Abstract
Neuropathic pain resulting from spinal hemisection or selective spinal nerve ligation is characterized by an increase in membrane-bound TNF alpha (mTNFα) in spinal microglia without detectable release of soluble TNF alpha (sTNFα). In tissue culture, we showed that full length transmembrane cleavage-resistant TNFα (crTNFα) construct can act through cell-cell contact to activate neighboring microglia. We undertook the current study to test the hypothesis that mTNFα expressed in microglia might also affect the phenotype of primary sensory afferents, by determining the effect of crTNFα expressed from COS-7 cells on gene expression in primary DRG neurons. Co-culture of DRG neurons with crTNFα-expressing COS-7 cells resulted in a significant increase in the expression of voltage gated sodium channel isoforms NaV1.7 and NaV1.8, and voltage gated calcium channel subunit CaV3.2 at both mRNA and protein levels, and enhanced CCL2 expression and release from the DRG neurons. Exposure to sTNFα only produced an increase in CCL2 expression and release. Treatment of the cells with an siRNA against TNFR2 significantly reduced crTNFα-induced gene expression changes in DRG neurons while administration of CCR2 inhibitor had no significant effect on crTNFα-induced increase in gene expression and CCL2 release in DRG neurons. Taken together, the results suggest that mTNFα expressed in spinal microglia can facilitate pain signaling by up-regulating expression of cation channels and CCL2 in DRG neurons in a TNFR2 dependent manner.
Introduction
Tumor necrosis factor alpha (TNFα) is a member of the superfamily of type II transmembrane proteins that is expressed in a full-length membrane bound form (mTNFα) that can be cleaved by the inducible TNFα converting enzyme (TACE) to release the diffusible peptide sTNFα [12]. Animal models of neuropathic pain are characterized by neuroimmune activation in the spinal cord associated with increased expression of TNFα in spinal microglia [6; 17; 19]. We previously observed in models both of neuropathic pain resulting from spinal hemisection and after spinal nerve ligation that the increase in TNFα mRNA is accompanied by an increase in mTNFα expression without detectable release of sTNFα in the spinal cord [10; 18]
In a subsequent study we found that exposure of microglia to substance P (SP) increases the expression of mTNFα without any increase in expression of TACE, and without release of sTNFα. Co-culture of COS-7 cells expressing a mutant TNFα resistant to cleavage by TACE (crTNFα) with microglial cells led to microglial cell activation through direct cell-cell contact [26]. These results suggested a novel pathway through which release of SP by primary afferents activates microglial expression of mTNFα, establishing a feed-forward loop in glia that might contribute to the establishment of chronic pain.
In order to explore whether microglial expression of mTNFα might also affect the phenotype of primary afferents, in the current study we used co-culture of COS-7 cells expressing crTNFα with primary DRG neurons in vitro to determine the effect of crTNFα on the expression of genes whose products are implicated in the pathogenesis of chronic neuropathic pain: the cation channel isoforms NaV1.7 NaV1.8, CaV3.2 and CCL2 [3; 5; 14; 15; 22; 23]. We found that co-culture of DRG neurons with crTNFα-expressing COS-7 cells, but not exposure of the neurons to sTNFα, resulted in an increase in the expression of the voltage gated sodium channel isoforms NaV1.7 and NaV1.8, and the voltage gated calcium channel isoform CaV3.2. Knockdown of the TNFα receptor TNFR2 in DRG neurons using siRNA but not knockdown of the TNFα receptor TNFR1, abrogated the effect of crTNFα on the neuronal phenotype. Taken together, these results indicate a previously unrecognized mechanism through which microglial activation in the spinal cord may contribute to the development of a pro-nociceptive phenotype in primary afferents.
1. Materials and Methods
2.1. Plasmids
Plasmid pGFP-crTNFα which expresses a crTNFα-GFP fusion protein has been described previously [26]. Plasmid pAcGFP1, which expresses control protein green fluoresent protein (GFP) under the control of cytomegalovirus immediate early promoter, was purchased from Clontech (Mountain View, CA).
1.1. Cell culture
COS-7 cells, a derivative of African Green Monkey Kidney cells, which do not express endogenous TNFα [26], were maintained and grown in low glucose Dulbecco’s modified eagle essential medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Atlanta Biologics, Atlanta, GA) and 100 units/ml penicillin in a 5% CO2 atmosphere [26]. Primary dorsal root ganglion (DRG) neurons were dissociated from DRGs dissected from 17-day rat embryos and cultured in Neurobasal medium (Invitrogen) supplemented with B27, Glutamax I, Albumax, Pstrep, and 7.0S nerve growth factor [1]. Co-culture of primary DRG neurons with COS-7 cells was conducted in the same medium as used for primary DRG neuron culture.
