Abstract
Objectives
To develop an assay for anti-HE4 antibodies and assess such antibodies in sera from women with increased epidemiologic risk for ovarian cancer (infertility) and patients with ovarian cancer in comparison to controls.
Methods
An ELISA was developed to measure antibodies to recombinant full length HE4 and cut-off values were determined for different levels of specificity (up to 99%).
Results
Infertile women more frequently had anti-HE4 antibodies than controls (23% at 98% specificity, p<0.001) with antibodies most frequent in women with POF (31%) and ovulatory dysfunction (47%). There was also an increased frequency of anti-HE4 antibodies in patients with ovarian cancer (14% at 97% specificity, p<0.01), but more women with certain types of infertility have anti-HE4 antibodies than women with ovarian cancer. Most patients with ovarian cancer have circulating HE4 antigen, which may interfere with detection of antibodies, while the level of HE4 antigen in sera from infertile women was not higher than in normal controls. There was a statistically significant correlation between antibodies to HE4 and antibodies to mesothelin in the same patients.
Conclusions
Women with certain types of infertility, which have increased risk to develop ovarian cancer, and women with ovarian cancer more frequently than controls have antibodies to HE4, a biomarker for ovarian cancer. The antibodies may reflect a tumor-promoting Th2 type of inflammation.
Introduction
Immunological mechanisms play a key role in the etiology and progression of many cancers, and many tumor antigens induce an antibody response in cancer patients [1-6], including antigens expressed by ovarian cancer (OvC) [7]. Autoimmunity appears to increase the risk for cancer [3, 6, 8, 9], and recent studies indicate that inflammation of the Th2 type, which involves antibody forming cells, plays a key role in carcinogenesis and tumor progression [10-14].
Infertile women more frequently than controls have antibodies to antigens expressed by both OvC and the normal ovary [7, 15]. This is noteworthy since they are at higher risk for OvC [16, 17], and it has been hypothesized that autoimmunity, including antibodies to antigens in the ovary and/or Fallopian tube, facilitates the development of OvC [1, 18, 19].
Most OvC overexpress mesothelin which is one of the best characterized antigens of OvC [20]. Antibodies to mesothelin have been detected in women with OvC [21, 22] and in sera from women with pelvic inflammatory disease [22], a condition associated with an increased frequency of OvC [23]. Likewise, women with certain types of infertility have antibodies to mesothelin more frequently than controls [24]. HE4, human epididymis factor 4 [25], which is expressed by OvC, particularly its serous type [26, 27], and in lung adenocarcinoma [28] is attracting attention as a diagnostic marker which can complement CA125 since it is less often elevated in benign conditions and can be applied in combination with CA125 to aid diagnosis of women with a pelvic mass [20, 29-31]. It may also be a therapeutic target since it can be recognized by Th1 type lymphocytes, including cytolytic T cells [32].
We developed an ELISA to measure antibodies to recombinant HE4 and then applied it to investigate sera from infertile women and from women with OvC. As demonstrated here, antibodies to HE4 were more frequently observed in women with certain types of infertility and in women with OvC, although fewer women with OvC than infertile women had antibodies to HE4. There was a strong correlation between antibodies to both mesothelin and HE4 in the same subject.
Methods
Infertility patients
Infertility patient sera (n = 114) were collected from infertility clinics at Rush University Medical Center), the Center for Human Reproduction (courtesy of Dr. Carolyn Coulam) and the University of Ulm (courtesy of Dr. Cosima Brucker). They represented idiopathic premature ovarian failure (POF; n = 26), endometriosis (n = 23), ovulatory dysfunction (n= 17), and unexplained infertility (n=47). The c l i n i c a l evaluation of infertility included semen analysis, postcoital test, ovulation (luteal phase progesterone), tubal patency (open and unobstructed Fallopian tubes via hysterosalphingogram), and measurement of FSH and estrogen. Unexplained infertility is a diagnosis of exclusion when the patient has otherwise normal test results but has not been able to achieve a pregnancy. The average duration of infertility was 3.6 ± 1.5 (range 2–8) years. Prior treatment was minimal. The average number of prior in vitro fertilization treatment cycles was less than one per patient.
