Abstract
OBJECTIVES
Recent evidence suggests that functional deficiency in regulatory T cells (Tregs), an innate immuno-modulator, exacerbates brain damage after cerebral ischemia. We therefore evaluated the effect of Treg transfer in rodent models of ischemic stroke and further investigated the mechanism underlying Treg-afforded neuroprotection.
METHODS
We examined the therapeutic potential of Tregs and the mechanisms of neuroprotection in vivo in 2 rodent models of ischemic stroke and in vitro in Treg-neutrophil co-cultures using a combined approaches including cell-specific depletion, gene knockout mice, and bone marrow chimeras.
RESULTS
Systemic administration of purified Tregs at 2, 6 or even 24 hours after MCAO resulted in a marked reduction of brain infarct and prolonged improvement of neurological functions lasting out to 4 weeks. Treg-afforded neuroprotection was accompanied by attenuated blood-brain barrier (BBB) disruption during early stages of ischemia, decreased cerebral inflammation and reduced infiltration of peripheral inflammatory cells into the lesioned brain. Surprisingly, Tregs exerted early neuroprotection without penetrating into the brain parenchyma or inhibiting the activation of residential microglia. Rather, both in vivo and in vitro studies demonstrated that Tregs suppressed peripheral neutrophil-derived matrix metallopeptidase-9 production, thus preventing proteolytic damage of the BBB. In additions to its potent central neuroprotection, Treg treatment was shown to ameliorate post-stroke lymphopenia, suggesting a beneficial effect on immune status.
INTERPREATION
Our study suggests that Treg adoptive therapy is a novel and potent cell-based therapy targeting post-stroke inflammatory dysregulation and neurovascular disruption.
Introduction
Ischemic stroke elicits profound inflammatory response involving both innate and adaptive immunity1. Innate immune cells such as neutrophils and microglia/macrophages respond promptly to cerebral ischemia and migrate to the injury. Although these cells are indispensable for the clearance of debris and tissue remodeling2, 3, their overactivation releases a large number of cytotoxic molecules. They can also compromise blood-brain barrier (BBB) integrity by producing matrix metallopeptidases (MMPs), allowing the invasion of even more peripheral immune cells4, 5. In addition, cytokines and chemokines produced by activated inflammatory cells further recruit and activate additional immune cells, resulting in a vicious cycle that promotes long-lasting brain damage and worsens neurological deficits.
Members of the adaptive immune system, specifically T lymphocytes, also play a critical role in ischemic brain injury. Most subsets of T cells have been revealed to be detrimental to the ischemic brain6–8. However, regulatory T cells (Tregs), a specialized subpopulation of T cells, appear to be an endogenous protective mechanism that dampens cerebral inflammation following ischemic stroke9. In general, CD4+CD25+ Tregs negatively regulate the immune system and modulate inflammation induced by pathogens and injuries. For example, Tregs suppress effector T cells and other immune cells10,11 through either direct contact with the suppressed cell or release of the immunosuppressive cytokines TGF-β and IL-1012. Disruptions of Treg function exacerbate inflammation and autoimmunity13. Tregs increase in the blood several days after the onset of stroke both in patients14 and in experimental animals15. This is likely to be an evolutionarily adaptive response that blunts the impact of stroke because deletion of Tregs worsens both ischemic brain damage and functional outcomes9. However, the therapeutic potential of Treg transplantation after ischemic stroke has not been investigated. Even less is known about the precise mechanism through which Tregs protect the brain after stroke.
Using rodent models of focal transient ischemia, we show for the first time that intravenous injections of Tregs even up to 24h after ischemia resulted in a marked reduction of brain infarct and a prolonged improvement of neurological functions lasting out to 4 weeks. We further demonstrated that Treg-conferred early neuroprotection is mediated via BBB protection involving an inhibition of peripheral neutrophil-derived MMP9.
Materials and Methods
Ischemia models
All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Cerebral focal ischemia was induced in mouse by intraluminal occlusion of the left MCA for 60min as described in Supplementary Methods. Transient (120min) cerebral focal ischemia was induced in Sprague Dawley rats as described16. Experimental procedures were performed following criteria derived from STAIR group guidelines for preclinical evaluation of stroke therapeutics17. Cerebral blood flow (CBF) was measured to confirm the vascular occlusion. Animals that did not show a CBF reduction of at least 75% on laser-Doppler flowmetry were excluded (less than 10% of stroke animals) from further experimentation. Animals that died after ischemia induction were also excluded from experimental groups. Temperature was controlled during the ischemic period. Immediately after surgery, animals were randomly assigned to PBS, splenocyte, or Treg treatment groups. Investigators were blinded to treatment groups during cell transfer or vehicle injection and during all outcome assessments.
Isolation, labeling and adoptive transfer of Tregs
Single-cell suspensions were prepared from inguinal and axillary lymph nodes and spleens. CD4+CD25+ Treg populations were enriched by negative selection and positive selection with a regulatory T cell isolation kit (Mouse: Miltenyi Biotec; Rat: R&D system) according to the manufacturers' instructions. Two mice or 1 rat were needed to obtain 2×106 Tregs. The recipient mouse or rat received a tail vein injection of 2×106 freshly enriched Tregs or freshly isolated splenocytes in 0.2 ml PBS at 2, 6, or 24h after reperfusion. The 2×106 cell dose was chosen on the basis of our preliminary evaluation of the relationship between Tregs dose and their therapeutic effect (Supplementary Figure 1A). For Treg labeling and tracking, Tregs were incubated with 0.5 µM cell tracker orange CMTMR (Invitrogen) at 37 °C for 30 min before intravenous injection.
Irradiation and bone marrow transplantation
To construct bone marrow chimeric mice, 6-week old recipient male C57/B6 and MMP9−/− mice underwent lethal gamma irradiation (9.5 Gy), followed 6 hrs later by intravenously transplantation with bone marrow cells obtained from 6- to 8-week-old syngeneic donors (107 cells per recipient). Three groups were studied: (1) wild-type marrow transplanted into wild-type recipients, designated as WT/WT; (2) wild-type marrow transplanted to MMP9−/− recipients, designated as WT/MMP9KO; (3) MMP9−/− marrow transplanted to wild-type recipients, designated as MMP9KO/WT. Six weeks later, blood were obtained in every animal to verify hematopoietic cell reconstitution. Chimeras mice were subjected to brain ischemia seven weeks after irradiation.
Primary neutrophil culture and treatment
Primary mouse neutrophils from bone marrow and blood were isolated using EasySep Mouse Neutrophil Enrichment Kit (Stem Cell Technologies) according to the manufacturer’s instructions. To determine the influence of Tregs on neutrophil-derived MMP9, isolated Tregs were pre-activated with CD3/CD28 antibodies (BD Bioscience)18, 19 for 3 days and then plated into the lower chamber of 96-well Transwell permeable trays (Millipore) free of anti-CD3/CD28 stimulation. The enriched neutrophils (1×105 per well) were pre-stimulated with TNF-α (100 ng/ml, ebioscience) for 2h for MMP9 induction20, and then added to either the lower chamber or the upper chamber of 96-well Transwell trays. The Treg-neutrophil co-cultures were maintained at 37°C for another 24h. In all conditions, the medium was collected after 24h incubation to quantify the released proteases.
Statistical Analysis
Results were presented as mean ± SEM. The difference in means between 2 groups was assessed by the 2-tailed Student's t test. Differences in means among multiple groups were analyzed using 1- or 2-way ANOVA with time or treatment as the independent factors. When ANOVA showed significant differences, pair-wise comparisons between means were tested by post hoc Bonferroni/Dunn tests. In all analyses, p < 0.05 was considered statistically significant. See Supplementary Material for full description of all the experimental procedures.
