Abstract
Purpose
To investigate the association of spontaneous drusen regression in intermediate age-related macular degeneration (AMD) with changes on fundus photography and fundus autofluorescence (FAF) imaging.
Design
Prospective observational case series.
Methods
Fundus images from 58 eyes (in 58 patients) with intermediate AMD and large drusen were assessed over 2 years for areas of drusen regression which exceeded the area of circle C1 (diameter 125μm; Age-Related Eye Disease Study grading protocol). Manual segmentation and computer-based image analysis were used to detect and delineate areas of drusen regression. Delineated regions were graded as to their appearance on fundus photographs and FAF images, and changes in FAF signals were graded manually and quantitated using automated image analysis.
Results
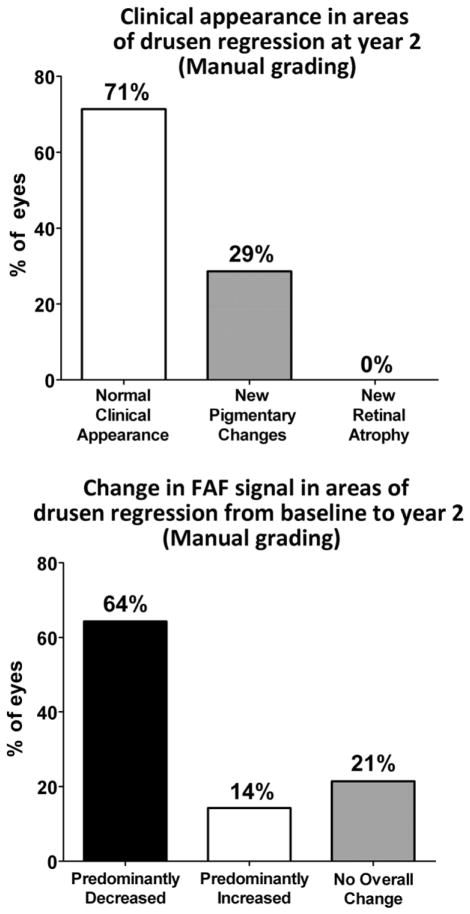
Drusen regression was detected in approximately half of study eyes using manual (48%) and computer-assisted (50%) techniques. At year 2, the clinical appearance of areas of drusen regression on fundus photography was mostly unremarkable, with a majority of eyes (71%) demonstrating no detectable clinical abnormalities, and the remainder (29%) showing minor pigmentary changes. However, drusen regression areas were associated with local changes in FAF that were significantly more prominent than changes on fundus photography. A majority of eyes (64–66%) demonstrated a predominant decrease in overall FAF signal, while 14–21% of eyes demonstrated a predominant increase in overall FAF signal.
Conclusions
FAF imaging demonstrated that drusen regression in intermediate AMD was often accompanied with changes in local autofluorescence signal. Drusen regression may be associated with concurrent structural and physiological changes in the outer retina.
Introduction
The presence of large soft drusen is a clinical hallmark of intermediate age-related macular degeneration (AMD)3 that is associated with an increased risk of progression to advanced AMD.4 Large soft drusen exhibit structural dynamism by demonstrating growth,5 as well as partial or complete regression, which can occur either spontaneously,6–8 or in response to laser photocoagulation.9–12 What cellular mechanisms underlie drusen regression and how retinal function and AMD progression are affected by its occurrence are incompletely understood.13–16 Whether drusen regression can constitute a useful surrogate endpoint in clinical trials on the prevention of AMD progression also remains a topic of some debate.17, 18
The purpose of the current study is to examine the incidence of drusen regression in eyes with intermediate AMD and to discover whether drusen regression in the short-term may be associated with local changes in the overlying retina. As large soft drusen abut the adjacent retinal pigment epithelium (RPE) layer, alterations in drusen size per se, and/or the processes that drive or accompany drusen regression, may influence the health and functioning of adjacent RPE cells. Fundus autofluoresence (FAF) imaging which relies primarily on the fluorescence generated from bisretinoid compounds accumulated in RPE cells19, 20 has been demonstrated to reveal clinically significant alterations in the anatomical and physiological state of the outer retina.21, 22 In the current study, we employed this modality to discover whether the events surrounding drusen regression were accompanied by changes in the outer retina. Our findings here are relevant to the significance of drusen regression in the natural history of AMD and may be relevant to the mechanisms by which large soft drusen confer risk on AMD progression.
Methods
Study Subjects
The present study is a prospective observational case series derived from fundus images obtained from participants in the Age-Related Eye Disease Study 2 (AREDS2; ClinicalTrials.gov Identifier #NCT00345176) protocol. Sixty participants were enrolled at the National Eye Institute site for the AREDS2 study between March 2007 and July 2008 who met the following enrollment criteria at the baseline visit: (1) age between 50 to 85 years, and (2) presence of large drusen (≥125 μm in diameter) in both eyes, or large drusen in one eye and advanced AMD (defined as the presence of choroidal neovascularization, central geographic atrophy, or disciform scarring) in the fellow eye. Participants were characterized by multimodal imaging at baseline and at 2 years. As the present study aims to examine drusen changes in eyes with intermediate AMD over this time period, eyes that contained or developed advanced AMD were identified and excluded from analysis. Eyes that were (1) without advanced AMD at baseline, and (2) did not progress to advanced AMD by the year 2 visit were identified as eligible eyes. Each study participant was permitted to contribute at most one eye to the analysis; when both eyes were eligible, one of the two eyes was randomly selected. This inclusion process resulted in a total of 58 eyes in 58 patients that constituted the population of study eyes analyzed in the present study. Informed consent was obtained from all study participants. The study design was prospectively approved by the National Institutes of Health Combined Neuroscience Institutional Review Board. Informed consent was obtained from all participants, and the study was conducted in accordance with Health Insurance Portability and Accountability Act regulations and adhered to the tenets of the Declaration of Helsinki.
Fundus imaging
Retinal imaging of study eyes was performed with color fundus photography, red-free monochromatic fundus photography, and modified fundus camera-based fundus autofluorescence (mFC FAF) imaging at study baseline and at the year 2 follow-up visit. Participants had their pupils dilated to 6 mm or larger with two sets of 2.5% phenylephrine and 1% tropicamide ophthalmic drops prior to imaging. Images were captured using a retina camera with a 30° magnification setting according to a modified 3-standard fields color fundus photography; Field 2 of the macula was used for image analysis. Color fundus photos were obtained using an ultra-high resolution (2392 × 2048 pixels) color camera (Ophthalmic Imaging Systems; Sacramento, CA). Monochromatic red-free fundus photographs were captured using a 12-bit ultra-sensitive grayscale CCD (1376 × 1036 pixels; Ophthalmic Imaging Systems; Sacramento, CA) and a Topcon factory-installed green filter (550-nm to 580-nm). FAF images were captured using a Topcon 50-EX retinal camera (Topcon Medical Systems; Oakland, NJ) adapted for autofluorescence imaging using the modifications described by Spaide23 and connected to an OIS WinStation™ 5000 digital capture system. Band-pass filters for excitation (550 to 600nm) and emission (660 to 800nm) were used for FAF imaging. Color images were stored in 24-bit RGB color format (8-bits for each of red, green and blue channel data). Monochromatic red-free and mFC FAF images were stored in 8-bit grayscale format.