Patients with idiopathic POF experienced menopause at an average age of 26.6 ± 9.1 years and had elevated, menopausal day 3 FSH levels (i.e., > 10 mIU/mL). Endometriosis patients were obtained from the infertility clinic or from the gynecology clinic and had surgically confirmed endometriosis without other conditions. Hormone levels were not available for the latter group. For endometriosis patients obtained through the infertility clinic, day 3 FSH was in the normal range. Ovulatory dysfunction was defined as oligomenorrhea (35–90 days between cycles) or anovulation (no evidence of ovulation) and excluded polycystic ovary syndrome [33]. Women with ovulatory dysfunction had slightly elevated but w i t h i n normal day 3 FSH levels. All samples were collected after documenting informed consent according to a protocol approved by the human institutional review board for human subjects.
Cancer Patients
We measured antibodies in archived sera from 92 patients, which were collected at the time of primary diagnosis and were obtained from the University of Washington Gynecologic Oncology Tissue Bank. All samples were collected after documenting informed consent according to a protocol approved by the human institutional review board for human subjects. The diagnosis of OvC was confirmed by histopathology in all patients. Patients with advanced OvC were treated with 6 to 8 cycles of standard platinum and taxane-based regimens.
Serum preparation
Venous blood (10 mL) was withdrawn from each participant into a red top tube, and serum was separated, aliquoted and stored at −80°C.
Recombinant HE4
Recombinant protein is generated by amplifying a cDNA fragment encoding the Human Epididymis Protein 4 (HE4) by high fidelity PCR. The amplified DNA fragment containing the HE4 cDNA is digested with Age I and Sbf I, and cloned into the CIHDpa mammalian expression vector (by replacing the IFN-γ with the HE4 fragment). The CIHDpa vector, provided by Dr. S.K. Ng, utilizes destabilizing sequences on a selection marker for improved recombinant protein productivity in CHO-DB44 cells [34]. CHO cells are transfected with the vector, the supernatant collected and adjusted to pH 8 with carbonate-bicarbonate buffer), after which it is filtered with 0.2um filters (Nalgene, Rochester, NY) and concentrated with 10kDa centrifugal filters (Millipore, Billerica, MA). The CHO supernatant is stored at 4°C before use.
An affinity column was prepared by coupling the 3D8 MAb provided by Fujirebio Diagnostics, Inc. to CNBr-activated Sepharose 4B (Sigma, St. Louis, MO) at 4°C overnight. The column was blocked with 1M Tris-HCl (Sigma) for 2 hour at 4°C and then washed with PBS. The concentrated supernatant was filtered with 0.2um filters immediately before purification to remove impurities and added to the column, which was then washed with PBS. The HE4 antigen was eluted using 0.1M Glycine-HCl pH 2.7 after which pH was neutralized with 2M Tris (Sigma). The buffer was changed to PBS using a centrifugal filter with 10,000 MWCO (Millipore, Billerica, MA), after which antigen concentration was determined using Pierce BCA Protein Assay (Thermo Scientific, Rockford, IL). Two equally prepared pools of HE4 antigen were used for testing sera from infertile women vs sera from patients with OvC, since we did not have sufficient amount of antigen from the first pool to test both types of sera.
Control sera to establish ‘cutoffs’ in ELISA assays
We established cut-offs for each antigen pool by assaying sera, diluted 1:20 or 1:100, from two control groups (131 for the first pool and 71 for the second one). The first pool of control sera included 10 sera from healthy women without a history of diagnosed infertility or autoimmune disease and without a prior history of cancer, which had been collected at Rush Medical Center, and 121 sera from age matched healthy women which had been obtained from Dr O. Nilsson at Fujirebio Diagnostics, Inc (FDI) and used as part of FDI’s normal controls for assay development. An additional group of assay control sera was from 18 healthy women. As controls for the second set of experiments we used 71 still available samples from the FDI control group so there was overlap between the two control sets used to determine a cutoff value. Each subject provided one serum sample and all tests were done on coded samples. All control sera, as well as all sera from patients, were collected according to protocols approved by the relevant Institutional Review Boards.
