Abstract
Mutations in the tail domain of dynein heavy chain (DYNC1H1) cause two closely related human motor neuropathies, dominant spinal muscular atrophy with lower extremity predominance (SMA-LED) and axonal Charcot-Marie-Tooth (CMT) disease, and lead to sensory neuropathy and striatal atrophy in mutant mice. Dynein is the molecular motor carrying mitochondria retrogradely on microtubules, yet the consequences of dynein mutations on mitochondrial physiology have not been explored. Here, we show that mouse fibroblasts bearing heterozygous or homozygous point mutation in the tail domain of dynein, similar to human mutations, show profoundly abnormal mitochondrial morphology associated with loss of mitofusin 1. Furthermore, heterozygous dynein mutant mice display mitochondrial dysfunction in multiple tissues and mitochondria progressively increase in size and invade sarcomeres in muscles. As a likely consequence of systemic mitochondrial dysfunction, dynein mutant mice develop hyperinsulinemia and hyperglycemia and progress to glucose intolerance with age. Last, similar defects in mitochondrial morphology and mitofusin levels are observed in fibroblasts from patients with SMA-LED. Our results show that dynein function is required for the maintenance of mitochondrial morphology and function with ageing and suggest that mitochondrial dysfunction contributes to dyneindependent neurological diseases, such as SMA-LED.
Introduction
Cytoplasmic dynein (later referred as dynein) is the major molecular motor involved in retrograde transport along microtubules. Multiple indirect evidence point to dynein being involved in neurodegenerative diseases (1, 2) and most recent work identified mutations in the dynein heavy chain gene (dync1h1) leading to human neurological diseases. Initially, de novo mutations close to or in the motor domain of DYNC1H1 were identified in patients with major mental retardation (3, 4). In parallel, a cluster of mutations in the tail domain of DYNC1H1 were shown to lead to hereditary motor neuropathies. Firstly, the H306R mutation leads to dominant axonal Charcot Marie Tooth (CMT) disease (5). Secondly, K671E, Y971C and I584L mutations cause dominant spinal muscular atrophy with lower extremity predominance (SMA-LED) (6). Interestingly, point mutations in the same tail domain of DYNC1H1 were identified in three mouse lines (7, 8) and lead to striatal atrophy and sensory neuropathy in the absence of motor neuron involvement (7–11). From a molecular point of view, tail-domain DYNC1H1 mutations impair the processivity of the dynein motor, leading to a mild, but detectable decrease in run-length of the motor (12) and diminished retrograde axonal transport in vivo (13). In homozygous animals, these mutations lead to abnormal development of the central nervous system and perinatal death (7, 14). In heterozygous mice, however, development appears normal yet dynein transport activity is mildly compromised (7, 14). How these mild decreases in dynein activity might lead to late-onset neuropathies is unknown.
A compelling candidate mechanism for the pathogenicity of tail domain DYNC1H1 mutations would be interference with dynein-dependent mitochondrial trafficking, leading to mitochondrial dysfunction and subsequent neurodegeneration. Indeed, dynein represents the major molecular motor carrying mitochondria towards the perinuclear region and multiple in direct evidence suggests that dynein might be involved in mitochondrial function (15).
Firstly, dynein appears strongly associated with mitochondria during the interphase (16), and is involved in a proper localisation of mitochondria in cells (17). Secondly, dynein is thought to drive dysfunctional mitochondria at sites of autophagocytic degradation (18, 19, 20) and interference with dynein leads to abnormally localized and morphologically abnormal mitochondria (21). Finally, a number of hereditary sensory-motor neuropathies are caused by mutations in genes involved in mitochondrial morphology and transport. In particular, mutations in mitofusin 2 (mfn2) lead to axonal CMT2A, a clinical phenotype also observed with some dync1h1 mutations. Mutant MFN2 leads to abnormal mitochondrial distribution, and to decreased mitochondrial transport in both anterograde and retrograde direction (22–24). Despite this constellation of indirect evidence, it remains unknown whether tail domain mutations of DYNC1H1 lead to mitochondrial abnormalities. Here, we provide in vitro and in vivo evidence that tail domain mutations lead to a late-onset mitochondrial pathology with systemic consequences.
Results
Cramping mutation leads to abnormal mitochondrial morphology in fibroblasts
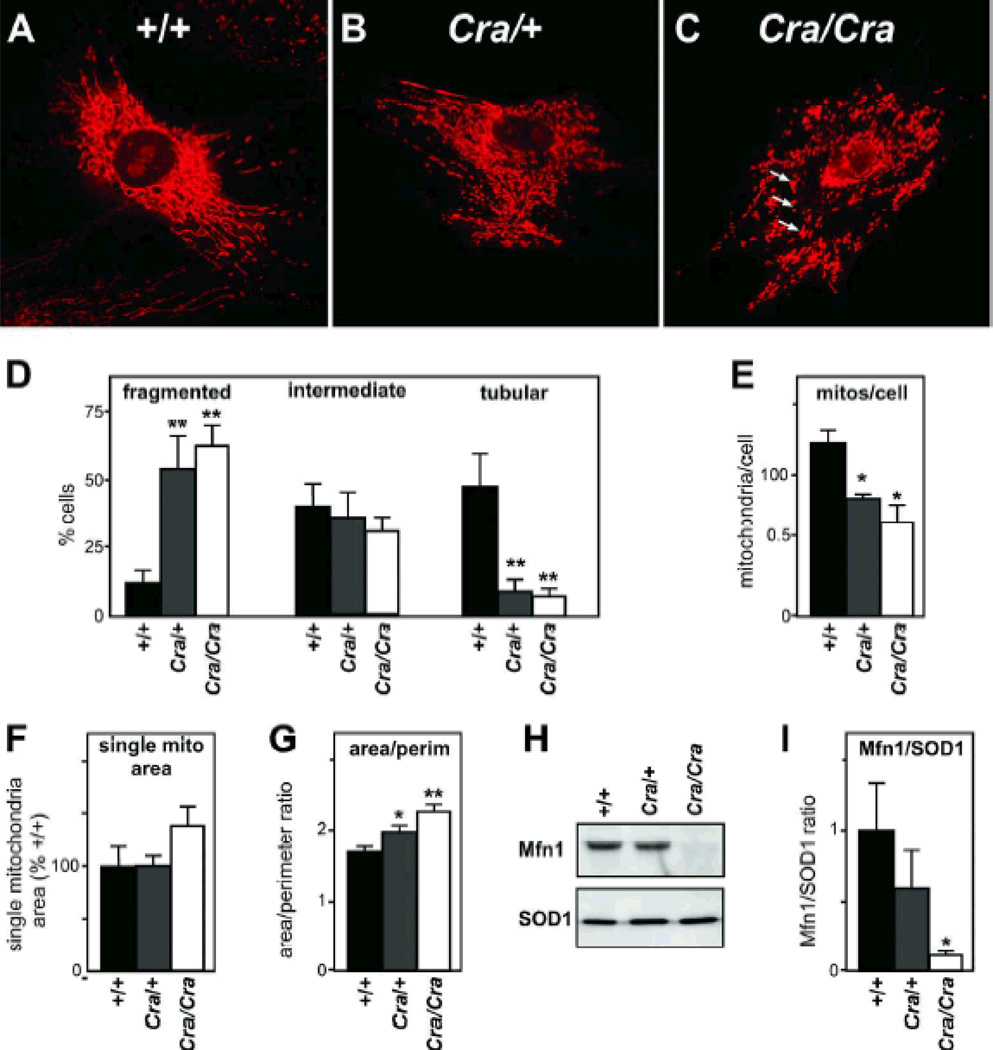
To determine whether tail domain dynein mutations might lead to mitochondrial morphological abnormalities, we stained with Mitotracker cultured mouse embryonic fibroblasts (MEFs) obtained from embryos bearing the Cramping mutation (later abbreviated Cra) in the dync1h1 gene (7, 10, 11, 25). The mitochondrial networks of both Cra/+ and Cra/Cra MEFs appeared profoundly disrupted (figure 1A–C). Most MEFs with a Cra/Cra genotype displayed fragmented mitochondrial morphology and the appearance of mitochondrial aggregates resembling mitoaggresomes (26, 27) (arrows in Figure 1C), while +/+ MEFs showed extensive tubular morphology of the mitochondrial network (Figure 1D).