Image processing
For each study eye, fundus images from all three imaging modalities (color fundus photography, monochromatic red-free fundus photography, and mFC FAF) at baseline and year 2 were spatially registered using the ImageJ imaging processing software (version 1.44f; National Institutes of Health; Bethesda, MD) using the TurboReg plugin (version June 19, 2008; Biomedical Imaging Group, Swiss Federal Institute of Technology Lausanne; Lausanne, Switzerland) employing bilinear transformation. Color RGB images were registered by splitting RGB channel data as an RGB stack, selection of landmarks in the green channel, and recombining RGB channel data to create a registered color RGB image. The accuracy of image registration was individually confirmed by two independent reviewers (BT, MI) and the registration refined to achieve accuracy between imaging modalities.
To facilitate the longitudinal comparison of fundus images, registered images from each imaging modality at baseline and year 2 were normalized with respect to each other using tools within ImageJ, which included (1) the automatic window and level adjustment tool and (2) the rolling paraboloid background subtraction tool24 in order to achieve normalized grayscale distributions in longitudinal images. Pre- and postprocessing images were independently reviewed by three graders (BT, MI, NK) to verify the absence of image obscuration or artifact.
Manual segmentation and grading of areas of drusen regression
Registered color and red-free fundus images at baseline and year 2 were inspected, and areas of drusen regression at year 2 (relative to baseline) were identified. Areas of drusen regression greater than, or equal in size to, circle C1 (of diameter 125 μm, as described in the AREDS AMD grading protocol)25 were circumscribed by planimetry using the draw tool in ImageJ to delineate regions of interest (ROIs) within which drusen regression was observed. Region of interest (ROI) outlines were overlaid onto color fundus photographs and monchromatic red-free images at year 2, and the overall clinical appearance of these ROIs was graded according to one of the three following categories: (1) No observable clinical feature distinct from background, (2) development of new pigmentary changes at year 2, or (3) development of new retinal or RPE atrophy at year 2. The same ROIs were also overlaid onto mFC FAF images at baseline and year 2. The FAF patterns within the ROIs were compared between baseline and year 2, and changes in overall FAF signal within the ROIs at year 2 (relative to baseline) were manually graded according to one of the three following categories: (1) predominant decrease in the overall level of autofluorescence, (2) predominant increase in the overall level of autofluorescence, or (3) no overall change in the overall level of autofluorescence
Computer-assisted delineation of areas of drusen regression
A semi-automated method of image processing was performed in parallel to manual segmentation and grading. In order to automate the delineation of areas of drusen regression in individual study eyes, monochromatic red-free fundus images at baseline and year 2, which had been registered and normalized, were subjected to image subtraction using ImageJ. The image calculator tool was employed to perform pixel-by-pixel image subtraction of the year 2 image from the baseline image. As areas of drusen appear brighter than the normal fundus background, areas of drusen regression appeared on the subtraction image as areas of preferentially increased pixel intensity (Fig. 1, top row). Standardized automated thresholding using isodata binarization 26 was applied to the subtraction image to sharply delineate general areas of drusen regression (Fig. 1, bottom left). Specific areas of drusen regression were individually identified and tagged using the automated particle analysis tool in ImageJ; ROIs with areas smaller than the area of circle C1 were excluded. The remaining areas were used to define drusen-regression ROIs (Fig. 1, bottom middle) and characterized in the subsequent analysis of changes in FAF pattern (Fig. 1, bottom right).
Figure 1. Computer-assisted delineation of areas of drusen regression over two years in eyes with intermediate age-related macular degeneration.
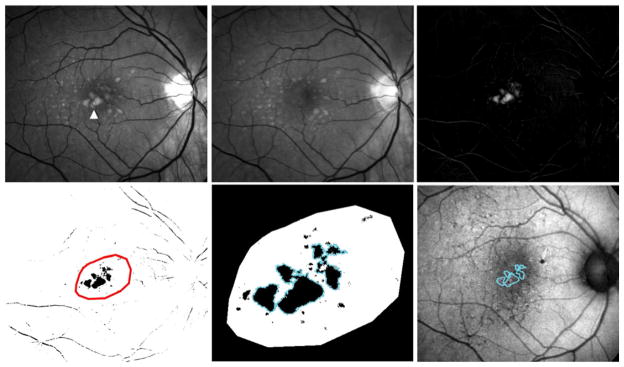
(top left, top middle) Normalized red-free monochromatic images of a study eye with intermediate age-related macular degeneration and large drusen captured at baseline (top left) and at year 2 (top middle). Multiple large drusen at the center of the macula (arrowhead) visible at baseline have undergone regression and cannot be visualized at year 2. (top right) Subtraction image between baseline and year 2 that generates an image of the regressing drusen. (bottom left) Inversion and binarization of the image shown in (top right). The areas of relevant drusen regression were highlighted manually for further processing (red outline). (bottom middle) Expanded image of the inset shown in (bottom left) demonstrating automated delineation of individual regions-of-interest (ROIs) of drusen regression (blue outline). ROIs of areas smaller than the area of circle C1 were excluded. (bottom right) Drusen regression ROIs (blue outlines) were superimposed on co-registered autofluorescence images captured at baseline and year 2 for further grading and analysis.
Computer-assisted quantification of fundus autofluorescence in fundus autofluorescence images
We developed a semi-automated method to quantitatively characterize changes in FAF within areas of drusen regression. As manual grading of areas of hyperautofluorescence and hypoautofluorescence in FAF images relies on comparisons that the grader makes between these areas and the overall level of “normal background” autofluoresence, our computer-assisted algorithm simulated this comparison by first measuring the grayscale level of FAF in areas that were considered to be of “normal background” autofluoresence. In this process, two graders (BT, MI) independently inspected mFC FAF images at baseline and year 2 and then manually delineated three separate ROIs that were representative of “normal background” autofluorescence (Fig. 2, top left). The use of this reference accounted for individual variations in macular pigment distribution, which generally varies with distance from the fovea.27–29 The two graders were masked to whether an image was at “baseline” or “follow-up.” The criteria used for the delineation of these “background” ROIs were that at both baseline and at year 2, these areas should: 1) contain uniform levels of background FAF; 2) be located outside areas of drusen, drusen regression, and other retinal pathology; and 3) be selected in areas at the same approximate radial distance from the fovea as the relevant drusen regression ROIs for that study eye. The background ROIs were independently reviewed by a third grader (NK or WW) to verify conformity to these criteria. The distribution of grayscale levels of all the pixels located in these “background” ROIs within a given eye was represented in a histogram (Fig. 2, top right), and the mean ± standard deviation (SD) for this distribution was calculated.
