Abstract
Aims
Chemotherapy-associated cognitive impairment often follows cancer chemotherapy. We explored chemotherapy-induced DNA damage in the brain cells of mice treated with 5-fluorouracil (5FU), an antineoplastic agent, to correlate the extent of DNA damage to behavioral functioning in an autoshaping-operant mouse model of chemotherapy-induced learning and memory deficits (Foley et al. 2008).
Main methods
Male, Swiss-Webster mice were injected once with saline or 75 mg/kg 5FU at 0, 12, and 24 h and weighed every 24 h. Twenty-four h after the last injection, the mice were tested in a two-day acquisition and retention of a novel response task for food reinforcement. Murine brain cells were analyzed for the presence of single- and double-strand DNA breaks by the single cell gel electrophoresis assay (the Comet assay).
Key findings
We detected significant differences (p<0.0001) for all DNA damage characteristics (DNA “comet” tail shape, migration pattern, tail moment and Olive moments) between control mice cohort and 5FU-treated mice cohort: tail length – 119 vs. 153; tail moment – 101 vs. 136; olive moment – 60 vs. 82, correspondingly. We found a positive correlation between increased response rates (r=0.52, p<0.05) and increased rate of errors (r=0.51, p<0.05), and DNA damage on day 1. For all 15 mice (saline-treated and 5FU-treated mice), we found negative correlations between DNA damage and weight (r=−0.75, p<0.02).
Significance
Our results indicate that chemotherapy-induced DNA damage changes the physiological status of the brain cells and may provide insights to the mechanisms for cognitive impairment after cancer chemotherapy.
Keywords: Acquisition, Autoshaping, Chemotherapy, Cognition, DNA damage, 5-Fluorouracil
INTRODUCTION
The effects of chemotherapeutic agents on learning and memory behavior remain poorly understood, although recently established rodent models provide a powerful experimental approach to the “chemo-fog” problem (Seigers and Fardell 2011). Several recent studies discuss hypothetical molecular and cellular mechanisms for chemotherapy-induced learning and memory deficits (Dietrich et al. 2006; Briones and Woods 2011; Janelsins et al. 2012) but, to date, no studies have addressed chemotherapy-induced DNA damage per se in brain cells in relationship to learning and memory. While damage of cellular DNA can occur by spontaneous hydrolysis, alkylation, or bioactive molecules such as toxins, many chemotherapeutic agents are targeted against DNA, and the compromised DNA integrity is the major mechanism of their antineoplastic activity (Figg and McLeod 2004).
Treatment of cells with one of the most widely used anticancer drugs, 5-fluorouracil (5FU), leads to the incorporation of abnormal fluorouracil and uracil residues into DNA causing FU/A and U/A mismatches, and eventually DNA damage (Krynetskaia et al. 2008). The well-known adverse effect of this chemotherapeutic agent is substantial toxicity against normal cells, and the damage introduced into DNA of brain cells is hypothesized either to account for, or accompany the cognitive impairment associated with conventional chemotherapy.
In this study, we set out to test a hypothesis that DNA damage is a molecular marker that is related to learning and memory changes we have observed in mice treated with 5FU in variations of the autoshaping learning and memory task (Foley et al. 2008; Walker 2010; Walker et al. 2011). As the first step toward this goal, we established a simple and robust Comet assay to monitor the physical integrity of DNA in the murine brain cells following treatment with 5FU. Formation of single- and double-strand breaks in the genomic DNA of control (saline-treated) and 5FU-treated mice was evaluated by the single cell gel electrophoresis assay (the Comet assay). In this assay, analysis of electrophoretic mobility at neutral pH reveals accumulation of double-strand breaks, while alkaline gel electrophoresis is sensitive to formation of both double- and single-strand breaks in the DNA backbone. The semi-quantitative assessment of DNA degradation after in situ cell lysis was performed by comparing DNA migration patterns in murine brain samples and human cultured cells (non-small cell lung cancer cells A549) treated with 5FU or hydrogen peroxide. Next, we estimated the DNA integrity in mice treated with 5FU after testing the mice in a protocol previously used to demonstrate acquisition and retention impairment in mice (Foley et al. 2008). These experiments demonstrated that the evaluation of DNA damage could be a useful tool in assessing the adverse effects of chemotherapeutic agents in mice.
