Abstract
Body mass index (BMI) may not accurately or adequately reflect body composition or its role in the development of cardiovascular disease (CVD). Ectopic adipose depots may provide a more refined representation of the role of adiposity in CVD. Thus, we examined the association of pericardial and intra-thoracic fat with coronary artery calcium (CAC). Nearly 600 white men and women, as well as Filipina women and African-American women, all without known CVD, had abdominal and chest computed tomography (CT) scans at two time points about four years apart from which CAC presence, severity and progression, as well as pericardial and intra-thoracic fat volumes were obtained. Logistic and linear regression models with staged adjustment were used to assess associations of pericardial and intra-thoracic fat with CAC presence, severity and progression. After adjustment for age, BMI, sex/ethnic group, ever smoking, and lipids, each standard deviation higher increment of intra-thoracic fat, but not pericardial fat, was significantly associated with 3.84-fold higher odds of prevalent CAC (95% CI (1.54, 9.58), p=0.004) and a 38.4% higher CAC score (95% CI (3.5%, 90.0%), p=0.03). Neither pericardial nor intra-thoracic fat were associated with CAC progression. Contrary to previous reports, pericardial fat was not associated with the presence, severity or progression of CAC. We did, however, demonstrate a significant association between intra-thoracic fat and both the presence and severity of CAC. Studies measuring fat in the thoracic cavity may consider defining intra-thoracic fat as a separate entity from pericardial fat.
Introduction
Obesity is a well-established risk factor for cardiovascular disease (CVD)(1), and is also associated with subclinical measures of CVD, such as coronary artery calcium (CAC)(2, 3). However, overall anthropometric measures such as body mass index (BMI) may not be the best indicators of obesity(1, 4, 5) since they intrinsically result in misclassification with respect to tissue types and do not accurately or adequately reflect the distribution, location, and complex biology of adipose tissue. Regional ectopic measures of adiposity may provide a more refined representation of the role of adiposity in CVD and how adiposity contributes to the CVD risk profile(1). For example, central adiposity (i.e. visceral fat), rather than BMI, appears to be a better predictor of mortality among those with coronary artery disease (CAD) (6), as well as a better predictor of CAC progression(7). Moreover, ectopic adipose depots around the heart, such as pericardial or intra-thoracic fat, may be more strongly related to clinical or subclinical CVD than overall measures of adiposity, or even other adipose depots such as abdominal visceral fat (8, 9). In this regard, recent evidence indicates that epicardial and pericardial fat, may be independent, significant sources of inflammatory cytokines (9, 10, 11, 12, 13), thereby increasing the risk for CVD due to local inflammatory processes.
Pericardial fat has been associated with both prevalent(14) and incident(15, 16) CVD. In previous studies, pericardial and intra-thoracic fat were strongly correlated with both traditional risk factors and inflammatory cytokines(14, 17). Studies have also found pericardial fat associated with CAC presence(18, 19), presence of coronary plaques(20), and coronary artery stenosis(21). On the other hand, intra-thoracic fat has been less well-defined and studied. In the Framingham Heart Study, intra-thoracic fat, exclusive of pericardial fat, was associated with abdominal aortic calcium, but not with CAC(18); however, Dey et al found thoracic fat, which included pericardial fat, was significantly associated with CAC(22).
These previous studies of pericardial and intra-thoracic fat have included participants of predominantly Northern European (Caucasian) descent, coronary plaque presence only, or smaller sample sizes, and did not examine CAC severity or progression. Thus, using an ethnically diverse cohort of White men and women (n=343), Filipina women (n=117), and African-American women (n=138), we sought to determine whether pericardial and intra-thoracic fat were associated with CAC presence, severity, and progression independent of traditional cardiovascular risk factors and visceral fat. We specifically examined whether these associations differed by ethnicity or sex, and whether adipocytokines mediated these associations. Additionally, we assessed the associations of traditional and novel cardiovascular risk factors, including adipocytokines, with pericardial and intra-thoracic fat.
Methods
Study Participants
The Rancho Bernardo Study (RBS) is a prospective cohort study of community-dwelling middle to upper class adults of predominantly Northern European ancestry (Caucasian) established between 1972 and 1974, when 82% of the adult residents of a San Diego, California suburb first participated in a survey of heart disease risk factors. The master list for the survey was determined from a reverse telephone directory, census and maps provided by the developer, and a roster of community center membership. Participants were recruited by telephone and seen in random order over the two-year period. Approximately half of participants were retirees at the time of the survey, and the average age was 64. The participants of this study have been followed periodically since then, with the eighth examination (Visit 8) taking place between 2001-02. Further details of recruitment and inclusion criteria for the original cohort can be found elsewhere(23, 24). At that time, participants ≥ 55 years old who were free of known cardiovascular disease had electron beam computed tomography (CT) scans of the chest performed so that the extent of coronary artery calcium (CAC) could be determined. These CT scans also included slices in the abdomen near the umbilicus. Known cardiovascular disease was defined by use of Minnesota codes, Rose angina questionnaire, physician diagnosed cardiovascular disease, or previous coronary artery bypass graft or percutaneous transluminal coronary angiography.
Additionally, a group of Filipina women and African-American women from the UCSD Filipino Women’s Health Study and the Health Assessment Study of African-American Women (HASAAW) cohort were originally recruited in 1994-1999 as ethnic comparison groups to the Rancho Bernardo study using the same research protocol, staff, and diagnostic laboratories. Filipina women were recruited in 1995-99 to study the prevalence of several common chronic diseases such as type 2 diabetes, osteoporosis, CVD and hypertension, among this ethnic group, and included community dwelling women aged 50-69 who self identified as Filipina. Participants were primarily from the Mira Mesa area of north San Diego, a middle class community only ~10 miles from where the original white RB participants were recruited. Filipina participants were recruited by both workplace and community volunteers. More detailed inclusion criteria for the Filipina women have been described elsewhere(25). African-American women were originally recruited also to study the prevalence of chronic diseases as a comparison to the white RB women, and were recruited as a convenience sample from San Diego City and County agencies, active and retired employees from the University of California, San Diego and San Diego State University, women’s organizations, and churches. Participants were age 45-87, self-identified as African-American ethnicity, and had to be available in the San Diego for the next 5 years for follow-up studies. Participants were also chosen to be similar socio-economic status to the RB study participants. More detailed inclusion criteria for the African-American women have been described elsewhere(26).