Sandwich assay for Anti-HE4 Antibodies
The purified HE4 antigen was diluted in PBS at 0.66 ug/mL and incubated for 1 hour at 37°C to coat the wells of a 96-well Medisorp ELISA plate (Thermo Scientific, Rockford, IL). In addition, PBS was added to separate wells as a negative control. After blocking for 2 hours with 3% BSA/PBS, the plate was washed with wash buffer (0.002 M imidazole, 0.02% Tween 20, 0.5 mM EDTA, 160 mM NaCl). Serum samples were diluted to 1:20 and 1:100 with 3% BSA/PBS was added to both HE4 antigen-coated and background wells and incubated at room temperature for 1 hour. After washing the plate with wash buffer, 1:6,000 diluted horseradish peroxidase–conjugated goat anti-human IgG antibody (Invitrogen, Carlsbad, CA) is added to each well and incubated for 1 hour at room temperature. After washing the plate with wash buffer, SureBlue TMB Microwell Peroxidase Substrate (KPL, Gaithersburg, MD) was added to each well and incubated for 15 min at room temperature before the interaction was terminated by adding the TMB stop solution (KPL, Gaithersburg, MD). OD450 is measured with a Dynatech MR5000 plate reader (Dynatech Laboratories). For each serum sample, optical density values in wells without antigen were subtracted from the optical density value in wells plated with HE4. Data were analyzed either by comparison of optical density values or using a cutoff value to classify an antibody result as positive. The cutoff value was equivalent to the mean optical value (M) for the normal controls plus 1, 2 or 3 SD.
Determination of HE4 antigen in serum and urine
The commercially available kit developed by Fujirebio Diagnostics, Inc. was applied, following manufacturer’s instructions and using 150 pM as the recommended cut-off when assaying sera. Urine samples were evaluated as previously described with the data normalized to creatinine; under the conditions of the tests, an OD value above 2.0 is considered positive.
Statistics
Statistical significance was determined according to Fisher’s exact test, Graphpad software, two-tailed p-values was applied when determining the correlation between antibodies to mesothelin and HE4. The Spearman correlation coefficient comparing anti-mesothelin and anti-HE4 was estimated using SPSS software with p<0.05 considered significant.
Results
Infertile women have antibodies to HE4 more often than controls
Based on the average optical density values (OD), women with infertility more frequently had antibodies to HE4 than controls (Table 1). At a serum dilution of 1:20 and a cutoff value of M+2SD (98% confidence level), 26 subjects from the combined group of 113 infertile women (23%) had antibodies to HE4 (p<0.001), including 8 of 26 (31%) women with POF (p<0.001), 8 of 17 women (47%) with ovulatory dysfunction (p<0.001) and 6 of 47 (13%) women with unexplained infertility (p<0.05); the differences for the POF and ovarian dysfunction groups were still significant at a cutoff value of M+3SD (98% confidence level). Four of 23 women with endometriosis (17%) had anti-HE4 antibodies at a cutoff value of M+2 SD (p<0.01) when sera were tested at a dilution of 1:20. At a dilution of 1:100, significant differences versus controls were detected only for the POF and ovulatory dysfunction groups. The HE4 antigen, detected with the FDI kit using the standard value of 150 pM as the cut-off, was not detected in sera from 31 healthy control subjects, 25 women with POF, 30 women with unexplained infertility or 13 women with endometriosis. In contrast, most patients with OvC have increased levels of HE4 antigen in serum and urine [20].
Table 1.