1.2. Transfection
COS-7 cells were transfected with pGFP-crTNFα or pAcGFP1 using lipofectamine 2000 as previously described [26]. To knock down the expression of TNFR1 or TNFR2 in primary DRG neurons, cells were transfected with control siRNA or siRNA specific to rat TNFR1 or TNFR2 (ON-TARGET plusSMARTpool; Dharmacon, Chicago, IL) using lipofectamine 2000 (Invitrogen). One day before transfection, culture medium was changed and cells cultured in antibiotics-free neuronal medium and incubated in a 37°C and 5% CO2 atmosphere overnight. siRNA was diluted by Opti-Mem I (Invitrogen) (250 pmole of siRNA diluted into 0.1 ml by opti-Mem I for transfection of one-well cells) and equal amount of 1: 25 diluted lipofectamine 2000 by Opti-Mem I added into diluted siRNA. The mixture was incubated at RT for 20 min and pre-warmed Opti-Mem I (0.2 ml per well-cell transfection) added into the complex. 0.3 ml of siRNA-lipofectamine 2000 mixture was applied to cells per well after DRG cells was washed by 1 ml of pre-warmed Opti-Mem I. Three days after transfection, cells were harvested for determination of TNFR1 and TNFR2 protein levels. To test the effect of knock-down of TNFR1 or TNFR2 by siRNA on crTNFα-induced up-regulation of gene expression in DRG neurons, 2 days after siRNA transfection, COS-7 cells transfected with plasmid DNA 4 hrs after transfection were added onto DRG cells and co-cultured cells harvested for determination of gene expression and CCL2 release 1 day after co-culture.
1.3. Western blot
Cells were harvested using a scraper and collected by centrifugation, then washed in 1 × PBS and re-suspended in RIPA buffer supplemented with a protease inhibitor cocktail (Sigma, St. Louis, MO) and incubated on ice for 10 min. The cell suspension was sonicated, and the disrupted cells incubated on ice for 10 min. Supernatant was collected by centrifugation at 10,000 RPM at 4° C for 10 min. Protein concentrations in lysates were measured by the BCA method (Thermo Scientific, Rockford, IL), and the proteins separated on 4–20% gradient SDS–PAGE gel (Invitrogen) and transferred onto a polyvinylidene difluoride membrane (Millipore, Medford, MA). Immunoblots were incubated with the primary antibody: anti-NaV1.7 or 1.8 (Millipore), anti-CaV3.2 (Sigma), anti-TNFR1, anti-TNFR2 (Santa Cruz Biotechnology, Santa Cruz,CA) or anti-β-actin (Sigma) and subsequently incubated with an HRP-conjugated secondary antibody. Protein bands were visualized using an enhanced chemiluminescent substrate (Thermo Scientific). The amount of protein was quantitated using the chemiluminescence values obtained (ChemiDoc, Bio-Rad, Hercules, CA) and protein levels normalized to β-actin and compared to the control group.
1.4. Quantitative PCR (qPCR)
Total RNA was isolated from cell pellets using RNeasy Plus kit from Qiagen (Germantown, MD) and RNA concentrations measured by spectrophotometry. cDNA was synthesized from 2 µg of total RNA with poly(T) as the primer using the superscript first strand synthesis system (Invitrogen). qPCR was performed using SYBRE green mix (Bio-Rad) under the following conditions: 1 cycle of 95°C/3 min; 40 cycles of 95°C/20s and 60°C/30s. Primers used for qPCR were as follows. CCL2: upper, 5’-ATG CAG TTA ATG CCC CAC TC-3’; lower, 5’-TTC CTT ATT GGG GTC AGC AC-3’. NaV1.7: upper, 5’- GCC ATG GAC CCC TAT GAG TA-3’; lower, 5’-CAA TCT GAA TGA CCG CAG AA-3’. NaV1.8: upper, 5’-CGA GCT CGA GGA AGA TAT GG-3’; lower, 5’- GCC TGG TGG TTT TCA CAC TT-3’. CaV3.2: upper, 5’-CAG AGC TTC CTG GAC AAA CC-3’; lower, 5’-GGG AGG GCT CAT CTT CTT CT-3’. β-actin upper, 5’-AGC AGA TGT GGA TCA GCA AG-3’; lower, 5’-TTT GCG CAA GTT AGG TTT TG-3’. mRNA levels were normalized to β-actin and the relative mRNA levels compared to the control group.