African-American women and Filipina women age 55 or older and free of known cardiovascular disease in 2001-2002 (using the same CVD criteria stated above for the RB participants) were also invited to have CT scans performed at this time, which also included the abdomen. Using these scans and appropriate software for making the measurements, data on pericardial fat, intra-thoracic fat and CAC was available on a total of 598 participants: 343 Caucasian men and post-menopausal women, 117 post-menopausal Filipina women and 138 post menopausal African-American women.
At the tenth examination (Visit 10) (2005-06), CT scans were repeated on Caucasian, Filipina and African-American participants who returned. Participants were mailed invitation letters to have a follow-up CT scan at the V10 exam, regardless of current CVD status. From these scans, we generated data on CAC progression, which was available on 452 participants: 275 Caucasians, 92 Filipinas, and 85 African-Americans. Of the 146 participants who did not have V10 data, 65 refused for various reasons, 18 died, 2 were ineligible, 2 had unrecoverable CT scans at the V10 exam, and 59 were either lost to follow-up or did not return for unknown reasons.
The RBS, as well as the additional UCSD Filipino Women’s Health and HASAAW studies, were approved by the Institutional Review Board of the University of California, San Diego; participants gave written informed consent.
Measurement of Coronary Artery Calcium
An Imatron C 150 ultrafast computed tomography scanner (Imatron, San Francisco, California) that produced contiguous thin-section sections was used to obtain the images of the chest and abdomen. Scans were electrocardiographically triggered to the R-R interval, and images were obtained at end-diastole during a single breath-hold. CAC was scored according to the Agatston method(27) by specially trained technicians. Further details on CAC measurement can be found elsewhere(28).
Computed Tomography Measurement of Adiposity
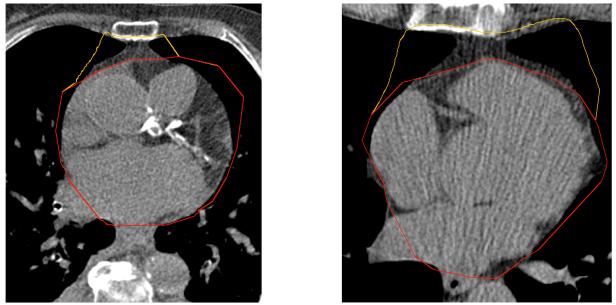
Intra-thoracic, total thoracic, and pericardial adipose tissue volumes were measured from the Visit 8 computed tomography (CT) scans by three experienced CT analysts on networked workstations running muscle segmentation software (MIPAV 4.1.2, National Institutes of Health). Fifteen CT slices of 3mm thickness were selected originating from the right coronary artery, 4 slices above the right coronary artery and 10 slices below. The anterior border of the total thoracic fat volume was defined by the interior margin of the chest wall and the posterior border by the aorta and bronchus. The pulmonary vessels were excluded. The anterior border of the pericardial volume was defined by the line of the fibrous pericardium and the posterior border was shared with the thoracic volume. Intra-thoracic fat volume was defined as the total thoracic fat volume minus the pericardial fat volume. Fat was discerned from other tissue types by the voxel count that fell within the threshold of 190 and 30 Hounsfield units. The volume of fat tissue was established by the product of slice thickness and the area of fat determined by voxel count, and reported in cm3. Figure 1 shows the total thoracic area and the division into pericardial and intra-thoracic areas measured in this study. With the non-contrast CT scans and MIPAV software, epicardial fat cannot be precisely quantified in an efficient manner. Thus, we did not measure it in this study.
Figure 1.
Figure 1 shows two example CT images of segmentation of the pericardial and intra-thoracic fat areas in our study with MIPAV software. The yellow line encloses the intra-thoracic fat area and red line encloses the pericardial fat area. The image on the left shows an example nearer the top of the slice sequence, closer to the apex of the heart, while the image one the right shows an example further down in the slice sequence, at nearer the bottom of the heart. Fifteen total CT slices, each of 3mm thickness, were used to calculate pericardial and intra-thoracic fat.
Visceral fat was obtained from CT scans at Visit 8 (2001-02) and was measured using the same software as for pericardial fat. Briefly, using a cross-sectional scan, the umbilicus was located and a region of interest was manually drawn around the inner most border of the abdominal muscle wall following the parietal peritoneum. Fat area was again differentiated from other tissue types by the voxel count that fell within the threshold of −190 and −30 Hounsfield units, and visceral fat is reported in cm2.
Measurement of Covariates and Adipocytokines
Information on age, sex, smoking status, alcohol use, physical activity, medical history, and medication use was obtained via standard self-administered or interviewer-administered questionnaires. Physical activity was assessed by asking whether the participant currently exercised three or more times per week. This simple question was indirectly validated by showing a positive association with HDL-cholesterol and an inverse association with resting pulse rate. Weight and height were measured with participants wearing light clothing and no shoes. Body mass index was calculated using weight in kilograms divided by height in meters squared. Systolic and diastolic blood pressures were measured twice in seated subjects at rest for at least 5 minutes, and the average of 2 readings was used, with the Hypertension Detection and Follow-up Program protocol(29).
Morning fasting blood samples were obtained by venipuncture after a requested 12-hour fast. Fasting plasma glucose was analyzed using a glucose oxidase method in a hospital diagnostic laboratory. Fasting total cholesterol and triglycerides were measured by enzymatic methods using the ABA-200 Biochromatic analyzer (Abbott Laboratories, Abbott Park, IL). High-density cholesterol (HDL) was determined by precipitation analysis using a protocol from the Lipid Research Clinic. Low-density cholesterol (LDL) was calculated with the (30)Friedewald equation.
