Abstract
Multiple lines of evidence suggest that serotonin type 1A (5-HT1A) receptor dysfunction is involved in the pathophysiology of mood disorders, and that alterations in 5-HT1A receptor function play a role in the mechanisms of antidepressant and mood stabilizer treatment. The literature is in disagreement, however, as to whether 5-HT1A receptor binding abnormalities exist in bipolar disorder (BD). We acquired PET images of 5-HT1A receptor binding in 26 unmedicated BD subjects and 37 healthy controls using [18F]FCWAY, a highly selective 5-HT1A receptor radio-ligand. The mean 5-HT1A receptor binding potential (BPP) was significantly lower in BD subjects compared to controls in cortical regions where 5-HT1A receptors are expressed post-synaptically, most prominently in the mesiotemporal cortex. Post-hoc assessments involving other receptor specific binding parameters suggested that this difference particularly affected the females with BD. The mean BPP did not differ between groups in the raphe nucleus, however, where 5-HT1A receptors are predominantly expressed pre-synaptically. Across subjects the BPP in the mesiotemporal cortex was inversely correlated with trough plasma cortisol levels, consistent with preclinical literature indicating that hippocampal 5-HT1A receptor expression is inhibited by glucocorticoid receptor stimulation. These findings suggest that 5-HT1A receptor binding is abnormally reduced in BD, and this abnormality may particularly involve the postsynaptic 5-HT1A receptor system of individuals with a tendency toward cortisol hypersecretion.
Keywords: 5-HT1A receptor, Positron-emission tomography, Bipolar disorder, (18F)FCWAY
1. Introduction
Multiple lines of evidence suggest that abnormal serotonin type 1A (5-HT1A) receptor function is involved in the pathophysiology of mood disorders, and that alterations in the function of the 5-HT1A receptor are relevant to the mechanisms of antidepressant (Blier et al., 1997; Drevets, 1999; Lemonde et al., 2003) and mood stabilizing treatment (reviewed in Savitz et al., 2009).
Positron emission tomography (PET) has been used to study 5-HT1A receptor bindng in mood disorders, with most studies targeting major depressive disorder (MDD). Relatively fewer PET studies have assessed 5HT1A receptor binding in BD. Reduced 5-HT1A receptor binding in unmedicated depressed BD subjects was first suggested by a pilot PET study using the 5-HT1A radioligand [carbonyl-11C]WAY-100635. This series found that both depressed BD subjects and MDD subjects with a family history of BD had lower 5-HT1A binding than controls in the raphe and mesiotemporal cortex (MTC) (Drevets et al., 1999, 2007). Partly consistent with these results Moses-Kolko et al. (2007), found that the 5-HT1A receptor BPND (calculated by dividing regional BPP by VT in the reference region) was decreased from 20% to 28% in the MTC, anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC) in 9 unmedicated women with post-partum depression (of whom 4 had BD) versus 9 post-partum, non-depressed controls. In contrast, a more recent study showed increased 5-HT1A binding (using the outcome measure BPF) in unmedicated-depressed BD subjects (Sullivan et al., 2009). Finally, a study of euthymic BD subjects receiving mood stabilizing medications found no abnormality in 5-HT1A receptor binding using the outcome measure BPND (Sargent et al., 2010).
In post-mortem studies of BD (Gray et al., 2006), reported that 5-HT1A receptor density was reduced in the prefrontal cortex (PFC) in BD subjects versus controls. Moreover Lopez-Figueroa et al. (2004), demonstrated a reduction in 5-HT1A mRNA expression in the hippocampus and dorsolateral PFC in MDD subjects versus controls, and reported that similar (although non-significant) reductions were evident in a smaller sample of BD subjects. The post-mortem data suggest that BD is associated with reduced 5-HT1A receptor antagonist binding in regions where 5-HT1A receptors are expressed post-synaptically.