1.5. Enzyme-linked immunosorbent assay (ELISA)
The amount of CCL2 released from DRG neurons was determined using a commercially available ELISA (Thermo Scientific). This ELISA is specific for the measurement of natural and recombinant rat CCL2 with a detection sensitivity of ≤ 5pg/mL.
1.6. Statistical analysis
All experiments were conducted in triplicate. The statistical significance of the difference between groups was determined by Student t-test in one parameter experiments and by ANOVA analysis in multiple comparisons. The significance of difference between groups in multiple comparisons was corrected using Bonferroni’s method. Results are expressed as mean ± SEM.
2. Results
2.1. Co-culture with crTNFα-expressing COS-7 cells induces expression of voltage gated cation channels and CCL2 in DRG neurons
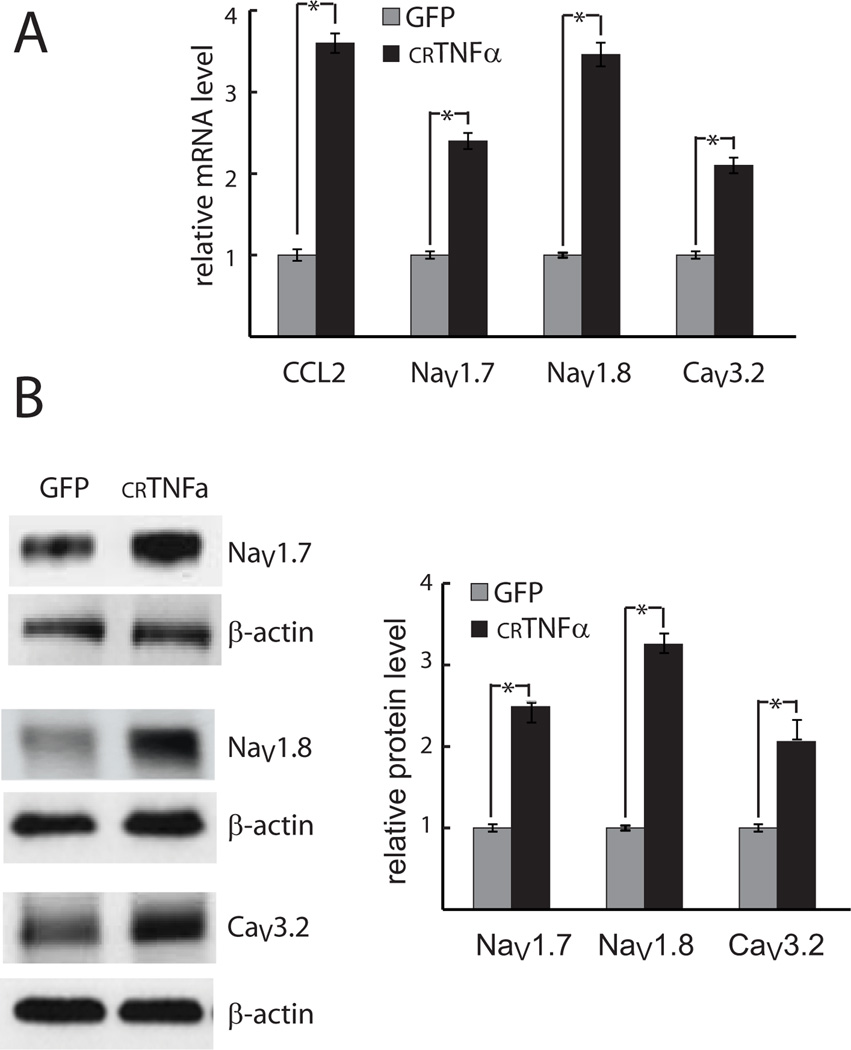
COS-7 cells in 6-well plates were transfected with the crTNFα expression plasmid or control GFP-expressing plasmid. 4 hrs later 1.5 × 105 COS-7 cells suspended in DRG neuron culture medium were placed onto primary DRG neurons (3 × 105 cells per well). Cells were harvested after 1-day co-culture. DRG neurons exposed to crTNFα-expressing COS-7 cells showed an increase in NaV1.7, NaV1.8, CaV3.2 and CCL2 mRNA expression (Fig.1A) and NaV1.7, NaV1.8, CaV3.2 protein levels (Fig. 1B). Co-culture with crTNFα-expressing COS-7 cells also enhanced the release of CCL2 from those neurons into the medium (109 ± 5.5 ng/ml observed in co-culture of DRG neurons with COS-7 cells expressing crTNFα versus 42 ± 2.2 ng/ml in co-culture of DRG neurons with COS-7 cells expressing GFP).