Comorbidities included type 2 diabetes and hypertension. Diabetes was defined as self-report of physician diagnosis, anti-diabetes medication use, fasting glucose ≥ 126 mg/dL, or 2-h post-challenge glucose ≥ 200 mg/dl. Hypertension was defined as self report of physician diagnosis, and anti-hypertensive medication use, or systolic blood pressure ≥ 140 mmHg, or diastolic blood pressure ≥ 90 mmHg.
Adipocytokines were measured from EDTA plasma (fasting) obtained at the Visit 7 (1992-1996) for Caucasians and 1994-1999 visit for Filipina and African-American women, and stored at −70°C. Adiponectin and leptin were measured via radioimmunoassay (Millipore Linco Research, St Charles, MO) with intra and inter-assay coefficients of variation (CVs) ranging from 1.97% - 7.32% for adiponectin and 4.37% - 6.43% for leptin. Interleukin (IL)-6 and tumor necrosis factor (TNF)-α were measured via ELISA (R&D Systems, Minneapolis, MN) with intra- and inter-assay CVs for IL-6 of 4.9% - 10.5%, and for TNF-α of 6.0% 10.0%.
Statistical Analysis
To examine the univariate association of characteristics and risk factors across ethnic and sex groups, ANOVA, chi-square or Kruskal-Wallis tests were used as appropriate. Age and BMI-adjusted least squares means (95% CI) were obtained from ANOVAs for each sex/ethnic group. Multivariate linear regression was used to determine risk factors and covariates that were independently associated with pericardial and intra-thoracic fat.
In order to examine the linearity of pericardial and intra-thoracic fat with CAC presence, amount and progression, generalized additive models (GAMs) with a cubic B-spline function were used to create splines. As these associations were approximately linear, pericardial and intra-thoracic fat were then modeled as per standard deviation increment in all further modeling.
Staged multivariate logistic regression models were used to examine the association of pericardial and intra-thoracic fat with CAC presence and CAC progression. Staged multivariate proportional odds models were used to determine the association of fat with CAC as an ordinal outcome. CAC presence was defined as Agatston score at Visit 8 > 0. Ordinal CAC was defined as Agatston score at Visit 8 categorized as 0, 1-100, 101-300, >300. CAC progression was defined using the Hokanson method(31), and participants were defined as having a progression of CAC if the square root of (Visit 10 CAC volume - Visit 8 CAC volume) ≥ 2.5 cm3. The proportional odds assumption was tested for all models using the score test. The first stage of adjustment for all models included age and sex/ethnic groups, then adding BMI, ever smoking, and exercise ≥ 3 times per week in the second stage. Prevalent hypertension, diabetes, and lipid levels were added in the third stage. Finally, adipocyokine levels and visceral fat were added to determine whether any mediation of the associations existed.
Staged multivariate linear regression models were used to determine the association of pericardial and intra-thoracic fat with CAC amount (or severity) and CAC change as continuous outcomes. CAC amount was defined as ln(Agatston score at Visit 8 + 1), and CAC change was defined as ln((Visit 10 Agatston score – Visit 8 Agatston score)/Visit 8 Agatston score). Since these outcomes were natural log transformed, we used the transformation (eβ – 1)*100 to convert the beta coefficients into percents. The corresponding confidence intervals were similarly converted to percents. Staging took place as described above for the multivariate regression models.
In models adjusted for age, BMI, ever smoking, exercise, prevalent hypertension, prevalent diabetes and lipids, interactions between sex and pericardial and intra-thoracic fat, as well as ethnic group and pericardial and intra-thoracic fat were tested for CAC presence and amount. Interactions were tested on an additive scale in linear regression models for CAC amount and on a multiplicative scale in logistic regression models for CAC presence.
To examine whether pericardial or intra-thoracic fat substantially added to the prediction of CAC presence beyond traditional risk factors, c-statistics, the net reclassification improvement (NRI)(32) and the integrated discrimination improvement (IDI)(32) metrics were calculated for any significant associations.
P-values <0.05 were considered statistically significant and SAS V 9.2 (Cary, NC) was used for all analyses, except GAMs and splines, which were performed in SPlus V 8.1 (Tibco Software, Inc, Seattle, WA).
Results
Characteristics and Ethnic/Sex Differences in Pericardial and Intra-thoracic Fat
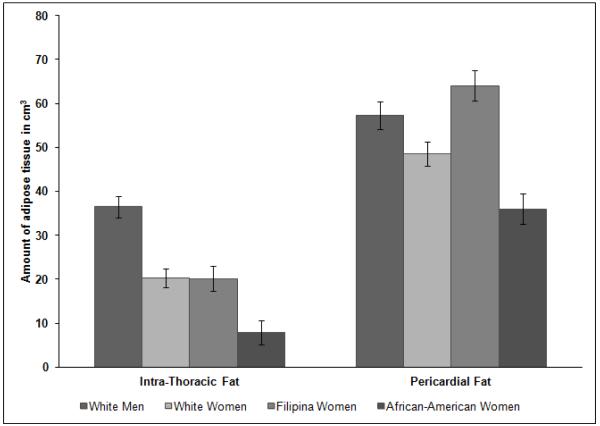
In the cross-sectional analyses, characteristics and risk factors differed significantly across sex/ethnic groups for all factors except hypertension (Table 1). In particular, visceral fat, CAC presence and CAC amount were highest in white men, followed by Filipina women, white women, and then African-American women. Figure 2 shows the volume of pericardial and intra-thoracic fat adjusted for age and body size (BMI) by sex/ethnic groups. All sex/ethnic groups differed significantly on pericardial fat (all pairwise comparisons p<0.005). For intra-thoracic fat, all sex/ethnic groups differed significantly (p<0.001), except white women and Filipina women (p=0.95).
Table 1.