Here we measure 5-HT1A receptor binding in a sample of 26 unmedicated BD subjects and 37 healthy controls using PET and the highly selective 5-HT1A radioligand [18F]trans-4-fluoro-N-(2-[4-(2-methoxyphenyl) piperazino]-ethyl)-N-(2-pyridyl) cyclohexanecarboxamide (FCWAY) (Lang et al., 1999). The a priori hypothesis of reduced 5-HT1A BP was tested in brain regions, including the MTC, ACC, posterior cingulate cortex (PCC), anterior insula (AI), and parieto-occipital cortex (POC), where 5-HT1A binding abnormalities were identified in previous post-mortem and PET studies of MDD and BD (Drevets et al., 1999; Lopez et al., 1998; Sargent et al., 2000). Finally, we tested for the first time the hypothesis that the mesiotemporal cortical 5-HT1A receptor BPP would correlate inversely with the trough cortisol level, a sensitive indicator of hypersecretion in mood disorders (Deuschle et al., 1997).
2. Experimental procedures
2.1. Participants
Subjects were informed about the purpose of the study and risks involved and gave written consent as approved by the NIH Combined CNS Institutional Review Board and the NIH Radiation Safety Committee. Twenty-six subjects (19 female; mean age 32.9±9.5 years, range 21-54 years) meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition-Text Revision (DSM-IV-TR) criteria for bipolar I, most recent episode depressed (N =9) or for bipolar II disorder, depressed (N = 17), as established by interviews conducted by a psychiatrist and by the Structured Clinical Interview for DSM-IV-TR (First et al., 2002) participated. In addition, 37 psychiatrically healthy volunteers who were group-matched to the BD subjects for gender and age (26 female; mean age 32.7±9.4 years, range 20-59 years) were imaged. Subjects had been free of psychotropic medication or medication likely to affect cerebral blood flow or monoamine neurotransmitter function for at least 3 weeks prior to scanning. Additional exclusion criteria were having a lifetime history of substance dependence (excluding nicotine) or substance abuse within 6 months of scanning (by DSM-IV-TR criteria), major medical or neurological disorders and pregnancy. Depression severity was rated using the Montgomery-Asberg Depression Rating Scale (MADRS). The Young Mania Rating Scale (YMRS) was used to assess the presence of mania symptoms on the day of PET scanning. Anxiety symptoms were also assessed on the day of the PET scan using the Hamilton anxiety rating scale (HAM-A).
2.2. Trough cortisol measurements
On a separate day within one week of PET scanning, subsets of the BD (N=16) and healthy control (N=14) subjects underwent hourly serial blood sampling from 8:00 pm to 9:00 am the following morning. Samples were immediately centrifuged and then frozen and stored as plasma at −70 °C until the time of assay when all samples were run in a single batch. Plasma cortisol concentrations were measured using solid-phase 2-site chemiluminescent immunometric assay. Trough cortisol values were calculated for each subject as the mean value between 11 pm and 2 am.
2.3. PET image acquisition, modeling, and processing
PET scans were acquired with a GE Advance tomograph (35 contiguous slices, 4.25 mm plane separation; reconstructed resolution = 7 mm full-width at half-maximum in all planes). A transmission scan was performed to enable attenuation correction of the emission scans. A 120-min dynamic emission scan then was initiated following intravenous bolus administration of 8 mCi of FCWAY. An arterial input function was derived from radial artery samples, as detailed in the Supplementary methods. Individual dynamic PET images were corrected for motion and attenuation, co-registered with the anatomical MR image, and partial volume corrected as detailed in the Supplementary methods.
2.4. MR anatomical imaging
Whole brain anatomical images were obtained with a GE Signa Scanner (3.0 T) and an MPRAGE sequence (TE=2.98 ms, TR=7.5 ms, inversion time=725 ms, voxel size=0.9 × 0.9 × 1.2 mm3). Non-brain tissues were removed from the images using either a combination of the Brain Extraction Tool (BET; FMRIB, University of Oxford, UK) (Smith, 2002) and manual editing, or the AFNI tool 3dSkullStrip (Analysis of Functional NeuroImages, NIMH, NIH, Besthesda, MD). The resulting whole brain images were segmented into gray matter, white matter, and cerebrospinal fluid (CSF) components using the FMRIB automated segmentation tool (FAST) (Zhang et al., 2001), and binary mask images were created for each component.
