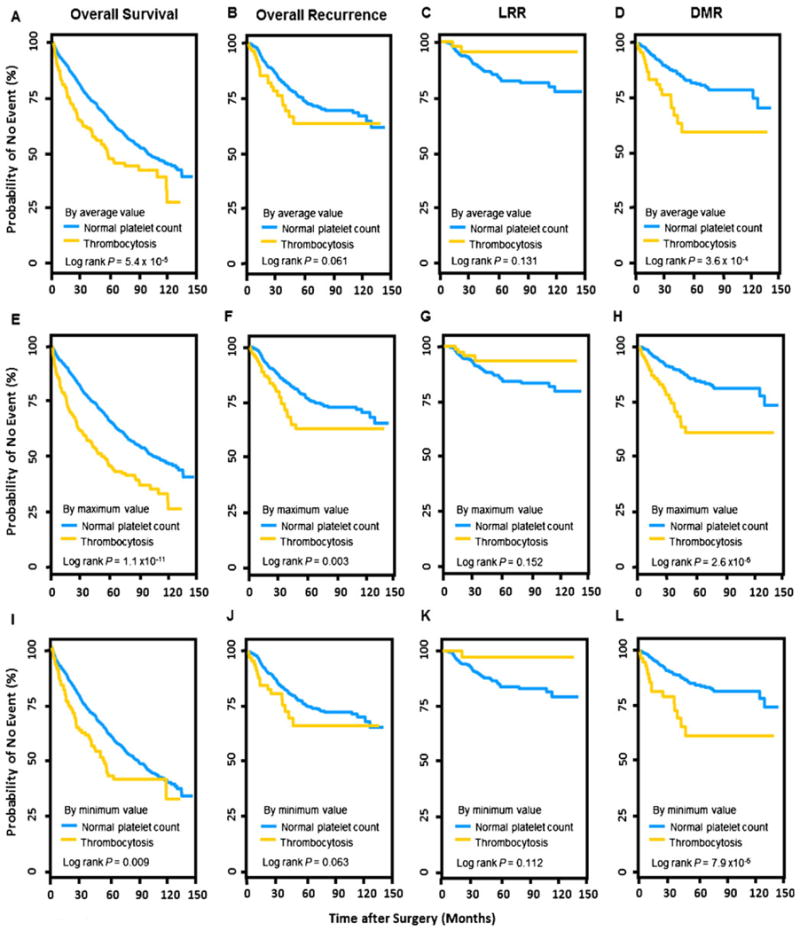
Kaplan–Meier curves of the analyses between platelet count and four clinical endpoints including overall survival, overall recurrence, LRR, and DMR, evaluated by the average (a to d), maximum (e to h), or minimum (i to l) values of platelet count levels measured within 1 month before surgery. LRR locoregional recurrence, DMR distant metastatic recurrence
Dose-Dependent Effects of Preoperative Platelet Count on CRC Outcomes
For all subsequent analyses, we used the maximum platelet count value which appeared to have the best predictive capacity. In addition, the analyses were restricted to overall survival and DMR that were validated by bootstrap resampling. The analyses in Table 2 were conducted based on a clinically determined cut-off (400×109/L) that separated the patients into high and normal groups. We further evaluated whether the effects were dose dependent using a fractional polynomial regression model [30]. For both overall survival and DMR analyses, we noticed an apparent trend of increasing HRs along with increasing platelet counts (Supplementary Fig. 1). The curve slope of the DMR analysis (Supplementary Fig. 1A) was steeper and the confidence interval was wider than the survival analysis (Supplementary Fig. 1B), likely due to the smaller number of patients in the DMR than the survival analysis (710 vs. 1,513).
Stratified Analyses of the Effects of Preoperative Platelet Count on CRC Outcomes
We conducted stratified and interaction analyses of the effects of the maximum value of platelet count on overall survival and DMR by all the host variables including age, gender, ethnicity, smoking status, drinking status, primary tumor site, tumor stage, tumor grade, chemotherapy, and radiation therapy. For both overall survival and DMR, a significant effect conferred by thrombocytosis retained in the majority of the stratified analyses (Supplementary Table 1). In the overall survival analysis, a significant interaction (P for interaction=0.038) was observed between maximum platelet count value and tumor grade. In the DMR analysis, significant interactions were observed between maximum platelet count value and gender (P for interaction=0.016) and drinking status (P for interaction=0.023) (Supplementary Table 1).