Figure 1. abnormal mitochondrial morphology and loss of Mfn1 in Cramping mutant MEFs.
A-C- Representative microphotograph of Mitotracker staining in +/+ (A), Cra/+ (B) and Cra/Cra (C) MEFs. Note the fragmentation of the mitochondrial network and the perinuclear aggregates resembling mitoaggresomes (arrows in C). D- Fraction of cells presenting with fragmented (left), intermediate (middle) or tubular (right) mitochondrial network. n=9–12 embryos per condition, at least 25 cells were analyzed per embryo; **, p<0.01 vs corresponding +/+, ANOVA followed by Newman-Keuls post-hoc test.
E-G- Semi-automated morphometric analysis of mitochondrial shape determining the number of individual mitochondria (E), the surface of individual mitochondria (F), and the surface to perimeter (S/P) ratio of individual mitochondria as an index of the shape. The lower is the S/P ratio, the closer to circle is the mitochondria. Note that the dync1h1 mutation leads to decreased individualized mitochondria and increased S/P ratio demonstrating more compact mitochondria. *, p<0.05; **, p<0.01 vs corresponding +/+, ANOVA followed by Newman-Keuls post-hoc test.
H-I Representative western blotting showing Mitofusin 1 (Mfn1) and SOD1 levels in +/+, Cra/+ and Cra/Cra MEFs. n=3 embryos per genotype analyzed. Panel I presents the quantification of the experiments. *, p<0.05; vs corresponding +/+, ANOVA followed by Newman-Keuls post-hoc test.
We performed quantitative morphometric analysis using a semi-automated method previously described (28) and showed that the number of individual mitochondria were decreased in both dynein mutant genotypes in a dose dependent manner (Figure 1E), while the surface of individual mitochondria tended to increase in Cra/Cra MEFs as compared with +/+ MEFs (figure 1F). The surface to perimeter ratio, an index of mitochondrial shape, increased in Cra/+ MEFs and Cra/Cra MEFs suggesting that mitochondria were more compact in these cells (Figure 1G). Consistent with mitochondrial morphological abnormalities, we observed decreased levels of mitofusin 1 in MEFs derived of Cra/Cra but not of Cra/+ embryos (figure 1H–I). We did not detect mitofusin 2 protein in cultured MEFs. In all, the Cramping mutation leads to fragmented mitochondria and loss of mitofusin 1 in homozygous MEFs.
Cramping mice develop progressive mitochondrial dysfunction in muscle and adipose tissue
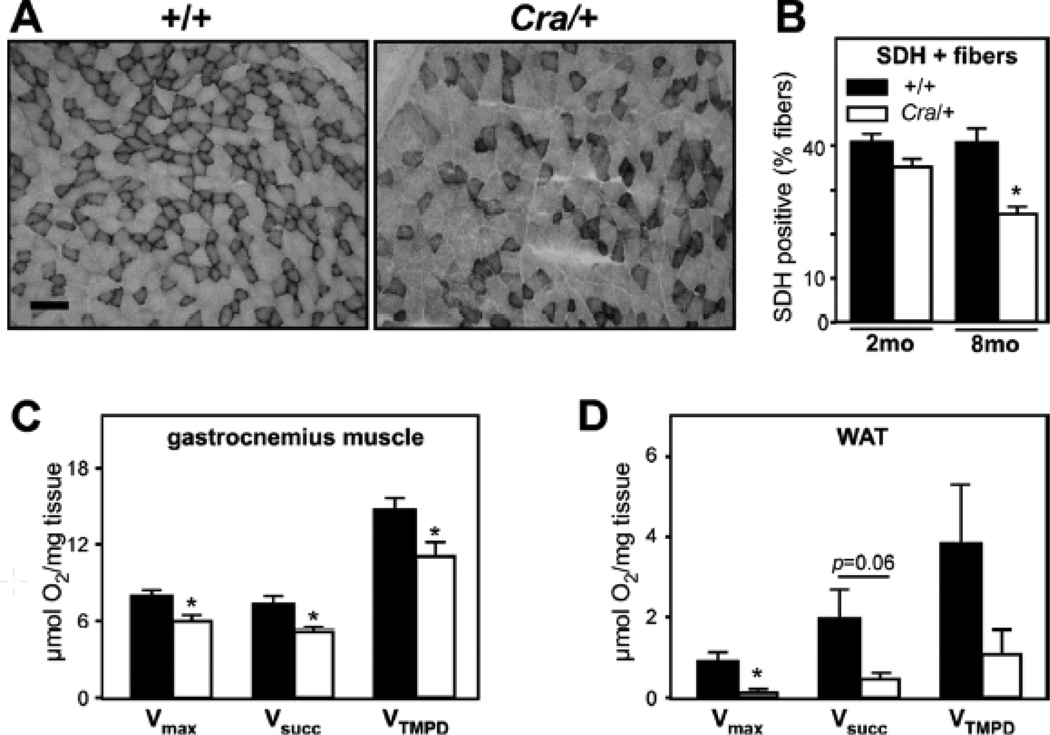
We next sought to determine whether these morphological abnormalities in dynein mutant cells translated into mitochondrial dysfunction in vivo. To this end, we focused our studies on skeletal muscle as this tissue is among the most affected in mitochondrial diseases. In the gastrocnemius muscle of Cra/+ mice, we observed a progressively decreased density of SDH-positive muscle fibers suggestive of a progressive decrease in mitochondrial activity (Figure 2A–B). Consistently, saponin-skinned muscle fibers of Cra/+ mice showed decreased maximal respiration rates of mitochondria (Figure 2C, left panel). Maximal mitochondrial respiration (Vmax, complexes I, III, IV) stimulated by ADP was decreased by more than 20%. Similarly respiration elicited by succinate, a complex II substrate (VSUCC, complexes II, III, IV) and individual complex IV activity (VTMPD) were significantly reduced (Figure 2C). Decreased mitochondrial respiration was also observed in white adipose tissue (WAT), with up to 80% decreased maximal mitochondrial respiration in tissue explants (figure 2D). Thus, the Cramping dynein mutation leads to significant mitochondrial disease, progressing with age.