MATERIAL AND METHODS
Subjects
Male, Swiss-Webster mice weighing 29–42 g were used (Ace Animals, Philadelphia, PA), Mice were group-housed in a vivarium maintained at 21°C with humidity set at 30–40% under a 12 h light/dark cycle. The mice had access to food and water ad libitum until behavioral testing. After at least a week long acclimation period in the vivarium, the mice were injected i.p. with either saline (n=7) or 75 mg/kg 5FU (n=8) at timepoints 0, 12, and 24h as shown on the flow chart of the experimental procedures on Fig. 1. This dosing regimen was based on allometric techniques to convert relevant human doses and dose schedules to comparable mouse doses and schedules (Boxenbaum 1982; Tang and Mayersohn 2005) and the pharmacokinetics of 5FU in plasma and tumor cells (Peters et al. 1993). Twenty-four h prior to the first day of testing in the autoshaping-operant procedure, mice were food-restricted, weighed, and then separated into individual cages with water available ad libitum. Immediately after the second behavioral testing session (72 h after the first injection), mice were euthanized, and their brains excised. Mice were weighed prior to injections at 0, 24, and 48 h and prior to behavioral testing at 72 h. All mice were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of Temple University and the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources, National Academy Press 1996; NIH publication No. 85–23, revised 2011). The highest standards of animal welfare were maintained throughout these studies and the experiments were designed to reduce the number of mice required by using a repeated measures procedure to measure behavior and by using cryopreserved tissues for replicated analysis of control brain cells.
Figure 1.
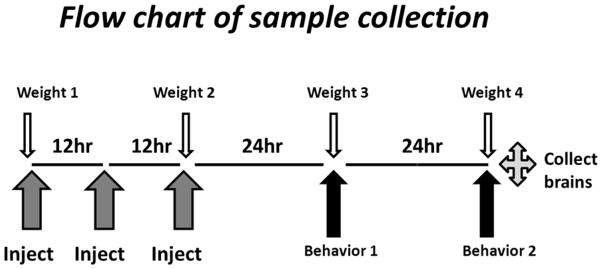
Flow chart of the experimental design. Upward arrows designate drug injections, and behavioral experiments, as indicated. Downward arrows indicate the time points for weighing mice. After 72 h, mice were sacrificed, and neural cells isolated and analyzed as described in Methods.
Isolation of brain cells
Brain cells for the Comet assay analysis were prepared as described (Singh 1998). Briefly, the brain was immersed in ice-cold PBS containing 200 μM N-1-butyl-alpha-phenytrone, washed 4×20 ml PBS, and dispersed using a hand-held tissue press (BioSpec Products, Inc., Bartlesville, OK). The tissue pieces were washed 4×20 ml PBS, and dispersed into single-cell suspension in 5 ml cold PBS with 5 ml pipette. Cells were filtered through 40 um nylon cell strainer (Fisher Scientific, Pittsburgh, PA). Cell count and viability were determined using ViaCount reagent (Guava, CA). Cells were concentrated by centrifugation, and cryopreserved at 10 mln/ml in DMEM medium supplemented with10% FBS and containing 10% DMSO, in liquid nitrogen.
Comet assay of DNA damage
Frozen cells were thawed in a water bath at 37°C, immediately transferred to 15 ml cold DMEM medium supplemented with 50% FBS and 10% dextrose, and centrifuged at 200×g for 10 min at +4°C. Cell pellets were resuspended in cold 1×PBS at density 1×106 cells/ml. The slide preparation and the Comet assay were performed according to a manufacturer’s protocol (Trevigen Inc., Gaithersburg, MD). A549 non-small cell lung cancer cells (ATCC, NIH) treated with 0.2 mM H2O2 for 20 min at 37°C were used as positive control, and untreated A549 cells used as negative control in each Comet assay analysis. Each slide contained three agarose gel-imbedded samples. Briefly, 200 ul melted LMA agarose was mixed with 20 ul cells, and loaded onto FLARE slides (Trevigen Inc., Gaithersburg, MD). Slides were left to solidify at 4°C in the dark for 35 minutes, and incubated in the lysis solution at 4°C overnight. Slides were washed (3×5min) by 1×FLARE Washing buffer 1, and equilibrated in the alkaline electrophoresis solution for 20 min. Alkaline electrophoresis was performed in freshly prepared alkaline electrophoresis solution (0.3 M NaOH, 1 mM EDTA, pH 12.1) at 20V for 40 min. Slides were washed with water (2×10min), fixed with 70% ethanol, and air-dried. DNA staining was performed with the SYBR Green I staining solution for 40 min at room temperature. Images were recorded by Nikon Eclipse 50 epifluorescent microscope equipped with a CCD camera.