Distributions of Preoperative Platelet Count over Time Before Surgery
We then compared the maximum platelet count values measured within 1 month before surgery for each patient by different clinical outcomes. In line with the findings of the main effects analysis that high platelet count conferred unfavorable survival and DMR, we observed a significantly higher platelet count in deceased than living patients (P<0.0001, Fig. 3a, left panel) and in DMR than LRR (P=0.004) or NR (P<0.0001) patients (Fig. 3a, right panel). However, no significant difference was observed between LRR and NR patients (P=0.567) (Fig. 3a, right panel). These analyses using the maximum values were consistent with those using the average values (Table 1). Furthermore, we assessed the distributions of seven time points of maximum platelet count over the period of 1 year before surgery and noticed a persistently higher level of platelet count in deceased than living patients (P=0.001, Fig. 3b, left panel) and in DMR than LRR (P<0.0001) or NR patients (P=0.0004) patients (Fig. 3b, right panel). Consistently, there was no difference between LRR and nonrecurrent patients (P=0.979, Fig. 3b, right panel).
Fig. 3.
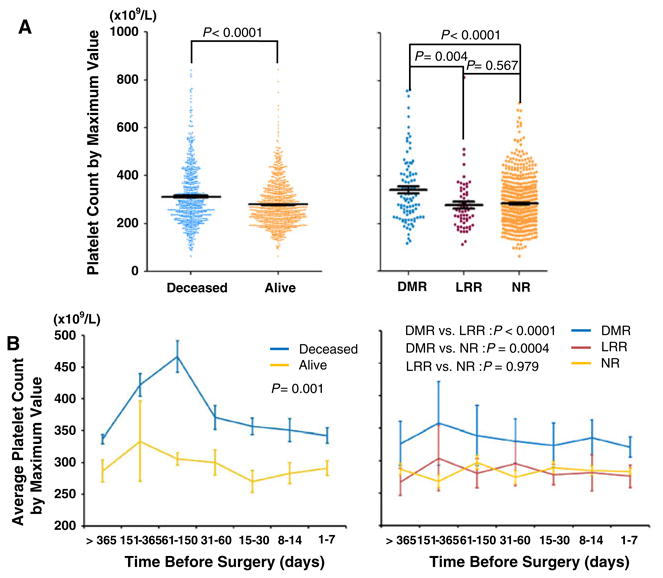
The distributions of the maximum value of platelet count by CRC clinical outcomes. a Comparisons of platelet count values between deceased and alive patients (left panel) and between DMR, LRR, and NR patients (right panel). b Comparisons of average value of platelet count measured in different time periods before surgery between deceased and alive patients (left panel) and between DMR, LRR, and NR patients (right panel). The platelet count values were presented as average ± standard error. LRR locoregional recurrence, DMR distant metastatic recurrence, NR no recurrence
Time-Dependent Effects of Preoperative Platelet Count on 5-Year CRC Survival and Metastatic Recurrence After Surgery
Finally, we analyzed time-dependent effects of preoperative platelet count on the 5-year overall survival and metastatic recurrence after surgery, using the flexible parametric modeling framework adjusting all major host variables (Fig. 4). We found that the increased risk of death by preoperative platelet count persisted over the 5-year period, and the risk decreased over time and became nonsignificant (the lower limit of confidence interval reached 1) at about over 3 years after surgery (Fig. 4a). The effects on metastasis also persisted over the 5-year period, but the risk increased over time after an initial U shape decrease at 15 months after surgery (Fig. 4b). Although the confidence interval increasingly widened along with time which was mostly due to the smaller number of patients with longer follow-up, its lower limit did not reach 1 until approximately four and half years, indicating the observation remained statistically significant across most of the 5-year period (Fig. 4b). These results were consistent with the main effects analysis and strongly corroborated the role of preoperative platelet count in predicting CRC prognosis, especially distant metastasis.