Figure 2. mitochondrial dysfunction in muscle and white adipose tissue in Cramping mice.
A- Representative photomicrographs showing muscle sections of +/+ and Cra/+ mice of 8 months of age stained for succinate deshydrogenase activity (SDH). Note the decrease in SDH positive fibers in dynein mutant muscles. Scale bar: 100Sµ.
B- Quantification of the experiments presented in A for +/+ and Cra/+ mice of 2 months (2mo) or 8 months (8mo) of age *, p<0.05 vs +/+, n=4 per group.
C-D- Maximal mitochondrial respiration (Vmax) and evaluation of mitochondrial respiratory chain complexes (VSucc: complexes II, III, and IV; VTMPD: complex IV) of saponin-skinned fibers of skeletal muscle (C) and WAT explants (D) of +/+ and Cra/+ mice at 8 months of age. *P<0.05 vs +/+ (n=9 mice per group). When the p value was close to significance, value was indicated.
Cramping mutation leads to mitochondrial ultra-structural abnormalities and abnormal muscle energy metabolism
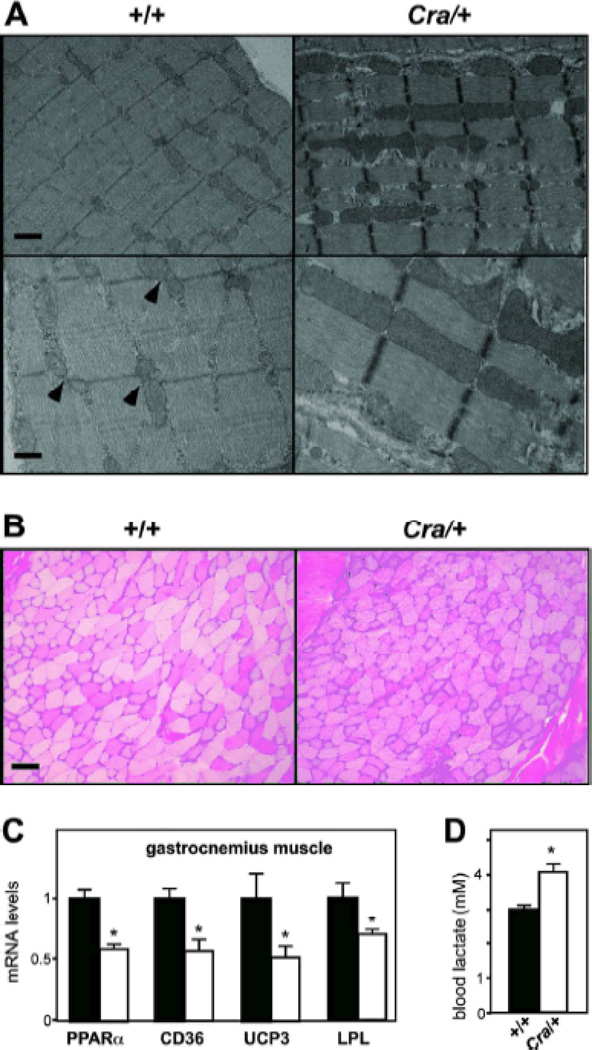
Ultrastructural abnormalities of mitochondria are usually observed upon mitochondrial disease. In electron micrographs of glycolytic gastrocnemius muscle of wild type mice, we observed pairs of mitochondria in the I-band on both sides of the Z-band (figure 3A, arrowheads) similar to what has been reported in the tibialis anterior muscle (29). In 8-months old Cra/+ mice however, this very regular organisation was profoundly perturbed. We observed intense mitochondrial proliferation, with U-shaped, giant, elongated mitochondria that disrupted the alignment of sarcomeres (figure 3A). Mitochondria occupied 3.2 +/− 1.1 % of glycolytic gastrocnemius muscle surface and 17.2+/−4.8% in 8 months old Cra/+ mice (n=4, p<0.05, Student’s t-test) Such a mitochondrial proliferation was not observed in 4-months old mice (results not shown), showing again that the defect was progressively developing with aging. Dysfunction of energy metabolism in muscle was further shown by increased glycogen accumulation on muscle sections stained with PAS (figure 3B), by consistent downregulation of genes involved in lipid uptake and catabolism, including PPARα, CD36, LPL and UCP3 (Figure 3C) and by increased steady states of blood lactate in Cra/+ mice (Figure 3D). In all, the Cramping mutation leads to progressive mitochondrial abnormalities in muscle, that are consistent with alterations in muscle energy metabolism.
Figure 3. ultrastructural and metabolic abnormalities in Cramping muscle.
A- Representative electron micrographs of 8 months old +/+ and Cra/+ gastrocnemius muscle at low (upper panels) and high (lower panels) magnification. Note the regular disposition of +/+ mitochondria (arrowheads) and the largely increased mitochondrial area in Cra/+ muscles. Mitochondria also invades sarcomeres in Cra/+ muscles. Scale bar: 1Sm.
B- Representative photomicrographs showing muscle sections of +/+ and Cra/+ mice of 8 months of age stained for PAS. Note the prominent glycogen accumulation in dynein mutant muscles. Scale bar: 100Sm. C- mRNA levels of PPARα, CD36, mitochondrial uncoupling protein 3 (UCP3) and lipoprotein lipase (LPL) in the gastrocnemius muscle of +/+ and Cra/+ mice at 4 months of age. *P<0.05 vs +/+ (n=8 mice per group). D- Blood lactate levels in +/+ and Cra/+ mice of 4 months of age. *P<0.05 vs +/+ (n=5 mice per group).