Behavioral testing
Apparatus
Twelve mouse experimental chambers (21.6 cm × 17.8 cm × 12.7 cm, Model ENV-307W, MED Associates, St. Albans, VT) were used. Each chamber was housed within a sound-attenuating enclosure and connected to a computer-driven interface (Model SG-502, MED Associates, St. Albans, VT). One wall of the chamber contained three receptacles: one large dipper hole in the center (ENV 313M) and two smaller nose-poke holes on the left and right (ENV 313W). A dipper lever and dipper well were located behind the center dipper hole. The opposite wall featured a house light that illuminated the chamber during the session. Each chamber was also equipped with an audible tone device (Sonalert, 2900 Hz, Mallory Sonalert, Indianapolis, IN, USA). Nose-poke responses into each hole were detected by photocell head entry detectors (ENV 303HD) and recorded.
Autoshaping-operant procedure
Twenty-four hours after food restriction, the mice began the acquisition phase (day 1) of the autoshaping-operant procedure (Foley et al. 2008). Mice were weighed and placed inside the experimental chambers for a 15 min habituation period. A solution of 50:50 vanilla-flavored Ensure:water was located in the dipper well just under the dipper nose poke hole. At the start of the session, the house light illuminated the chamber and a tone on a variable-time schedule (mean of 45 s, range 4–132 s) was sounded for 6 s or until a nose-poke response occurred. If the mouse made a dipper-hole, nose-poke response during the tone, a dipper lever with 0.01 ml of a solution was presented and the tone was turned off. If no dipper-hole response was made during the tone, there were no consequences. Each session lasted for 2 h or until 20 reinforced nose-pokes were recorded. After the session, mice were fed 4 g of food and returned to their cages until testing again 24 h later. On day 2, all mice were placed back into the chambers for the retention test.
Data Acquisition and Statistical Analysis
In the behavioral tests, each response into the center nose-poke hole in concert with the tone was recorded as a correct, reinforced response. A general measure of speed of acquisition and retention was the total number of responses in the dipper nose-poke hole during the session – regardless of the presence or absence of the tone – divided by the total session time (s) for each mouse. The latency to earn the 10th reinforcer and the number of responses made during the absence and presence of the tone were recorded. In addition, the number of responses into the left and right nose-poke holes was also recorded and divided by the total session time (s) for each mouse. This response-rate measure was taken as an assessment of as errors in discrimination. Body weights between the saline- and 5FU-treated mice across the four time points was analyzed using a two-way ANOVA with treatment and time as the two factors while comparisons between saline- and 5FU-treated mice on these behavioral measures were determined by unpaired Student t-tests (Prism 5 for Windows, Version 5.01). Pearson and partial correlations between behavior or physiological factors and the olive tail moment were determined by IBM SPSS (v.20 for Windows).
For the DNA studies, five images were acquired from each spot, and image analysis of 20–25 cells from each image was performed using ImageJ 1.45s software (NIH). Images were analyzed with CometScore freeware (TriTek, Sumerduck, VA). Tail length was determined as head diameter subtracted from comet length, tail moment was defined as percent of DNA in tail multiplied by tail length, Olive tail moment was defined as summation of tail intensity profile values multiplied by their relative distances to the head center, divided by total comet intensity. Two sample t-test, Mann-Whitney U test, and one-way ANOVA were used for statistical evaluation, as implemented in Statistica 7 package (StatSoft, Tulsa , OK).