Antibodies to HE4 in sera from women with various types of infertility compared to normal controls.
| Positive sera at dilution 1:20 | Positive sera at dilution 1:100 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serum donor | n | M+2SD | M+3SD | n | M+2SD | M+3SD | ||||
| N | % pos | n | % pos | n | % pos | n | % pos | |||
| Normal controls | 131 | 3 | 2 | 1 | 1 | 82 | 1 | 1 | 1 | 1 |
| Unexplained Infertility. | 47 | 6 | 13 | 3 | 6 | 37 | 0 | 0 | 0 | 0 |
| POF | 26 | 8 | 31*** | 6 | 24*** | 15 | 2 | 13* | 2 | 13* |
| Ovulatory dysfunction | 17 | 8 | 47** | 8 | 47** | 5 | 2 | 40** | 1 | 20 |
| Endometriosis | 23 | 4 | 17** | 0 | 0 | 22 | 0 | 0 | 0 | 0 |
|
| ||||||||||
| ALL | 113 | 26 | 23*** | 17 | 15*** | 79 | 4 | 5 | 2 | 3 |
p<0.05
p<0.01
p<0.001
There is a correlation between antibodies to HE4 and to mesothelin
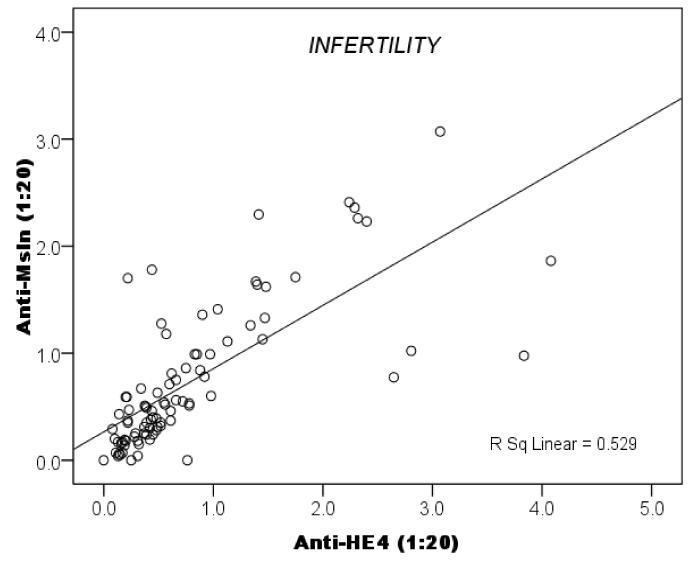
The samples tested for antibodies to HE4 had previously been tested for antibodies to mesothelin [24]. We next investigated whether women forming antibodies to one antigen (mesothelin or HE4) were more likely to also have antibodies to the other antigen. Data summarized in Fig. 1 show with high statistical significance (p<0.0001) that the occurrence of antibodies to HE4 and to mesothelin was correlated within individuals.
Fig. 1.
Correlation between antibodies to HE4 and mesothelin in sera from infertile women.
Patients with OvC have antibodies to HE4 although less frequently than infertile women
After cut-off values were established for a second pool of recombinant HE4 antigen by testing 71 control sera at 1:20 (mean OD value = 0.390 and SD= 0.655 at a 1:20 dilution), and 1:100 (mean OD value = 0.020 and SD = 0.501), we tested archived sera from 92 patients with OvC who had been studied at the University of Washington. As shown in Table 2, antibodies to HE4 were detected more frequently in OvC patients than controls and the difference was greater when the sera were tested at a dilution of 1:100; however, only 13 of 92 OvC patients (14%) were positive at M+2 SD (97% specificity). There was no statistically significant difference between patients who had stage I/II as compared to stage III/IV disease (Table 2). Thirtyseven of the OvC patients had ascites which contained OvC cells. In all cases, the level of HE4 antigen was high in ascites, serum and urine. The ascites from 7 of these 37 patients had high levels of anti-HE4 antibodies.
Table 2.