Fig. 4.
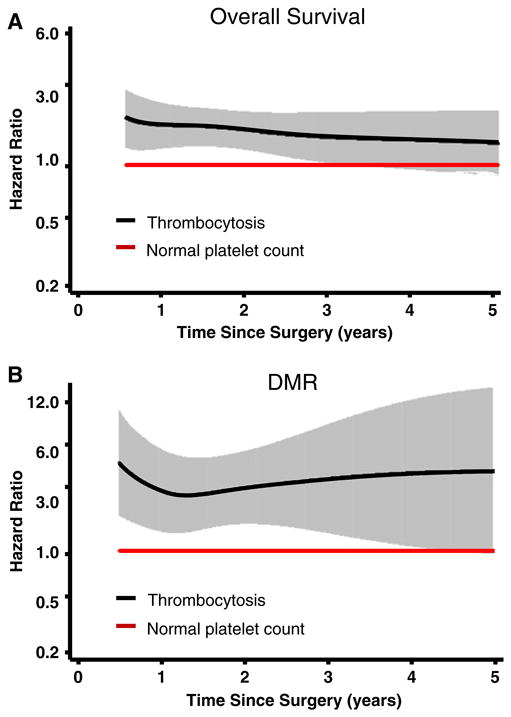
Time-dependent effect of platelet count on CRC clinical outcomes. Flexible parametric modeling was used to predict the effect by platelet count on the 5-year a overall survival and b distant metastatic recurrence (DMR) after surgery. The analyses were adjusted for age, gender, ethnicity, smoking status, drinking status, primary tumor site, tumor stage, tumor grade, chemotherapy, and radiation therapy. Solid lines, hazards ratios; shaded areas, 95 % confidence intervals
Discussion
In this study, we evaluated the prognostic value of preoperative platelet count in surgically resected CRC patients. Our data converged on the conclusion that that thrombocytosis patients with a clinically high platelet count measured within 1 month before surgery had a significantly unfavorable overall survival and were more likely to develop distant metastasis after surgery.
Platelets are made from mature bone marrow megakaryocytes and play an important role in arresting hemorrhage after tissue trauma or vascular injury [31]. Abnormal platelet count is generally classified into primary thrombocytosis (thrombocythemia), which is commonly caused by myeloproliferative disorders, and secondary thrombocytosis (reactive thrombocytosis), which is commonly a result of external causes such as chronic inflammation, cancers, and surgery-related tissue damage [29, 32]. The abnormal platelet count observed in our study mainly refers to reactive thrombocytosis caused by CRC. Reactive thrombocytosis has been linked to the advanced stage, tumor aggressiveness, and prognosis in a few published retrospective studies of different malignancies with relatively small populations and mixed findings [19–26]. Our results indicated that thrombocytosis determined by the maximum value of pre-operative platelet count was a strong predictor of CRC clinical outcomes. The maximum value of platelet count was more conservative than the average value, since patients with only one test of abnormally high platelet count were defined as having thrombocytosis. In comparison, patients in the normal group were those who had persistently normal values of platelet count. The comparison between the results using the maximum and average value indicated that patients with only one test showing an abnormally high platelet count were at an increased risk of death and metastasis. The use of persistently normal platelet count to determine thrombocytosis has also been reported in studies to predict the prognosis of other cancers [21, 33].