RESULTS
Behavioral testing and DNA analysis in mice cohorts
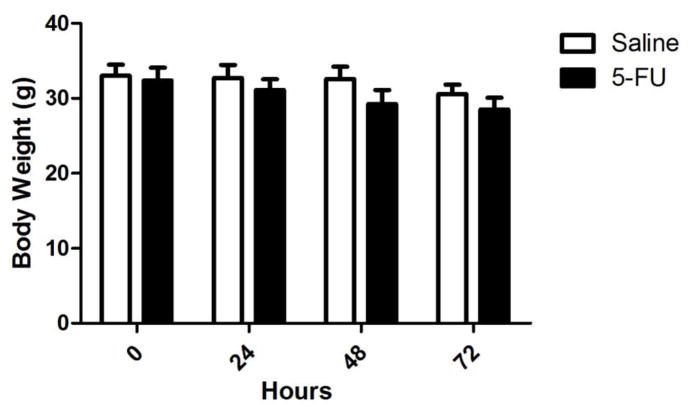
Over the course of 72 h and the three injections of 75 mg/kg 5FU or saline (see Flow chart of sample collection, Figure 1), the mice in the 5FU group lost 3.9 ± 0.85 g while the mice in the saline group lost only 2.4 ± 0.61 g. However, this trend for weight loss was not significant either between the two groups or across the four different time points. Between 24 and 48 h, the mice are food restricted prior to behavioral testing and then receive 3 g of chow after testing at 48 h. This experimental constraint for behavioral testing actually over-represents the actual amount of weight lost due to injections and chemotherapy (Figure 2). Otherwise, the mice were healthy and there was no evidence of any adverse effects from the 5FU treatment. The saline-treated mice responded 0.068 ± 0.026 responses/s on day 1 which significantly increased to 0.25 ± 0.061 responses/s on day 2 indicating that the response was learned and retained over the course of the two days (t(6)=4.201, p <0.01). The 5FU-treated mice, however, responded 0.10 ± 0.033 responses/s on day 1 which was not significantly different than the response rate of 0.18 ± 0.079 responses/s on day 2 indicating this group of mice failed to demonstrate the same degree of acquisition and retention of the novel nose-poke response although the 5FU-treated mice nearly doubled their response rates from day 1 to 2.
Figure 2.
Changes in mouse body weights after saline (open bars) or 5FU (closed bars) treatment on at the beginning of the experiment (0 h), the last day of injections (24 h), and the first (48 h) and second day (72 h) of behavioral testing. Although the 5FU-treated mice lost 3.9 ± 0.85 g while the mice in the saline group lost 2.4 ± 0.61 g over the course of the 4 days, this difference between groups was not statistically significant nor was the difference between the body weights on day 4 different from the body weights at baseline.
For the saline-treated mice, the number of correct responses made in the absence and presence of the tone was 280/12 on day 1 and 524/18, respectively. For the 5FU-treated mice, the number of correct responses made in the absence and present of tone was 332/14 on day 1 and 367/15 on day 2, respectively. Although the mice in either group are not discriminating between responding during the tone and not responding in the absence of the tone at this early point of two days of training, the saline-treated mice are making significantly more responses on day 2 (t=2.76, p<0.03) in the correct nose poke hole whereas the difference between day 1 and 2 was not significant for the 5FU-treated mice. There were no differences in the latencies to respond for 10 reinforcers between the two groups. The saline-treated mice decreased their response rates in the incorrect nose poke holes from 0.0044 ± 0.0016 responses/s on day 1 to 0.0019 ± 0.00060 responses/s on day 2. The 5FU-treated mice also decreased their error rates from 0.0054 ± 0.0020 responses/s on day 1 to 0.0014 ± 0.00032 responses/s on day 2 indicating that both groups of mice were able to ascertain the correct nose poke hole from the incorrect nose-poke holes by day 2.
Following excision, murine brains were washed with 1×PBS with 200 uM PBN, to prevent radical-induced damage to DNA (Singh 1998). After tissue dispersion, cells were evaluated by flow cytometry, and inspected microscopically. Most cells were viable (70–75% viability) as tested by dye-exclusion assay, and round-shaped. Total number of isolated cells was enough for preparing 3–5 cryo-preserved batches (10 mln/vial); cells were kept in liquid nitrogen for future experiments.
