Abstract
Autophagy describes the degradation of unnecessary or dysfunctional cellular components through the lysosomal machinery. Autophagy is essentially required to prevent accumulation of cellular damage and to ensure cellular homeostasis. Indeed, impaired autophagy has been implicated in a variety of different diseases. We examined the role of autophagy in inflammatory bone loss. We demonstrated that autophagy is activated by the pro-inflammatory cytokine tumor necrosis factor (TNF/TNFα) in osteoclasts of patients with rheumatoid arthritis (RA). Autophagy induces osteoclast differentiation and stimulates osteoclast-mediated bone resorption in vitro and in vivo, thereby highlighting autophagy as a novel mediator of TNF-induced bone resorption.
Keywords: TNFα, arthritis, autophagy, bone resorption, osteoclasts
Autophagy is characterized by the formation of a phagophore around the target structure and subsequent fusion of the completed autophagosome with lysosomes. Although initially discovered as a mechanism for cell survival during nutrient starvation, autophagy exerts other functions that are crucial for cellular homeostasis. Autophagy prevents accumulation of cell damage and premature aging, and contributes to the elimination of pathogens from infected cells. Considering the crucial role of autophagy for cellular homeostasis, it may not be surprising that deregulated autophagy has been implicated in the pathogenesis of several diseases including cancer, infections, neurodegeneration and cardiovascular diseases.
We analyzed the role of autophagy in the pathogenesis of rheumatoid arthritis and associated inflammatory bone loss. RA is a chronic inflammatory disease that results in severe destruction of articular cartilage and bone. Osteoclasts are the major cellular mediators of bone degradation and are essentially required for local bone erosions and systemic osteoporosis in RA. TNF is considered as a key player in the pathogenesis of RA. It is abundantly expressed in the synovium of inflamed joints and stimulates osteoclastogenesis. This is exemplified by the phenotype of mice overexpressing human TNF (TNF tg) that present with increased numbers of osteoclasts and destructive arthritis closely resembling RA in humans.
We first analyzed the expression of several proteins with crucial roles in autophagy and demonstrated that the levels of BECN1 and ATG7, which are essential for the initiation and the elongation of the phagophore during autophagy, are increased in osteoclasts of RA patients. Given the key role of TNF in RA, we hypothesized that TNF might stimulate autophagy in osteoclasts. Indeed, TNF induces the expression of ATG7 and BECN1 in murine osteoclasts in vitro. Moreover, TNF also stimulates the conversion of the microtubule-associated protein1 light chain 3 (LC3)-I into LC3-II, providing further evidence that TNF stimulates autophagy in osteoclasts. We also confirmed the stimulatory effects of TNF on autophagy in vivo by demonstrating increased expression of ATG7 and BECN1 in osteoclasts of TNF tg mice compared with wild-type littermates.
We next investigated whether autophagy is required for TNF-induced osteoclastogenesis. Indeed, inhibition of autophagy either by knockdown of ATG7 or by treatment with bafilomycin A1 strongly impairs osteoclast differentiation with reduced numbers of multinucleated, ACP5/TRAP-positive osteoclasts and impaired expression of osteoclast-associated genes, like NFATC1, OSCAR, ACP5 and CTSK. In addition to our findings on osteoclast differentiation, a recent elegant study by DeSelm and coworkers demonstrated that ATG proteins also regulate osteoclast function as they direct secretory lysosomes to the plasmalemma for subsequent fusion and extracellular secretion. Consistently, inhibition of autophagy strongly reduces bone resorption. Conversely, activation of autophagy by overexpression of BECN1 in osteoclast precursor cells induces osteoclastogenesis with elevated mRNA levels of osteoclast-associated genes and increased bone resorption in vitro.