The most notable and innovative finding of this study is that high platelet count was significantly associated with distant metastatic, but not locoregional recurrence of CRC. Previous evidence has suggested that platelets play a prominent role in the process of cancer metastasis. For example, it was reported that disseminated tumor cells can induce platelet aggregation which promotes their survival in the circulation system by protecting and cloaking circulating tumor cells from physical damage and immune clearance [34, 35]. The formation of metastasis in distant organs essentially depends on the stimulation of growth factors and generation of neovessels. Various studies have demonstrated that platelet releasates such as platelet-derived growth factor, vascular endothelial growth factor, angiopoetins, and platelet-derived lysophosphatidic acid are major sources of growth factors supporting tumor growth and tumor microenvironment angiogenesis [36–38]. A recent animal study reported that direct platelet–tumor cell contact synergistically activates the TGFB/SMAD and NFKB pathways in colon adenocarcinoma cells, resulting in augmented EMT in tumor cells and subsequently enhanced metastasis [18]. Inhibition of these two pathways solely in platelets suppresses metastasis in vivo, indicating that platelet may be a potential intervention target for metastasis prevention [18]. However, despite the strong evidence from these basic studies, no hospital cohort-based epidemiological study has been reported to determine whether preoperative platelet count may directly predict the metastatic recurrence in cancer patients. In the present study, we demonstrated for the first time using a hospital cohort-based approach that preoperative platelet count was significantly associated with metastasis after surgery. Moreover, the lack of association between platelet count and locoregional recurrence (Table 2) further underscored the role of platelets in CRC metastasis, which was highly consistent with the findings of basic studies that platelets mainly function to promote the survival of tumor cells after they are disseminated into the circulation system.
Another potentially important finding of this study was that the time-dependent effects of platelet count on CRC death and metastasis after surgery exhibited different patterns. The high risk for death conferred by high platelet count decreased over time after surgery (Fig. 4a), but increased steadily for metastasis after a U shape decrease at 15 months after surgery (Fig. 4b). Clinically, it is commonly recognized that the long-term risks of death and recurrence decrease with time after treatments, consistent with the result of Fig. 4a. However, patients might develop distant metastasis many years post-treatments [39]. It has been suggested that metastasis is an inefficient process during which a small set of disseminated cells may remain latent for an extended period but undergo genetic and epigenetic changes and interact with host microenvironments to stimulate tumor regrowth in distant organs [39]. Since platelets have been shown to interact with tumor cells to promote CRC metastasis [18], it is worthwhile to further determine the longitudinal values of platelet count after surgery to identify time-dependent patterns that are consistent with the result of Fig. 4b. Additional longitudinal studies or clinical trials with extended follow-up are warranted to answer these questions.
There are several strengths of our study. Our study has a relatively large population with more than 1,500 CRC patients from a single institute. The analyses were restricted to platelet count measured within 1 month before surgery to minimize the influences of non-CRC causes on platelet count, because factors including surgery, chemotherapy, radiation therapy, or other major tissue damages may also affect platelet levels [40]. Furthermore, we analyzed platelet count of different time points over 1 year before surgery and obtained similar results (Fig. 3). The study was focused on the comprehensive analysis of a single variable and, thus, did not have the multiple comparison issue. The findings were highly statistically significant in both the Cox regression and the log rank analyses with strict internal validation using bootstrap resampling, suggesting the possibility of false-positive findings unlikely. Our study also has limitations. We were not able to assess the effect of variability of platelet counts measured at different time points of individual patients on CRC prognosis. This limitation is mainly due to the fact that this study was based on a retrospective analysis of archived data but not a prospectively designed cohort, and thus, longitudinal data points were not available for each patient and not comparably distributed in different patients and different time points. Future studies with prospective and longitudinal patient populations are needed to address this issue and further confirm our findings. Also, the results of some stratified analyses had a limited number of patients and the results of those analyses should to be interpreted with caution. Collectively, our data, although statistically significant and biologically plausible, need to be further substantiated in more rigorous studies using large independent and prospective populations.
In summary, we ascertained that preoperative platelet count independently predicted the overall survival and distant metastasis of CRC patients after surgery. Although platelet functions in cancer metastasis as suspected in basic studies, this is the first hospital cohort-based study suggesting that platelet count may directly predict the distant metastasis in CRC patients. Since clinically high platelet count has been reported to be present in up to 57 % of cancer patients, the finding of our study is significant at the population level [41]. Bootstrap validation suggested the findings are unlikely to be false positive, although additional validations using independent populations are still warranted. Preoperative laboratory blood tests of various clinical variables including platelet count are highly automatically and routinely used in CRC management. If our findings are validated, platelet count may serve as a cost-effective and noninvasive marker that might be incorporated with other clinical variables to build up prediction models for CRC survival and metastasis. Meanwhile, platelets are not only merely a prognosis predictor, but also play an indispensable functional role in promoting cancer metastasis. Thus, it remains an important question as to whether reducing preoperative platelet counts can help prevent metastatic recurrence after surgery in CRC patients.