Results
Tregs reduce brain damage after transient focal cerebral ischemia
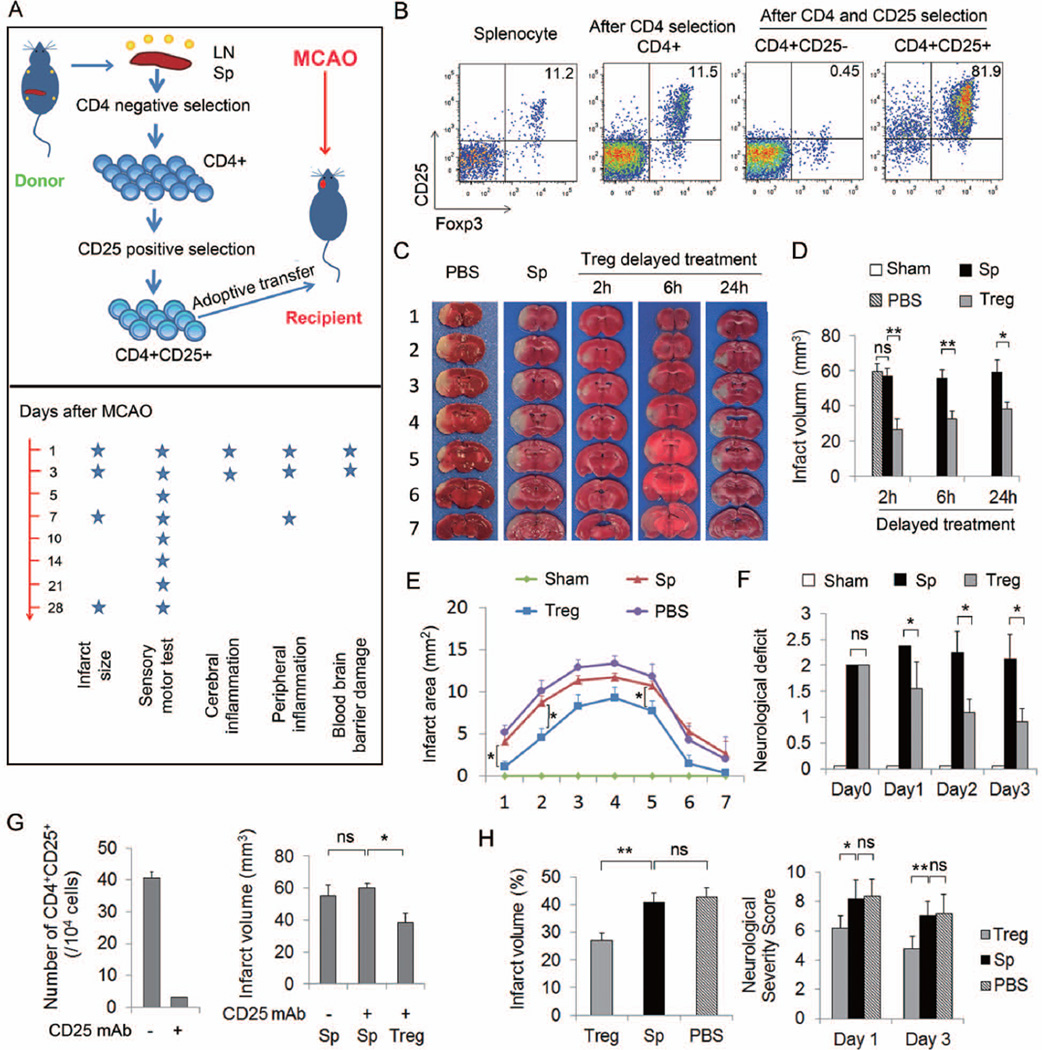
Recent studies that depleted Tregs from the circulation prior to ischemic challenge suggest a beneficial effect of Tregs on late phase ischemic brain injury9. However, conflicting findings suggest that Treg depletion fails to affect brain infarct volume21. To confirm a direct effect of Tregs on cerebral ischemia and to further evaluate their therapeutic value, we isolated Tregs from donor mice and injected them into recipient mice after MCAO (Figure 1A). Flow cytometry demonstrated that the isolated CD4+CD25+ Tregs were more than 95% enriched, with 82% of them expressing the Treg immunophenotypic marker Foxp3 (Figure 1B). Transient focal ischemia lasting 60 min was induced by MCAO under CBF monitoring before, during and after MCAO. Animals were randomly assigned to Tregs, splenocyte, or PBS treatment groups. Retrospectively, we found no statistical difference in CBF reduction during and after MCAO among the various experimental groups (Supplementary Figure 1B). Treg treatment at 2, 6, or 24h post-ischemia significantly attenuated brain infarct (Figure 1C–1E) and reduced neurological deficits (Figure 1F) compared to PBS or splenocyte-treated animals. Mice with splenocyte or PBS treatment developed similar brain infarct volumes as well as cerebral inflammation (Supplementary Figure 2) after MCAO. Splenocyte-treated animals were therefore used in most experiments as controls. The maximal protection was elicited with early Treg transfer 2h after MCAO, resulting in approximately 50% reduction of infarct volume. All subsequent experiments used this optimal post-MCAO administration regimen. Treg treatment did not affect CBF during reperfusion up to 24h as measured by 2-D laser speckle imaging techniques (Supplementary Figure 1C).
Figure 1. Adoptive transfer of Tregs confers neuroprotection against focal cerebral ischemia.
(A) Scheme for experimental design. Tregs or splenocytes were isolated from pooled spleens and lymph nodes of donors and injected intravenously (2×106 cells/animal) into recipients at 2, 6 or 24 h after MCAO. Time line for parameter measurements is indicated. (B) Representative flow cytometry plots of CD25 and Foxp3 expression on splenocyte, CD4+ T cells after negative selection, CD4+CD25− and CD4+CD25+ subsets of T cells after double selection. (C–E) Treg-afforded protection in mice at 3d after 60 min MCAO. (C) Representative TTC stained coronal sections showed a smaller cerebral infarct in a mouse with adoptively-transferred Tregs than in a splenocyte-transferred or PBS-treated mouse. (D) Infarct volumes in mice treated with Tregs at 2h (n=7/group), 6h (n=8–9/group) or 24h (n=6–7/group) after MCAO were significantly reduced. (E) Infarct areas of 7 consecutive coronal sections, 1 mm apart, throughout the MCA territory in mice that received treatments at 2h after MCAO (n=7/group). (F) Treg treatment with 2h delay improved neurological deficits in mice over 3d after MCAO compared to splenocyte- or PBS-treatment (n=7/group). (G) Exogenous Tregs protected the ischemic brain in the absence of endogenous Tregs. Mice were injected intraperitoneally (i.p). with either PBS (control) or 300 µg of CD25-specific antibody (CD25 mAb) 2d prior to MCAO. Left: Flow cytometry analysis showing that endogenous Tregs were depleted in anti-CD25 mAb treated mice. Right: Infarct volume was measured 3d after MCAO. (H) Treg-afforded protection in rats at 3d after MCAO. Left: Infarct volume in rats treated with Tregs, splenocytes or PBS at 2h after 120 min of MCAO. Right: Treg-treated rats demonstrated reduced neurological severity scores at 1d and 3d after MCAO compared to splenocyte- or PBS-treated rats (n=5/group). Data are mean ± SE. *P<0.05, **P<0.01.
To disentangle the role and interaction of transplanted Tregs with endogenous Tregs, we depleted the endogenous Treg population with a specific CD25 antibody 2d prior to MCAO (Figure 1G). Therapeutic numbers of exogenous Tregs protected against early stage ischemic brain injury even in the absence of endogenous Tregs.
We also tested the effect of Treg transfer in a rat model of stroke. Consistent with results in mice, rats with Treg post-treatment developed a significantly smaller infarct than splenocyte- or PBS-treated animals, which was accompanied by a reduced neurological severity score (Figure 1H).
Tregs improve functional outcomes and confer prolonged protection after cerebral ischemia
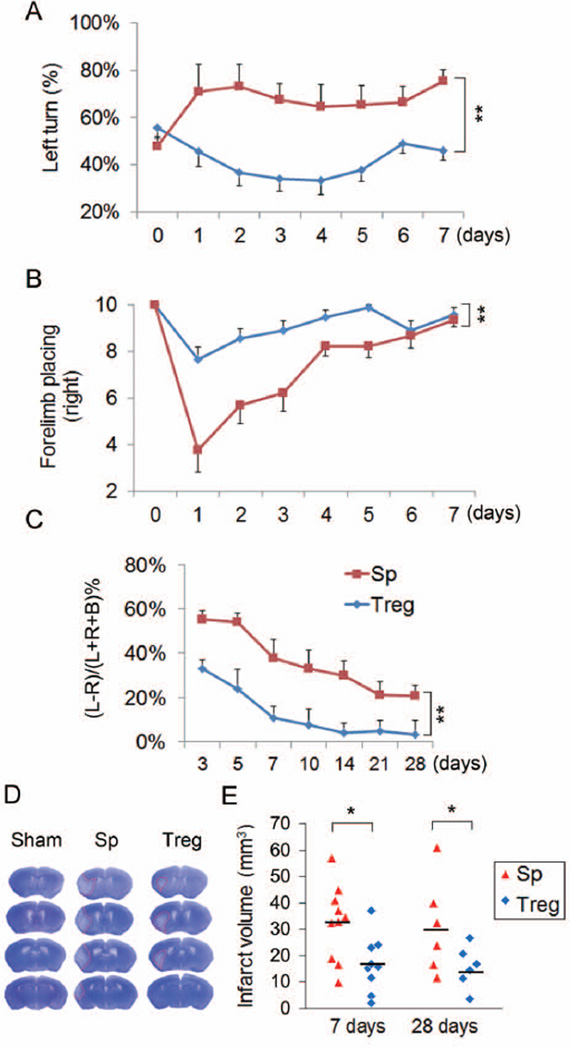
Post-ischemic sensorimotor dysfunction significantly improved during both the acute and late recovery period after MCAO in Treg-treated mice compared to splenocyte-treated animals, as assessed by the corner test, forelimb placing test and the cylinder test up to 28d following MCAO (Figure 2A–2C). Cresyl violet staining and MAP2 immunostaining revealed profoundly reduced infarct volumes at 7d and 28d after MCAO, respectively (Figure 2D–2E). Collectively, these results confirmed that Treg treatment actually reduced cerebral tissue loss, rather than simply delaying cell death, and improved long-term neurological function after cerebral ischemia.