Antibodies to HE4 in archived sera from patients with OvC and controls.
| Positive sera at dilution 1:20 |
Positive sera at dilution 1:100 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum donor | n | M+2SD | M+3SD | M+1SD | M+2SD | M+3SD | |||||
|
| |||||||||||
| n | % pos | n | % pos | n | % pos | n | % pos | n | % pos | ||
| Controls | 71 | 3 | 4 | 1 | 1 | 3 | 4 | 2 | 3 | 2 | 3 |
| All OvC patients | 92 | 9 | 10 | 8 | 9* | 20 | 22*** | 1 | 14** | 1 | 12* |
| 3 | 1 | ||||||||||
| OvC stage I/II | 17 | 2 | 12 | 1 | 6 | 4 | 27* | 3 | 20* | 2 | 13 |
| OvC stage III/IV | 75 | 6 | 8 | 4 | 5 | 16 | 21** | 9 | 12* | 8 | 11* |
p<0.05
p<0.01
p<0.001
Table 3 presents data from 13 patients with advanced (stage III or IV) OvC for which we concomitantly assayed sera for anti-HE4 antibodies and antigen and urine for HE4 antigen. All patients had clinical evidence of tumor when the samples were obtained, and the serum and urine samples were obtained on the same day for each patient. Four patients had anti-HE4 antibodies detectable at levels above M+3 SD, while the other 9 patients did not have higher antibody levels than controls. Eight patients were positive for HE4 antigen in serum and an additional 3 patients were positive for HE4 antigen in urine. Urine samples from the 4 patients who had anti-HE4 antibodies were positive for HE4, as were serum samples from 3 of them.
Table 3.
Summary of anti-HE4 antibodies and HE4 in serum and urine from patients with clinical evidence of stage III or IV OvC at the time of assay.
| Patient | Anti-HE4 antibodies (mean OD) # | HE4 antigen (pM) | ||
|---|---|---|---|---|
| Dilution 1:20 | Dilution 1:100 | Serum | Urine | |
| 1 | 0.63 | 0.57 | 439* | 11.5* |
| 2 | 0.29 | 0.18 | 180* | 11.9* |
| 3 | 0.03 | 0 | 116 | 7.0* |
| 4 | 3.51* | 3.65* | 90 | 9.1* |
| 5 | 1.70* | 2.61* | 652* | 16.9* |
| 6 | 0.01 | 0.09 | 665* | 46.4* |
| 7 | 0.10 | 0.21 | 492* | 15.6* |
| 8 | 0.30 | 0.16 | 71 | 1.5 |
| 9 | 0.65 | 0.39 | 73 | 2.2* |
| 10 | 2.69* | 1.38* | 176* | 8.1* |
| 11 | 0.30 | 0.23 | 337* | 4.9* |
| 12 | 0 | 0 | 82 | 1.7 |
| 13 | 2.49* | 2.93* | 205* | 2.2* |
Cut-off values were the same as for the data presented in Table 2 and adsorption values corresponding to M+2 SD are indicated (*).
Sera were scored as positive for HE4 antigen using the 150 cutoff established for the FDI kit. Urine samples were assayed as described [42] and scored as positive (M+2SD) at a cut-off value of 2.0.
Discussion
We have developed an ELISA to detect antibodies to recombinant full length HE4 protein and show that sera from women with infertility involving ovarian dysfunction (POF, ovulatory dysfunction, unexplained infertility) have antibodies to HE4 more frequently than healthy controls, as does a subgroup of women with OvC. We previously reported that sera from the same group of infertile women often contain antibodies to mesothelin [24]. We now demonstrate that there was a strong correlation between antibodies to both of these antigens in specimens from the same patient, suggesting that antibodies to antigens expressed by epithelium from ovary and Fallopian tube and overexpressed in OvC may identify high risk individuals and serve as complementary biomarkers to antigen alone. Antibodies to HE4, but not to mesothelin [24], were found in women with endometriosis although less frequently than in women with ovarian dysfunction. In cancer, antibodies usually reflect the expression of the corresponding antigen in the tumor [35]. It is not clear whether HE4 is expressed in endometriosis, since expression of HE4 was found in one study [29] but not in another [36]. It is expressed in endometrioid ovarian tumors [37], the most common tumor type associated with endometriosis.