Fig. 1. crTNFα increases expression of genes in DRG neurons.
Primary DRG neurons were co-cultured with crTNFα or control (GFP-expressing) COS-7 cells. The expression of voltage-gated channel subunits and CCL2 in DRG neurons was determined at the mRNA (A) and protein (B) levels. * p < .05.
2.2. The effect of crTNFα on neuronal gene expression is distinct from the effect of sTNFα on the same cells
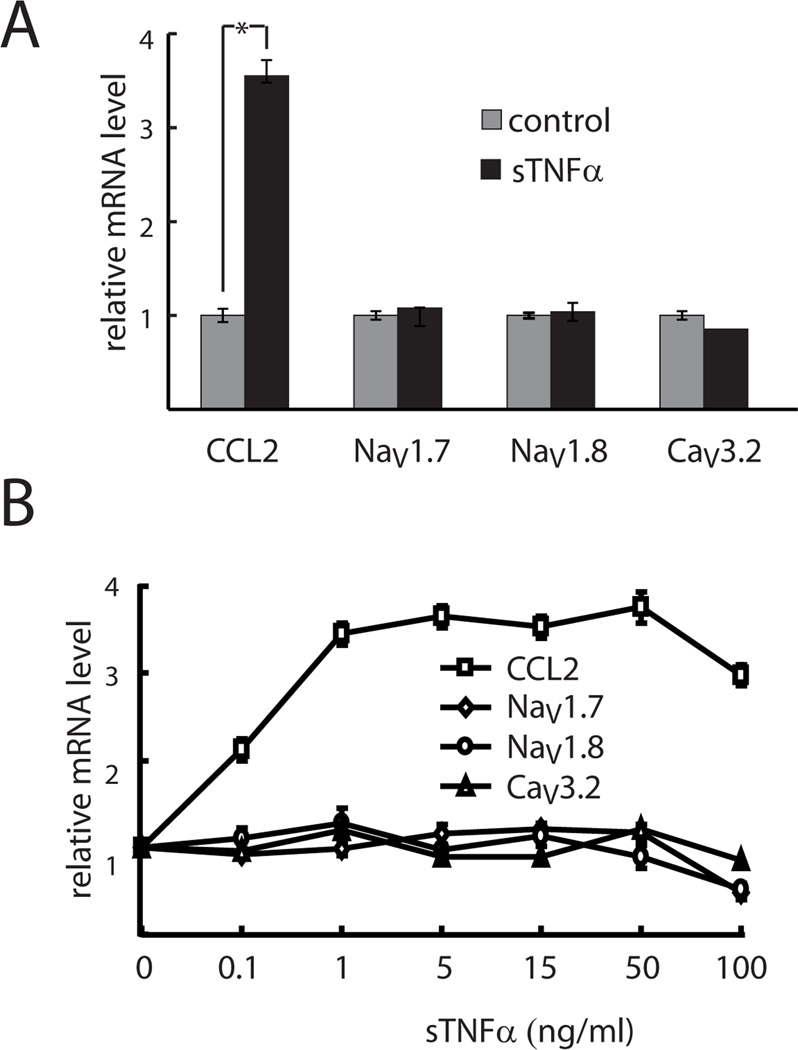
In order to assess whether the effect of crTNFα was specific to the transmembrane form of the cytokine, primary DRG neurons were exposed to 15 ng/ml of sTNFα for 15 hrs. Preliminary studies indicate that the effect of exposure to sTNFα plateaued after 15 hrs (data not shown). Exposure of DRG neurons to sTNFα substantially increased CCL2 mRNA level (Fig.2A) and enhanced the release of CCL2 from DRG neurons into the medium compared with no treatment (49 ± 1.7 versus 19 ± 0.9 ng/ml), but in contrast to the effect of co-culture with crTNFα-expressing COS-7 cells, there was no change in the mRNA expression of NaV1.7, NaV1.8, or CaV3.2 in DRG neurons exposed to sTNFα (Fig. 2A). sTNFα dose experiments indicated 0.1 ng/ml sTNFα induced much less CCL2 mRNA expression (Fig. 2B) (P< .005) and CCL2 release relative to sTNFα treatment of higher concentrations (28 ± 1.5 versus 47 ± 2.8 – 50.5 ± 3.2 ng/ml released into the medium). 100 ng/ml sTNFα resulted in less NaV1.7 and NaV1.8 mRNA expression compared with sTNFα treatment of lower doses (P<.005) (Fig. 2B). But identical results in terms of CCL2 and voltage gated cation channel mRNA expression and CCL2 release (47 ± 2.8 – 50.5 ± 3.2 ng/ml) were found in doses ranging from 1 to 50 ng/ml of sTNFα (Fig. 2B).