Figure 2. Tregs confer long-term neuroprotection against cerebral ischemia.
Tregs or splenocytes were transferred intravenously into recipient mice at 2h after MCAO. Brain infarct and sensorimotor functions were assessed up to 28d after MCAO. (A–B) Acute sensorimotor dysfunction at 1–7d after ischemia was significantly improved in Treg-treated mice as assessed by the corner test (A, n=10/group) and forelimb placing test (B, n=10/group). Corner test performance was expressed by the percentage of left turns out of 10 turn trials. The performance in forelimb placing test was expressed as the number of successful placing responses on the impaired right forelimb out of 10 placing trials. (C) Treg-treated mice demonstrated improved long-term sensorimotor performance as assessed by cylinder test up to 28d after ischemia (n=6/group). The number of left, right, or both forepaw contacts were counted, and the performance asymmetry was expressed as (left−right)/(left+right+both)×100% paw use in 5 min. (D) Representative cresyl violet stained brain sections obtained at 7d after MCAO showed smaller infarct in a Treg-treated mouse than in a splenocyte-transferred mouse. (E) Infarct volume determined at 7d (n=10/group) and 28d (n=6/group) after ischemia with cresyl violet staining and MAP2 staining, respectively, was significantly reduced in Treg-treated mice. Data are mean ± SE. *P<0.05, **P<0.01.
Tregs attenuate cerebral inflammation and reduce the early infiltration of peripheral immune cells into the ischemic brain prior to their own central nervous system (CNS) infiltration
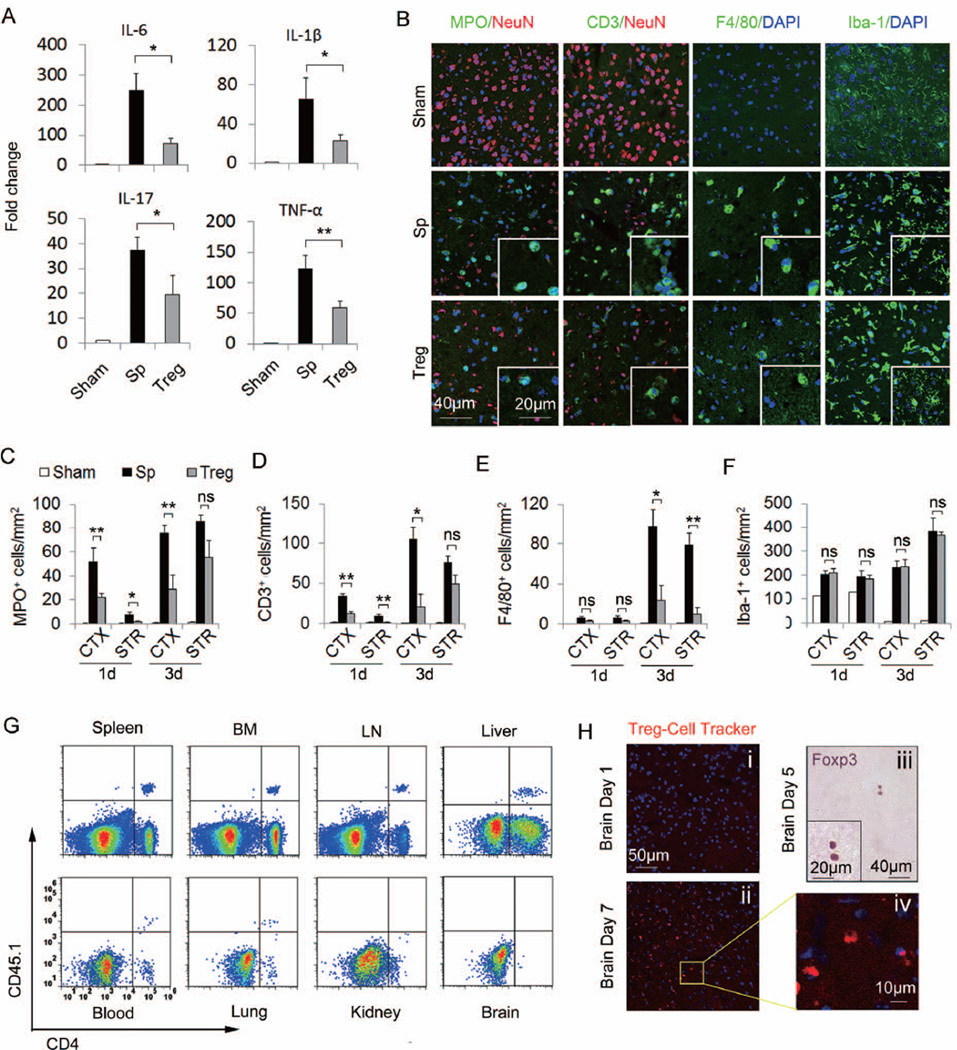
Adoptive transfer of Tregs has been shown to suppress inflammatory responses in several disease models11, 22–23. To investigate the effect of Tregs on post-ischemic cerebral inflammation, we measured multiple inflammatory markers in the ischemic hemisphere at 24h after MCAO. The mRNA levels of IL-6, IL-1β, IL-17, and TNF-α were substantially increased in splenocyte-treated MCAO mice and these increases were significantly blunted by Treg treatment (Figure 3A). IL-10 and TGF-β, two main anti-inflammatory mediators released by Tregs, were also upregulated at 24h post-MCAO. Unexpectedly, Treg treatment did not change the expression of these two cytokines in the brain (Supplementary Figure 3).
Figure 3. Tregs attenuate post-ischemic inflammation and reduce the early infiltration of peripheral immune cells into the brain ahead of their own CNS infiltration.
(A) Quantitative real-time PCR for mRNA expression of IL-6, IL-1β, IL-17, and TNF-α in the ischemic hemispheres from animals with 60 min MCAO and 24h reperfusion (n=6/group). IL-6, IL-1β, IL-17, and TNF-α mRNA were significantly decreased in Treg-treated mice compared to splenocyte-treated mice. (B) Representative immunofluorescent staining of MPO, CD3, F4/80, and Iba-1 on brain sections obtained 3d after MCAO. (C-F) Time courses for the infiltration of MPO+ neutrophilic granulocytes (C), CD3+ T cells (D), F4/80+ macrophages (E) and the activation of Iba-1+ microglial cells (F) in the ischemic brains of Treg-treated mice compared to splenocyte-treated and sham-operated mice (n=6/group). (G) Adoptively transferred CD45.1+CD4+ Tregs present in the spleen, bone marrow (BM), lymph node (LN), liver, blood and lung, but not in the brain or kidney at 1d after MCAO. Plots are representative of four animals. (H) Delayed brain infiltration of Tregs after stroke. Cell tracker-labeled Tregs were observed in the brain at 7d (ii, iv) but not 1d (i) after MCAO. (iii) Immunohistochemical staining of Foxp3 in brain sections at 5d after MCAO. Images are representative of sections from four animals. Data are mean ± SE. *P<0.05, **P<0.01.
Local microglia and infiltrated peripheral immune cells are the major sources of inflammatory cytokines in the injured brain. We then investigated whether Tregs impede the recruitment of peripheral inflammatory cells and/or stabilize resident microglia in the ischemic penumbra (Supplementary Figure 4A), which is a zone of reversible ischemia and salvageable in first few hours after ischemic stroke onset. Neutrophil, T cell, and macrophage infiltration was prominent at 1–3d after MCAO in splenocyte-treated mice, but remarkably attenuated in Treg-treated mice (Figure 3B–3E, Supplementary Figure 4B–4C). In contrast, an amelioration of microglial activation was not observed until 7d post-MCAO (Figure 3B and 3F, Supplementary Figure 5). These results suggest that the early anti-inflammatory effects of Tregs after ischemia may largely be attributed to their inhibition of peripheral immune cell infiltration rather than to a significant impact on CNS resident microglia.