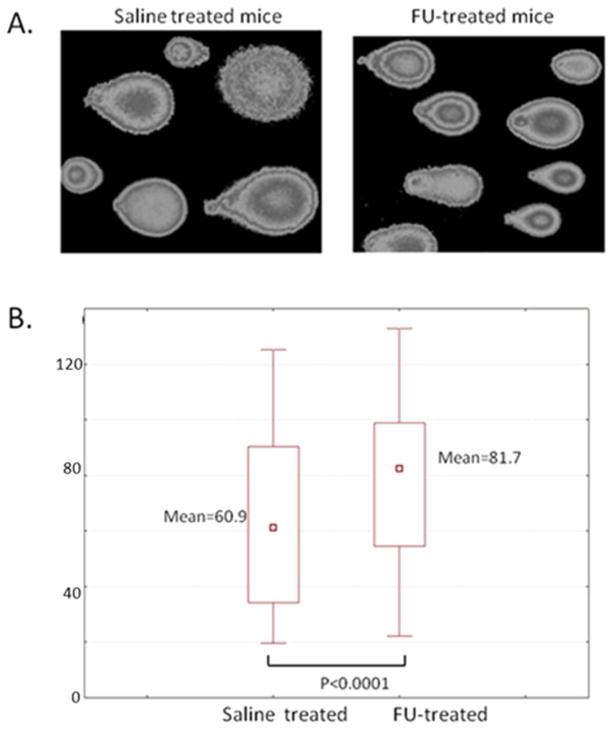
Assessment of the DNA “comet” tail shape, migration pattern, tail moment and Olive moments were used for semi-quantitative estimation of DNA damage in whole brain cells (Table 1). Analysis of Comet assay images revealed that the DNA migration patterns differed between mice treated with saline and those treated with 5FU (Figure 3). Our data indicate that relatively short-term treatment with 5FU at therapeutically relevant concentrations resulted in accumulation of DNA damage, as evidenced by higher electrophoretic mobility and larger tail moment values in the cells obtained from 5FU-treated mice (Table 1). The Comet assay demonstrated that a significant difference (p<0.0001) exists for DNA damage characteristics between the control mice cohort and the 5FU-treated mice cohort including tail length, tail moment, and Olive moment.
Table 1.
Migration patterns of DNA as determined by a single cell electrophoresis after in situ lysis (Comet assay) in two cohorts of mice (saline-treated, n=7 vs. 5FU-treated, n=8) (p value by t-test).
| Treatment | Saline | 5FU | P value |
|---|---|---|---|
| Tail length | 119 | 153 | <0.0001 |
| Tail moment | 101 | 136 | <0.0001 |
| Olive moment | 60 | 82 | <0.0001 |
Figure 3.
Evaluation of DNA damage in the murine brain cells by alkaline Comet assay. Panel A: Epifluorescence microscopy images of the Comet assay slides after single-cell electrophoresis in alkaline conditions. Panel B: Quantitative analysis of images shown in Panel A using CometScore software (TriTek, Sumerduck, VA), as described in Materials and Methods section.
Correlations between DNA damage and behavioral or physiological functioning
Using the entire cohort of mice (n=15), we found a statistically significant correlation between the level of DNA damage and the response rate to make correct nose poke responses while the mice were learning the novel response task (r=0.52, p<0.05) and the rate of errors (r0.51, p<0.05) on day 1 (Figure 4A and 4B). Therefore, the greater the level of DNA damage, the faster the mice responded to earn the food reward and to make errors. This relationship may be related to the observation that a negative correlation was found between the body weight of mice on the last day of the experiment and DNA damage (Figure 4C). There was a negative correlation (r=−0.75, p<0.001) between beginning body weights at baseline (0 h) and eventual DNA damage. This inverse relationship between beginning body weights and DNA damage remained throughout the experiment so that by the last day of behavioral testing at 72 h, the correlation between weight and DNA damage was also significant (r=−0.60, p<0.02). There were no correlations between weight loss and DNA damage or between beginning body weight and degree of weight loss (n=15). In other words, the smaller mice had greater DNA damage independent of treatment or the amount of weight lost. When body weight was used as a covariate using a partial correlation, the relationship between DNA damage and the rate of errors remained significant (r=0.54, p<0.05) but the correlation between DNA damage and the rate of responding in the correct nose poke hole was not. This analysis suggests that the increased response rates observed are not directly related to the DNA damage but more likely related to the body weight of the mice.
Figure 4.
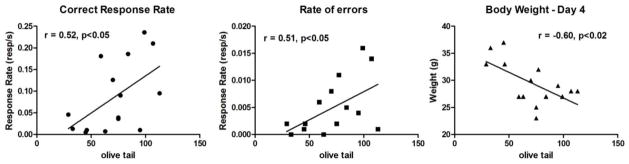
Linear regression analyses of the relationships between olive tail (DNA damage) and correct response rates on day 1 (Panel 4A), rate of errors on day 1 (Panel 4B), and body weights on day 4 (Panel 4C). Higher DNA damage was observed in mice with low body weight, in mice treated with saline or 5FU at day 4.