Figure 2. Computer-assisted measurement of autofluorescence changes in areas of drusen regression over two years in eyes with intermediate age-related macular degeneration.
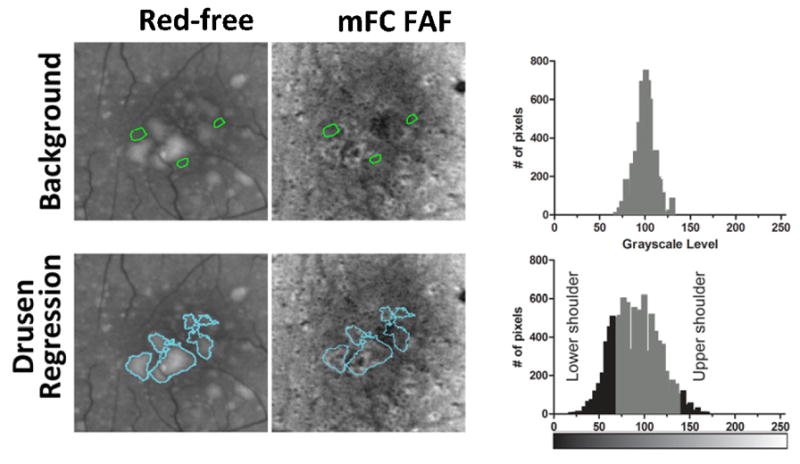
(top left, top middle) Areas containing background levels of autofluorescence and not involving areas of drusen or pigmentary changes were manually delineated following the inspection of red-free and fundus autofluorescence (FAF) images captured at baseline and year 2 (green outlines). These were referred to as “background” regions-of-interest (ROIs). (top right) The distribution of grayscale levels of all pixels located within “background” ROIs was plotted as a frequency histogram, and the mean±standard deviation (SD) for this distribution was calculated. (bottom left, bottom middle) Drusen regression ROIs defined from red-free fundus photographs were then superimposed on corresponding FAF images. (bottom right) The distribution of grayscale levels of all pixels located within all drusen regression ROIs was plotted as a frequency histogram. Pixels with a grayscale level that was greater than (Mean background grayscale level + 3 SD background grayscale level) were considered to have increased FAF levels (upper shoulder in distribution histogram), while pixels with a grayscale level that was less than (Mean background grayscale level - 3 SD background grayscale level) were considered to have decreased FAF levels (lower shoulder).
The drusen regression ROIs defined earlier for the same study eye were then superimposed onto mFC FAF images from baseline and year 2 for characterization of the patterns of FAF located within these ROIs relative to background levels (Fig. 2, bottom left). We defined a given pixel within the ROI to have “increased FAF signal” when its grayscale level exceeded that of the mean background grayscale level by greater than three times the standard deviation (SD) of the background grayscale distribution (ie. when pixel grayscale level > [mean background grayscale level + 3 SD]). Conversely, a pixel had “decreased FAF signal” when pixel gray level < [mean background gray level - 3 SD] (Fig. 2, bottom right). In this way, the numbers of pixels with (1) increased FAF (upper shoulder of histogram) and (2) decreased FAF (lower shoulder of histogram) were computed in the drusen regression ROIs of each eye for baseline and year 2 mFC FAF images separately. The absolute and percentage changes in areas of increased and decreased autofluorescence signal were then calculated between baseline and year 2. If the changes in the areas of increased and decreased FAF were relatively equal (±5%), the overall change in FAF was categorized as “no overall change in the overall level of autofluorescence.” If the change in the area of either decreased or increased FAF predominated, then the overall change in FAF was categorized as either “predominantly decreased” or “predominantly increased,” respectively.
Results
Prevalence of drusen regression in eyes with intermediate age-related macular degeneration
Study eyes (n= 58) were assessed for the presence of significant drusen regression between baseline and year 2. Two methods of analysis were performed in parallel: (1) manual segmentation and (2) computer-assisted delineation based on image subtraction. On manual segmentation of baseline and year 2 follow-up fundus images of 58 study eyes, 28 eyes (48%) were identified as demonstrating at least one area of drusen regression exceeding circle C1 (125 μm) in area. In comparison, computer-assisted delineation of drusen regression identified a similar proportion of eyes (50%, n=29/58) as having met this criterion. Agreement between the two methods was very good (Cohen’s κ=0.91±0.05), with 27 eyes being similarly identified as having significant drusen regression by both methods. One eye identified by manual segmentation was not identified by computed-assisted delineation, while two eyes identified by computed-assisted grading were not identified by manual segmentation. These three discordant eyes were “borderline” cases in which the largest area of drusen regression was close to the area of circle C1.
Manual grading of clinical and fundus autofluorescence changes in areas of drusen regression
For study eyes which were identified on manual segmentation to have met criteria for drusen regression (n=28), multimodal fundus images (color, red-free, mFC-FAF) captured at baseline and year 2 were registered and inspected in parallel. Figures 3–5 illustrate representative examples of drusen regression and their appearance on multimodal imaging in analyzed eyes. These representative individual examples illustrate that retinal areas of drusen regression appeared clinically unremarkable on color and monochromatic photography in the majority of cases. In these areas that were previously occupied by drusen, the retina either had a normal clinical appearance or developed small amounts of new pigment clumping. However, on FAF imaging, these same areas typically demonstrated clear changes in the local patterns of autofluorescence that were considerably more prominent than the clinically observable changes.
Figure 3. Example of drusen regression in intermediate age-related macular degeneration in Study Participant 1.
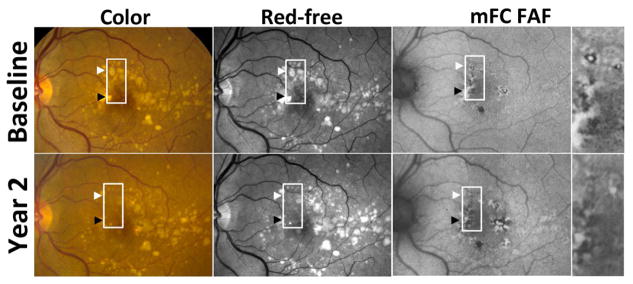
Color (top left, bottom left), red-free monochromatic (top middle, bottom middle) fundus photographs, and fundus autofluorescence (FAF) (top right, bottom right) images at baseline (top row) and year 2 (bottom row) demonstrate areas of drusen regression in the macula (highlighted by arrowheads and box). These areas of drusen regression were not associated with pigmentary change or atrophy on either color fundus (bottom left) or red-free (bottom middle) photographs, and these areas had a normal appearance and were not significantly different from neighboring areas that were unaffected by drusen at baseline and year 2. However, these areas of drusen regression contained regions of abnormal (increased and decreased) FAF signal that were significantly different in appearance and pattern from “background” FAF signal. The change in FAF patterns in drusen regression areas in this study eye was judged as demonstrating an overall decrease in FAF signal (insets from top right and bottom right), in which areas of predominantly increased FAF signal were replaced by areas of predominantly decreased FAF signal.