In line with a non-CNS targeting mechanism for Treg action, the infiltration of Foxp3+ Tregs into brain parenchyma was delayed until 5d after stroke (Figure 3H). Using the CD45.1 congenic marker, exogenously transferred Tregs were detected by flow cytometry in the spleen, lymph nodes, bone marrow, lung, liver and blood, but not in the brain or kidney of MCAO recipients at 1d post-MCAO (Figure 3G). These transferred CD45.1+CD4+ Tregs showed high expression of CD25 and Foxp3 (Supplementary Figure 6C). Similarly, cell tracker-labeled Tregs were found in multiple peripheral organs in the absence of trafficking into the brain parenchyma of MCAO recipients at 1d after MCAO (Figure 3H, Supplementary Figure 6A). Taken together, these data verify that the transferred Tregs are located outside the brain while exerting their anti-inflammatory effects by inhibiting peripheral immune cell infiltration into the CNS.
Tregs do not exacerbate post-stroke immunosuppression
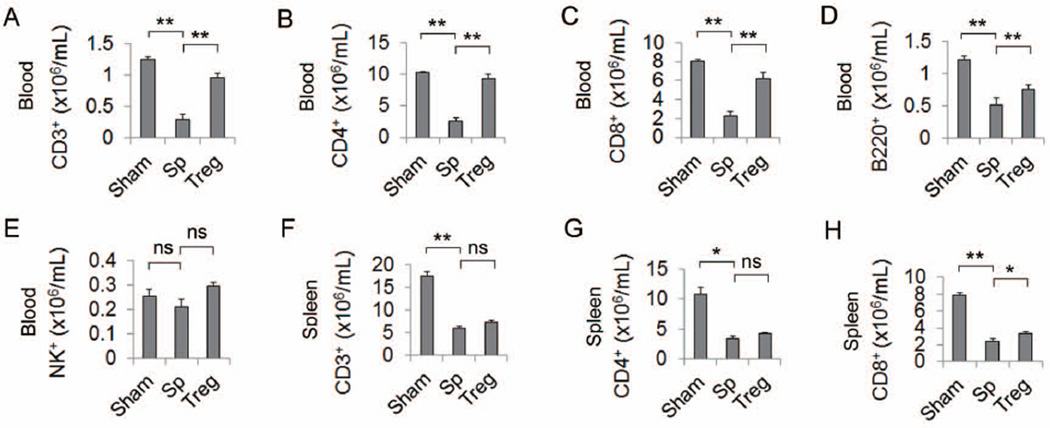
The negative effect of Tregs on some immune responses raises the concern that Treg therapy might further inhibit the already suppressed immune system after stroke24. We thus performed differential blood cell counting by flow cytometry to evaluate the effect of Treg transfer on stroke-induced immunosuppression. Treg treatment preserved the otherwise significantly reduced blood CD3+ T lymphocytes (Figure 4A) and B220+ B lymphocytes (Figure 4D) following MCAO. Further analysis of the T cell subpopulation showed a significantly increased number of CD4+ T helper cells and CD8+ cytotoxic T cells in Treg-treated groups (Figure 4B–4C). Tregs had no effect on blood NK1.1+ cells (Figure 4E). A similar trend was observed in spleen T lymphocyte populations (Figure 4F–4H). Our results indicate that Treg treatment did not exacerbate post-stroke immunosuppression.
Figure 4. Tregs did not exacerbate post-stroke immunosuppression.
(A–E) Treg treatment did not exacerbate the immunosuppression in the blood after MCAO. At 5d after stroke, blood cells were isolated and stained for CD3+ T cells (A), CD4+ T helper cells (B), CD8+ T cytotoxic cells (C), B220+ B cells (D), and NK1.1+ NK cells (E). Flow cytometric analysis revealed that the decrease of CD3, CD4, CD8 and B cells after stroke in the splenocyte-treated mice was reversed by Treg treatment. n=6/group. (F–H) Treg treatment did not worsen the loss of T cells in the spleen after stroke. The number of CD3+ (F), CD4+ (G) and CD8+ (H) T cells were significantly decreased in the spleen at 5d after stroke in the splenocyte-treated mice. Treg treatment did not worsen the T cell loss. n=6/group. Data are mean ± SE. *P<0.05, **P<0.01.
Tregs preserve BBB integrity after ischemia
The pathology of stroke involves disruption of the BBB and ensuing communication between peripheral immune cells and the brain. We investigated whether Tregs inhibited peripheral immune cell infiltration after cerebral ischemia via BBB protection. The extravasation of two tracers, cadaverine Alexa-555 or BSA Alexa-555, into the brain parenchyma was prominent at 24h after MCAO but reduced by Treg treatment (Figure 5A). Fluorescence quantification revealed significantly lower tracer levels in brain lysates from Treg-treated mice compared to splenocyte-treated controls (Figure 5B–5C). Similarly, the intracranial leakage of plasma-derived IgG was lessened in Treg-treated mice (Figure 5A, 5D–5F). Importantly, Treg-treated animals shown significantly reduced IgG extravasation (Supplementary Figure 7C) and inflammatory cell infiltration (Supplementary Figure 7D–7F) compared to untreated animals even when the cerebral lesion volumes were matched across groups by titrating MCAO intervals (Supplementary Figure 7A–7B). These data suggest that the BBB protection and anti-inflammatory effects in Treg-treated animals are not simply the consequences of reduced infarct volume.
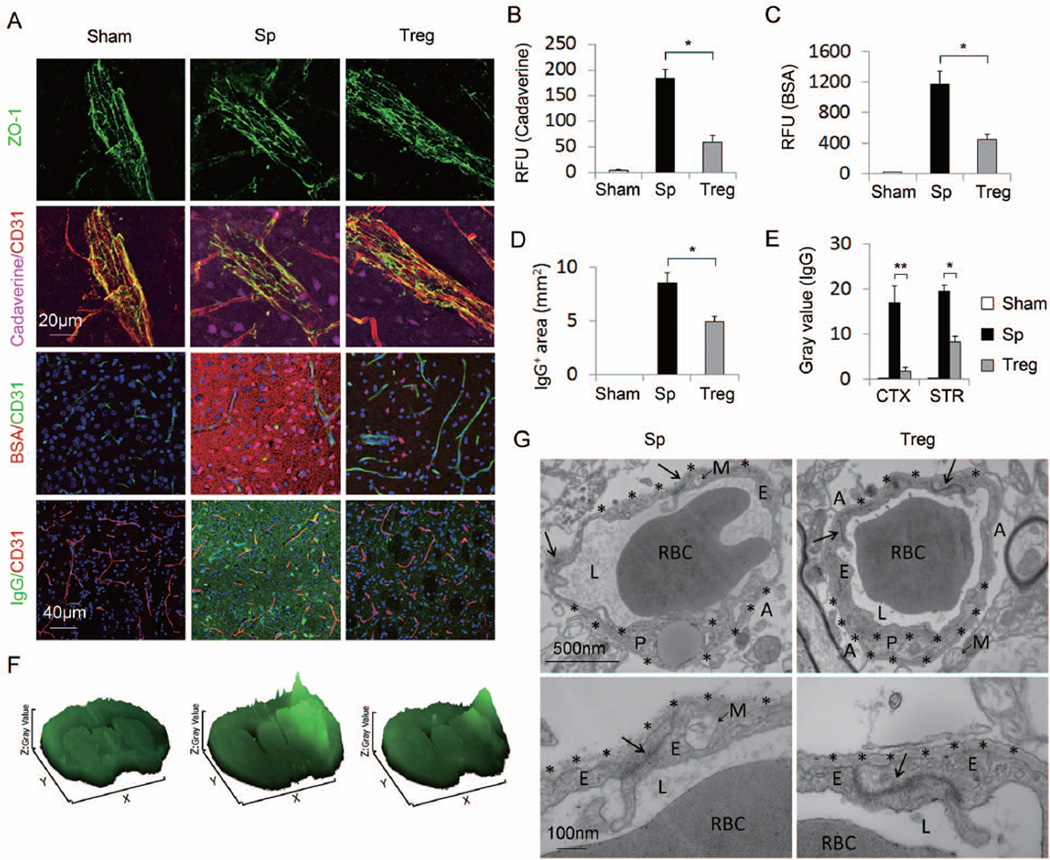
Figure 5. Tregs preserve blood-brain barrier integrity after MCAO.