Characteristics by Ethnic and Gender groups
| White Men n=156 |
White Women n=187 |
Filipina Women n=117 |
African-American Women n=138 |
p-valuea | |
|---|---|---|---|---|---|
| Age, years | 68.0 ± 7.4 | 65.9 ± 6.1 | 63.7 ± 6.3 | 60.6 ± 7.4 | <0.001 |
| Ever Smoking | 84 (54%) | 95 (51%) | 12 (10%) | 65 (47%) | <0.001 |
| Exercise ≥ 3x/week | 125 (80%) | 138 (74%) | 82 (70%) | 87 (63%) | 0.01 |
| Body Mass Index, kg/m2 | 26.8 ± 3.5 | 25.7 ± 4.6 | 25.7 ± 3.1 | 29.7 ± 5.7 | <0.001 |
| Visceral Fat, cm2 | 156.1 ± 65.4 | 108.0 ± 56.9 | 123.8 ± 53.6 | 96.1 ± 44.0 | <0.001 |
| Systolic Blood Pressure, mmHg | 131.1 ± 18.9 | 128.8 ± 19.6 | 133.8 ± 20.6 | 134.5 ± 22.4 | 0.008 |
| Diastolic Blood Pressure, mmHg | 78.2 ± 8.0 | 75.2 ± 8.1 | 79.3 ± 9.7 | 77.2 ± 10.4 | <0.001 |
| Prevalent Hypertensionb | 84 (54%) | 87 (47%) | 63 (54%) | 76 (55%) | 0.37 |
| Prevalent Diabetesb | 19 (12%) | 8 (4%) | 39 (33%) | 15 (11%) | <0.001 |
| Fasting Plasma Glucose, mg/dL | 106.3 ± 18.3 | 100.4 ± 27.1 | 107.9 ± 36.6 | 96.7 ± 27.4 | <0.001 |
| Triglycerides, mg/dLc | 104.5 (78.0, 147.5) | 123.0 (84.0, 164.0) | 142.0 (94.0, 196.0) | 77.0 (58.0, 112.0) | <0.001 |
| Total Cholesterol, mg/dL | 198.4 ± 31.3 | 215.8 ± 32.9 | 219.3 ± 40.5 | 206.4 ± 34.9 | <0.001 |
| HDL cholesterol, mg/dL | 50.6 ± 12.0 | 66.0 ± 16.7 | 53.1 ± 11.9 | 62.7 ± 15.9 | <0.001 |
| LDL cholesterol, mg/dL | 123.6 ± 29.4 | 123.3 ± 30.5 | 133.0 ± 34.6 | 123.1 ± 35.5 | 0.004 |
| Interleukin-6, pg/mLc | 1.46 (1.06, 2.27) | 1.43 (1.08, 2.04) | 1.36 (1.09, 2.12) | 1.81 (1.21, 2.87) | <0.001 |
| Tumor Necrosis Factor-α, pg/mL ‡ | 1.06 (0.88, 1.26) | 1.00 (0.84, 1.25) | 0.98 (0.80, 1.34) | 1.01 (0.80, 1.42) | <0.001 |
| Adiponectin, ug/mLc | 7.96 (6.03, 11.02) | 13.27 (10.73, 17.08) | 6.40 (3.80, 10.00) | 7.60 (5.40, 10.80) | <0.001 |
| Leptin, ng/mL ‡ | 5.86 (4.24, 9.12) | 14.36 (9.35, 21.55) | 14.20 (10.80, 18.40) | 15.90 (12.10, 21.00) | <0.001 |
| CAC prevalence (CAC>0, V8) | 138 (88%) | 118 (63%) | 80 (68%) | 78 (57%) | <0.001 |
| CAC, Agatston units (V8)† | 184.9 (37.7, 637.6) | 13.2 (0, 119.4) | 21.4 (0, 140.0) | 3.5 (0, 64.7) | <0.001 |
P-value by ANOVA, chi-square or Kruskal-Wallis test as appropriate
Prevalent hypertension defined as physician diagnosis of high blood pressure, use of anti-hypertensive medications, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg; prevalent diabetes defined as physician diagnosis, fasting plasma glucose > 126 mg/dL, and/or use of diabetes medication (pill or insulin)
Median (Quartile 1, Quartile 3)
Figure 2.
Figure 2 displays the age and BMI adjusted mean levels of pericardial and intra-thoracic by sex/ethnic groups. The x-axis displays the adipose tissue type, and each bar (varying gray-tone colors) represent the sex/ethnic groups. African-American women have the darkest gray/black bars, followed by white men, Filipina women, and then white women.
Among white men, the Spearman correlation of pericardial fat with intra-thoracic fat was 0.68. Among white women, the Spearman correlation of pericardial fat with intra-thoracic fat was 0.66, for Filipina women the correlation was 0.51, and for African-American women the correlation was 0.54. Visceral fat exhibited strong correlations with pericardial fat and intra-thoracic fat, which were 0.65 and 0.68, respectively, for white men (both p<0.001), 0.68 and 0.72, respectively, for white women (both p<0.001), 0.45 and 0.60, respectively, for Filipina women (both p<0.001), and 0.52 and 0.64, respectively, for African-American women (both p<0.001).
Association of Cardiovascular Risk Factors with Pericardial and Intra-thoracic Fat
Age, ethnic/sex group, body mass index, ever smoking, LDL cholesterol, and triglycerides were significantly independently associated with pericardial fat (Table 2), while HDL cholesterol and IL-6 were marginally associated (p=0.07, 0.06, respectively). Similar results were found for intra-thoracic fat, although ever smoking, HDL cholesterol and IL-6 did not seem to play as large of a role.
Table 2.