Figure 5. Example of drusen regression over two years in intermediate age-related macular degeneration in Study Participant 3.
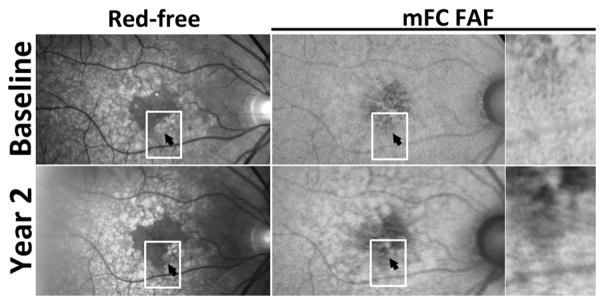
Red-free monochromatic (top left, bottom left) fundus photographs and fundus autofluorescence (FAF) (top right, bottom right) images at baseline (top row) and year 2 (bottom row) demonstrate two adjacent areas of drusen regression in the macula (highlighted by arrowhead and box). While the area of drusen regression appeared clinicallly unremarkable and devoid of pigmentary change or atrophy, FAF imaging demonstrated a predominant decrease in FAF in the corresponding areas of drusen regression.
Areas of drusen regression, delineated as “regions of interest” (ROIs), were overlaid on color fundus and red-free photographs and graded (Fig. 6, top). In the majority of graded eyes (71%, n = 20/28 eyes), these areas had a clinical appearance that was devoid of retinal pathology and indistinguishable from neighboring fundus areas uninvolved with AMD lesions (drusen or pigmentary changes). A minority of eyes (29%; n = 8/28 eyes) had some evidence of new pigmentary changes that were not apparent at baseline. None of the graded eyes was noted to have clinically evident geographic atrophy or neovascular change in the areas of drusen regression.
Figure 6. Manual grading of clinical and fundus autofluorescence changes in areas of drusen regression over two years in intermediate age-related macular degeneration.
Study eyes that met criteria for drusen regression (n = 28) were manually graded for (top) the clinical appearance within the areas of drusen regression at year 2 and (bottom) the overall change in fundus autofluorescence signal within the same areas between baseline and year 2.
These manually outlined “drusen-regression” ROIs were also overlaid onto mFC-FAF images at baseline and year 2, and the FAF changes within these ROIs were qualitatively graded (Fig. 6, bottom). In a majority of eyes (64%, n = 18/28), these ROIs were judged to demonstrate a predominant decrease in FAF signal as drusen regressed in these areas. About 14% of eyes (n = 4/28) were judged to have a predominant increase in FAF signal, and 21% (n = 6/28) had no net overall change in FAF signal.
Computer-assisted grading of fundus autofluorescence changes in areas of drusen regression
In parallel to the manual grading of FAF changes, we developed a computer-assisted, quantitative method of characterizing FAF changes that are associated with drusen regression. FAF signals located within computer-defined “drusen regression” ROIs were normalized with respect to background, and differences in grayscale levels between baseline and year 2 images were calculated and analyzed. In each study eye demonstrating drusen regression of size greater than circle C1 (diameter 125μm; Age Related Eye Disease Study AMD grading protocol), as assessed by computer-assisted grading (n = 29), the total areas of increased and decreased FAF signal located within the “drusen regression” ROIs were calculated separately and plotted on a single graph (Fig. 7, top). A majority of points were found to lie on the positive (right) side of the vertical axis (i.e. x>0), indicating that most eyes at the year 2 time point demonstrated an expansion of areas of decreased FAF in the loci where drusen regression occurred. On the other hand, changes in the areas of increased FAF signal were smaller and more mixed, with points falling close to and on either side of the horizontal axis, indicating moderate and mixed changes in the area of increased FAF in the loci where drusen regression occurred.
Figure 7. Computer-assisted grading of fundus autofluorescence changes in areas of drusen regression over two years in intermediate age-related macular degeneration.
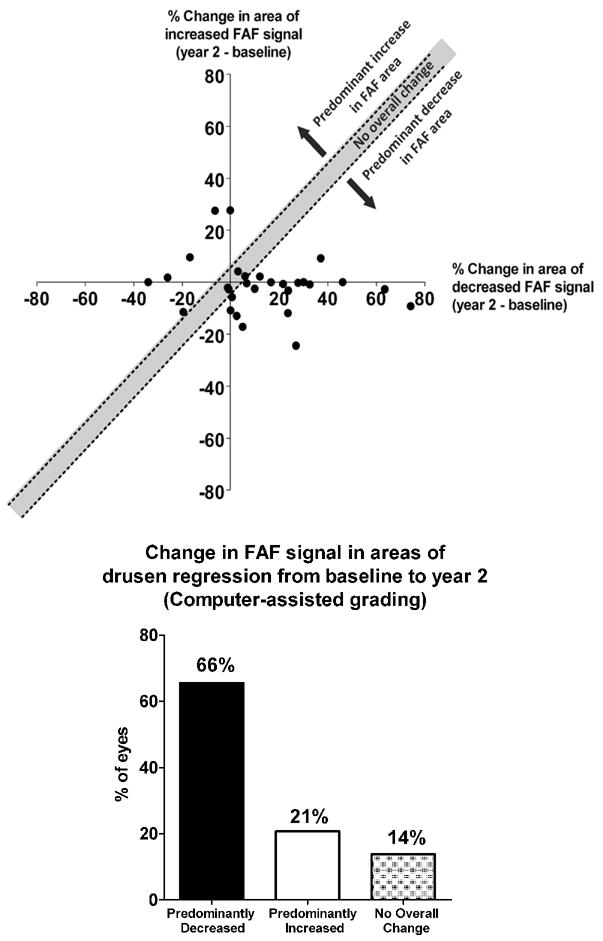
Study eyes that met image-processing criteria for drusen regression (n = 29) were evaluated for changes in fundus autofluorescence (FAF) signals in drusen-regression regions of interest (ROIs). The areas of decreased FAF and increased FAF within drusen-regression ROIs in each eye were determined at baseline and at year 2, and the changes from baseline to year 2 calculated and plotted on a common graph. (top) Graph showing the percentage change in the area of increased FAF (y-axis) plotted against the percentage change in the area of decreased FAF (x-axis) with each eye represented as an individual point (n = 29). Eyes that demonstrated changes in area that were well-matched between increased FAF and decreased FAF (±5%) are represented by points that lie within the y=x (±5%) zone (unshaded region) which indicates “no overall changes” in general FAF. Eyes in which the change in the area of increased FAF exceeded the change in the area of decreased FAF change are represented by points lying above the unshaded zone (green region) and were considered to have “predominantly increased FAF.” Eyes in which the change in the area of decreased FAF exceeded the change in the area of increased FAF change are represented by points lying below the unshaded zone (red region) and were considered to have “predominantly decreased FAF.” (bottom) The distribution of eyes demonstrating different overall changes in FAF as defined in (top) shows that a majority of eyes (66%) exhibited significant decreases in overall FAF in the regions where drusen have regressed.