Fig. 2. Exposure of DRG neurons to sTNFα does not produce the same changes in gene expression.
Effect of sTNFα on the expression of voltage-gated channel subunits and CCL2 mRNA in DRG neurons. A: Effect of 15 ng/ml sTNFα. B. Effect of sTNFα doses. * p < .05.
2.3. The effect of crTNFα on neuronal gene expression is mediated through TNFR2
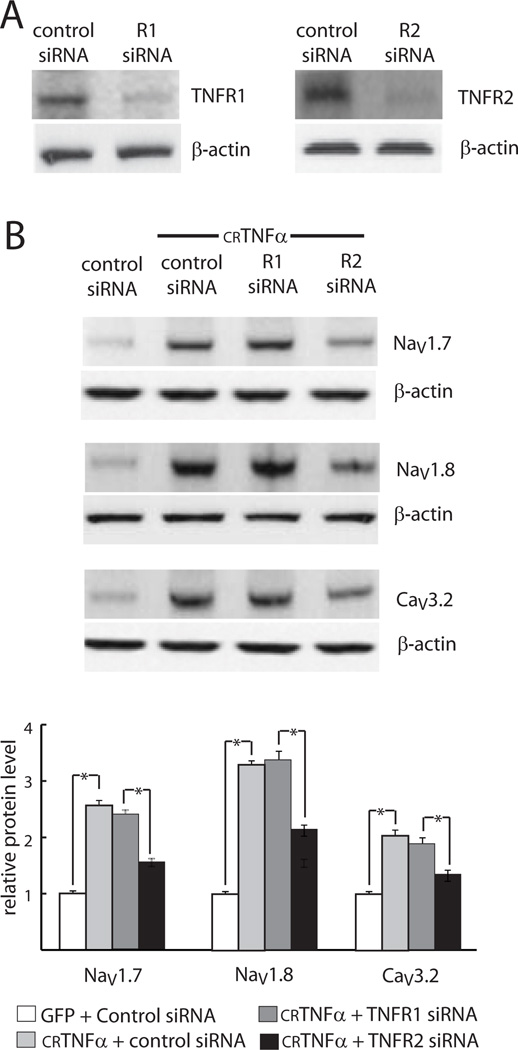
TNFα receptors TNFR1 and TNFR2 have different affinities for forms mTNFα and sTNFα, as well as distinct downstream activation pathways. In order to determine the receptor or receptors involved in mediating the effect of crTNFα on DRG neurons, we tested the effect of knockdown of TNFR1 or TNFR2 by siRNA on crTNFα-induced gene expression in DRG neurons. We first confirmed that siRNA specific to TNFR1 or TNFR2 silenced the expression of TNFR1 and TNFR2 effectively as evidenced by much lower protein levels of TNFR1 (~70 ± 4 % knockdown) and TNFR2 (~75 ± 4.5 % knock-down) observed in DRG neurons receiving target specific siRNA compared with those observed in cells treated with control siRNA (Fig. 3A). To determine which receptor is responsible for the effect of crTNFα on DRG neurons, DRG neurons 2 days after siRNA transfection were co-cultured with COS-7 cells expressing ether control GFP or crTNFα for 24 hrs. Co-culture of DRG neurons receiving control siRNA with crTNFα-expressing COS-7 cells resulted in increased expression of NaV1.7 and NaV1.8 and CaV3.2 protein (Fig. 3B) and CCL2 release (105 ± 6 versus 42 ± 2.7 ng/ml) in DRG neurons compared with co-culture with COS-7 cells expressing GFP, but the effect of co-culture on voltage-gated channel protein expression (Fig. 3B) and CCL release (75 ± 3.5 versus 105 ± 6 ng/ml) was significantly reduced in neurons treated with the TNFR2 siRNA compared with control siRNA. However, up-regulation of gene expression and increase in CCL2 release (99 ± 5.5 versus 105 ± 6 ng/ml) in DRG neurons induced by crTNFα were not impaired by the treatment of TNFR1-specific siRNA compared with control siRNA (Fig. 3B).
Fig. 3. crTNFα acts through TNFR2.
Effect of knock-down of TNFR1 and TNFR2 by siRNA on crTNFα-induced up-regulation of gene expression in DRG neurons. A: Knockdown of TNFR1 and R2 by siRNA (Western blot). B: Protein levels of voltage-gated channel subunits. * p < .05.