DISCUSSION
In addition to clinical findings that cancer chemotherapy can induce cognitive deficits (e.g., McDonald et al. 2012), preclinical in vivo and in vitro studies reveal 5FU and methotrexate can produce learning, memory, and cognitive deficits and cytotoxicity (Dietrich et al. 2006; Han et al. 2008; Fardell et al. 2010; Fardell et al. 2011). Recently our laboratory has taken advantage of a high-throughput learning and memory screen called autoshaping in mice (Vanover and Barrett 1998) which we modified to include predominantly instrumental as opposed to predominantly Pavlovian learning to study chemotherapy-induced learning and memory deficits in mice (Foley et al. 2008; Walker 2010; Walker et al. 2011). This behavioral model provides us with an avenue for exploring the molecular events associated with chemotherapy-induced damage in brain at the cellular level. Many conventional chemotherapeutic agents are targeted against DNA, and the antitumor activity of these drugs is due to cytotoxic effects induced by activating DNA damage response in the cancer cells (Bouwman and Jonkers 2012). As a consequence, multiple signal and regulatory pathways are up- or down-regulated leading to altered or abrogated physiological functions.
In this study, we set out to test a hypothesis that DNA-damaging effects of chemotherapy may contribute to chemotherapy-associated cognitive impairments in cancer patients. Our goal was to define a molecular marker of abrogated or impaired functioning of neural cells associated with effects of chemotherapeutic agents. If identified, such a marker would allow developing better, more precisely directed chemotherapeutic regimens that improve quality of life of cancer patients, and reduce the burden of chemotherapy.
Similar to many other antimetabolite drugs, 5FU exerts its cytotoxic effect after anabolic activation of a prodrug to cytotoxic metabolites (Longley et al. 2003). While the metabolic pathways leading to anabolic activation or catabolic inactivation of 5FU are well characterized, the exact mechanism of cytotoxicity still remains a matter of discussion (An et al. 2007; Pettersen et al. 2007; Grogan et al. 2011). The cytotoxic effect of 5FU misincorporated into DNA is hypothesized to be mediated by complex biochemical mechanisms including compromised DNA integrity and chromatin modification, rather than by direct inhibition of DNA replication (An et al. 2007; Kunz et al. 2009).
Following metabolic activation, 5FU is incorporated into DNA and is further processed by DNA repair machinery. Several studies in mice demonstrated that incorporation of 5FU into genomic DNA correlated with 5FU cytotoxicity. In mice, the major enzyme involved in detection and removal of 5FU from DNA is Smug1 DNA glycosylase, while in humans the enzyme uracil-DNA-glycosylase is the major enzyme that removes 5FU from DNA. Nevertheless, the downstream effects of 5FU are probably the same in these two species. Biochemical processing of 5FU incorporated into DNA includes its removal, and initiation of DNA damage response utilizing the components of the base excision repair and mismatch repair systems (Cortazar et al. 2011; Kemmerich et al. 2012). Accumulation of single- and double-strand breaks in DNA is a clear indication of 5FU cytotoxic effect (Kunz et al. 2009).
The Comet assay provides a convenient, semi-quantitative method to detect DNA damage in the whole cells. This technique directly estimates DNA damage in individual cells by determining the length of DNA migration in the electric field, after cell lysis in situ. This DNA migration pattern is derived from broken DNA fragments that migrate away from the head or nucleus region. Although precautions were taken to avoid oxidative damage of DNA in the process of cell isolation, we still noticed a relatively high level of DNA damage in the control (saline-treated) cohort. The reasons for that are unclear but may be related to unaccounted for factors such as age, diet, or variations in the level of DNA glycosylases , e.g. Smug1, Tdg, Ung, or Mbd4 (Kemmerich et al. 2012).
In our experiments, a strong factor associated with DNA damage was smaller body weight, independent of treatment condition, an observation that still remains to be elucidated. Although a negative correlation existed between body weight at 0h and 72h and DNA damage, there was no such relationship for weight loss and DNA damage. The heavier mice at the beginning of the experiment were less likely to show DNA damage suggesting that whatever factors contribute to larger body weights in mice (slight variations in age, genetics, dominance, rate of metabolism, etc.,) were protective when it came to DNA damage. The 5FU treatment failed to significantly alter body weight over the course of the 72 h of injections and testing although the 5FU-treated mice lost approximately 1.5 g more weight than the saline-treated mice. However, despite the lack of significant differences between the groups, the relationship still exists across both treatment groups, that the smaller saline- or 5FU-treated mice displayed greater DNA damage.