Cramping mice develop late onset glucose intolerance
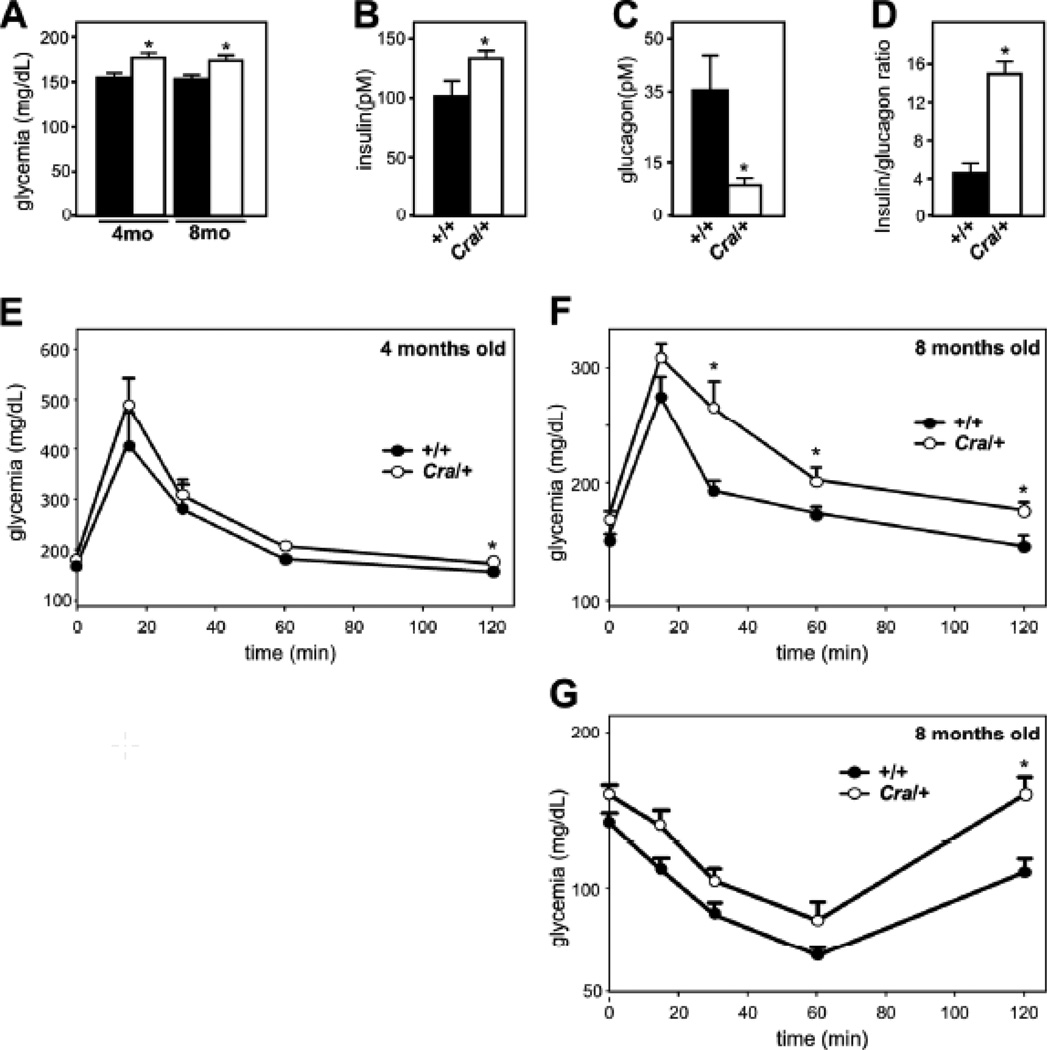
The observed widespread mitochondrial dysfunction might result in abnormal glucose homeostasis, paving the way for insulin resistance (30). Indeed, blood glucose levels were increased in 4 and 8 months old Cra/+ mice, but not earlier (Figure 4A and not shown). The hyperglycaemia was accompanied by increased insulin levels (Figure 4B) and decreased glucagon levels in 4 months old Cra/+ mice (Figure 4C), leaving these animals with a 3-fold increase in the insulin/glucagon ratio (Figure 4D), that could precede onset of type 2 diabetes. Consistent with this, intra-peritoneal glucose tolerance test (IPGTT) revealed glucose intolerance in 8 months (Figure 4F), but not 4 months old Cra/+ mice (Figure 4E).
Figure 4. abnormal glucose homeostasis in Cramping mice.
A- Glycemia (F) in +/+ and Cra/+ mice of 4 (4mo) and 8 (8mo) months of age. n=8 per group. *P<0.05 vs corresponding +/+.
B-D- Insulin (C), glucagon (D) and insulin/glucagon ratio (E) in +/+ and Cra/+ mice of 4 months of age. n=8 per group. *P<0.05 vs +/+.
E-G Glucose (F-G) and insulin (H) tolerance test in +/+ and Cra/+ mice of 4 months (F) and 8 months (G-H) of age. N=8 per group. *, p<0.05 vs corresponding +/+ in Student’s t-test.
Moreover, insulin action was short-lived in 8 months old Cra/+ mice as detected using an insulin tolerance test (Figure 4G). In all, dynein mutant mice showed abnormal glucose homeostasis and developed features of type 2 diabetes with age, in parallel with mitochondrial dysfunction.
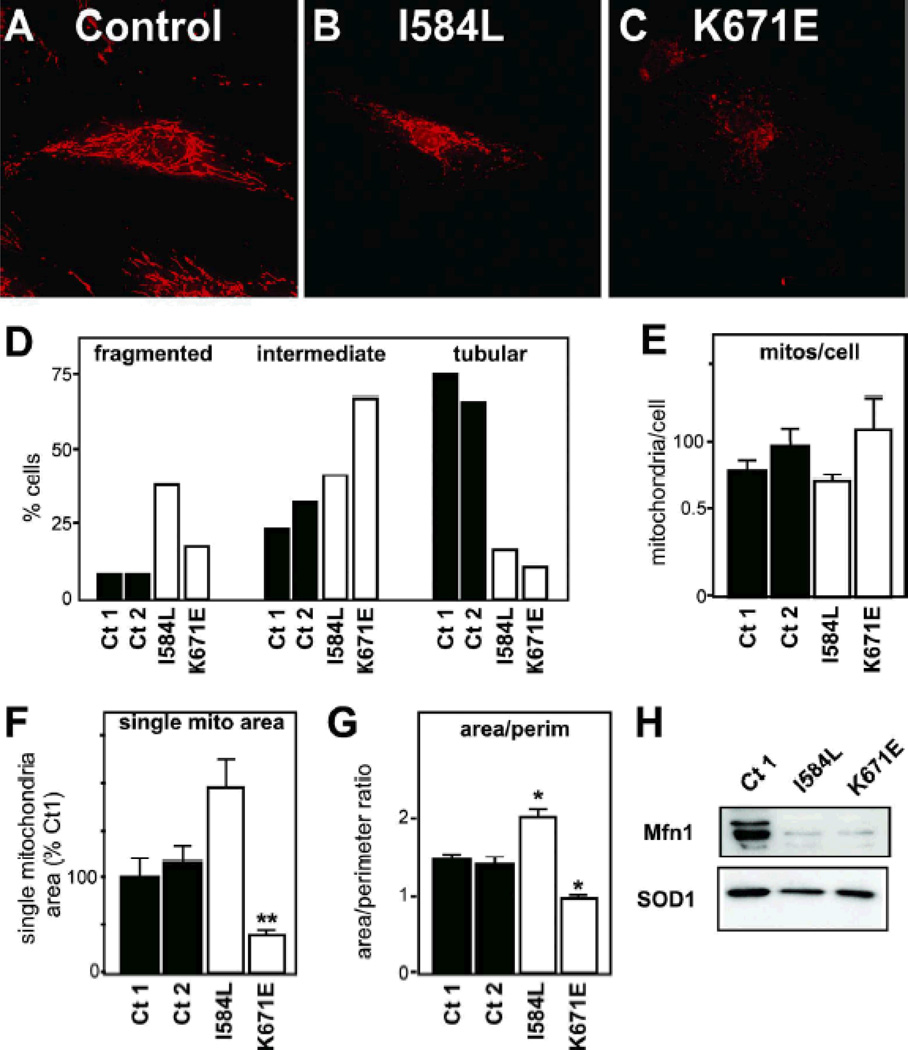
SMA-LED associated mutations in dynein lead to mitochondrial morphological defects in fibroblasts
Our previous results had shown that a tail-domain mutation in mouse dynein is sufficient to lead to systemic mitochondrial dysfunction in mice. To test the validity of our findings in patients, we studied human fibroblasts bearing the SMA-LED associated mutations K671E and I584L in the dynein heavy chain gene along with two healthy controls. Cells from K671E and I584L patients showed intensely fragmented mitochondrial network as compared with healthy controls (Figure 5A–D). Morphometric analysis of the mitochondrial network showed that the two mutations in dync1h1 had different effects on mitochondrial morphology. I584L mutant cells displayed a trend towards an increased area of individual mitochondria (p=0.06), and an increased area/perimeter ratio, in a manner similar to Cra/+ cells (Figure 5E–G). Contrasting with this, individual mitochondria were much smaller in K671E cells as compared with either controls. As a consequence, the area/perimeter ratio decreased in these cells (Figure 5E–G). Levels of mitofusin 1 were potently decreased in both dync1h1 mutants as compared with the controls (Figure 5H), suggesting that loss of mitofusin 1 might be involved in the mitochondrial defects observed upon tail domain mutations of dynein.