(A) Representative Z-stack confocal images showed that the disruption of the tight junction protein ZO-1 (Top panel), the brain penetration of intravenously injected tracers, Cadaverine-Alexa-555 and BSA-Alexa-555 (middle two panels), and the endogenous IgG extravasation into the brain (bottom panel) at 1d after MCAO were attenuated by Treg treatment compared to splenocyte-treated controls. (B) Cadaverine-Alexa-555 (950Da) was injected intravenously at 22h post-MCAO. Fluorescence intensities in brain lysates from the infarct area were measured after 2h of circulation (n=5/group). (C) BSA-Alexa-555 (66 KDa) was injected intravenously at 8h post-MCAO. Fluorescence intensities in brain lysates were measured after 16h of circulation (n=5/group). RFU shows relative fluorescence units per 0.5 gram of tissue. (D-F) Tregs ameliorated IgG extravasation after MCAO. (D) Quantification of endogenous IgG positive area determined by immunohistochemical staining of mouse IgG (n=5/group). (E) Quantification of gray values of IgG immunostaining (n=5/group). (F) Surface plot images generated from immunostaining of mouse IgG. (G) Transmission electron microscopy performed at 48h after stroke to observe the integrity of the BBB. Tight junctions (arrows) were dramatically disrupted and basement membranes (stars) were broken down in the splenocyte-treated MCAO animals. Treg treatment elicited prominent protection of BBB ultrastructures. Images are representative of brain sections from four animals per group. RBC, red blood cell; L, vascular lumen; E, endothelial cell; P, pericyte; A, astrocyte end-feet; M, mitochondria. Data are mean ± SE. *P<0.05, **P<0.01.
The integrity of tight junction complexes is associated with paracellular impermeability of the BBB25. We assessed the continuity of such complexes using the ZO-1 junctional marker (Figure 5A). ZO-1 was expressed in a continuous manner in intact animals but was largely disrupted in the infarct areas 24h after MCAO. Treg adoptive therapy retained the integrity of ZO-1 expression. The ultrastructure of tight junctions was further observed with transmission electron microscopy (Figure 5G). Ischemic injury resulted in abnormalites in intercellular tight junctions, manifested by lower electron density and less well-defined basement membranes. Treg treatment after ischemia elicited prominent protection of the ultrastructure of tight junctions and basement membranes. These experiments lead to the solid conclusion that the adoptively transferred Tregs ameliorated the BBB disruption in the early phase after stroke.
Tregs confer protection against MCAO by blunting a rise in MMP9
MMP9 rises in the brain and plasma quickly after ischemic stroke and disrupts the BBB 26–29. We thus investigated whether Treg treatment could inhibit this increased MMP9 production after MCAO. Gel zymography demonstrated a significant increase in pro-MMP9 and activated MMP9 at 24h after ischemic onset in the plasma and, to a lesser extent, in the brain. Remarkably, Treg treatment abolished the ischemia-induced MMP9 elevation almost to sham levels (Figure 6A–6C). Quantitative ELISA assays further confirmed the inhibitory effect of Tregs on plasma MMP9 production after ischemia (Figure 6D). The inhibition of central MMP9 expression by Tregs was also confirmed by immunofluorescent staining (Figure 6E). At 24h after MCAO, MMP9 immunostaining was observed mainly around CD31+ blood vessels in the ischemic zone. Treg treatment reduced the amount of perivascular MMP9. The length of MMP9 positive blood vessels, which was defined by double labeling of MMP9 with CD31, was significantly decreased in Treg-treated brains compared to control MCAO brains (Figure 6F). In addition to blood and brain, we also observed the upregulation of MMP9 in the spleen 24h after ischemia (Supplementary Figure 8A). Treg treatment dramatically reduced MMP9 staining in the spleen.
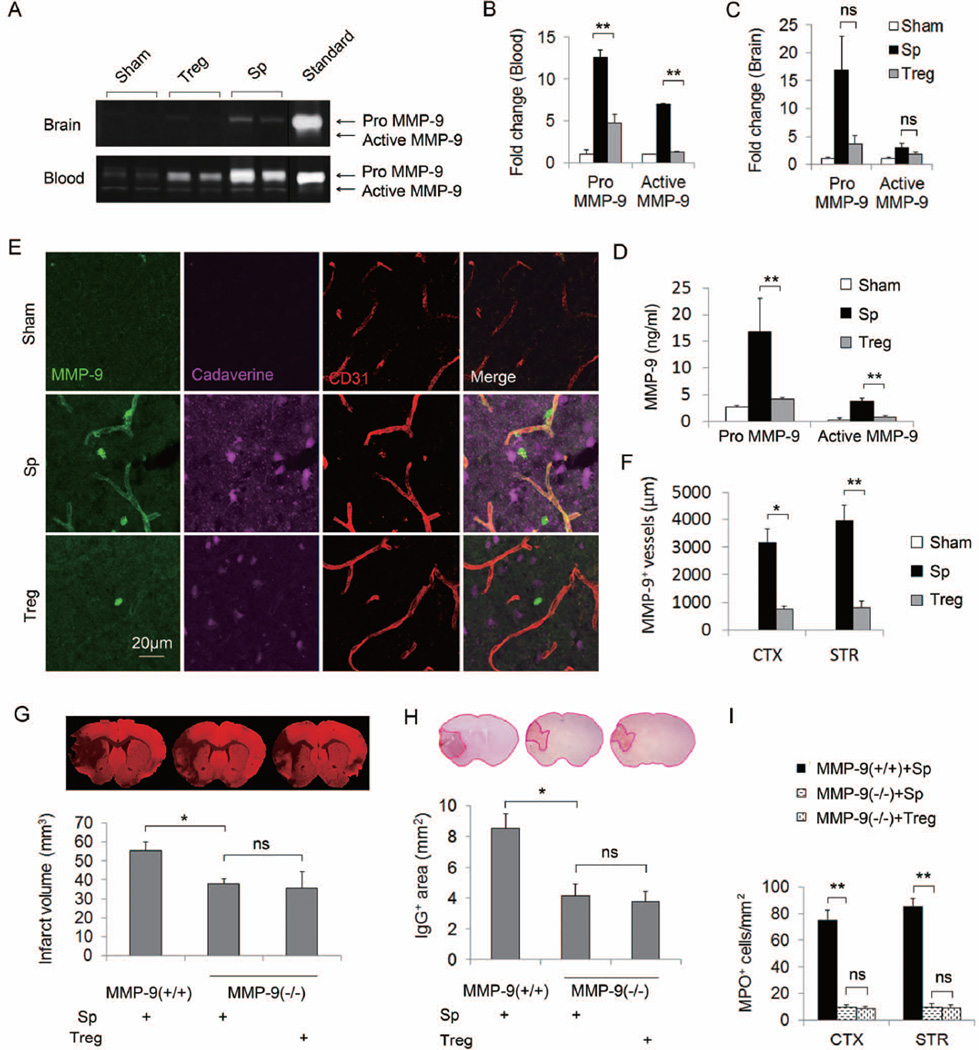
Figure 6. Tregs confer protection against MCAO by ameliorating a rise in MMP9 production.
(A–F) Tregs ameliorated MMP9 production after MCAO. Plasma and brain tissue were obtained at 24h after MCAO from splenocyte- or Treg-treated mice or sham-operated mice. (A) Representative zymogram comparing brain and plasma MMP9 levels among different treatments. (B–C) Quantified densitometry of MMP9 zymography bands in plasma samples (B, n=5/group) and in brain lysates (C, n=5/group). (D) Plasma pro- and active MMP9 levels quantified by ELISA. (E) Representative Z-stack confocal image of MMP9 and CD31 double immunostaining. MMP9 immunostaining was observed in the brain parenchyma and around blood vessels in the ischemic zone. The increase in brain MMP9 is accompanied by the prominent leakage of cadaverine-Alexa-555 into the brain parenchyma. MMP9 expression in the brain infarct and tracer leakage after MCAO was abolished by Treg treatment. (F) Quantification of the length of MMP9+/CD31+ blood vessels in the brain (n=4/group). (G–I) Treg-conferred neuroprotection was abolished in MMP9 deficient mice. (G) Brain infarcts as measured by MAP2 staining in wild type and MMP9 deficient mice treated with splenocytes or Tregs. (H) Quantification of IgG leakage determined by positive area of mouse IgG immunohistochemical staining (n=6/group). (I) The number of infiltrated MPO+ neutrophils at 3d after MCAO. Brain infarct, IgG leakage, and neutrophil infiltration were significantly decreased in MMP9−/− compared to wild type mice. Treg treatment failed to confer further protection in MMP9−/− mice. Data are mean ± SE. *P<0.05, **P<0.01.
To verify a causal connection between MMP9 and Treg-afforded neuroprotection, we subjected MMP9−/− mice to MCAO followed by splenocyte or Treg treatment. Consistent with previous reports26, MMP9 deficient mice exhibited significant albeit smaller infarct volumes (Figure 6G), which was accompanied by reduced IgG extravasation through the BBB (Figure 6H). Notably, Treg-treated and splenocyte-treated MMP9−/− mice demonstrated a comparable volume of infarct (Figure 6G), BBB damage (Figure 6H) and neutrophil infiltration (Figure 6I, Supplementary Figure 9) early after MCAO.