2.5. Image analysis
Regions of interest (ROI's) in structures with abundant postsynaptic 5-HT1A receptor concentrations were defined on a template image. The location of each region was superimposed on the MRI registered to the stereotaxic space of the template, and adjusted to match individual subject anatomy. Regions were defined in the MTC, ACC, posterior cingulate cortex (PCC), anterior insula (AI), and parietooccipital cortex (POC), as described by Neumeister et al. (2004). The ROI then were transformed from the stereotaxic space of the template back to the original space of the subject's MRI and applied to the VT image. The VT value for each ROI was defined as the mean VT for all gray matter voxels within that ROI.
Because the [18F]fluoride secondary metabolite of FCWAY is largely taken up by bone in FCWAY images, binding estimates from the cerebral cortical surface are confounded by errors in the correction for signal spillover from the skull. Thus, some other regions of potential interest in MDD and BD (e.g., OFC, temporal polar cortex) could not be examined using this method.
The anatomical boundaries of the raphe nucleus are not clearly evident in MR images, so the ROI for measuring radioactivity in this structure was defined directly on PET images. A reference tissue ROI was defined in the cerebellar white matter, which is virtually devoid of 5-HT1A receptors (Parsey et al., 2005). Although some studies have previously used cerebellar cortex, cerebellar white matter has lower specific binding than cerebellar gray matter and would therefore be a more appropriate reference region (Hall et al., 1997; Parsey et al., 2005). Details of placement of both the raphe ROI and reference tissue ROIs are provided in the Supplementary methods. Several choices of dependent variable are available to describe radiotracer binding in PET studies. The distribution volume, VT, estimates the sum of free and non-specifically bound radiotracer in the region (binding that is “non-displaceable” by a receptor specific ligand), plus radiotracer specifically bound to 5-HT1A receptors. VND is the non-displaceable binding, presumed to be equivalent to the distribution volume of the cerebellar reference region. Binding potential (BP) estimates specific binding to target receptors relative to some reference concentration. We chose to calculate VT and BPP as our primary outcome measures, but include BPF and BPND as secondary outcome measures to facilitate comparison with the literature. Briefly, BPP is a measure of BP relative to total plasma concentration, calculated as VT ROI–VND. Details of the various BP parameters and a discussion of the rationale for choice of particular parameters are given in the Supplementary methods.
2.6. Statistical analysis
Because we expected that the regional values would be correlated, we carried out a linear mixed model with region as a repeated measure and a compound symmetry covariance matrix. Diagnosis, gender and age were fixed effects. All two-way interactions were included in the initial model and removed if they were found to be insignificant. The false discovery rate (FDR) correction for multiple comparisons over 11 regions was applied. Post-hoc tests of un-weighted marginal means corrected for model effects were performed to assess the difference between diagnostic groups in each region. We examined VT and BPP, as well as BPF and BPND in this manner. We calculated Spearman correlation coefficients of mesiotemporal cortical VT and BPP with trough cortisol levels for whom these values were obtained. We corrected for 2 comparisons, left and right lateralized MTC, given that our primary hypothesis was specific to MTC. All other regions were examined and corrected for 9 comparisons. The difference between average trough cortisol values between groups was evaluated using an independent samples t-test.
To investigate possible sources of bias in the BP measures, general linear models were performed to detect group differences in fp and VT in the cerebellar reference region. Similar to the mixed model above, all two-way interactions were initially included in the model and iteratively removed if they were non-significant.
3. Results
Demographic and clinical characteristics of the sample appear in Table 1. Note that some rating scores were not obtained for some subjects; the number of subjects included in the mean for each scale is given. Clinical ratings for the BD subjects indicated that the mean depression severity was in the moderate to severe range, while the YMRS indicated only minimal mania symptoms, and the HAM-A indicated only mild anxiety symptoms. Of the 26 BD subjects, 23 met DSM-IV-TR criteria for a current major depressive episode and three for partial remission from the most recent depressive episode. Three subjects were treatment naïve, and for two subjects we were unable to unequivocally establish the date of their last psychiatric medication, although the entrance criterion of at least 3 unmedicated weeks clearly had been met. There was no significant difference in mean age or mean trough cortisol level between groups by independent samples t-tests, and no significant difference in gender composition between groups by chi-square testing (p>0.05).
Table 1.