Risk Factors Associated with Pericardial Fat and Intra-Thoracic Fata
| Pericardial Fat Beta (95% CI) |
p-value | Intra-Thoracic Fat Beta (95% CI) |
p-value | |
|---|---|---|---|---|
| Age, years | 0.36 (0.11, 0.60) | 0.004 | 0.31 (0.11, 0.50) | 0.002 |
| Sex/Ethnic Group | ||||
| White Men | ref | -- | ref | -- |
| White Women | −9.93 (−15.62, −4.25) | 0.001 | −17.79 (−22.33, −13.25) | <0.001 |
| Filipina Women | 5.25 (−0.53, 11.04) | 0.07 | −17.88 (−22.49, −13.26) | <0.001 |
| African-American Women | −20.12 (−25.63, −14.61) | <0.001 | −28.60 (−33.00, −24.21) | <0.001 |
| Body Mass Index, kg/m2 | 1.76 (1.27, 2.26) | <0.001 | 1.29 (0.90, 1.69) | <0.001 |
| Ever Smoking | ||||
| Yes | 3.84 (0.47, 7.20) | 0.03 | 2.01 (−0.67, 4.69) | 0.14 |
| No | ref | -- | ref | -- |
| Exercise ≥ 3x/week | ||||
| Yes | −2.66 (−6.32, 1.01) | 0.16 | −0.71 (−3.63, 2.22) | 0.64 |
| No | ref | -- | ref | -- |
| Prevalent Hypertensionb | ||||
| Yes | 1.25 (−2.10, 4.60) | 0.47 | 2.05 (−0.62, 4.73) | 0.13 |
| No | ref | -- | ref | -- |
| Prevalent Diabetesb | ||||
| Yes | −3.05 (−8.10, 2.00) | 0.25 | −2.26 (−6.29, 1.76) | 0.27 |
| No | ref | -- | ref | -- |
| HDL cholesterol, mg/dL | −0.12 (−0.24, 0.01) | 0.07 | −0.06 (−0.16, 0.04) | 0.27 |
| LDL cholesterol, mg/dL | 0.07 (0.02, 0.12) | 0.005 | 0.04 (0.00, 0.08) | 0.03 |
| Triglycerides, mg/dL | 0.05 (0.03, 0.08) | <0.001 | 0.02 (0.00, 0.05) | 0.03 |
| Interleukin-6, pg/mL | 0.79 (−0.03, 1.60) | 0.06 | 0.24 (−0.41, 0.89) | 0.47 |
| Tumor Necrosis Factor-α, pg/mL | 0.40 (−1.36, 2.17) | 0.65 | −0.34 (−1.74, 1.07) | 0.64 |
| Adiponectin, ug/mL | 0.23 (−0.10, 0.56) | 0.17 | 0.04 (−0.22, 0.30) | 0.78 |
| Leptin, mg/mL | 0.04 (−0.20, 0.28) | 0.75 | 0.16 (−0.03, 0.35) | 0.10 |
In a multivariate linear regression model
Prevalent hypertension is defined as hypertension defined as physician diagnosis of high blood pressure, use of anti-hypertensive medications, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg; prevalent diabetes defined as physician diagnosis, fasting plasma glucose > 126 mg/dL, use of diabetes medication (pill or insulin)
Associations of Pericardial and Intra-thoracic Fat with CAC presence, amount and progression
Pericardial fat and intra-thoracic fat per standard deviation were significantly associated with CAC presence in minimally adjusted models (Table 3); however, after further adjustment, only intra-thoracic remained significantly associated with CAC presence. A similar pattern was observed for the associations of pericardial and intra-thoracic fat with CAC as an ordinal outcome (Table 3). Pericardial and intra-thoracic fat were not significantly associated with CAC progression as a binary outcome. Adipocytokines did not appear to substantially mediate any of these associations, and further adjustment for visceral fat did not appear to affect any associations substantially.
Table 3.
Association of pericardial fat and intra-thoracic fat with CAC prevalence and progression
| Prevalent CACa OR (95% CI) n=598 |
p-value | CAC ordinala OR (95% CI) n=598 |
p-value | CAC progressiona OR (95% CI) n=452 |
p-value | |
|---|---|---|---|---|---|---|
| Pericardial Fat b | ||||||
| Age, race/gender | 1.54 (1.22, 1.97) | 0.0005 | 1.30 (1.10, 1.53) | 0.002 | 1.11 (0.88, 1.40) | 0.39 |
| +BMI, Smoking, Exercise | 1.40 (1.07, 1.82) | 0.01 | 1.17 (0.98, 1.41) | 0.08 | 0.98 (0.75, 1.27) | 0.86 |
| +HTN, Diabetes, Lipids | 1.28 (0.97, 1.70) | 0.08 | 1.06 (0.88, 1.29) | 0.52 | 0.94 (0.71, 1.25) | 0.67 |
| +Adipocytokines | 1.28 (0.95, 1.72) | 0.11 | 1.04 (0.86, 1.27) | 0.69 | 1.00 (0.74, 1.34) | 0.97 |
| +Visceral Fat | 1.20 (0.84, 1.71) | 0.32 | 1.00 (0.79, 1.26) | 0.99 | 1.09 (0.77, 1.55) | 0.64 |
| Intra-Thoracic Fat b | ||||||
| Age, race/gender | 4.07 (2.14, 7.74) | <0.001 | 2.16 (1.52, 3.06) | <0.001 | 1.58 (0.95, 2.62) | 0.08 |
| +BMI, Smoking, Exercise | 3.41 (1.70, 6.81) | 0.0005 | 1.85 (1.26, 2.72) | 0.002 | 1.30 (0.73, 2.29) | 0.37 |
| +HTN, Diabetes, Lipids | 2.79 (1.39, 5.60) | 0.004 | 1.62 (1.09, 2.41) | 0.02 | 1.16 (0.64, 2.10) | 0.64 |
| +Adipocytokines | 2.94 (1.40, 6.18) | 0.004 | 1.69 (1.11, 2.57) | 0.02 | 1.10 (0.59, 2.09) | 0.75 |
| +Visceral Fat | 3.84 (1.54, 9.58) | 0.004 | 1.95 (1.16, 3.28) | 0.01 | 1.70 (0.76, 3.79) | 0.19 |
Prevalent CAC is CAC in Agatston units >0 at Visit 8; CAC ordinal is CAC in Agatston units at Visit 8 categorized 0, 1-100, 101-300, >300; CAC progression is defined by Hokanson method, i.e. sqrt(Visit 10 CAC volume – Visit 8 CAC volume) ≥ 2.5 cm3 is considered progression
Per standard deviation higher – pericardial fat 22.7 cm3, intra-thoracic fat 19.5 cm3
For CAC severity, in models adjusted for age and sex/ethnic groups, each standard deviation (SD) greater pericardial fat and intra-thoracic fat were associated with a 30.6% and 54.0% higher CAC amount, respectively (Table 4). Further adjustment for lifestyle factors, comorbidities, and lipids levels attenuated the pericardial fat results to non-significance. However, the association of intra-thoracic fat with CAC amount remained statistically significant after adjustment for these factors, and did not appear to be mediated by adipocytokine levels or substantially attenuated by adjustment for visceral fat. Pericardial fat and intra-thoracic fat were not significantly associated with change in CAC amount (Table 4).