2.4. The effect of crTNFα on neuronal gene expression is not mediated through induction of CCL2 release
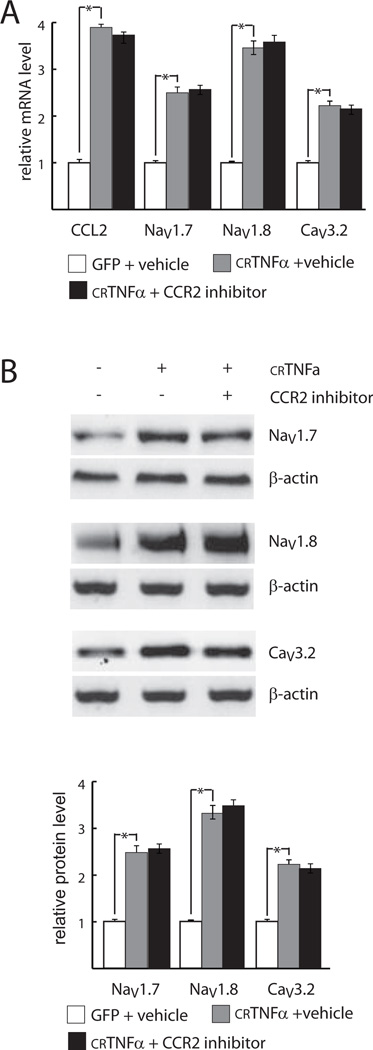
In addition to the observed effect on voltage gated ion channel gene expression, crTNFα stimulated the expression and release of CCL2 from DRG neurons. In order to determine whether CCL2 acting through CCR2 might be responsible for the changes in expression of voltage-gated channels, DRG neurons were treated with 20 nM CCR2 inhibitor [4; 24] (Santa Cruz Biotechnologies) or vehicle (DMSO) and after 4 hrs of inhibitor treatment co-cultured with COS-7 cells expressing GFP or crTNFα. One day later the cells were harvested for determination of the NaV1.7, NaV1.8, CaV3.2 and CCL2 mRNA, NaV1.7, NaV1.8, CaV3.2 protein levels and CCL2 release. The effect of co-culture with crTNFα-expressing COS-7 cells on the expression of voltage gated cation channels and CCL2 mRNA (Fig. 4A), the protein levels of NaV1.7, NaV1.8, CaV3.2 (Fig. 4B) in DRG neurons were not significantly affected by the presence of the CCR2 inhibitor. The CCR2 inhibitor did not influence crTNFα -induced CCL2 release into the medium compared with vehicle treatment (102 ± 4.8 ng/ml in the presence of CCR2 inhibitor versus 106 ± 6.5 ng/ml in the absence of the inhibitor).
Fig. 4. The effects of crTNFα are not mediated through CCL2/CCR2.
Effect of CCR2 inhibition on crTNFα-induced up-regulation of gene expression in DRG neurons. A. mRNA. B. Protein. * p < .05.
3. Discussion
In this study, we found that: 1) contact with crTNFα-expressing COS-7 cells, but not exposure to sTNFα, enhances the expression of voltage-gated channel subunits NaV1.3, NaV1.8 and CaV3.2 at the mRNA and protein levels in DRG neurons; 2) exposure to both crTNFα and sTNFα upregulates CCL2 mRNA expression in DRG neurons and results in release of CCL2 from those cells; 3) the increase in voltage-gated subunit expression is independent of CCL2/CCR2 signaling; and 4) the effect of crTNFα on the DRG neuronal phenotype is mediated through TNFR2.
Chronic pain following nerve injury is characterized by spontaneous pain and by peripheral sensitization resulting in allodynia: a phenomenon in which normally innocuous stimuli are perceived as painful, and hyperalgesia, a phenomenon in which normally painful stimuli perceived as more painful than usual. Both spontaneous pain and peripheral sensitization reflect reduced thresholds for activation of peripheral sensory nerves, an effect that is caused in part by alterations in voltage gated channels that are the critical determinants of neuronal excitability [3; 5; 14; 15; 22].