We also scored eligible study eyes according to the predominant change in overall FAF in the areas of drusen regression. We considered eyes with approximately equal changes (±5%) in the areas of increased and decreased FAF to have “no net change” in overall FAF in drusen regression ROIs. This is represented in the unshaded zone between the lines y=x+5% and y=x−5% in Figure 7. Points located above this unshaded zone were considered to have increased overall FAF (i.e. predominant expansion of areas of increased FAF), and those located below this unshaded zone were considered to have decreased overall FAF (i.e. predominant expansion of areas of decreased FAF). This overall change in FAF signal across drusen regression ROIs is analogous to the quantity of overall FAF change estimated by manual grading (Fig. 6, bottom). The distribution of points in these three zones is tallied in Fig. 7, bottom. The majority of eyes (65%, n=19/29) were classified as showing overall decreased AF, while a minority (21%, n=6/29) were classified as showing overall increased FAF, with a smaller proportion (14%, n = 4/29) classified as showing “no change.”
Discussion
In the present study, we were motivated to discover the incidence of drusen regression in eyes with intermediate AMD, and to use FAF imaging to elucidate whether clinical changes at the level of the outer retina were detectable in areas of drusen regression. Detectable local change in FAF signal would indicate that drusen regression is accompanied by a tissue response at the level of the adjacent outer retina. The features of the FAF alterations may provide clues as to the nature of associated cellular changes and help interpret structural and functional consequences of drusen regression studied using other modalities.
The phenomenon of spontaneous drusen regression has been previously described for a variety of drusen types, including large soft drusen in intermediate AMD,3, 6, 7 basal laminar drusen,30 large drusenoid pigment epithelial detachments,31 and small hard drusen.32 Spontaneous drusen regression can occur concurrently with the development or growth of drusen in the same eye, such that total drusen area in the macula may remain relatively stable despite considerable flux in individual drusen.8 Inducing drusen regression in eyes with intermediate AMD using laser treatment has been hypothesized to diminish AMD progression risk,33,34 but its efficacy has not been uniformly demonstrated.35–37 Spontaneous drusen regression, on the other hand, coupled with the development of pigmentary changes and calcified drusen, has been associated with the long-term evolution of geographic atrophy, leading to the hypothesis that the spontaneous regression of large soft drusen can constitute a precursor event leading to RPE atrophy.15
In addition to its effect on the RPE atrophy, the influence of drusen regression on local photoreceptor structure and function has also been investigated. In a recent study, local retinal sensitivity in the eyes of 14 patients demonstrating soft drusen regression was characterized using photopic and scotopic fine matrix mapping.16 While scoptic and phototopic sensitivities were generally lower in these eyes relative to control eyes without AMD, no significant difference was found in the local sensitivities between areas with drusen regression versus unaffected neighboring areas. The anatomic integrity of photoreceptors overlying areas of drusen regression examined using spectral-domain optical coherence tomography also did not reflect deleterious changes in the inner segment/outer segment (IS/OS) layer but on the contrary, showed intact IS/OS junction integrity.14 Taken together, these investigations of drusen regression reflect current uncertainty on short- and long-term consequences of drusen regression.
The present study demonstrates a computer-assisted method of analyzing longitudinal color fundus images that are registered, normalized (leveled, background-subtracted using a rolling paraboloid method, and contrast-enhanced), digitally subtracted, and automatically segmented by isodata thresholding) for drusen regression. The methods employed for delineation of drusen regression are easily implemented using open source software (NIH ImageJ) and are similar to other described methods using iterative38 and fitted-polynomial39 leveling of macular background, Otsu thresholding,40 and digital subtraction of drusen segmentation images8. These methods have also demonstrated close correlation with manual grading of drusen41.
With regards to analysis of FAF images, differences in FAF signal between images acquired longitudinally have been challenging to quantify, due to differences in exposure, gain, and operator variability22. The present study analyzed longitudinal, registered, and normalized fundus FAF images for changes in FAF signal associated with drusen regression. We employed large-scale normalization of FAF images involving leveling and rolling paraboloid background subtraction, followed by local normalization using background regions that were similar in eccentricity to regions of drusen regression. This allowed for quantitative comparison of changes in AF signal over two years. A method to facilitate quantitative, longitudinal comparison of FAF images using an internal reference has been described42, and would be useful for the detection of FAF changes in a large sampling area, but because of the regions of drusen regression were relatively small (compared to the field of view), our study implemented normalization using local background regions in order to measure local change in AF signal over time. Selection of background regions according to the criteria mentioned in the methods involved manual selection and relied on the assumption that the distribution of macular pigment shows radial symmetry. Previously described methods to have automated this process8, 38–41, and future studies may investigate their application in FAF quantification. In disease states where macular pigment does not exhibit radial symmetry, such as in idiopathic macular telangiectasia, finer adjustments for macular pigment may be required43.
We followed patients with intermediate AMD on an annual basis and monitored study eyes for the occurrence of drusen regression exceeding the area of AREDS circle C1 (diameter 125μm; Age Related Eye Disease Study AMD grading protocol). We found that drusen regression of this magnitude is a prevalent and ongoing process in eyes with intermediate AMD eyes (occurring in about 50% of study eyes over a 2-year period) as ascertained by both manual segmentation and computer-assisted subtractive image analysis. The short-term consequences of drusen regression are often not obvious on clinical examination; on fundus photographs, the majority of retinal areas involved with drusen regression demonstrated a “normal clinical appearance” and was not distinguished by appearance from other drusen-free areas. In a minority of study eyes (about one-third), some degree of mild new hyperpigmentary or hypopigmentary change was detectable in drusen regression areas. However, FAF imaging revealed that areas of drusen regression were associated with short-term local changes in FAF signal which were more common and pronounced. By both manual grading and computer-assisted grading, a majority (78–86%) of eyes demonstrated changes in overall FAF signal in drusen regression areas. In these cases, predominant decreases in FAF signal were found more frequently than predominant increases (by a 3:1 – 4.5:1 ratio). These findings indicate that spontaneous drusen regression, even in the short-term, may not be completely innocuous and likely involve subclinical anatomical or physiological changes located at the level of the photoreceptors and RPE cells.