To further determine if the beneficial effects of exogenous Tregs are attributable to the MMP9 alteration in peripheral or CNS cells, MCAO was performed in animals that had been subjected to lethal irradiation and bone marrow transplantation. The success of hematopoietic cell reconstitution was confirmed by white blood cell counts (not shown) and measuring MMP9 levels in the blood at 24h after MCAO (Table 1). Tregs showed significant protection in wild-type or MMP9−/− mice that were reconstituted with wild-type bone marrow (WT/WT and WT/MMP9KO), whereas Tregs had no protective effect in wild-type mice that were reconstituted with MMP9−/− bone marrow (MMP9KO/WT). These results suggest that the Treg-conferred neuroprotection in the acute phase of ischemic brain injury is due to the inhibition of MMP9 expression in bone marrow-derived peripheral hematopoietic cells.
Table 1.
The beneficial effects of exogenous Tregs are attributable to periphery-derived MMP9.
| Chimeras | Donor mice |
Recipient mice |
Treatment | n | Blood MMP9 (ng/ml) |
Infarct volume (mm3) |
|---|---|---|---|---|---|---|
| WT/WT | Wild type | Wild type | Sp | 5 | 19.32 ± 5.43 | 60.14 ± 7.12 |
| Treg | 6 | 4.78 ± 7.97a,c | 28.83 ± 0.41a,d | |||
| WT/ MMP9KO |
Wild type | MMP9KO | Sp | 5 | 17.21 ± 2.87 | 58.42 ± 5.11 |
| Treg | 5 | 3.73 ± 0.63a,c | 35.45 ± 5.84b,d | |||
| MMP9KO/ WT |
MMP9KO | Wild type | Sp | 6 | 0.63 ± 0.19a,c | 39.81 ± 5.49b |
| Treg | 6 | 0.54 ± 0.20a,c | 35.98 ± 6.94b,d |
p<0.01 versus splenocyte-treated WT/WT
P<0.05 versus splenocyte-treated WT/WT
p<0.01 versus splenocyte-treated WT/MMP9KO
p<0.05 versus splenocyte-treated WT/MMP9KO
Neutrophil-derived MMP9 is a major target for Treg cerebroprotective action
It is becoming more widely accepted that neutrophils contribute to the MMP9 signatures after brain ischemia30. Our immunostaining demonstrated that MMP9-loaded neutrophils invaded the brain parenchyma early after MCAO (Figure 7A–7C). We also observed numerous MMP9+ neutrophils in the spleen (Supplementary Figure 8B). Some Gr1+ neutrophils were in close proximity to cell tracker-labeled Tregs in the red pulp area (Supplementary Figure 6B). Furthermore, the ELISA assay demonstrated a dramatic increase in MMP9 expression in blood neutrophils isolated from MCAO animals (Figure 7D). Treg treatment significantly reduced MMP9 production in blood neutrophils and inhibited the number of MMP9+ neutrophils in the brain after MCAO. Taken together, these data suggest that adoptively transferred Tregs may exert neuroprotection after cerebral ischemia via the inhibition of neutrophil-derived MMP9.
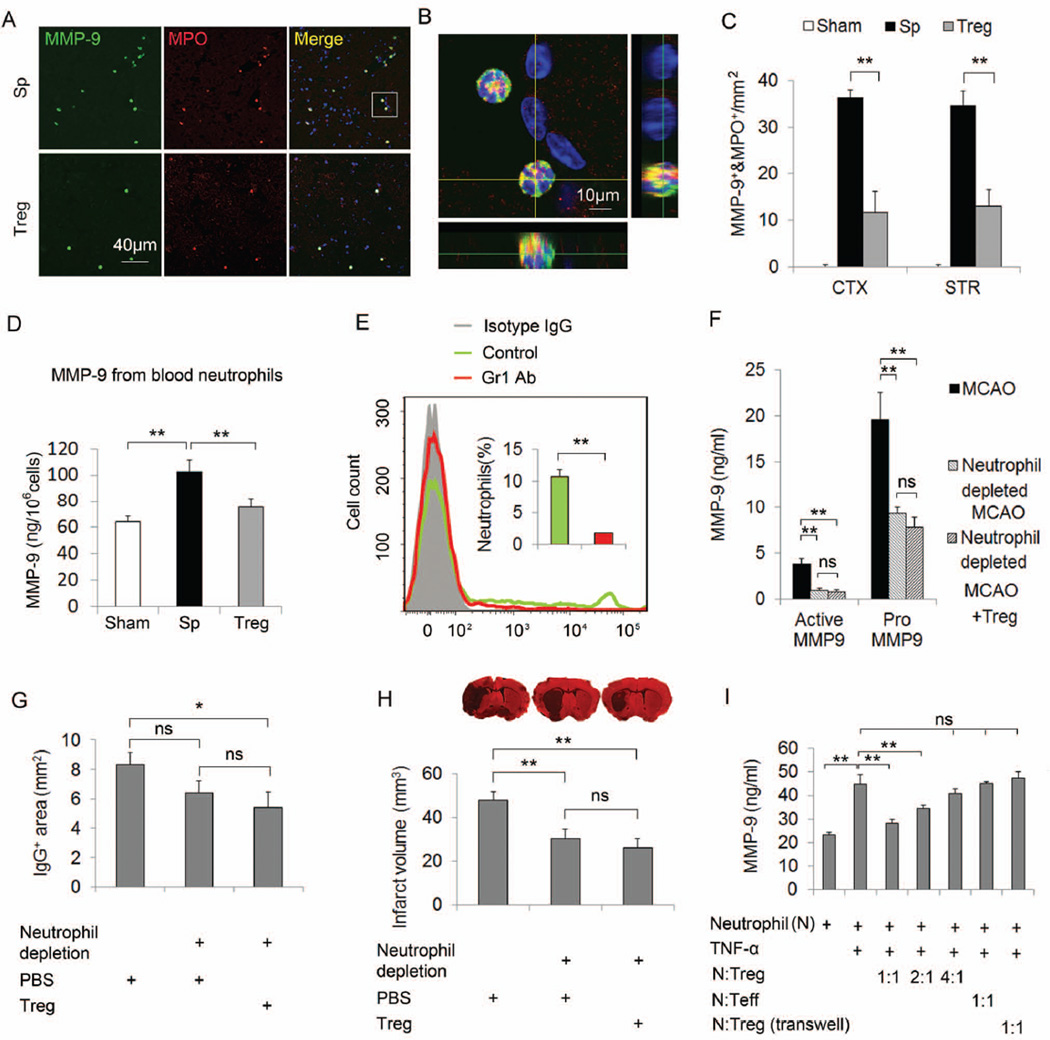
Figure 7. Neutrophil-derived MMP9 is a major target for Treg cerebroprotective actions.
(A–C) Tregs inhibit the infiltration of MMP9-loaded neutrophils into the brain at 3d after MCAO (A) Representative images of MMP9 and MPO double staining in brain sections. (B) A three-dimensional confocal image shows MMP9+/MPO+ cells. (C) Quantification of the number of MMP9+/MPO+ cells in the cortex and the striatum (n=4/group). (D) Quantification of blood neutrophil-derived MMP9 at 24h after MCAO (n=4/group). Neutrophils were isolated from the blood of splenocyte- or Treg-treated MCAO mice and sham mice at 24h after operation. MMP9 was measured in the neutrophil lysate by ELISA. Neutrophil-derived MMP9 was increased after stroke and this increase was significantly inhibited by Treg treatment. (E–H) Neutrophil depletion abolished Treg conferred protection. (E) Flow cytometry confirmed neutrophils were depleted in anti-Gr1 mAb (400 µg/mouse, ip) treated mice. (F) Plasma pro- and active MMP9 levels quantified by ELISA. (G) Quantification of IgG leakage determined by positive area of mouse IgG immunohistochemical staining (n=6/group). (H) Infarct volume defined by MAP2 staining (n=6/group). (I) Tregs inhibited TNF-α-induced MMP9 production from cultured neutrophils (n=6/group). Neutrophils isolated from blood and bone marrow were treated with TNF-α (100 ng/ml) for 2h and then co-cultured with or without CD3/CD28 antibody-primed Tregs or Teffs for 24h. The release of MMP9 in the culture medium was measured. Tregs, but not Teffs, inhibited the production of MMP9 from TNF-α-challenged neutrophils. Data are mean ± SE. *P<0.05, **P<0.01.
To confirm the importance of neutrophils in Treg-afforded protection, we used a Gr1-specific antibody to deplete neutrophils 1d before MCAO (Figure 7E). Consistent with a previous report31, neutrophil depletion resulted in a significant reduction in plasma MMP9 production (Figure 7F), BBB disruption (Figure 7G), and infarct volume (Figure 7H) at 1d after MCAO. Notably, Treg treatment failed to confer additional protection to neutrophil-depleted animals. These data suggest that neutrophil-derived MMP9 is an essential target for Treg cerebroprotective action.