There is substantial evidence to indicate that peripheral nerve injury results in activation of microglia in the spinal cord, and increased expression of inflammatory cytokines and chemokines by those cells including TNFα [16; 17; 25]}. But in our previous studies in models of neuropathic pain we found that the substantial increase in TNFα mRNA expression in the spinal cord after nerve injury is not accompanied by measurable release of sTNFα [10; 18]. This result correlates with the observation in microglial cells in vitro that exposure to substance P increases the expression of TNFα mRNA and full-length mTNFα protein, but does not cause increased expression of the TNFα cleaving enzyme (TACE) or release of sTNFα from those cells [26]. In our previous study we observed that full-length non-cleavable TNFα (crTNFα) localized in the cell membrane, acting through cell-cell contact, was fully capable of activating neighboring microglia, indicating one mechanism through which spread of sensitization might occur at the spinal level [10; 18]. The current study extends those results by indicating mTNFα expressed in the membrane of microglial cells, through cell-cell interactions with afferent nerve terminals, may modulate the expression of voltage-gated channels in the DRG neurons projecting to the dorsal horn.
What mechanism might be responsible for the differential effects of sTNFα and mTNFα that we observed? In other model systems it has been shown that sTNFα rapidly binds to TNFR1 with high affinity (Kd 19 pm) and a slow dissociation from the receptor once bound (t1/2=33 min), a process which efficiently activates TNFR1. The dissociation kinetics of sTNF from native TNFR2 is approximately 20 – 30 fold faster than from TNFR1 and the affinity significantly less than sTNF’s affinity for TNFR1 [7; 9]. It is not clear how the binding characteristics of membrane-bound TNF at TNFR1 and TNFR2 compare to the binding characteristics of sTNFα, but it is well-known that slight structural changes in the TNFα sequence can lead to dramatic changes in its binding characteristics to TNF receptors. In DRG neurons specific effects of sTNFα acting through TNFR1 have been reported [13], and distinct effects of mTNFα acting through TNFR2 have been identified in the immune system [2].
We demonstrated in this study that full-length uncleaved TNFα produces an increase not only in mRNA but also in protein levels of NaV1.3, NaV1.8 and CaV3.2 voltage-gated channel proteins in DRG neurons. In this study we have not directly assessed the function of these channels in cultured neurons, but all of these alterations by increasing the number of available channels would be expected to increase neuronal excitability and thus could serve to produce both spontaneous pain and the hypersensitive state characteristic of neuropathic pain.
Peripheral nerve hyperexcitability is characteristic of the hypersensitivity state that is observed in models of inflammatory pain, a process in which peripheral release of sTNFα and other cytokines have been shown to play an important role [17]. In the current study, we found that the effect of crTNFα on gene expression in DRG neurons is distinct from the effect of exposure of the same cells to sTNFα. By knockdown experiments we found evidence that the effect of crTNFα on neuronal gene expression is achieved through selective activation of the TNFα receptor TNFR2. This result is consistent with studies in immune and neuron cells that indicate that sTNFα preferentially activates TNFR1 [2; 11; 20; 21] while mTNFα typically acts through TNFR2 [8].
The observations in the current study indicating that mTNFα can activate DRG neurons to upregulate the expression of voltage-gated channel subunit proteins and the chemokine CCL2 through TNFR2 have potentially important implications for understanding mechanisms that would facilitate the persistence of neuropathic pain. Further studies will be required to explore this effect in vivo, and to determine whether selective block of this interaction may provide a novel therapy for the treatment of neuropathic pain.
Acknowledgements
These studies were supported by grants from the Department of Veterans Affairs (to MM and DJF) and the NIH NS038850 and NS069378.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing interests.
References
- 1.Chattopadhyay M, Krisky D, Wolfe D, Glorioso JC, Mata M, Fink DJ. HSV-mediated gene transfer of vascular endothelial growth factor to dorsal root ganglia prevents diabetic neuropathy. Gene Ther. 2005;12(18):1377–1384. doi: 10.1038/sj.gt.3302533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Oppenheim JJ. Contrasting effects of TNF and anti-TNF on the activation of effector T cells and regulatory T cells in autoimmunity. FEBS Lett. 2011;585(23):3611–3618. doi: 10.1016/j.febslet.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng KI, Wang HC, Chang LL, Wang FY, Lai CS, Chou CW, Tsai HP, Kwan AL. Pretreatment with intrathecal amitriptyline potentiates anti-hyperalgesic effects of post-injury intra-peritoneal amitriptyline following spinal nerve ligation. BMC Neurol. 2012;12:44. doi: 10.1186/1471-2377-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherney RJ, Mo R, Meyer DT, Nelson DJ, Lo YC, Yang G, Scherle PA, Mandlekar S, Wasserman ZR, Jezak H, Solomon KA, Tebben AJ, Carter PH, Decicco CP. Discovery of disubstituted cyclohexanes as a new class of CC chemokine receptor 2 antagonists. J Med Chem. 2008;51(4):721–724. doi: 10.1021/jm701488f. [DOI] [PubMed] [Google Scholar]