What may be the significance of these FAF changes that occur soon after drusen regression? As the background level of FAF signal is thought to originate primarily from the RPE layer, and areas of markedly decreased or absent FAF signal correspond to overt RPE cell loss (as in geographic atrophy),44, 45 changes constituting a predominant decrease in FAF signal may be interpreted as alterations leading towards incipient RPE cell damage and loss.46 This association potentially connects the biological mechanisms inducing drusen regression with those responsible for causing RPE injury in AMD. While the precise cellular mechanisms driving drusen regression are obscure, they seem to occur constitutively in eyes with AMD and in some cases, are prominent enough to induce rapid regression of very large drusen (e.g. drusenoid pigment epithelial detachments) over the time period of a few months.31 One hypothesized mechanism of drusen regression involves the role of inflammatory macrophages and microglia which are attracted to areas of drusen deposition and may clear drusen deposits by phagocytosis.47–49 It is possible that these immune cell aggregations may induce local inflammatory changes that result in RPE injury,50, 51 which in the early stages, is manifest as changes in FAF signal. These early deleterious RPE changes, while not easily detected on clinical examination in the short term, may lead to progressive atrophic changes leading to clinically-evident geographic atrophy in the long term.
While drusen regression was more often associated with decrements in FAF signal, a minority of study eyes showed an association with a predominant local increase in FAF signal. Increased FAF signal has been interpreted as arising from increases in either the amount or the photo-oxidation state of RPE lipofuscin, or from elevated bisretinoid synthesis by photoreceptors,52 which have been linked to photoreceptor and RPE dysfunction. In AMD as well as other retinal disorders, increases in FAF signal have been associated with physiological changes in retinal and RPE cells that are deleterious or pathological in nature.44, 53, 54 Junctional zones of geographic atrophy lesions demonstrating increased FAF signal contain structural abnormalities in RPE and photoreceptors as observed on optical coherence tomography,55 and correlate with local decrements in photoreceptor function as assessed by fundus perimetry.56
In an attempt to link these associations in a single timeline, our observations here suggest the possibility that drusen regression induces a transient increase in FAF signal in the adjacent outer retina that leads to a more prolonged phase of predominantly decreased FAF signal, which in the long term culminates in overt RPE atrophy. In this schema, the mechanisms underlying drusen regression may first induce increases in bisretinoid synthesis, accumulation, or photo-oxidation in photoreceptors and RPE cells. These effects can then lead to incipient RPE degeneration, which is reflected in a decrease of bisretinoid content and consequently locally decreased FAF signal. These degenerative changes then culminate in overt RPE loss that becomes clinically evident as depigmentation and atrophy.
The present study was limited to an examination of drusen regression over a fixed time interval (2 years). Although we observed that regions of drusen regression contained areas in which FAF changes were concentrated, slight changes in FAF signal were also observed in areas outside of these delineated areas. These can potentially be contributed to by regression of drusen prior to the study baseline visit (i.e. areas of pre-existing drusen regression), the formation of new drusen,57, 58 or other AMD-associated changes. We have excluded eyes progressing to advanced AMD in this analysis, so subretinal hemorrhage, fibrosis, and exudation, which can markedly change FAF signal, were not contributory in these cases.
The current findings indicate that close long-term follow-up of FAF signal in areas of drusen regression will be necessary to discover the precise sequence of cellular changes occurring in the aftermath of drusen regression. Correlation of such long-term FAF data with other imaging modalities (such as optical coherence tomography) will be helpful to further elucidate concurrent overlying retinal anatomical changes. Drusen regression may constitute an interesting and significant outcome measure in long-term clinical studies which can potentially reveal underlying mechanisms driving AMD progression.
Figure 4. Example of drusen regression over two years in intermediate age-related macular degeneration in Study Participant 2.
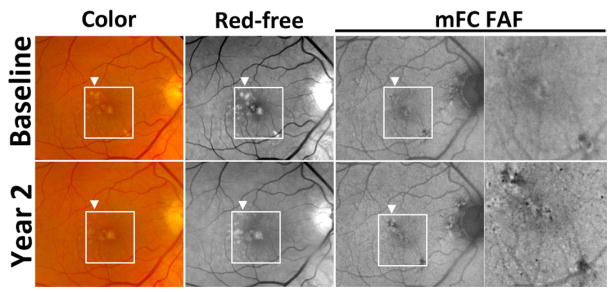
Color (top left, bottom left), red-free monochromatic (top middle, bottom middle) fundus photographs, and fundus autofluorescence (FAF) (top right, bottom right) images at baseline (top row) and year 2 (bottom row) demonstrate two adjacent areas of drusen regression in the macula (highlighted by arrowhead and box). While the areas of drusen regression are of normal appearance on red-free photography (bottom middle), subtle trace pigmentary changes were observed on color photography (bottom left). However, on autofluorescence imaging, distinct areas of decreased FAF signal were localized to areas of drusen regression (bottom right and inset) that were not present at the baseline visit (top right and inset).
Acknowledgments
Funding/Support: This research was funded by the National Eye Institute Intramural Research Program.
Fundus images analyzed in the current study were obtained from participants in the Age-Related Eye Disease Study 2 (AREDS2; ClinicalTrials.gov Identifier #NCT00345176) protocol.
Biography

Brian C. Toy, MD, is an ophthalmology resident at Stanford University. He graduated magna cum laude from the University of California, Berkeley (2007), with a BS in bioengineering, and received an MD with distinction from the University of California, San Francisco (2012). He completed a pre-doctoral fellowship at the National Eye Institute through the National Institutes of Health Clinical Research Training Program (2011). Dr. Toy’s research interests include retinal imaging, telemedicine, and safety net care.