Table 4.
Association of pericardial fat and intra-thoracic fat with amount of CAC and change in CAC
| CAC severityb Percent (95% CI) |
p-value | Change in CACb Percent (95% CI) |
p-value | |
|---|---|---|---|---|
| Pericardial Fat a | ||||
| Age, race/gender | 30.61 (7.53, 58.63) | 0.007 | −1.52 (−9.56, 7.23) | 0.72 |
| +BMI, Smoking, Exercise | 19.4 (−3.88, 48.33) | 0.11 | −2.06 (−11.14, 7.96) | 0.67 |
| +HTN, Diabetes, Lipids | 6.02 (−14.75, 31.84) | 0.60 | −3.16 (−12.33, 6.98) | 0.53 |
| +Adipocytokines | 3.96 (−16.9, 30.05) | 0.73 | −0.76 (−10.4, 9.9) | 0.88 |
| +Visceral Fat | −1.20 (−23.61, 27.80) | 0.93 | −1.36 (−12.39, 11.07) | 0.82 |
| Intra-Thoracic Fat a | ||||
| Age, race/gender | 54.04 (25.12, 89.64) | <0.001 | 0.09 (−8.31, 9.27) | 0.98 |
| +BMI, Smoking, Exercise | 44.98 (15, 82.77) | 0.002 | 0.08 (−9.41, 10.57) | 0.99 |
| +HTN, Diabetes, Lipids | 32.81 (5.52, 67.16) | 0.02 | −1.61 (−10.29, 7.90) | 0.73 |
| +Adipocytokines | 31.39 (3.32, 67.09) | 0.03 | −0.65 (−10.75, 10.59) | 0.90 |
| +Visceral Fat | 38.35 (3.49, 84.97) | 0.03 | 0.10 (−12.92, 15.08) | 0.99 |
CAC severity is defined as ln(Agatston score + 1) at Visit 8; Change in CAC defined as ln((Visit 10 Agatston score – Visit 8 Agatston score)/Visit 8 Agatston score)
Per standard deviation higher – pericardial fat 22.7 cm3, intra-thoracic fat 19.5 cm3
Since the association of intra-thoracic fat and CAC presence was significant and robust, we further calculated the difference in c-statistics, as well as the NRI and IDI for this model. With a base model containing traditional risk factors (age, sex/ethnic group, ever smoking, physical activity, BMI, hypertension, diabetes, HDL, LDL and triglycerides), the c-statistic was 0.7728, and with the addition of intra-thoracic fat, it was 0.7833 (p for the difference = 0.0668). The IDI was 0.0161 (p=0.0006) indicating improved average sensitivity. However, even using liberal risk cut points of 0.15, 0.25 and 0.35 which would be more appropriate for this sample given the large amount of CAC, adding intra-thoracic fat did not significantly improve reclassification (p=0.48).
We further examined interactions of sex and sex/ethnic group with each of pericardial fat and intra-thoracic fat for CAC presence; no interactions were significant (all p>0.22). We also examine interactions by sex in the white participants only in CAC presence models, which were not statistically significant (p=0.60 for pericardial fat, and p=0.39 for intra-thoracic fat). Finally we examined interaction by ethnicity in the women participants only, which were not statistically significant (p=0.69 for pericardial fat, and p=0.17 for intra-thoracic fat).
Discussion
In this ethnically diverse sample of men and women, we have shown that pericardial and intra-thoracic fat are associated with CAC presence and severity, but not with CAC progression or change in CAC between examinations. Significant independent factors associated with pericardial and intra-thoracic fat included traditional cardiovascular risk factors, and the significance of these factors overlapped well among these fat depots. IL-6 was marginally associated with pericardial fat, but not with intra-thoracic fat. Associations of intra-thoracic fat, but not pericardial fat, with CAC presence and severity remained significant even after adjustment for potential confounders and mediators. In particular, the association of pericardial fat with CAC presence and severity appeared to be attenuated somewhat by the addition of adipocytokines and visceral fat, whereas intra-thoracic fat was not.
There were significant differences in the amount of pericardial and intra-thoracic fat by sex/ethnic groups in our study. After adjusting for age and body size, Filipina women had the largest amount of pericardial fat followed by white men, white women and African-American women, differences which were all statistically significant. For intra-thoracic fat, White men had the largest amount, White women and Filipina women virtually the same amount, and African-American women the least. To our knowledge, this is the first quantification of pericardial and intra-thoracic fat in a Filipina sample. Despite these sex/ethnic differences, there were no differences in associations of pericardial or intra-thoracic fat with CAC by sex or ethnicity.
Our results are generally consistent with previous studies of pericardial or intra-thoracic fat and CAC. Ahmadi et al, in a study of 111 asymptomatic participants, found that each standard deviation (SD) increase in pericardial fat (defined as the difference between total thoracic fat and epicardial fat) was associated with a 2.6-fold greater odds of CAC ≥ 100 compared to CAC of 0(19). Dey et al obtained similar results for pericardial fat and CAC presence (CAC>0 versus CAC = 0) in a sample of 201 participants(22). In 1155 Framingham Heart Study (FHS) participants, pericardial fat per SD was associated with 1.21-fold greater odds of CAC presence after adjustment for traditional risk factors and visceral adipose tissue; however, intra-thoracic fat was not associated with CAC after this adjustment(18). Using somewhat different but still related outcome measures, previous studies have also found pericardial fat associated with calcified coronary plaque presence(20), and with coronary artery stenosis > 50%(21). Total thoracic fat (defined as pericardial + intra-thoracic fat) and intra-thoracic fat (defined as epicardial + pericardial adipose tissue) have been previously associated with CAC > 0(22, 33). In our study, we have additionally examined intra-thoracic fat (defined as adipose tissue outside the pericardium extending to the chest wall above the diaphragm), as well as CAC severity and CAC progression in a large multi-ethnic sample.