Demographic and clinical characteristics of the sample group. All values are given in the format mean (standard deviation).
| N | Age | # Female (%) | MADRS | YMRS | HAM-A | Time off meds (months)b | Age at onset | Trough cortisol (μg/dL)a | |
|---|---|---|---|---|---|---|---|---|---|
| Bipolar disorder | 26 | 33 (9.5) | 19 (73%) | 23 (10.0), N=25 | 7 (4.8), N=21 | 13 (6.3), N=25 | 19 (22.2), N=21 | 17 (6.3) | 2.4 (1.48), N=16 |
| Healthy control | 37 | 33 (9.4) | 26 (70%) | N/A | N/A | N/A | N/A | N/A | 2.4 (1.32), N=14 |
Three subjects were naïve to previous treatment, and the time off medication could not be accurately established for two subjects, although these subjects would have been medication free for at least three weeks.
The mean trough cortisol levels did not differ between groups (t=0.894, p=0.379).
The main effects of age (F=0.072, p=0.790) and diagnosis (F=1.55, p=0.219) on the reference region VT were not significant, but the main effect of gender on cerebellar VT showed a non-significant trend (F=3.82, p=0.055). Similarly, the main effects of age (F=2.30, p=0.135) and diagnosis (F=0.359, p=0.551) on fp were not significant, but the main effect of gender showed a non-significant trend (F=4.01, p=0.050).
3.1. Regional VT and BPP differences between groups
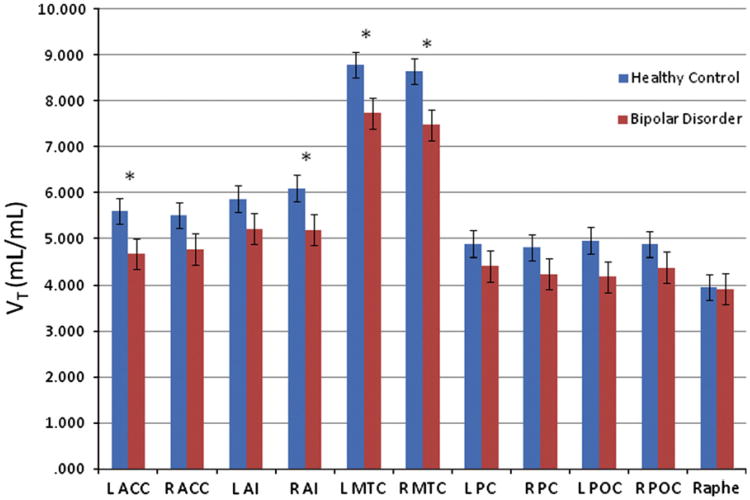
The mean model-adjusted VT values are shown in Figure 1 and Supplementary Table 1. The VT showed a significant main effect of region (F10,610=160, P<0.001), and a non-significant trend towards main effects of diagnosis (F1,60=3.63, p=0.062) and age (F1,60=3.76, p=0.057). There was a significant interaction of diagnosis and region (F10,610=1.96, p=0.036). The main effect of gender was non-significant (F1,60=0.016, p=0.899). Reductions in VT in the BD subjects versus the controls remained significant in the bilateral MTC after applying the FDR correction.
Figure 1.
The VT unweighted means and standard errors plotted for healthy and BD subjects in all regions of interest. MTC=mesiotemporal cortex; ACC=anterior cingulate cortex; PCC=posterior cingulate cortex; AI=anterior insula; POC=parieto-occipital cortex. *Difference between groups significant at p<0.05.