Footnotes
Contributions of Authors in these areas: Design of study (BT, NK, EC, WW); Conduct of study (NK, DC, CC, EY, WW); Analysis and interpretation of data (BT, NK, MI, WW); Writing of manuscript (BT, NK, MI, DC, CC, EY, WW); Final approval of version to be published (BT, NK, MI, DC, CC, EY, WW)
Financial Disclosures: BT was supported by the Clinical Research Training Program, a public-private partnership supported jointly by the National Institutes of Health and Pfizer Inc (via a grant to the Foundation for the National Institutes of Health from Pfizer). The sponsor or funding organization had no role in the design or conduct of this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rodriguez-Carmona M, Kvansakul J, Harlow JA, Kopcke W, Schalch W, Barbur JL. The effects of supplementation with lutein and/or zeaxanthin on human macular pigment density and colour vision. Ophthalmic Physiol Opt. 2006 Mar;26(2):137–147. doi: 10.1111/j.1475-1313.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 2.Schalch W, Cohn W, Barker FM, et al. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin - the LUXEA (LUtein Xanthophyll Eye Accumulation) study. Arch Biochem Biophys. 2007 Feb 15;458(2):128–135. doi: 10.1016/j.abb.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 3.Gass JD. Drusen and disciform macular detachment and degeneration. Arch Ophthalmol. 1973 Sep;90(3):206–217. doi: 10.1001/archopht.1973.01000050208006. [DOI] [PubMed] [Google Scholar]
- 4.Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005 Nov;123(11):1570–1574. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarks JP, Sarks SH, Killingsworth MC. Evolution of soft drusen in age-related macular degeneration. Eye (Lond) 1994;8 ( Pt 3):269–283. doi: 10.1038/eye.1994.57. [DOI] [PubMed] [Google Scholar]
- 6.Bressler NM, Munoz B, Maguire MG, et al. Five-year incidence and disappearance of drusen and retinal pigment epithelial abnormalities. Waterman study. Arch Ophthalmol. 1995 Mar;113(3):301–308. doi: 10.1001/archopht.1995.01100030055022. [DOI] [PubMed] [Google Scholar]
- 7.Klein R, Klein BE, Tomany SC, Meuer SM, Huang GH. Ten-year incidence and progression of age-related maculopathy: The Beaver Dam eye study. Ophthalmology. 2002 Oct;109(10):1767–1779. doi: 10.1016/s0161-6420(02)01146-6. [DOI] [PubMed] [Google Scholar]
- 8.Smith RT, Sohrab MA, Pumariega N, et al. Dynamic soft drusen remodelling in age-related macular degeneration. Br J Ophthalmol. 2010 Dec;94(12):1618–1623. doi: 10.1136/bjo.2009.166843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frennesson IC, Nilsson SE. Effects of argon (green) laser treatment of soft drusen in early age-related maculopathy: a 6 month prospective study. Br J Ophthalmol. 1995 Oct;79(10):905–909. doi: 10.1136/bjo.79.10.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Little HL, Showman JM, Brown BW. A pilot randomized controlled study on the effect of laser photocoagulation of confluent soft macular drusen. Ophthalmology. 1997 Apr;104(4):623–631. doi: 10.1016/s0161-6420(97)30261-9. [DOI] [PubMed] [Google Scholar]
- 11.Sarks SH, Arnold JJ, Sarks JP, Gilles MC, Walter CJ. Prophylactic perifoveal laser treatment of soft drusen. Aust N Z J Ophthalmol. 1996 Feb;24(1):15–26. doi: 10.1111/j.1442-9071.1996.tb01546.x. [DOI] [PubMed] [Google Scholar]
- 12.Figueroa MS, Regueras A, Bertrand J, Aparicio MJ, Manrique MG. Laser photocoagulation for macular soft drusen. Updated results. Retina. 1997;17(5):378–384. doi: 10.1097/00006982-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Abdelsalam A, Del Priore L, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv Ophthalmol. 1999 Jul-Aug;44(1):1–29. doi: 10.1016/s0039-6257(99)00072-7. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann KI, Gomez ML, Bartsch DU, Schuster AK, Freeman WR. Effect of change in drusen evolution on photoreceptor inner segment/outer segment junction. Retina. 2012 Sep;32(8):1492–1499. doi: 10.1097/IAE.0b013e318242b949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein ML, Ferris FL, 3rd, Armstrong J, et al. Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology. 2008 Jun;115(6):1026–1031. doi: 10.1016/j.ophtha.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Sallo FB, Rechtman E, Peto T, et al. Functional aspects of drusen regression in age-related macular degeneration. Br J Ophthalmol. 2009 Oct;93(10):1345–1350. doi: 10.1136/bjo.2008.150334. [DOI] [PubMed] [Google Scholar]
- 17.Maguire MG. Drusen volume as a study endpoint. Ophthalmology. 2012 Jul;119(7):1501–1502. doi: 10.1016/j.ophtha.2012.03.012. author reply 1502. [DOI] [PubMed] [Google Scholar]
- 18.Yehoshua Z, Wang F, Rosenfeld PJ, Penha FM, Feuer WJ, Gregori G. Natural history of drusen morphology in age-related macular degeneration using spectral domain optical coherence tomography. Ophthalmology. 2011 Dec;118(12):2434–2441. doi: 10.1016/j.ophtha.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995 Mar;36(3):718–729. [PubMed] [Google Scholar]
- 20.von Ruckmann A, Fitzke FW, Bird AC. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br J Ophthalmol. 1995 May;79(5):407–412. doi: 10.1136/bjo.79.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitz-Valckenberg S, Fleckenstein M, Scholl HP, Holz FG. Fundus autofluorescence and progression of age-related macular degeneration. Surv Ophthalmol. 2009 Jan-Feb;54(1):96–117. doi: 10.1016/j.survophthal.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina. 2008 Mar;28(3):385–409. doi: 10.1097/IAE.0b013e318164a907. [DOI] [PubMed] [Google Scholar]
- 23.Spaide RF. Fundus autofluorescence and age-related macular degeneration. Ophthalmology. 2003 Feb;110(2):392–399. doi: 10.1016/S0161-6420(02)01756-6. [DOI] [PubMed] [Google Scholar]
- 24.Sternberg S. Biomedical Image Processing. IEEE Computer. 1983;16(1):22–34. [Google Scholar]
- 25.Group A-REDSR. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol. 2001 Nov;132(5):668–681. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 26.Ridler TWCS. Picture thresholding using an iterative selection method. IEEE Transactions on System, Man, and Cybernetics. 1978;8(8):630–632. [Google Scholar]
- 27.Hammond BR, Jr, Wooten BR, Snodderly DM. Individual variations in the spatial profile of human macular pigment. J Opt Soc Am A Opt Image Sci Vis. 1997 Jun;14(6):1187–1196. doi: 10.1364/josaa.14.001187. [DOI] [PubMed] [Google Scholar]
- 28.Robson AG, Moreland JD, Pauleikhoff D, et al. Macular pigment density and distribution: comparison of fundus autofluorescence with minimum motion photometry. Vision Res. 2003 Jul;43(16):1765–1775. doi: 10.1016/s0042-6989(03)00280-3. [DOI] [PubMed] [Google Scholar]
- 29.Kilbride PE, Alexander KR, Fishman M, Fishman GA. Human macular pigment assessed by imaging fundus reflectometry. Vision Res. 1989;29(6):663–674. doi: 10.1016/0042-6989(89)90028-x. [DOI] [PubMed] [Google Scholar]