One difficulty in comparing results across studies of adipose tissue near and surrounding the heart is the differing methodology for measuring cardiac adipose tissue types. These differences include number and location of CT slices used, the origin CT slice used, the type of software used to quantify the adipose tissue, choice of segmentation of the adipose tissue compartments in the CT image, and differences in nomenclature, all of which can make a difference in the amount of pericardial and intra-thoracic fat accounted for or how these areas of adipose tissue are defined. Few, if any, studies have examined the association of intra-thoracic fat, as we have defined it, and CAC. For example, Ding et al(20) appear to have included a portion of what we deem intra-thoracic fat in their definition of pericardial fat. Huang et al(33) defined intra-thoracic fat as pericardial plus epicardial fat, where pericardial fat appeared to possibly include at least some portion of our definition of intra-thoracic fat. The FHS used a different type of software and scoring system than our study, but is likely most comparable in terms of defining for the areas of pericardial and intra-thoracic fat(18). Epicardial and pericardial fat have several differences, including different embryologic origins and local circulation, as well as possibly differing pathophysiologic roles in inflammation and CVD, which suggest they are distinct cardiac adipose tissue compartments(12, 34, 35).
Previous literature suggests that adipose tissue around the heart, specifically pericardial and/or epicardial fat, could be an independent significant source of adipocytokines (9, 10, 11, 12, 13). Mazurek et al found significantly higher levels of IL-6 and TNF-α in epicardial fat as compared to subcutaneous fat among 55 participants undergoing coronary artery bypass grafts (CABG)(11). Konishi et al found significantly more leukocyte antigen positive cells, indicating inflammation, in pericardial fat among autopsy cases with known CAD as compared to autopsy cases without CAD(10). In our study, after adjustment for adipocytokines, we did not observe substantial attenuation of the associations of pericardial fat or intra-thoracic fat with CAC presence, CAC severity or CAC progression, although there was a slightly more noticeable attenuation for pericardial fat with CAC severity and continuous change in CAC. Additionally, we did not observe substantial attenuation in the association of intra-thoracic fat with CAC after adjustment for visceral fat, indicating that intra-thoracic fat likely provides distinct information. Thanassoulis et al(17) found intra-thoracic fat more strongly associated with a metabolic risk profile and visceral fat. However, in our study, there are similar magnitudes of correlation of visceral fat with pericardial and intra-thoracic fat for all sex/ethnic groups, which is likely why we did not observe a strong attenuation when adding visceral fat to either pericardial fat or intra-thoracic fat models. Lipids appear to be one of the most strongly related factors to pericardial and intra-thoracic fat in our study, so it is not surprising that most of the attenuation, if any, occurs here.
Our study has several strengths, but also some limitations. We have a relatively large ethnically diverse sample of men and women, good quality CT images, and quantified intra-thoracic fat as well as pericardial fat. Since each of our ethnic groups had CT scans performed at the same location with the same CT scanners, and the CT scans were read by the same readers, we have consistent within study results with which to make ethnic and sex comparisons. We also have prospective measures for change in CAC and CAC progression, and have included CAC severity as an outcome. In terms of limitations, it is possible that we may have lacked sufficient power or duration for the CAC progression outcomes or power to detect interactions for sex/ethnic groups. However, associations of pericardial fat and intra-thoracic fat with CAC presence did not appear to differ among sex/ethnic groups in our study, with odds ratios for pericardial fat and CAC presence ranging from 1.10-1.44 and for intra-thoracic fat and CAC ranging from approximately 2-3 for the sex/ethnic groups, for example. Factors other than adipose tissue depots around the heart could be more important in determining CAC progression, and change in CAC is an inherently noisy measure. Additionally, while our sample is ethnically diverse, it is heavily weighted towards women. Our sample consists of participants without clinically overt CVD at baseline. Therefore, the results are not generalizable to a population with known CVD. We also did not quantify epicardial fat in the current study due to limitations of the CT scans and software. Finally, residual confounding or unknown confounders could play a significant role, as in any observational study.
Summary/Conclusions
In our multi ethnic sample, intra-thoracic fat was associated with CAC presence and severity, while pericardial fat was not. Neither cardiac adipose tissue depot was associated with CAC progression. Additional work to better elucidate the potential pathways for cardiac adipose tissue and adipocytokines will help to assess whether these types of adipose have a distinct, independent pathophysiologic role in cardiovascular and other diseases. Additionally, development of more standardized methodology, calculation, nomenclature and definitions for these particular adipose tissue depots so that studies can be effectively compared and/or meta-analyzed will further our knowledge of these adipose depots. Future studies may consider pericardial and intra-thoracic fat as separate entities for analysis given our findings. Finally, more studies with even larger and more ethnically diverse samples still need to be conducted.
Funding and Acknowledgements
This work was supported by R21HL089622 from the National Heart Lung and Blood Institute to CLW. GAL was supported by an American Heart Association award. The authors would like to thank the participants of the RB Study, the UCSD Filipino Women’s Health Study, and the HASAAW for their participation in the studies.