The acute and chronic treatment of 5FU to mice produces deficits in acquisition and/or retention in variations of this autoshaping procedure (Foley et al. 2008; Walker 2010; Walker et al. 2011). Similarly, after three injections of 5FU spaced at 12 h intervals, we observed an increase in the error discrimination rate, and a deficit in the ability to learn a novel response (i.e., a significant difference in response rates between day 1 and 2) in mice. Although these data indicate a retardation of the learning and/or retention of the novel response, all of the mice still learned the response and reduced their errors by day 2. Other indices of learning were not altered such as adjusted latency or any retention measures for response rates suggesting that this regimen of dosing and testing produced mild deficits. The increased rate of responding in the dipper well for the reward and the rate of responding in the incorrect nose-poke holes on day 1 both were correlated with the extent of DNA damage. Interestingly, when 5FU is administered as an acute dose, 30 min prior to testing, no changes are observed on day 1 but a decreased response rate in the correct dipper nose poke hole on day 2 (Foley et al. 2008) and when 5FU is administered weekly for 3 weeks, both acquisition and retention deficits are observed (Walker et al. 2011). However, when body weights were used as a covariate, only the rate of responding in the incorrect nose-poke hole (i.e., error rate) remained significant suggesting the increased response rate into the correct nose poke hole in the current study is more closely related to the differences in body weights. The fact that the DNA damage correlated with error rates on day 1 and not day 2 suggest that the learning or acquisition measures for a novel task were disrupted to a greater extent than the memory or retention measures for a recently learned task (i.e., 24 h ago). Taken together, these results demonstrate that the behavioral effects of 75 mg/kg 5FU using this dosing regimen are relatively modest and that the extent of global DNA damage in either saline- or 5FU-treated mice is disruptive to certain measures of acquisition. Future studies can examine relationships between the long term consequences of the DNA damage, the relationship of frequency of dosing and time of testing, the relevance of body weight, and the behavioral changes.
CONCLUSION
Our results support the hypothesis that chemotherapy-induced DNA damage of murine brain cells is associated with behavioral changes. DNA damage may change the gene expression status and functioning of neural cells, and therefore provides a hypothetical mechanism for cognitive impairment after pharmacological management of cancer.
Acknowledgments
The project was supported by Undergraduate Research Program, Temple University, Philadelphia, PA (KW, VC) and NIH CA129092 (EAW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An Q, Robins P, Lindahl T, Barnes DE. 5-Fluorouracil incorporated into DNA is excised by the Smug1 DNA glycosylase to reduce drug cytotoxicity. Cancer Res. 2007;67:940–5. doi: 10.1158/0008-5472.CAN-06-2960. [DOI] [PubMed] [Google Scholar]
- Bouwman P, Jonkers J. The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat Rev Cancer. 2012;12:587–598. doi: 10.1038/nrc3342. [DOI] [PubMed] [Google Scholar]
- Boxenbaum H. Interspecies scaling, allometry, physiological time, and the ground plan of pharmacokinetics. J Pharmacokinet Biopharm. 1982;10:201–227. doi: 10.1007/BF01062336. [DOI] [PubMed] [Google Scholar]
- Briones TL, Woods J. Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci. 2011;12:124. doi: 10.1186/1471-2202-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortazar D, Kunz C, Selfridge J, Lettieri T, Saito Y, MacDougall E, Wirz A, Schuermann D, Jacobs AL, Siegrist F, Steinacher R, Jiricny J, Bird A, Schar P. Embryonic lethal phenotype reveals a function of TDG in maintaining epigenetic stability. Nature. 2011;470:419–423. doi: 10.1038/nature09672. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardell JE, Vardy J, Logge W, Johnston I. Single high dose treatment with methotrexate causes long-lasting cognitive dysfunction in laboratory rodents. Pharmacol Biochem Behav. 2010;97:333–339. doi: 10.1016/j.pbb.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Fardell JE, Vardy J, Shah JD, Johnston IN. Cognitive impairments caused by oxaliplatin and 5-fluorouracil chemotherapy are ameliorated by physical activity. Psychopharmacology (Berl) 2011;220(1):183–93. doi: 10.1007/s00213-011-2466-2. [DOI] [PubMed] [Google Scholar]