Figure 5. abnormal mitochondrial morphology and loss of Mfn1 in SMA-LED fibroblasts.
A-C- Representative microphotograph of Mitotracker staining in healthy control (A), I584L (B) and K671E (C) dync1h1 mutant patients. Note the fragmentation of the mitochondrial network.
D- Fraction of cells presenting with fragmented (left), intermediate (middle) or tubular (right) mitochondrial network. At least 25 cells were analyzed per patient. The distribution of I584L dync1h1 and K671E dync1h1 mutant is different from both controls as judged from Chi2 tests.
E-G- Semi-automated morphometric analysis of mitochondrial shape determining the number of individual mitochondria (E), the surface of individual mitochondria (F), and the surface to perimeter (S/P) ratio of individual mitochondria as an index of the shape. The lower is the S/P ratio, the closer to circle is the mitochondria. *, p<0.05; **, p<0.01 vs healthy controls, ANOVA followed by Newman-Keuls post-hoc test.
H- Representative western blotting showing Mitofusin 1 (Mfn1) and SOD1 levels in healthy control, I584L and K671E dync1h1 mutant patients. n=3 experiments.
Discussion
We show here that tail domain mutations in the dynein heavy chain gene lead to accumulation of mitochondrial abnormalities and to a metabolic dysfunction that over time promotes insulin resistance and type 2 diabetes in mice. Our findings show that dynein is critical in the maintenance of mitochondrial function with age, and suggest that dysfunction of mitochondrial transport is a shared pathogenic event in human motor neuropathies such as SMA-LED and axonal CMTs.
Dynein and mitochondrial function
The first important result of this study is that tail domain mutations in dynein impair mitochondrial function over time. A number of previous studies linked mitochondrial physiology to either microtubules or dynein (16, 17, 19, 21, 31). These studies were all conducted in vitro and were either descriptive (16), or used indirect tools to interfere with dynein function, in particular dynamitin overexpression (21) or pharmacological drugs of uncertain specificity (31). Our current study, contrastingly, used different mutations in the dynein heavy chain gene to directly address the role of dynein in mitochondrial function in vitro and in vivo. In vitro, we showed that several dynein mutations, in mouse and in patient derivedcells, lead to an abnormal mitochondrial network. In vivo, we observed widespread mitochondrial dysfunction in several peripheral tissues, notably muscle and WAT, leading to increased circulating lactate as early as 4 months of age. Intriguingly, El-Kadi and collaborators reported roughly normal mitochondrial respiration in brain mitochondria of Loa/+ mice, a mouse bearing a F580Y mutation in dynein (32). Several points might explain such a discrepancy: first, El-Kadi and collaborators used mice of 120 days of age for their experiments, an age at which mitochondrial defects in Cramping mice are still subtle. Second, brain and spinal cord mitochondrial fractions were used in these experiments and this procedure is deleterious for mitochondria (33). The technique used here, saponin skinned muscle fibers, is known to assess mitochondrial function in a less destructive manner (33). Third, El-Kadi and collaborators did not measure mitochondrial respiration directly, but rather cytochrome-c oxidase activity, as a surrogate marker of mitochondrial respiratory activity.
Why are mitochondria dysfunctional in dynein mutant cells? A result that could provide insights into mechanisms is the decreased levels of Mfn1 in mouse and patient-derived fibroblasts. Ablation of mitofusins is able to profoundly alter mitochondrial network and decrease mitochondrial respiration in vitro in MEFs (34) in a manner analogous yet exacerbated to what observed in dynein mutant cells. The degradation of mitofusins is an early step in mitophagy (35) and dynein has been indirectly involved in mitophagy through its role in generation and/or clearing of mitochondrial aggregates (“mito-aggresomes”) (26, 27) similar to those observed in Cra/Cra MEFs. Thus, decreased Mfn1 levels might be the consequence of a block in mitophagy in dynein mutant fibroblasts. Further research is needed to ascertain a mechanistic link between dynein mutation, mitophagy dysfunction and decreased mitofusin levels.
Dynein and peripheral abnormalities in energy metabolism
A second major result of our studies is that dynein mutation lead to hyperglycemia, glucose intolerance, and accumulation of glycogen in muscle. Such a phenotype in glucose homeostasis is expected in view of the widespread mitochondriopathy (30), and is consistent with increased WAT deposition in Cramping mice (25). There are few reports of co-morbid diabetes and CMT (36–38) and no information is currently available regarding the energy metabolism of SMA-LED patients. Our studies however suggest that dynein mutations in humans might be associated with a systemic dysfunction of energy metabolism, potentially culminating in type 2 diabetes. These results also highlight that phenotypic expression of these diseases might be detectable in peripheral tissues. Such peripheral phenotypes are of high interest, for instance to provide a potential source for biomarkers of the disease.
Mitochondrial transport as a key to understand human motor neuropathies
Our study supports the view that at least a subset of human motor neuropathies, including SMA-LED and axonal CMT are associated with defective transport of mitochondria. Indeed, dynein is the major molecular motor carrying retrogradely mitochondria in cells, and we show here that disease relevant mutations in this gene profoundly modify mitochondrial morphology and respiration. Importantly Mfn2, neurofilament L or GDAP1, three proteins mutant in other forms of CMT disease, have also been shown to interfere with mitochondrial fission/fusion cycles and mitochondrial transport (22, 23, 39–43). In the specific case of Mfn2, CMT2A associated mutations appear to systematically impair the mitochondrial transport activity while some mutants retain fusion properties similar to wild type Mfn2 (23, 41). Among these genes, however, only dynein has direct motor activity, and we provide thus here a direct genetic link between transport defects and mitochondrial dysfunction in these diseases.
We would like to emphasize that while our results show that disease relevant mutations in dynein are sufficient to lead to a late-onset mitochondriopathy in mice, we do not show that this mitochondrial dysfunction is directly causing the degenerative phenotypes, in particular degeneration of proprioceptive and striatal neurons in Cramping mice (10, 11). Striatal neurons are however exquisitely sensitive to mitochondrial toxins and the mitochondrial dysfunction observed might be sufficient to underlie the observed phenotypes (44, 45).