It is noteworthy that antibodies to antigens expressed by OvC are commonly seen in women with certain types of infertility, since infertility has been related to autoimmunity and to an increased risk for cancer [15, 38]. The antibodies may reflect a Th2 type inflammation, a condition that appears to promote tumor growth and facilitate the escape of tumors from destruction by a Th1 type immune response [10-12, 14, 39]. It is unclear whether the antibodies themselves promote tumor growth or whether some other part of a Th2 type inflammation is responsible, e.g. certain lymphokines. Alternatively, the antibodies may reflect other molecular alterations, immunological dysfunction or risk factors that promote carcinogenesis. It will be important to learn whether the probability of developing OvC is different for women who have antibodies to HE4, mesothelin and other OvC antigens, i.e. whether detection of the antibodies facilitates the identification of women at increased risk for OvC. This may be investigated by using serum samples which have been ‘banked’ over time from a large group of women among whom some later developed OvC. Such samples are available [40] and have been used to investigate CA125, HE4 and other tumor antigens as aids for early detection of OvC [41].
Sera from OvC patients, including those with early disease, are positive for anti-HE4 antibodies less often than in women with certain types of infertility. The fact that an increased level of HE4 antigen as compared to healthy controls is present in most sera from OvC patients [20] but not in sera from infertile women, suggests that immune complexes can form and hide the presence of antibodies in patients with OvC. Likewise, anti-HE4 antibodies may interfere with the demonstration of HE4 antigen in serum. This may explain why testing urine rather than serum for HE4 is more sensitive for detection of OvC [20, 42], a situation similar to that for mesothelin [43, 44], since antibodies can be retained by the kidneys.
Large prospective studies are needed to confirm the association of antibodies to OvC antigens and risk of OvC and whether this association applies just to infertility or also to other risk factors for OvC. The relation between circulating HE4 antigen, anti-HE4 antibodies and the expression of a patient’s OvC and by her normal epithelial cells in the ovary and Fallopian tube needs to be further investigated. One may speculate that expression of HE4 in normal epithelial cells initiates a local Th2 type inflammation which includes the formation of anti-HE4 antibodies and that the situation is similar for other antigens as well. Alternatively, an inflammatory response may promote the expression of mesothelin, HE4 and certain other antigens expressed by normal and (at increased levels) by neoplastic ovarian epithelial cells.
HIGHLIGHTS.
Anti-HE4 antibodies more common in infertility
Correlate with antibodies to mesothelin
Acknowledgments
The authors thank Dr N. Kiviat for her generous support. They also thank Jade Jaffar and Jee Hang Tse for excellent technical help.
Grant support. Studies were supported by NIH 1RO1CA134487 (I Hellstrom), a grant from Fujirebio Diagnostics Inc (I. Hellstrom), NIH R01A1055060 (J. Luborsky), Ovarian Cancer SPORE (P50CA83636) Development Award (J. Luborsky), NIH RO1CA134487 (J. Luborsky, subcontract), Rush University Segal award (J. Luborsky), Wendy Feuer Ovarian Cancer Research Fund (E.Swisher)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest. There are no potential conflicts of interest.