- Figg WD, McLeod HL, editors. Handbook of Anticancer Pharmacokinetics and Pharmacodynamics. Totowa, NJ: Humana Press Inc; 2004. [Google Scholar]
- Foley JJ, Raffa RB, Walker EA. Effects of chemotherapeutic agents 5-fluorouracil and methotrexate alone and combined in a mouse model of learning and memory. Psychopharmacology (Berl) 2008;199:527–538. doi: 10.1007/s00213-008-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan BC, Parker JB, Guminski AF, Stivers JT. Effect of the thymidylate synthase inhibitors on dUTP and TTP pool levels and the activities of DNA repair glycosylases on uracil and 5-fluorouracil in DNA. Biochemistry. 2011;50:618–627. doi: 10.1021/bi102046h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R, Yang YM, Dietrich J, Luebke A, Mayer-Proschel M, Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol. 2008;7:12. doi: 10.1186/jbiol69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Mustian KM, Palesh OG, Mohile SG, Peppone LJ, Sprod LK, Heckler CE, Roscoe JA, Katz AW, Williams JP, Morrow GR. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Support Care Cancer. 2012;20:831–9. doi: 10.1007/s00520-011-1158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerich K, Dingler FA, Rada C, Neuberger MS. Germline ablation of SMUG1 DNA glycosylase causes loss of 5-hydroxymethyluracil- and UNG-backup uracil-excision activities and increases cancer predisposition of Ung-/-Msh2-/- mice. Nucleic Acids Res. 2012;40:6016–6025. doi: 10.1093/nar/gks259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krynetskaia N, Xie H, Vucetic S, Obradovic Z, Krynetskiy E. High mobility group protein B1 is an activator of apoptotic response to antimetabolite drugs. Mol Pharmacol. 2008;73:260–9. doi: 10.1124/mol.107.041764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz C, Focke F, Saito Y, Schuermann D, Lettieri T, Selfridge J, Schar P. Base excision by thymine DNA glycosylase mediates DNA-directed cytotoxicity of 5-fluorouracil. PLoS Biol. 2009;7:e91. doi: 10.1371/journal.pbio.1000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–8. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012;30:2500–8. doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters GJ, Lankelma J, Kok RM, Noordhuis P, van Groeningen CJ, van der Wilt CL, Meyer S, Pinedo HM. Prolonged retention of high concentrations of 5-fluorouracil in human and murine tumors as compared with plasma. Cancer Chemother Pharmacol. 1993;31:269–276. doi: 10.1007/BF00685670. [DOI] [PubMed] [Google Scholar]
- Pettersen HS, Sundheim O, Gilljam KM, Slupphaug G, Krokan HE, Kavli B. Uracil-DNA glycosylases SMUG1 and UNG2 coordinate the initial steps of base excision repair by distinct mechanisms. Nucleic Acids Res. 2007;35:3879–3892. doi: 10.1093/nar/gkm372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci Biobehav Rev. 2011;35:729–741. doi: 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Singh NP. A rapid method for the preparation of single-cell suspensions from solid tissues. Cytometry. 1998;31:229–232. doi: 10.1002/(sici)1097-0320(19980301)31:3<229::aid-cyto10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Tang H, Mayersohn M. A novel model for prediction of human drug clearance by allometric scaling. Drug Metab Dispos. 2005;33:1297–1303. doi: 10.1124/dmd.105.004143. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Barrett JE. An automated learning and memory model in mice: pharmacological and behavioral evaluation of an autoshaped response. Behav Pharmacol. 1998;9:273–283. [PubMed] [Google Scholar]
- Walker EA. Animal models. Adv Exp Med Biol. 2010;678:138–146. doi: 10.1007/978-1-4419-6306-2_18. [DOI] [PubMed] [Google Scholar]
- Walker EA, Foley JJ, Clark-Vetri R, Raffa RB. Effects of repeated administration of chemotherapeutic agents tamoxifen, methotrexate, and 5-fluorouracil on the acquisition and retention of a learned response in mice. Psychopharmacology (Berl) 2011;217:539–548. doi: 10.1007/s00213-011-2310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]