- 5.Dong XW, Goregoaker S, Engler H, Zhou X, Mark L, Crona J, Terry R, Hunter J, Priestley T. Small interfering RNA-mediated selective knockdown of Na(V)1.8 tetrodotoxin-resistant sodium channel reverses mechanical allodynia in neuropathic rats. Neuroscience. 2007;146(2):812–821. doi: 10.1016/j.neuroscience.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 6.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126(1):56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grell M, Becke FM, Wajant H, Mannel DN, Scheurich P. TNF receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. Eur J Immunol. 1998;28(1):257–263. doi: 10.1002/(SICI)1521-4141(199801)28:01<257::AID-IMMU257>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, Scheurich P. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83(5):793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 9.Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A. 1998;95(2):570–575. doi: 10.1073/pnas.95.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hao S, Mata M, Glorioso JC, Fink DJ. Gene transfer to interfere with TNFalpha signaling in neuropathic pain. Gene Ther. 2007;14:1010–1016. doi: 10.1038/sj.gt.3302950. [DOI] [PubMed] [Google Scholar]
- 11.He XH, Zang Y, Chen X, Pang RP, Xu JT, Zhou X, Wei XH, Li YY, Xin WJ, Qin ZH, Liu XG. TNF-alpha contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain. 2010;151(2):266–279. doi: 10.1016/j.pain.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005;115(1):1–20. doi: 10.1111/j.1365-2567.2005.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hensellek S, Brell P, Schaible HG, Brauer R, Segond von Banchet G. The cytokine TNFalpha increases the proportion of DRG neurones expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol Cell Neurosci. 2007;36(3):381–391. doi: 10.1016/j.mcn.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Jeon SM, Lee KM, Cho HJ. Expression of monocyte chemoattractant protein-1 in rat dorsal root ganglia and spinal cord in experimental models of neuropathic pain. Brain Res. 2009;1251:103–111. doi: 10.1016/j.brainres.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 15.Joshi SK, Mikusa JP, Hernandez G, Baker S, Shieh CC, Neelands T, Zhang XF, Niforatos W, Kage K, Han P, Krafte D, Faltynek C, Sullivan JP, Jarvis MF, Honore P. Involvement of the TTX-resistant sodium channel Nav 1.8 in inflammatory and neuropathic, but not post-operative, pain states. Pain. 2006;123(1–2):75–82. doi: 10.1016/j.pain.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi K, Yamanaka H, Yanamoto F, Okubo M, Noguchi K. Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury. Glia. 2012;60(10):1529–1539. doi: 10.1002/glia.22373. [DOI] [PubMed] [Google Scholar]
- 17.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115(1–2):71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Peng X, Zhou Z, Glorioso JC, Fink DJ, Mata M. Tumor necrosis factor alpha contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol. 2006;59:843–851. doi: 10.1002/ana.20855. [DOI] [PubMed] [Google Scholar]
- 19.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306(2):624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 20.Schafers M, Sommer C, Geis C, Hagenacker T, Vandenabeele P, Sorkin LS. Selective stimulation of either tumor necrosis factor receptor differentially induces pain behavior in vivo and ectopic activity in sensory neurons in vitro. Neuroscience. 2008;157(2):414–423. doi: 10.1016/j.neuroscience.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 21.Sommer C, Schmidt C, George A. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp Neurol. 1998;151(1):138–142. doi: 10.1006/exnr.1998.6797. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka T, Minami M, Nakagawa T, Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci Res. 2004;48(4):463–469. doi: 10.1016/j.neures.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Wilson-Gerwing TD, Stucky CL, McComb GW, Verge VM. Neurotrophin-3 significantly reduces sodium channel expression linked to neuropathic pain states. Exp Neurol. 2008;213(2):303–314. doi: 10.1016/j.expneurol.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia M, Sui Z. Recent developments in CCR2 antagonists. Expert Opin Ther Pat. 2009;19(3):295–303. doi: 10.1517/13543770902755129. [DOI] [PubMed] [Google Scholar]
- 25.Zheng W, Ouyang H, Zheng X, Liu S, Mata M, Fink DJ, Hao S. Glial TNFalpha in the spinal cord regulates neuropathic pain induced by HIV gp120 application in rats. Mol Pain. 2011;7:40. doi: 10.1186/1744-8069-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Z, Peng X, Hagshenas J, Insolera R, Fink DJ, Mata M. A novel cell-cell signaling by microglial transmembrane TNFalpha with implications for neuropathic pain. Pain. 2010;151(2):296–306. doi: 10.1016/j.pain.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]