References
- 1.Gnjatic S, et al. Seromic profiling of ovarian and pancreatic cancer. Proc Natl Acad Sci U S A. 2010;107(11):5088–93. doi: 10.1073/pnas.0914213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58(10):1535–44. doi: 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bei R, et al. A common repertoire of autoantibodies is shared by cancer and autoimmune disease patients: Inflammation in their induction and impact on tumor growth. Cancer Lett. 2009;281(1):8–23. doi: 10.1016/j.canlet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Stockert E, et al. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187(8):1349–54. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nesterova M, et al. Autoantibody biomarker opens a new gateway for cancer diagnosis. Biochim Biophys Acta. 2006;1762(4):398–403. doi: 10.1016/j.bbadis.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Tan EM, Zhang J. Autoantibodies to tumor-associated antigens: reporters from the immune system. Immunol Rev. 2008;222:328–40. doi: 10.1111/j.1600-065X.2008.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luborsky JL, et al. Anti-tumor antibodies in ovarian cancer. Am J. Reprod Immunol. 2005;54:55–62. doi: 10.1111/j.1600-0897.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez-Montagut T, et al. Immunity to melanoma: unraveling the relation of tumor immunity and autoimmunity. Oncogene. 2003;22(20):3180–7. doi: 10.1038/sj.onc.1206462. [DOI] [PubMed] [Google Scholar]
- 9.Uchi H, et al. Unraveling the complex relationship between cancer immunity and autoimmunity: lessons from melanoma and vitiligo. Adv Immunol. 2006;90:215–41. doi: 10.1016/S0065-2776(06)90006-6. [DOI] [PubMed] [Google Scholar]
- 10.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 11.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azar KK, T M, Yasuda H, Sakai A, Inoue M, Sasagawa T. Increased secretion patterns of interleukin-10 and tumor necrosis factor-alpha in cervical squamous intraepithelial lesions. Hum Pathol. 2004;35(11):1376–84. doi: 10.1016/j.humpath.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Feng Q, et al. Th2 type inflammation promotes the gradual progression of HPV-infected cervical cells to cervical carcinoma. Gynecol Oncol. 2012;127(2):412–9. doi: 10.1016/j.ygyno.2012.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edassery S, et al. Autoanigens in ovarian autoimmunity associated with unexplained infertility and premature ovarian failure. Fertil Steril. 2010;94:2636–2641. doi: 10.1016/j.fertnstert.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanderhyden BC. Loss of ovarian cancer fuanction and the risk for ovarian cancer. Cell Tissue Res. 2005;322:117–124. doi: 10.1007/s00441-005-1100-1. [DOI] [PubMed] [Google Scholar]
- 17.Smith E, XX X. Ovarian ageing, follicle depletion, and cancer: a hypothesis for the aetiology of epithelial ovarian cancer involving follicle depletion. Lancet Oncol. 2008;9:1108–1111. doi: 10.1016/S1470-2045(08)70281-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barua A, et al. Anti-ovarian and anti-tumor antibodies in women with ovarian cancer. Am J Reprod Immunol. 2007;57:243–249. doi: 10.1111/j.1600-0897.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- 19.Luborsky JL, et al. Anti-tumor antibodies in ovarian cancer. Am J Reprod Immunol. 2005;54(2):55–62. doi: 10.1111/j.1600-0897.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 20.Hellstrom I, Hellstrom KE. Two new biomarkers, mesothelin and HE4, for diagnosis of ovarian carcinoma. Expert Opinion Med Diagnosis. 2011;5:227–240. doi: 10.1517/17530059.2011.559459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho MH, et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res. 2005;11:3814–3820. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 22.Hellstrom I, et al. Anti-mesothelin antibodies and circulating mesothelin relate to the clinical state in ovarian cancer patients. Cancer Epidemiol Biomarkers Prev. 2008;17:1520–1526. doi: 10.1158/1055-9965.EPI-08-0039. [DOI] [PubMed] [Google Scholar]
- 23.Lin HW, et al. Risk of ovarian cancer in women with pelvic inflammatory disease: a population-based study. Lancet Oncol. 2011;112:900–904. doi: 10.1016/S1470-2045(11)70165-6. [DOI] [PubMed] [Google Scholar]