Supplementary Material
Acknowledgments
The work was supported by National Cancer Institute Grants CA153099, CA152703, and CA162201 and a Research Scholar Award from the V Foundation for Cancer Research.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12029-013-9491-9) contains supplementary material, which is available to authorized users.
Conflict of Interest: The authors declare that they have no conflict of interest.
Contributor Information
Shaogui Wan, Division of Population Science, Department of Medical Oncology, Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA 19107, USA.
Ronald E. Myers, Division of Population Science, Department of Medical Oncology, Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA 19107, USA
Bingshan Li, Center for Human Genetics Research, Department of Molecular Physiology and Biophysics, Vanderbilt University, Nashville, TN 37232, USA.
Terry Hyslop, Division of Biostatistics, Department of Pharmacology, and Experimental Therapeutics, Thomas Jefferson University, Philadelphia, PA 19107, USA.
Jack London, Department of Cancer Biology, Thomas Jefferson University, Philadelphia, PA 19107, USA.
Devjani Chatterjee, Department of Cancer Biology, Thomas Jefferson University, Philadelphia, PA 19107, USA.
Juan P. Palazzo, Department of Pathology, Thomas Jefferson University, Philadelphia, PA 19107, USA
Ashlie L. Burkart, Department of Pathology, Thomas Jefferson University, Philadelphia, PA 19107, USA
Kejin Zhang, Division of Population Science, Department of Medical Oncology, Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA 19107, USA.
Jinliang Xing, Department of Cell Biology, Fourth Military Medical University, Xi'an 710032, China.
Hushan Yang, Email: Hushan.Yang@jefferson.edu, Division of Population Science, Department of Medical Oncology, Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA 19107, USA.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D. Genetic prognostic and predictive markers in colorectal cancer. Nature reviews. Cancer. 2009;9:489–99. doi: 10.1038/nrc2645. [DOI] [PubMed] [Google Scholar]
- 3.Chau I, Allen MJ, Cunningham D, Norman AR, Brown G, Ford HE, et al. The value of routine serum carcino-embryonic antigen measurement and computed tomography in the surveillance of patients after adjuvant chemotherapy for colorectal cancer. J Clin Oncol: official j Am Soc Clin Oncol. 2004;22:1420–9. doi: 10.1200/JCO.2004.05.041. [DOI] [PubMed] [Google Scholar]
- 4.Arriola E, Navarro M, Pares D, Munoz M, Pareja L, Figueras J, et al. Imaging techniques contribute to increased surgical rescue of relapse in the follow-up of colorectal cancer. Dis Colon Rectum. 2006;49:478–84. doi: 10.1007/s10350-005-0280-9. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Moranta F, Salo J, Arcusa A, Boadas J, Pinol V, Bessa X, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J Clin Oncol: official j Am Soc Clin Oncol. 2006;24:386–93. doi: 10.1200/JCO.2005.02.0826. [DOI] [PubMed] [Google Scholar]
- 6.Safi F, Beyer HG. The value of follow-up after curative surgery of colorectal carcinoma. Cancer Detect Prev. 1993;17:417–24. [PubMed] [Google Scholar]
- 7.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 8.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature reviews Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 9.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature reviews Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 10.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nature reviews Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nature reviews Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 13.Nash GF, Turner LF, Scully MF, Kakkar AK. Platelets and cancer. Lancet Oncol. 2002;3:425–30. doi: 10.1016/s1470-2045(02)00789-1. [DOI] [PubMed] [Google Scholar]
- 14.Gupta GP, Massague J. Platelets and metastasis revisited: a novel fatty link. J Clin Invest. 2004;114:1691–3. doi: 10.1172/JCI23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erpenbeck L, Schon MP. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood. 2010;115:3427–36. doi: 10.1182/blood-2009-10-247296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nature reviews Cancer. 2011;11:123–34. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9:237–49. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 18.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial–mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–90. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monreal M, Fernandez-Llamazares J, Pinol M, Julian JF, Broggi M, Escola D, et al. Platelet count and survival in patients with colorectal cancer—a preliminary study. Thromb Haemost. 1998;79:916–8. [PubMed] [Google Scholar]