- 30.van de Ven JP, Boon CJ, Smailhodzic D, et al. Short-term changes of Basal laminar drusen on spectral-domain optical coherence tomography. Am J Ophthalmol. 2012 Sep;154(3):560–567. doi: 10.1016/j.ajo.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Cukras C, Agron E, Klein ML, et al. Natural history of drusenoid pigment epithelial detachment in age-related macular degeneration: Age-Related Eye Disease Study Report No. 28. Ophthalmology. 2010 Mar;117(3):489–499. doi: 10.1016/j.ophtha.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sparrow JM, Dickinson AJ, Duke AM, Thompson JR, Gibson JM, Rosenthal AR. Seven year follow-up of age-related maculopathy in an elderly British population. Eye (Lond) 1997;11 ( Pt 3):315–324. doi: 10.1038/eye.1997.67. [DOI] [PubMed] [Google Scholar]
- 33.Ho AC, Maguire MG, Yoken J, et al. Laser-induced drusen reduction improves visual function at 1 year. Choroidal Neovascularization Prevention Trial Research Group. Ophthalmology. 1999 Jul;106(7):1367–1373. doi: 10.1016/s0161-6420(99)00735-6. discussion 1374. [DOI] [PubMed] [Google Scholar]
- 34.Group CoA-RMDPTS. The Complications of Age-Related Macular Degeneration Prevention Trial (CAPT): rationale, design and methodology. Clin Trials. 2004 Feb;1(1):91–107. doi: 10.1191/1740774504cn007xx. [DOI] [PubMed] [Google Scholar]
- 35.Hsu J, Maguire MG, Fine SL. Laser prophylaxis for age-related macular degeneration. Can J Ophthalmol. 2005 Jun;40(3):320–331. doi: 10.1016/S0008-4182(05)80075-4. [DOI] [PubMed] [Google Scholar]
- 36.Friberg TR, Brennen PM, Freeman WR, Musch DC. Prophylactic treatment of age-related macular degeneration report number 2: 810-nanometer laser to eyes with drusen: bilaterally eligible patients. Ophthalmic Surg Lasers Imaging. 2009 Nov-Dec;40(6):530–538. doi: 10.3928/15428877-20091030-01. [DOI] [PubMed] [Google Scholar]
- 37.Group CoA-RMDPTR. Laser treatment in patients with bilateral large drusen: the complications of age-related macular degeneration prevention trial. Ophthalmology. 2006 Nov;113(11):1974–1986. doi: 10.1016/j.ophtha.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Smith RT, Nagasaki T, Sparrow JR, Barbazetto I, Klaver CC, Chan JK. A method of drusen measurement based on the geometry of fundus reflectance. Biomed Eng Online. 2003 Apr 18;2(1):10. doi: 10.1186/1475-925X-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith RT, Chan JK, Nagasaki T, Sparrow JR, Barbazetto I. A method of drusen measurement based on reconstruction of fundus background reflectance. Br J Ophthalmol. 2005 Jan;89(1):87–91. doi: 10.1136/bjo.2004.042937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith RT, Chan JK, Nagasaki T, et al. Automated detection of macular drusen using geometric background leveling and threshold selection. Arch Ophthalmol. 2005 Feb;123(2):200–206. doi: 10.1001/archopht.123.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith RT, Sohrab MA, Pumariega NM, et al. Drusen analysis in a human-machine synergistic framework. Arch Ophthalmol. Jan;129(1):40–47. doi: 10.1001/archophthalmol.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delori F, Greenberg JP, Woods RL, et al. Quantitative measurements of autofluorescence with the scanning laser ophthalmoscope. Invest Ophthalmol Vis Sci. 52(13):9379–9390. doi: 10.1167/iovs.11-8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Degli Esposti S, Egan C, Bunce C, Moreland JD, Bird AC, Robson AG. Macular pigment parameters in patients with macular telangiectasia (MacTel) and normal subjects: implications of a novel analysis. Invest Ophthalmol Vis Sci. Oct;53(10):6568–6575. doi: 10.1167/iovs.12-9756. [DOI] [PubMed] [Google Scholar]
- 44.Holz FG, Bellman C, Staudt S, Schutt F, Volcker HE. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001 Apr;42(5):1051–1056. [PubMed] [Google Scholar]
- 45.Schmitz-Valckenberg S, Jorzik J, Unnebrink K, Holz FG. Analysis of digital scanning laser ophthalmoscopy fundus autofluorescence images of geographic atrophy in advanced age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2002 Feb;240(2):73–78. doi: 10.1007/s00417-001-0413-3. [DOI] [PubMed] [Google Scholar]
- 46.Sunness JS, Ziegler MD, Applegate CA. Issues in quantifying atrophic macular disease using retinal autofluorescence. Retina. 2006 Jul-Aug;26(6):666–672. doi: 10.1097/01.iae.0000236472.56195.e9. [DOI] [PubMed] [Google Scholar]
- 47.Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol. 2010 Jul;94(7):918–925. doi: 10.1136/bjo.2009.165563. [DOI] [PubMed] [Google Scholar]
- 48.Killingsworth MC, Sarks JP, Sarks SH. Macrophages related to Bruch’s membrane in age-related macular degeneration. Eye (Lond) 1990;4 ( Pt 4):613–621. doi: 10.1038/eye.1990.86. [DOI] [PubMed] [Google Scholar]
- 49.Ma W, Coon S, Zhao L, Fariss RN, Wong WT. A2E accumulation influences retinal microglial activation and complement regulation. Neurobiology of aging. 2013 Mar;34(3):943–960. doi: 10.1016/j.neurobiolaging.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mettu PS, Wielgus AR, Ong SS, Cousins SW. Retinal pigment epithelium response to oxidant injury in the pathogenesis of early age-related macular degeneration. Molecular aspects of medicine. 2012 Aug;33(4):376–398. doi: 10.1016/j.mam.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Ma W, Zhao L, Fontainhas AM, Fariss RN, Wong WT. Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD. PLoS One. 2009;4(11):e7945. doi: 10.1371/journal.pone.0007945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sparrow JR, Yoon KD, Wu Y, Yamamoto K. Interpretations of fundus autofluorescence from studies of the bisretinoids of the retina. Invest Ophthalmol Vis Sci. 2010 Sep;51(9):4351–4357. doi: 10.1167/iovs.10-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleckenstein M, Charbel Issa P, Helb HM, Schmitz-Valckenberg S, Scholl HP, Holz FG. Correlation of lines of increased autofluorescence in macular dystrophy and pigmented paravenous retinochoroidal atrophy by optical coherence tomography. Arch Ophthalmol. 2008 Oct;126(10):1461–1463. doi: 10.1001/archopht.126.10.1461. [DOI] [PubMed] [Google Scholar]
- 54.Cukras CA, Wong WT, Caruso R, Cunningham D, Zein W, Sieving PA. Centrifugal expansion of fundus autofluorescence patterns in Stargardt disease over time. Arch Ophthalmol. 2012 Feb;130(2):171–179. doi: 10.1001/archophthalmol.2011.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fleckenstein M, Charbel Issa P, Helb HM, et al. High-resolution spectral domain-OCT imaging in geographic atrophy associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008 Sep;49(9):4137–4144. doi: 10.1167/iovs.08-1967. [DOI] [PubMed] [Google Scholar]
- 56.Schmitz-Valckenberg S, Bultmann S, Dreyhaupt J, Bindewald A, Holz FG, Rohrschneider K. Fundus autofluorescence and fundus perimetry in the junctional zone of geographic atrophy in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004 Dec;45(12):4470–4476. doi: 10.1167/iovs.03-1311. [DOI] [PubMed] [Google Scholar]
- 57.Delori FC, Fleckner MR, Goger DG, Weiter JJ, Dorey CK. Autofluorescence distribution associated with drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000 Feb;41(2):496–504. [PubMed] [Google Scholar]
- 58.Smith RT, Chan JK, Busuoic M, Sivagnanavel V, Bird AC, Chong NV. Autofluorescence characteristics of early, atrophic, and high-risk fellow eyes in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006 Dec;47(12):5495–5504. doi: 10.1167/iovs.05-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]