To further verify the direct effect of Tregs on MMP9 production from neutrophils, we co-cultured TNF-α-pretreated neutrophils with or without CD3 and CD28 antibody-primed Tregs (Figure 7I). MMP9 production was prominent in neutrophils co-cultured with T effective cells or without T cells. Tregs markedly inhibited TNF-α-induced MMP9 production from neutrophils when incubated at 1:1 or 1:2 ratios of Tregs:neutrophils; however, they failed to inhibit the production of neutrophil elastase, another important protease in neutrophils or PMA-induced superoxide production (Supplementary Figure 10), suggesting a specific inhibitory effect of Tregs on neutrophil MMP9 production. Interestingly, Tregs cultured into the transwells lost their inhibition on MMP9 production from neutrophils, which suggests a mechanism involving direct cell-cell interactions.
Discussion
As a result of numerous failed clinical trials, the Stroke Therapy Academic Industry Roundtable (STAIR) collaborators have established multiple criteria to assess neuroprotective strategies for viability and promise in clinical applications17. Our results qualify Treg therapy as a promising neuroprotective treatment as defined by STAIR. First, Treg treatment initiated within 2h after ischemia exerted potent protection against brain damage in two rodent transient focal MCAO models. Second, Tregs were protective out to one month after the stroke and improved both short- and long-term neurological functions. Third, Tregs show an extended therapeutic time window of up to at least 24h following ischemia. In addition, our ongoing research on long-term effect of Tregs revealed that Treg treatment not only alleviated grey matter injury, but also protected white matter from ischemia (not shown). Recent advances in expanding Tregs ex vivo32, 33 or in vivo34 to achieve therapeutic amount further enable their clinical translation.
Consistent with a previous report9, we found that Tregs inhibit cerebral inflammation in the brain as early as 1d after ischemia. The dampened cerebral inflammation was accompanied by a reduced invasion of peripheral immune cells. Although such a generalized inhibitory impact of Tregs on peripheral immune cell trafficking might be explained by their antigen-nonspecific function9, the lack of a simultaneous impact on CNS microglia strongly suggests an alternative mechanism involving the preservation of BBB function after ischemia. In line with this notion, Treg-treated MCAO mice showed reduced BBB leakage and preserved tight junction structures. Of note, a recent research in amyotrophic lateral sclerosis reported that Tregs may augment M2 microglial polarization and shift the balance of microglia responses from cytotoxicity toward neuroprotection35, suggesting that Treg treatment may change microglial properties without affecting their number. Our current results, however, do not support a direct effect of Tregs on microglial properties at 1 day after stroke due to the absence of Tregs infiltration and the lack of increase in Treg-derived anti-inflammatory cytokines (IL-10 and TGF-β) in the brains of Treg-treated animals.
Given our observation that the infiltration of Tregs into the brain was somewhat delayed relative to their early protection against cerebral inflammation, BBB disruption, and brain damage, Tregs must exert early neuroprotection through peripheral means, by either releasing protective mediators into the circulation or targeting other peripheral cells that in turn influence the brain infarct. Liesz and colleagues suggested that IL-10 is a critical mediator utilized by endogenous Tregs to protect the brain, but only at late stages after cerebral ischemia9. However, in our study, exogenous Treg treatment did not change IL-10 levels in the brain. Indeed, our data show that transfer of Tregs derived from IL-10-deficient mice, either in the presence or absence of endogenous Tregs, still protected against ischemic brain injury at 3d after MCAO (Supplementary Figure 11). Thus, although IL-10 may be important for the protection elicited by endogenous Tregs against late stages of ischemic injury, it is unlikely a direct mediator for exogenous Treg-afforded neuroprotection during early stages of ischemic brain injury. In addition, cerebral levels of TGF-β, another Treg-derived anti-inflammatory cytokine, was not affected by Treg treatment. All of this evidence disputes a direct ‘protective mediator’ theory for early Treg action.
In considering the candidates for peripheral cells that are targeted by Tregs, we noted that Tregs inhibited MMP9 production in the blood and the brain as early as 1d after ischemia. Further in vitro and in vivo studies identified a novel mechanism of Treg action involving potent suppression of MMP9 producing neutrophils. It has been reported in the transient MCAO model of stroke that MMP9 levels went up in the peripheral blood as early as 2h after MCAO, maximized at 4h, and then gradually subsided but still can be observed until 72h. The increase of brain MMP9 is relatively delayed compare to plasma MMP9, beginning at 4h, maximizing at 24h and remaining at high level until 72h36. Neutrophils are major sources of blood MMP9 and their infiltration into the ischemic brain enhances central MMP9 levels through releasing the MMP9 proform5, 37. Recent data reveals that reperfusion after tPA treatment promotes the degranulation of human neutrophils and release of MMP938. The neutrophil-derived MMP9 has been shown to be important in post-ischemic BBB breakdown, leukocyte infiltration and brain damage5. The specific inhibition of neutrophil-derived MMP9 by Tregs would thus explain the drastic decrease of MMP9 in the blood and in the brain of Treg-treated animals and the Treg-afforded neuroprotection after cerebral ischemia/reperfusion. In support of a neutrophil-Treg interaction in vivo, infused Tregs were observed in the bone marrow pool, circulating pool and the marginating pools (spleen, liver and lung) of neutrophils at 1d after MCAO. Immunostaining further demonstrated neutrophils in contact with Tregs in the spleen. Furthermore, we showed that neutrophils and MMP9 are critical for Treg-afforded neuroprotection because Tregs lost their early protective effects in peripheral MMP9 deficient or neutrophil-depleted mice. Therefore, although the involvement of other MMP9 secreting cells, such as endothelial cells and macrophages, in the action of Tregs cannot be excluded, our results strongly suggest that neutrophils are a novel direct target for Tregs after ischemic insults.
A post-stroke alteration in the systemic immune system is the immunosuppression characterized by loss of leukocytes and impairment of cell-mediated immunity24, which predisposes stroke victims to infectious complications. It was important to show that Treg treatment did not exacerbate post-stroke immunosuppression from the perspective of future clinical translation of this therapy. Instead, Treg-treated animals showed preserved lymphocyte populations in the blood and spleen after MCAO. This may be the consequence of less activated immune systems and fewer exhausted peripheral immune cells. It thus seems that Treg treatment benefits post-stroke immune status while restricting inflammatory overactivation, in line with its modulatory role in immune homeostasis. However, our study only assessed the short-term effect of Tregs on the number of peripheral immune cells. Further immunologic studies would be necessary to assess the long-term effect of Treg treatment on post-stroke immune functions.
In conclusion, we reports that Tregs protect the brain against ischemic/reperfusion injury and that this effect is associated with reduced inflammatory responses in the brain. Furthermore, Tregs attenuated BBB disruption following ischemia/reperfusion and subsequent infiltration of peripheral inflammatory cells. We also characterized a neuroprotective mechanism whereby Tregs inhibit neutrophil-derived MMP9 (Figure 8). Our study suggests that Treg adoptive transfer is a novel and potent cell-based therapy specifically targeting post-stroke inflammatory dysregulation and neurovascular disruption.
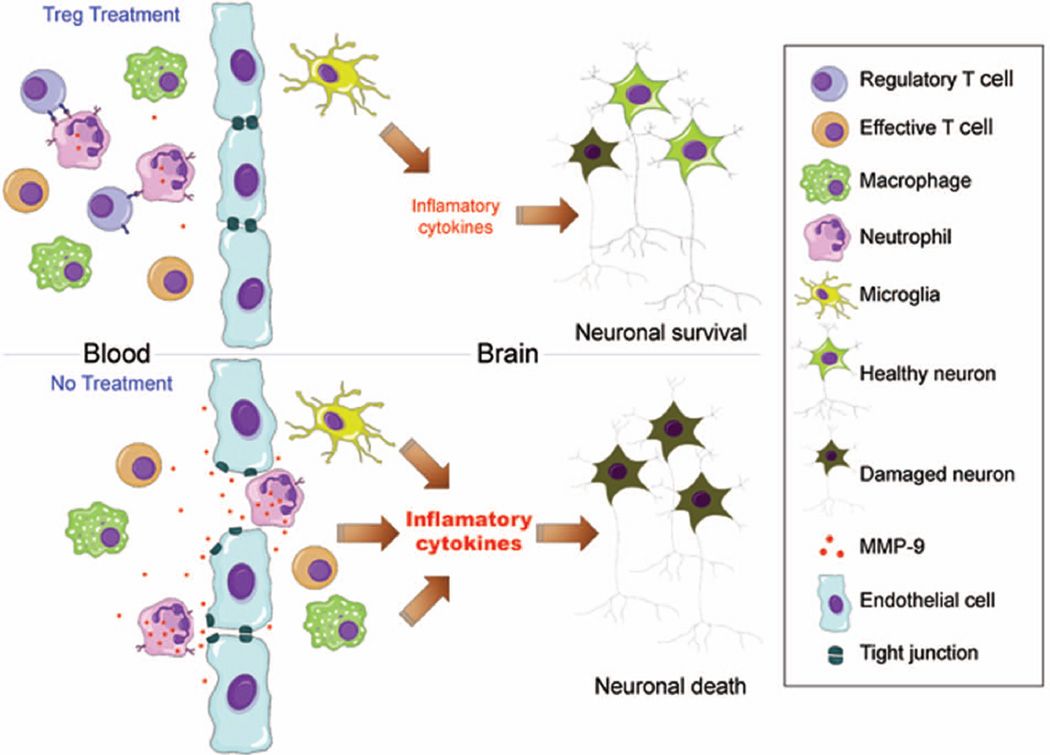
Figure 8. Scheme illustrating potential pathways of Tregs reduction of brain infarct size via blood brain barrier protection involving neutrophil-derived MMP9.