Moreover, if mitochondrial dysfunction is the primary pathological event in these diseases, the relative heterogeneity in the clinical picture that is observed between the different mutations (SMA, CMT, proprioceptive neuropathy, striatal atrophy) and species (human, mouse) might be explained by species and cell-type specific requirements for mitochondrial energy metabolism (46) and are very commonly observed in mitochondrial diseases. Interestingly, mutations in Mfn2 (47) and OPA1 (48) were shown to lead to widespread mitochondriopathies with mitochondrial DNA instability. Thus, our current results provide a rationale for dync1h1 genetic screening in families with such clinical phenotypes. In summary, we show here that the mutations in dynein relevant for SMA-LED and axonal CMT compromise mitochondrial morphology and function and are associated with systemic perturbations of glucose homeostasis.
Materials and methods
Patients
The clinical characteristics of patients with K671E and I584L mutations were previously reported, as was the derivation of fibroblasts from the I584L patient (6). After obtaining informed consent in compliance with the IRB of Washington University, dermal fibroblasts were obtained via skin biopsy from a patient with a K671E mutation and cultured as previously reported (6).
Animals
Heterozygous Cra/+ mice used were F1 generation of a C57Bl6/C3H crossing and were identified by tail DNA genotyping as described previously (7). Wild-type littermates were used as controls. Mice were maintained at 23°C with a 12h light/dark cycle and had food and water ad libitum. For biochemical analysis, animals were sacrificed and tissues were quickly dissected, frozen in liquid nitrogen and stored at −80°C until use. All animal experiments were performed under the supervision of authorized investigators and followed current EU regulations.
Murine embryonic fibroblasts cultures
For MEF isolation, embryos were isolated from 13-day-pregnant mice and washed with phosphate-buffered saline (PBS). The head and visceral tissues were removed from isolated embryos and used for genotyping. The remaining tissues were washed in PBS, minced using a pair of scissors, transferred into a 1mM EDTA and 28,05 U/mL trypsin solution and incubated at 37°C for 15 min. After trypsinization, mechanical dissociation was performed by pipetting up and down several times to improve tissue dissociation. Cells were collected by centrifugation and resuspended in DMEM containing 10% FCS and penicillin and streptomycin. Resulting suspension from each embryo was plated separately onto 100 mm dishes at 37°C with 5% CO2. Medium was changed 24h after plating to remove dead cells and non-adhering fragments. MEFs were used within three passages.
Mitochondrial morphology
Cells were stained with MitoTracker Red CMXRos (Invitrogen) at 100nM for 30 minutes. After staining, cells were washed with PBS, fixed with 4% formaldehyde for 15 minutes, rinsed with PBS and mounted for observation. Immunofluorescence staining was monitored with a laser scanning microscope (LSM 510, Carl Zeiss, Thornwood, NY) equipped with a Plan-Apochromat 63x oil immersion lens. Mitotracker was excited using the 543-nm line of the helium/neon laser and emission signals were filtered with an LP585 filter. Pictures were taken in order to identify the entire cell mitochondrial network and mitochondria morphology analyses were performed using ImageJ macro created as described before by Dagda and collaborators (28). Morphological parameters were calculated for a minimum of ten cells per embryo, with 9–12 embryos studied per condition.
Histological techniques
For muscle histology, isopentane frozen samples were cut on a Leica CM 3050S cryostat into slices 16 Sm thick and processed for SDH and PAS staining using standard pathological stainings. For electron microscopy, after intra-cardiac perfusion of PFA 4% in phosphate buffer (0.1M pH7.4), the glycolytic part of the gastrocnemius muscle was dissected and postfixed by immersion in 2.5% glutaraldehyde and 2.5% paraformaldehyde in cacodylate buffer (0.1 M, pH 7.4), washed in cacodylate buffer for further 30 minutes. The samples were postfixed in 1% osmium tetroxide in 0.1M cacodylate buffer for 1 hour at 4°C and dehydrated through graded alcohol (50, 70, 90, 100%) and propylene oxide for 30 minutes each. Samples were oriented longitudinally and embedded in Epon 812. Semithin sections were cut at 2 Sm and ultrathin sections were cut at 70 nm and contrasted with uranyl acetate and lead citrate and examined at 70 kV with a Morgagni 268D electron microscope. Images were captured digitally by Mega View III camera (Soft Imaging System).
RT-qPCR
Frozen tissues were placed into tubes containing a 5 mm stainless steel bead (Qiagen, Courtaboeuf, France) and 1 ml of Trizol reagent (Invitrogen, Paisley, UK) and homogenized using a TissueLyser (Qiagen). RNA was prepared on tissue homogenates following Trizol manufacturer’s instructions. RNA reverse transcription and SYBR Green real-time PCR assays were performed using the Bio-Rad (Biorad, Marnes la Coquette, France) iCycler kits and protocols. PCR conditions were 3 min at 94°C, followed by 40 cycles of 45 s at 94°C and 10 s at 60°C.
Mitochondrial respiration
The mitochondrial respiration in skeletal muscle was studied in saponin-skinned fibers in order to keep mitochondria in their architectural environment. We also studied mitochondrial respiration in WAT by homogenisation of this tissue before respiration measurement. After dissection, fibers were permeabilized using 50 µg/mL of saponin during 30 minutes. Oxygraphic measurements were performed in a 3-mL water-jacketed oxygraphic cell (Strathkelvin Instruments, Glasgow, Scotland) equipped with a Clark electrode. After the determination of the basal oxygen consumption (V0), the maximal respiration rates were measured at 22.1°C under continuous stirring in the presence of saturating amount of adenosine diphosphate (ADP) as a phosphate acceptor (Vmax). Complex I was blocked with amytal (0.02 mM) and then complex II was stimulated with succinate (25 mM). After that, N, N, N′, N′-tetramethyl-p-phenylenediamine dihydrochloride (TMPD, 0.5 mM) and ascorbate (0.5 mM) were added as an artificial electron donor to cytochrome c.
Metabolite and Hormone Assays
Plasma concentrations of insulin and glucagon (Linco Research, St. Charles, MO) were determined as indicated by the manufacturer. Intraperitoneal glucose tolerance tests were performed by injecting 2 g/kg of glucose into overnight-fasted mice. Glycemia was measured every 15 min with a glucometer (Glucomen, Menarini, Rungis, France).
Western blotting
At confluence, cells were washed with PBS and scraped in protein extraction buffer containing 250 mM sucrose, 1 mM EDTA, 2% SDS, 1 mM DTT, 10 mM Tris-HCl, 0,01% protease inhibitor cocktail and 0,01% phosphatase inhibitor cocktails (Sigma, Sigma-Aldrich, Lyon, France). Protein quantification was carried out using a BCA assay kit (Interchim, Montlucon, France). Equal amounts of soluble proteins were denaturated by boiling, resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS- PAGE) and transferred to a nitrocellulose membrane. After using a chemiluminescent blocker (Millipore, Billerica, MA), membranes were probed with primary antibodies (mitofusin-1: ABC41,Millipore, Billerica, MA; SOD1: 574597, Merck, Darmstadt, Germany) and secondary antibodies (antirabbit HRP: BI2407, P.A.R.I.S, Compiegne, France; anti-sheep HRP: 713-035-174, Jackson Immunoresearch, Suffolk, United Kingdom). To ensure that equal amounts of protein were loaded, SOD1 immunoreactivity was used rather than tubulin or actin. Indeed, these two latter proteins showed decreased levels in homozygous Cra/Cra MEFs precluding them to be used as loading controls. The protein bands were detected by chemiluminescence using an ECL Lumina Forte (Millipore, Billerica, MA) and a chemiluminescence detector (Bio-Rad, Hercules, CA).