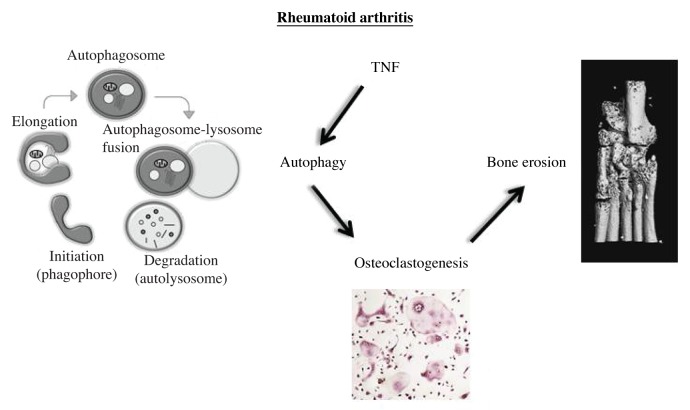
The stimulatory effects of TNF on autophagy and the crucial role of autophagy for osteoclastogenesis stimulated us to investigate whether inhibition of autophagy may prevent TNF-induced inflammatory bone loss in vivo (Fig. 1). We selectively inactivated autophagy in monocytic cells by transplanting TNF tg mice with bone marrow cells from Atg7fl/fl × Lyz2/LysM Cre+ mice (Atg7fl/fl × Lyz2 Cre+ → TNF tg mice). Atg7fl/fl × Lyz2 Cre+ → TNF tg mice are not protected from TNF-induced inflammation, displaying similar levels of paw swelling and loss of grip strength and a comparable extent of inflammation on histology as seen in Atg7fl/fl × Lyz2 Cre- → TNF tg control mice. However, inactivation of autophagy in monocytic cells reduces osteoclastogenesis and prevents structural damage. The number of osteoclasts and the osteoclast-covered area are strongly decreased in Atg7fl/fl × Lyz2 Cre+ → TNF tg mice. The eroded area on histology is also significantly reduced and micro CT (µCT) demonstrated that Atg7fl/fl × Lyz2 Cre+ → TNF tg mice are largely protected from TNF-induced local bone destruction.
Figure 1. Proposed model of autophagy in the pathogenesis of rheumatoid arthritis and associated bone loss. The stimulatory effects of TNF on autophagy and the crucial role of autophagy for osteoclastogenesis drive the bone erosion in TNF tg mice.
Atg7fl/fl × Lyz2 Cre+ → TNF tg mice also demonstrate less cartilage damage with reduced chondrocyte apoptosis and ameliorated proteoglycan loss compared with Atg7fl/fl × Lyz2 Cre- → TNF tg control mice. As autophagy is selectively inhibited in monocytic cells in Atg7fl/fl × Lyz2 Cre+ → TNF tg, the observed cartilage protection in Atg7fl/fl × Lyz2 Cre+ → TNF tg mice is likely mediated by decreased release of soluble mediators from infiltrating monocytic cells. The levels of the monocyte/macrophage-derived cytokines interleukin (IL)1B and IL6 are both decreased in serum of Atg7fl/fl × Lyz2 Cre+ → TNF tg mice compared with Atg7fl/fl × Lyz2 Cre- → TNF tg mice. Inhibition of autophagy in infiltrating monocytes and resident macrophages may thus impair decrease fibroblast activation and protect chondrocytes by impaired release of IL1 and IL6 from monocytic cells.
Our findings highlight a key role of autophagy in TNF-induced bone loss that may have translational implications. Although selective inhibitors of autophagy are not available for clinical application, several drugs in clinical use do have concomitant inhibitory effects on autophagy. Among those drugs are chloroquine and hydroxychloroquine, which can prevent bone erosions and are established therapies of RA. Chloroquine and hydroxychloroquine inhibit autophagy by raising the intralysosomal pH, which impairs protein degradation within the autolysosome. Although not exclusively mediated by inhibition of autophagy, the antiresorptive effects of chloroquine and hydroxychloroquine support the concept that inhibiting autophagy may be a promising therapeutic approach in the prevention of bone resorption in RA.
In summary, our study identifies autophagy as a crucial mediator of TNF-induced osteoclastogenesis, thereby demonstrating a central role of autophagy in inflammatory bone loss.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/25467