Adoptive transferred Tregs inhibit MMP9 production from peripheral neutrophils, resulting in reduced MMP9 levels in the circulation and in the brain early after ischemic brain injury. Reduce MMP9 leads to preserved blood brain barrier integrity, which in turn inhibits the cerebral infiltration of peripheral inflammatory cells, including T effective cells, neutrophils and macrophages. As a consequence, Tregs treatment mitigates post-stroke neuroinflammation, and protects against the expansion of the cerebral infarct.
Supplementary Material
Acknowledgments
We thank Dr. A. Planas (Institut d'Investigacions Biomèdiques de Barcelona (IIBB)-Consejo Superior de Investigaciones Científicas , Institut d'Investigacions Biomèdiques August Pi i Sunyer, Barcelona, Spain) and Dr. R. K. Leak (Duquesne University, Pittsburgh, USA) for detailed comments on the manuscript and helpful discussions. We are grateful for technical assistance from Benjamin Matta, Brian Rosborough, Yibei Zhu, Xin Gao and Gang Li. This work was supported by the Veterans Health Administration (GRECC pilot grant to J.C), the National Institutes of Health Grants (NS36736, NS43802, and NS45048 to J.C) and grants from the American Heart Association (10POST4150028 to X.H, 10SDG2560122 to F.Z.). B.S was supported by Chinese Natural Science Foundation grants (8107094). We thank S. Giegel for editorial assistance.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nature medicine. Jul;17(7):796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stirling DP, Liu S, Kubes P, Yong VW. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J Neurosci. 2009 Jan 21;29(3):753–764. doi: 10.1523/JNEUROSCI.4918-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao X, Sun G, Zhang J, et al. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007 Apr;61(4):352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- 4.Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006 Apr;37(4):1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- 5.Gidday JM, Gasche YG, Copin JC, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. American journal of physiology. 2005 Aug;289(2):H558–H568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006 May 2;113(17):2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 7.Shichita T, Sugiyama Y, Ooboshi H, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009 Aug;15(8):946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 8.Kleinschnitz C, Schwab N, Kraft P, et al. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. 2010 May 6;115(18):3835–3842. doi: 10.1182/blood-2009-10-249078. [DOI] [PubMed] [Google Scholar]
- 9.Liesz A, Suri-Payer E, Veltkamp C, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009 Feb;15(2):192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 10.Taams LS, van Amelsfort JM, Tiemessen MM, et al. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum Immunol. 2005 Mar;66(3):222–230. doi: 10.1016/j.humimm.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson's disease. Journal of leukocyte biology. 2007 Nov;82(5):1083–1094. doi: 10.1189/jlb.0507296. [DOI] [PubMed] [Google Scholar]
- 12.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nature reviews. 2008 Jul;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J Exp Med. 2005 Nov 21;202(10):1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan J, Greer JM, Etherington K, et al. Immune activation in the peripheral blood of patients with acute ischemic stroke. J Neuroimmunol. 2009 Jan 3;206(1–2):112–117. doi: 10.1016/j.jneuroim.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Offner H, Subramanian S, Parker SM, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006 Jun 1;176(11):6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R, Xue YY, Lu SD, et al. Bcl-2 enhances neurogenesis and inhibits apoptosis of newborn neurons in adult rat brain following a transient middle cerebral artery occlusion. Neurobiology of disease. 2006 Nov;24(2):345–356. doi: 10.1016/j.nbd.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Saver JL, Albers GW, Dunn B, Johnston KC, Fisher M. Stroke Therapy Academic Industry Roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke. 2009 Jul;40(7):2594–2600. doi: 10.1161/STROKEAHA.109.552554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan MS, Boesteanu A, Reed AJ, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001 Apr;2(4):301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 19.Hombach AA, Kofler D, Hombach A, Rappl G, Abken H. Effective proliferation of human regulatory T cells requires a strong costimulatory CD28 signal that cannot be substituted by IL-2. J Immunol. 2007 Dec 1;179(11):7924–7931. doi: 10.4049/jimmunol.179.11.7924. [DOI] [PubMed] [Google Scholar]
- 20.Chakrabarti S, Zee JM, Patel KD. Regulation of matrix metalloproteinase-9 (MMP-9) in TNF-stimulated neutrophils: novel pathways for tertiary granule release. J Leukoc Biol. 2006 Jan;79(1):214–222. doi: 10.1189/jlb.0605353. [DOI] [PubMed] [Google Scholar]
- 21.Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. CD4+FoxP3+ regulatory T-cells in cerebral ischemic stroke. Metab Brain Dis. 2011 Mar;26(1):87–90. doi: 10.1007/s11011-010-9226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright GP, Notley CA, Xue SA, et al. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc Natl Acad Sci U S. A. 2009 Nov 10;106(45):19078–19083. doi: 10.1073/pnas.0907396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishimaru N, Yamada A, Kohashi M, et al. Development of inflammatory bowel disease in Long-Evans Cinnamon rats based on CD4+CD25+Foxp3+ regulatory T cell dysfunction. J Immunol. 2008 May 15;180(10):6997–7008. doi: 10.4049/jimmunol.180.10.6997. [DOI] [PubMed] [Google Scholar]
- 24.Prass K, Meisel C, Hoflich C, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. The Journal gof experimental medicine. 2003 Sep 1;198(5):725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008 Jan 24;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Asahi M, Wang X, Mori T, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001 Oct 1;21(19):7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosell A, Ortega-Aznar A, Alvarez-Sabin J, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke; a journal of cerebral circulation. 2006 Jun;37(6):1399–1406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- 28.Park KP, Rosell A, Foerch C, et al. Plasma and brain matrix metalloproteinase-9 after acute focal cerebral ischemia in rats. Stroke; a journal of cerebral circulation. 2009 Aug;40(8):2836–2842. doi: 10.1161/STROKEAHA.109.554824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, Fagan SC. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC neuroscience. 2006;7:56. doi: 10.1186/1471-2202-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008 Apr;39(4):1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo Y, Onodera H, Shiga Y, et al. Correlation between myeloperoxidase-quantified neutrophil accumulation and ischemic brain injury in the rat. Effects of neutrophil depletion. Stroke. 1994 Jul;25(7):1469–1475. doi: 10.1161/01.str.25.7.1469. [DOI] [PubMed] [Google Scholar]
- 32.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. The Journal of experimental medicine. 2004 Jun 7;199(11):1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood. 2007 Jan 15;109(2):827–835. doi: 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- 34.Webster KE, Walters S, Kohler RE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009 Apr 13;206(4):751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beers DR, Henkel JS, Zhao W, et al. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain. 2011 May;134(Pt 5):1293–1314. doi: 10.1093/brain/awr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koh SH, Chang DI, Kim HT, et al. Effect of 3-aminobenzamide, PARP inhibitor, on matrix metalloproteinase-9 level in plasma and brain of ischemic stroke model. Toxicology. 2005 Oct 15;214(1–2):131–139. doi: 10.1016/j.tox.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Justicia C, Panes J, Sole S, et al. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab. 2003 Dec;23(12):1430–1440. doi: 10.1097/01.WCB.0000090680.07515.C8. [DOI] [PubMed] [Google Scholar]
- 38.Cuadrado E, Ortega L, Hernandez-Guillamon M, et al. Tissue plasminogen activator (t-PA) promotes neutrophil degranulation and MMP-9 release. Journal of leukocyte biology. 2008 Jul;84(1):207–214. doi: 10.1189/jlb.0907606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.