Statistical Analysis
Statistical comparisons were accomplished with the unpaired Student t test, unless otherwise indicated, or ANOVA followed by the post hoc Newman–Keuls multiple comparisons test using PRISM version 5 for MacOS X software (GraphPad, San Diego).
Acknowledgements
We thank patients and families. Annie PICCHINENNA, Marie-Jo RUIVO, Sylvie DIRRIG-GROSCH and Stéphane DIETERLE provided technical support for this study. We thank the “plate-forme d’imagerie” from IFR37/INSERM/UDS for technical assistance with confocal imaging.
This work was supported by the Agence Nationale de la Recherche [ANR JCJC Dynemit to L.D.]; Association pour la recherche sur la SLA et les autres maladies du motoneurone (ARSla) [to F.R. and J.P.L. ]; Thierry Latran Foundation [1st call to J.P.L., 4th call to L.D. ]; Association pour la recherche et le développement de moyens de lutte contre les maladies neurodégénératives (AREMANE); the European Community’s Health Seventh Framework Programme (FP7/2007–2013) [No 259867 to J.P.L.]; Association française de lutte contre les myopathies (AFM) [to J.P.L.]; Helmholtz Institute [RNA dysmetabolism in ALS and FTD, WP2, to L.D., P.W. and A.C.L.]; Muscular Dystrophy Association [to M.E.S. ]; the Charcot Marie Tooth Association[to M.E.S.] and National Institute of Health (NIH) [U54NS065712 to M.E.S., NS055980 and NS069669 to R.H.B. ]. L.D. is supported by a Mercator Professorship (DFG, 2011–2012) and a contrat d’interface INSERM/AP-HP. JLGDA is recipient of a Chaire d’excellence INSERM/Université de Strasbourg. R.H.B. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund.
Abbreviations
- CMT
Charcot Marie Tooth disease
- Cra
Cramping allele of the dync1h1 gene (Y1055C mutation)
- IPGTT
intra-peritoneal glucose tolerance test
- LPL
lipoprotein lipase
- MEF
Mouse embryonic fibroblast
- MFN
Mitofusin
- PPARα
peroxisome proliferation activated receptor, alpha
- SMA-LED
Spinal Muscular Atrophy with lower extremity dominance
- UCP3
uncoupling protein 3
Footnotes
Conflicts of interest
There are no conflicts of interest relative to the current manuscript.
References
- 1.Eschbach J, Dupuis L. Cytoplasmic dynein in neurodegeneration. Pharmacol Ther. 2011;130:348–363. doi: 10.1016/j.pharmthera.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Chevalier-Larsen E, Holzbaur EL. Axonal transport and neurodegenerative disease. Biochim Biophys Acta. 2006;1762:1094–1108. doi: 10.1016/j.bbadis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Willemsen MH, Vissers LE, Willemsen MA, van Bon BW, Kroes T, de Ligt J, de Vries BB, Schoots J, Lugtenberg D, Hamel BC, et al. Mutations in DYNC1H1 cause severe intellectual disability with neuronal migration defects. J Med Genet. 2012;49:179–183. doi: 10.1136/jmedgenet-2011-100542. [DOI] [PubMed] [Google Scholar]
- 4.Vissers LE, de Ligt J, Gilissen C, Janssen I, Steehouwer M, de Vries P, van Lier B, Arts P, Wieskamp N, del Rosario M, et al. A de novo paradigm for mental retardation. Nat Genet. 2010;42:1109–1112. doi: 10.1038/ng.712. [DOI] [PubMed] [Google Scholar]
- 5.Weedon MN, Hastings R, Caswell R, Xie W, Paszkiewicz K, Antoniadi T, Williams M, King C, Greenhalgh L, Newbury-Ecob R, et al. Exome sequencing identifies a DYNC1H1 mutation in a large pedigree with dominant axonal Charcot-Marie-Tooth disease. Am J Hum Genet. 2011;89:308–312. doi: 10.1016/j.ajhg.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harms MB, Ori-McKenney KM, Scoto M, Tuck EP, Bell S, Ma D, Masi S, Allred P, Al-Lozi M, Reilly MM, et al. Mutations in the tail domain of DYNC1H1 cause dominant spinal muscular atrophy. Neurology. 2012;78:1714–1720. doi: 10.1212/WNL.0b013e3182556c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hafezparast M, Klocke R, Ruhrberg C, Marquardt A, Ahmad-Annuar A, Bowen S, Lalli G, Witherden AS, Hummerich H, Nicholson S, et al. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- 8.Chen XJ, Levedakou EN, Millen KJ, Wollmann RL, Soliven B, Popko B. Proprioceptive sensory neuropathy in mice with a mutation in the cytoplasmic Dynein heavy chain 1 gene. J Neurosci. 2007;27:14515–14524. doi: 10.1523/JNEUROSCI.4338-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilieva HS, Yamanaka K, Malkmus S, Kakinohana O, Yaksh T, Marsala M, Cleveland DW. Mutant dynein (Loa) triggers proprioceptive axon loss that extends survival only in the SOD1 ALS model with highest motor neuron death. Proc Natl Acad Sci U S A. 2008;105:12599–12604. doi: 10.1073/pnas.0805422105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braunstein KE, Eschbach J, Rona-Voros K, Soylu R, Mikrouli E, Larmet Y, Rene F, De Aguilar JL, Loeffler JP, Muller HP, et al. A point mutation in the dynein heavy chain gene leads to striatal atrophy and compromises neurite outgrowth of striatal neurons. Hum Mol Genet. 2010;19:4385–4398. doi: 10.1093/hmg/ddq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupuis L, Fergani A, Braunstein KE, Eschbach J, Holl N, Rene F, Gonzalez De Aguilar JL, Zoerner B, Schwalenstocker B, Ludolph AC, et al. Mice with a mutation in the dynein heavy chain 1 gene display sensory neuropathy but lack motor neuron disease. Exp Neurol. 2009;215:146–152. doi: 10.1016/j.expneurol.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Ori-McKenney KM, Xu J, Gross SP, Vallee RB. A cytoplasmic dynein tail mutation impairs motor processivity. Nat Cell Biol. 2010;12:1228–1234. doi: 10.1038/ncb2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perlson E, Jeong GB, Ross JL, Dixit R, Wallace KE, Kalb RG, Holzbaur EL. A switch in retrograde signaling from survival to stress in rapid-onset neurodegeneration. J Neurosci. 2009;29:9903–9917. doi: 10.1523/JNEUROSCI.0813-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ori-McKenney KM, Vallee RB. Neuronal migration defects in the Loa dynein mutant mouse. Neural Dev. 2011;6:26. doi: 10.1186/1749-8104-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anesti V, Scorrano L. The relationship between mitochondrial shape and function and the cytoskeleton. Biochim Biophys Acta. 2006;1757:692–699. doi: 10.1016/j.bbabio.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Kim S, Sun X, Lee JH, Cho H. Cell cycle-dependent mitochondrial biogenesis and dynamics in mammalian cells. Biochem Biophys Res Commun. 2007;357:111–117. doi: 10.1016/j.bbrc.2007.03.091. [DOI] [PubMed] [Google Scholar]