- 24.Luborsky JL, et al. Autoantibodies to mesothelin in infertility. Cancer Epidemiol Biomarkers Prev. 2011;20:1970–1978. doi: 10.1158/1055-9965.EPI-11-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirchhoff C, et al. A major human epididymis-specific cDNA encodes a protein with sequence homology to extracellular proteinase inhibitors. Biol Reprod. 1991;45:350–357. doi: 10.1095/biolreprod45.2.350. [DOI] [PubMed] [Google Scholar]
- 26.Schummer M, et al. Comparative hybridization of an array of 21,500 ovarian cDNAs for the discovery of genes overexpressed in ovarian carcinomas. Gene. 1999;238(2):375–85. doi: 10.1016/s0378-1119(99)00342-x. [DOI] [PubMed] [Google Scholar]
- 27.Hellstrom I, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63(13):3695–700. [PubMed] [Google Scholar]
- 28.Bingle L, et al. WFDC2 (HE4): a potential role in the innate immunity of the oral cavity and resiratory tract in the development of adenocarcinomas of the lung. Respiratory Res. 2006;7:61–80. doi: 10.1186/1465-9921-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore RG, et al. Utility of a novel serum tumor biomarker HE4 in patients with endometrioid adenocarcinoma of the uterus. Gynecol Oncol. 2008;110:196–201. doi: 10.1016/j.ygyno.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore RG, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108:402–408. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Moore RG, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112:40–46. doi: 10.1016/j.ygyno.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei H, et al. Silencing of the TGFbeta1 gene increases the immunogenicity of cells from human ovarian carcinoma. J. Immunother. 2012;35:267–275. doi: 10.1097/CJI.0b013e31824d72ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broekmans F, Fauser B. Diagnostic criteria for polycystic ovarian syndrome. Endocrine. 2006;30:3–11. doi: 10.1385/ENDO:30:1:3. [DOI] [PubMed] [Google Scholar]
- 34.Ng SK, Wang D, Mg Y. Application of destabilizing sequences on selection marker for improved recombinant protein productivity in CHO-DG44. Metab Eng. 2007:304–316. doi: 10.1016/j.ymben.2007.01.001. Epub 2007 Feb 14. [DOI] [PubMed] [Google Scholar]
- 35.Reuschenbach M, von Knebel Doeberitz M, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58:1535–1544. doi: 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huhtinen K, et al. Serum HE4 concentrations differentiatesd malignant ovarian tumours from ovarian endometriotic cysts. Brit J Cancer. 2009;100:1315–1319. doi: 10.1038/sj.bjc.6605011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobel M, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLOS Medicine. 2008;5(12):e232. doi: 10.1371/journal.pmed.0050232. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luborsky J. Ovarian autoimmune disease and ovarian autoantibodies. J. Womens Health Gend Based Med. 2002;11:585–599. doi: 10.1089/152460902760360540. [DOI] [PubMed] [Google Scholar]
- 39.DeNardo DG, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thornquist M, et al. Statistical design and monitoring of the Carotene and Retinol Efficacy Trial (CARET) Control Clin Trials. 1993;14:308–324. doi: 10.1016/0197-2456(93)90228-6. [DOI] [PubMed] [Google Scholar]
- 41.Anderson GL, et al. Assessing lead time of selected ovarian cancer biomarkers: a nested case-control study. J. Nat. Cancer Inst. 2010;102:26–38. doi: 10.1093/jnci/djp438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hellstrom I, et al. Detection of the HE4 protein in urine as a biomarker for ovarian neoplasms. Cancer Letters. 2010;296:43–48. doi: 10.1016/j.canlet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badgwell D, et al. Urinary mesothelin provides greater sensitivity for early stage ovarian cancer than serum mesothelin, urinary hCG free beta subunit and urinary hCG beta core fragment. Gynecol Oncol. 2008;106:490–497. doi: 10.1016/j.ygyno.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hellstrom I, Hellstrom KE. SMRP and HE4 as biomarkers for ovarian carcinoma when used alone and in combination with CA125 and/or each other. Adv Exp Med Biol. 2008;622:15–21. doi: 10.1007/978-0-387-68969-2_2. [DOI] [PubMed] [Google Scholar]