- 20.Sasaki K, Kawai K, Tsuno NH, Sunami E, Kitayama J. Impact of preoperative thrombocytosis on the survival of patients with primary colorectal cancer. World J Surg. 2011;36:192–200. doi: 10.1007/s00268-011-1329-7. [DOI] [PubMed] [Google Scholar]
- 21.Symbas NP, Townsend MF, El-Galley R, Keane TE, Graham SD, Petros JA. Poor prognosis associated with thrombocytosis in patients with renal cell carcinoma. BJU Int. 2000;86:203–7. doi: 10.1046/j.1464-410x.2000.00792.x. [DOI] [PubMed] [Google Scholar]
- 22.Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366:610–8. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gucer F, Moser F, Tamussino K, Reich O, Haas J, Arikan G, et al. Thrombocytosis as a prognostic factor in endometrial carcinoma. Gynecol Oncol. 1998;70:210–4. doi: 10.1006/gyno.1998.5078. [DOI] [PubMed] [Google Scholar]
- 24.Lopes A, Daras V, Cross PA, Robertson G, Beynon G, Monaghan JM. Thrombocytosis as a prognostic factor in women with cervical cancer. Cancer. 1994;74:90–2. doi: 10.1002/1097-0142(19940701)74:1<90::aid-cncr2820740116>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Sagman U, Maki E, Evans WK, Warr D, Shepherd FA, Sculier JP, et al. Small-cell carcinoma of the lung: derivation of a prognostic staging system. J Clin Oncol: official j Am Soc Clin Oncol. 1991;9:1639–49. doi: 10.1200/JCO.1991.9.9.1639. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen LM, Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. EurRespir j:official J Eur Soc Clin Respir. 1996;9:1826–30. doi: 10.1183/09031936.96.09091826. [DOI] [PubMed] [Google Scholar]
- 27.Henderson AR. The bootstrap: a technique for data-driven statistics. Using computer-intensive analyses to explore experimental data. Clin Chim Acta Int J Clin Chem. 2005;359:1–26. doi: 10.1016/j.cccn.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Kaushansky K. Historical review: megakaryopoiesis and thrombopoiesis. Blood. 2008;111:981–6. doi: 10.1182/blood-2007-05-088500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schafer AI. Thrombocytosis. N Engl J Med. 2004;350:1211–9. doi: 10.1056/NEJMra035363. [DOI] [PubMed] [Google Scholar]
- 30.Royston P. A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med. 2000;19:1831–47. doi: 10.1002/1097-0258(20000730)19:14<1831::aid-sim502>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–94. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 32.Skoda RC. Thrombocytosis. Hematology Am Soc Hematol Educ Program. 2009:159–67. doi: 10.1182/asheducation-2009.1.159. [DOI] [PubMed] [Google Scholar]
- 33.O'Keefe SC, Marshall FF, Issa MM, Harmon MP, Petros JA. Thrombocytosis is associated with a significant increase in the cancer specific death rate after radical nephrectomy. J Urol. 2002;168:1378–80. doi: 10.1016/S0022-5347(05)64453-9. [DOI] [PubMed] [Google Scholar]
- 34.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–85. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 35.Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–300. [PubMed] [Google Scholar]
- 36.Mohle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci U S A. 1997;94:663–8. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10:355–62. doi: 10.1016/j.ccr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, et al. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004;114:1714–25. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griesshammer M, Bangerter M, Sauer T, Wennauer R, Bergmann L, Heimpel H. Aetiology and clinical significance of thrombocytosis: analysis of 732 patients with an elevated platelet count. J Intern Med. 1999;245:295–300. doi: 10.1046/j.1365-2796.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- 41.Sierko E, Wojtukiewicz MZ. Platelets and angiogenesis in malignancy. Semin Thromb Hemost. 2004;30:95–108. doi: 10.1055/s-2004-822974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.