- 17.Boldogh IR, Pon LA. Mitochondria on the move. Trends Cell Biol. 2007;17:502–510. doi: 10.1016/j.tcb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J Cell Sci. 2004;117:2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- 19.Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17:2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maday S, Wallace KE, Holzbaur EL. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol. 2012;196:407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varadi A, Johnson-Cadwell LI, Cirulli V, Yoon Y, Allan VJ, Rutter GA. Cytoplasmic dynein regulates the subcellular distribution of mitochondria by controlling the recruitment of the fission factor dynamin-related protein-1. J Cell Sci. 2004;117:4389–4400. doi: 10.1242/jcs.01299. [DOI] [PubMed] [Google Scholar]
- 22.Baloh RH, Schmidt RE, Pestronk A, Milbrandt J. Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J Neurosci. 2007;27:422–430. doi: 10.1523/JNEUROSCI.4798-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci. 2010;30:4232–4240. doi: 10.1523/JNEUROSCI.6248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Detmer SA, Vande Velde C, Cleveland DW, Chan DC. Hindlimb gait defects due to motor axon loss and reduced distal muscles in a transgenic mouse model of Charcot-Marie-Tooth type 2A. Hum Mol Genet. 2008;17:367–375. doi: 10.1093/hmg/ddm314. [DOI] [PubMed] [Google Scholar]
- 25.Eschbach J, Fergani A, Oudart H, Robin JP, Rene F, Gonzalez de Aguilar JL, Larmet Y, Zoll J, Hafezparast M, Schwalenstocker B, et al. Mutations in cytoplasmic dynein lead to a Huntington's disease-like defect in energy metabolism of brown and white adipose tissues. Biochim Biophys Acta. 2011;1812:59–69. doi: 10.1016/j.bbadis.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J Cell Biol. 2010;189:671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dagda RK, Cherra SJ, 3rd, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:92–103. doi: 10.1038/nrendo.2011.138. [DOI] [PubMed] [Google Scholar]
- 31.Arany Z, Wagner BK, Ma Y, Chinsomboon J, Laznik D, Spiegelman BM. Gene expression-based screening identifies microtubule inhibitors as inducers of PGC-1alpha and oxidative phosphorylation. Proc Natl Acad Sci U S A. 2008;105:4721–4726. doi: 10.1073/pnas.0800979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Kadi AM, Bros-Facer V, Deng W, Philpott A, Stoddart E, Banks G, Jackson GS, Fisher EM, Duchen MR, Greensmith L, et al. The legs at odd angles (Loa) mutation in cytoplasmic dynein ameliorates mitochondrial function in SOD1G93A mouse model for motor neuron disease. J Biol Chem. 2010;285:18627–18639. doi: 10.1074/jbc.M110.129320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T, Tranqui L, Olivares J, Winkler K, Wiedemann F, et al. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem. 1998;184:81–100. [PubMed] [Google Scholar]
- 34.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion. 2003 doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol. 2010;191:1367–1380. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Celik M, Forta H, Parman Y, Bissar-Tadmouri N, Demirkirkan K, Battaloglu E. Charcot-Marie-Tooth disease associated with Type 2 diabetes mellitus. Diabet Med. 2001;18:685–686. [PubMed] [Google Scholar]
- 37.Ota T, Osawa K. Marked improvement of glycaemic control with pioglitazone in a Type 2 diabetic patient associated with Charcot-Marie-Tooth disease. Diabet Med. 2003;20:420–421. doi: 10.1046/j.1464-5491.2003.009173.x. [DOI] [PubMed] [Google Scholar]
- 38.Koc F, Sarica Y, Yerdelen D, Baris I, Battaloglu E, Sert M. A large family with Charcot-Marie-Tooth Type 1a and Type 2 diabetes mellitus. Int J Neurosci. 2006;116:103–114. doi: 10.1080/00207450500341431. [DOI] [PubMed] [Google Scholar]
- 39.Niemann A, Wagner KM, Ruegg M, Suter U. GDAP1 mutations differ in their effects on mitochondrial dynamics and apoptosis depending on the mode of inheritance. Neurobiol Dis. 2009;36:509–520. doi: 10.1016/j.nbd.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Niemann A, Ruegg M, La Padula V, Schenone A, Suter U. Ganglioside induced differentiation associated protein 1 is a regulator of the mitochondrial network: new implications for Charcot-Marie-Tooth disease. J Cell Biol. 2005;170:1067–1078. doi: 10.1083/jcb.200507087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Misko AL, Sasaki Y, Tuck E, Milbrandt J, Baloh RH. Mitofusin2 mutations disrupt axonal mitochondrial positioning and promote axon degeneration. J Neurosci. 2012;32:4145–4155. doi: 10.1523/JNEUROSCI.6338-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gentil BJ, Minotti S, Beange M, Baloh RH, Julien JP, Durham HD. Normal role of the low-molecular-weight neurofilament protein in mitochondrial dynamics and disruption in Charcot-Marie-Tooth disease. FASEB J. 2012;26:1194–1203. doi: 10.1096/fj.11-196345. [DOI] [PubMed] [Google Scholar]
- 43.Tradewell ML, Durham HD, Mushynski WE, Gentil BJ. Mitochondrial and axonal abnormalities precede disruption of the neurofilament network in a model of charcot-marie-tooth disease type 2E and are prevented by heat shock proteins in a mutant-specific fashion. J Neuropathol Exp Neurol. 2009;68:642–652. doi: 10.1097/NEN.0b013e3181a5deeb. [DOI] [PubMed] [Google Scholar]
- 44.Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, Storey E, Srivastava R, Rosen BR, Hyman BT. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci. 1993;13:4181–4192. doi: 10.1523/JNEUROSCI.13-10-04181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brouillet E, Hantraye P, Ferrante RJ, Dolan R, Leroy-Willig A, Kowall NW, Beal MF. Chronic mitochondrial energy impairment produces selective striatal degeneration and abnormal choreiform movements in primates. Proc Natl Acad Sci U S A. 1995;92:7105–7109. doi: 10.1073/pnas.92.15.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rouzier C, Bannwarth S, Chaussenot A, Chevrollier A, Verschueren A, Bonello-Palot N, Fragaki K, Cano A, Pouget J, Pellissier JF, et al. The MFN2 gene is responsible for mitochondrial DNA instability and optic atrophy 'plus' phenotype. Brain. 2012;135:23–34. doi: 10.1093/brain/awr323. [DOI] [PubMed] [Google Scholar]
- 48.Amati-Bonneau P, Valentino ML, Reynier P, Gallardo ME, Bornstein B, Boissiere A, Campos Y, Rivera H, de la Aleja JG, Carroccia R, et al. OPA1 mutations induce mitochondrial DNA instability and optic atrophy 'plus' phenotypes. Brain. 2008;131:338–351. doi: 10.1093/brain/awm298. [DOI] [PubMed] [Google Scholar]