References
- 1.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837–41. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- 2.Blaha MJ, Rivera JJ, Budoff MJ, et al. Association between obesity, high-sensitivity C-reactive protein >/=2 mg/L, and subclinical atherosclerosis: implications of JUPITER from the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:1430–1438. doi: 10.1161/ATVBAHA.111.223768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burke GL, Bertoni AG, Shea S, et al. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:928–935. doi: 10.1001/archinte.168.9.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okorodudu DO, Jumean MF, Montori VM, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond) 2010;34:791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 5.Flint AJ, Rimm EB. Commentary: Obesity and cardiovascular disease risk among the young and old--is BMI the wrong benchmark? Int J Epidemiol. 2006;35:187–189. doi: 10.1093/ije/dyi298. [DOI] [PubMed] [Google Scholar]
- 6.Coutinho T, Goel K, Correa de Sa D, et al. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol. 2011;57:1877–1886. doi: 10.1016/j.jacc.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 7.Kramer CK, von Muhlen D, Gross JL, et al. A prospective study of abdominal obesity and coronary artery calcium progression in older adults. J Clin Endocrinol Metab. 2009;94:5039–5044. doi: 10.1210/jc.2009-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konishi M, Sugiyama S, Sugamura K, et al. Association of pericardial fat accumulation rather than abdominal obesity with coronary atherosclerotic plaque formation in patients with suspected coronary artery disease. Atherosclerosis. 2010;209:573–578. doi: 10.1016/j.atherosclerosis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Yun CH, Lin TY, Wu YJ, et al. Pericardial and thoracic peri aortic adipose tissues contribute to systemic inflammation and calcified coronary atherosclerosis independent of body fat composition, anthropometric measures and traditional cardiovascular risks. Eur J Radiol. 2011 doi: 10.1016/j.ejrad.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 10.Konishi M, Sugiyama S, Sato Y, et al. Pericardial fat inflammation correlates with coronary artery disease. Atherosclerosis. 2010;213:649–655. doi: 10.1016/j.atherosclerosis.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 12.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Tadros TM, Massaro JM, Rosito GA, et al. Pericardial fat volume correlates with inflammatory markers: the Framingham Heart Study. Obesity (Silver Spring) 2010;18:1039–1045. doi: 10.1038/oby.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng VY, Dey D, Tamarappoo B, et al. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging. 2010;3:352–360. doi: 10.1016/j.jcmg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thanassoulis G, Massaro JM, Hoffmann U, et al. Prevalence, distribution, and risk factor correlates of high pericardial and intrathoracic fat depots in the Framingham heart study. Circ Cardiovasc Imaging. 2010;3:559–566. doi: 10.1161/CIRCIMAGING.110.956706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 19.Ahmadi N, Nabavi V, Yang E, et al. Increased epicardial, pericardial, and subcutaneous adipose tissue is associated with the presence and severity of coronary artery calcium. Acad Radiol. 2010;17:1518–1524. doi: 10.1016/j.acra.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Ding J, Kritchevsky SB, Harris TB, et al. The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 2008;16:1914–1919. doi: 10.1038/oby.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim TH, Yu SH, Choi SH, et al. Pericardial fat amount is an independent risk factor of coronary artery stenosis assessed by multidetector-row computed tomography: the Korean Atherosclerosis Study 2. Obesity (Silver Spring) 2011;19:1028–1034. doi: 10.1038/oby.2010.246. [DOI] [PubMed] [Google Scholar]
- 22.Dey D, Wong ND, Tamarappoo B, et al. Computer-aided non-contrast CT-based quantification of pericardial and thoracic fat and their associations with coronary calcium and Metabolic Syndrome. Atherosclerosis. 2010;209:136–141. doi: 10.1016/j.atherosclerosis.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrett-Connor E. The prevalence of diabetes mellitus in an adult community as determined by history or fasting hyperglycemia. Am J Epidemiol. 1980;111:705–712. doi: 10.1093/oxfordjournals.aje.a112948. [DOI] [PubMed] [Google Scholar]
- 24.Criqui MH, Barrett-Connor E, Austin M. Differences between respondents and non-respondents in a population-based cardiovascular disease study. Am J Epidemiol. 1978;108:367–372. doi: 10.1093/oxfordjournals.aje.a112633. [DOI] [PubMed] [Google Scholar]
- 25.Araneta MR, Wingard DL, Barrett-Connor E. Type 2 diabetes and metabolic syndrome in Filipina American women: a high-risk nonobese population. Diabetes Care. 2002;25:494–499. doi: 10.2337/diacare.25.3.494. [DOI] [PubMed] [Google Scholar]
- 26.Afghani A, Barrett-Connor E, Wooten WJ. Resting energy expenditure: a better marker than BMI for BMD in African-American women. Med Sci Sports Exerc. 2005;37:1203–1210. doi: 10.1249/01.mss.0000170080.87526.85. [DOI] [PubMed] [Google Scholar]
- 27.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 28.Barrett-Connor E, Laughlin GA, Connor C. Coronary artery calcium versus intima-media thickness as a measure of cardiovascular disease among asymptomatic adults (from the Rancho Bernardo Study) Am J Cardiol. 2007;99:227–231. doi: 10.1016/j.amjcard.2006.07.085. [DOI] [PubMed] [Google Scholar]
- 29.Abernethy J, Borhani NO, Hawkins CM, et al. Systolic blood pressure as an independent predictor of mortality in the Hypertension Detection and Follow-up Program. Am J Prev Med. 1986;2:123–132. [PubMed] [Google Scholar]
- 30.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 31.Hokanson JE, MacKenzie T, Kinney G, et al. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. AJR Am J Roentgenol. 2004;182:1327–1332. doi: 10.2214/ajr.182.5.1821327. [DOI] [PubMed] [Google Scholar]
- 32.Pencina MJ, D’Agostino RBS, D’Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207 12. [DOI] [PubMed] [Google Scholar]
- 33.Huang G, Wang D, Zeb I, et al. Intra-thoracic fat, cardiometabolic risk factors, and subclinical cardiovascular disease in healthy, recently menopausal women screened for the Kronos Early Estrogen Prevention Study (KEEPS) Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iacobellis G. Epicardial and pericardial fat: close, but very different. Obesity (Silver Spring) 2009;17:625. doi: 10.1038/oby.2008.575. author reply 626 7. [DOI] [PubMed] [Google Scholar]
- 35.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311–9. doi: 10.1016/j.echo.2009.10.013. quiz 1417 8. [DOI] [PubMed] [Google Scholar]