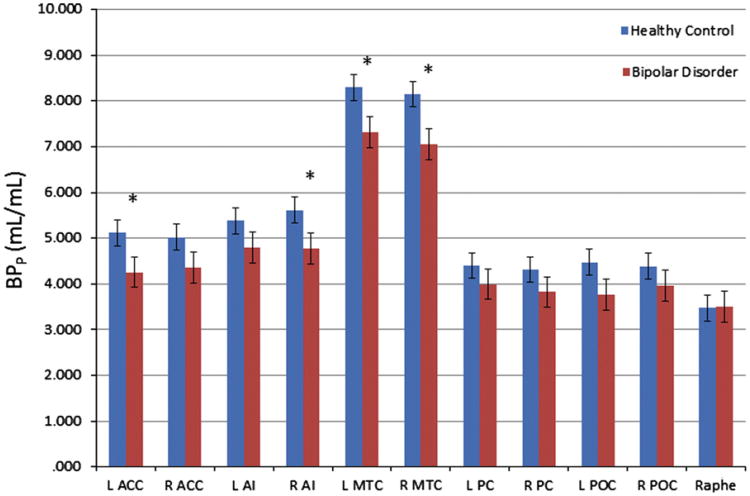
BPP showed a significant main effect of region (F10,610= 160, p<0.001), a non-significant trend toward an effect of diagnosis (F1,59=3.10, p=0.083) and age (F1,59=3.84, p=0.055), and a significant interaction of diagnosis and region (F10,610=1.96, p=0.036). Mean model-adjusted values for BPP, appear in Supplementary Table 1 and Figure 2. The main effect of gender was non-significant (F1,59=0.208, p=0.65). The reductions in mean BPP in the right and left MTC of the BD subjects remained significant after applying the FDR correction. The mean BPP in the left ACC and the right AI regions also were lower in the BD sample versus the control sample, but these differences did not remain significant after applying corrections for multiple testing.
Figure 2.
BPP estimated unweighted means and standard errors plotted for healthy and BD subjects in all regions of interest. Abbreviations as in Figure 1. *Difference between groups significant at p<0.05.
4. Results for secondary outcome measures
4.1. BPF and BPND
The BPF values showed a significant main effect of region (F10,610=170, p<0.001), and a trend towards a significant diagnosis-by-gender interaction (F10,610=3.88, p=0.054). The main effects of gender (F1,58=2.691, p=0.106), age (F1,58=1.521, p=0.223), and diagnosis (F1,58=0.227, p=0.636), and the interaction of diagnosis-by-gender-by-region (F30,590=1.06, p=0.382) were not significant. We conducted post-hoc tests for effects of diagnosis in each gender group, revealing a significant reduction in BPF in female subjects with BD compared to healthy females (p=0.021), but no difference in males. Post-hoc tests to assess regional specificity showed reductions between BD and healthy females in the bilateral MTC remained significant after applying the FDR correction for multiple comparisons (Table 2).
Table 2.
In secondary analyses, the unweighted estimated means for BPF derived from the mixed model for all regions in bipolar disordered versus healthy control samples for the females and males considered separately. Because these are estimated means calculated from the mixed model, standard errors are identical for all regions within group (male healthy: 3.36, BD: 4.18; female healthy: 2.17, BD: 2.52). MTC=mesiotemporal cortex; ACC=anterior cingulate cortex; PCC=posterior cingulate cortex; AI= anterior insula; POC=parieto-occipital cortex.
| BPF (units) | ||||
|---|---|---|---|---|
|
|
||||
| Healthy females | BD females | Healthy males | BD males | |
| L ACC | 44.03b | 36.65b | 36.34 | 37.65 |
| R ACC | 43.30c | 37.35c | 35.08 | 37.48 |
| L AI | 46.31 | 41.22 | 37.72 | 41.84 |
| R AI | 47.68b | 40.70b | 41.46 | 42.98 |
| L MTC | 73.94a | 61.92a | 58.85 | 68.25 |
| R MTC | 72.24a | 60.81a | 59.59 | 64.61 |
| L PC | 38.60 | 35.08 | 29.99 | 35.42 |
| R PC | 38.43 | 33.92 | 29.12 | 32.14 |
| L POC | 37.64 | 32.55 | 32.92 | 32.95 |
| R POC | 37.48 | 34.11 | 31.16 | 33.69 |
| Raphe | 31.01 | 30.76 | 22.74 | 28.61 |
Difference between groups significant at p<0.01.
Difference between groups significant at p<0.05.
Trend for significance at p<0.1.
BPND values showed significant main effects of region (F10,610 =124.3, p<0.001) and gender (F1,59 =7.576, p=0.008). There was also a significant gender-by-region interaction (F10,610 =6.05, p<0.001). Main effects of age (F1,59 =0.59, p=0.446) and diagnosis (F1,59 =0.151, p=0.666) were not significant.
4.2. Correlations between trough cortisol values, VT, and BPP
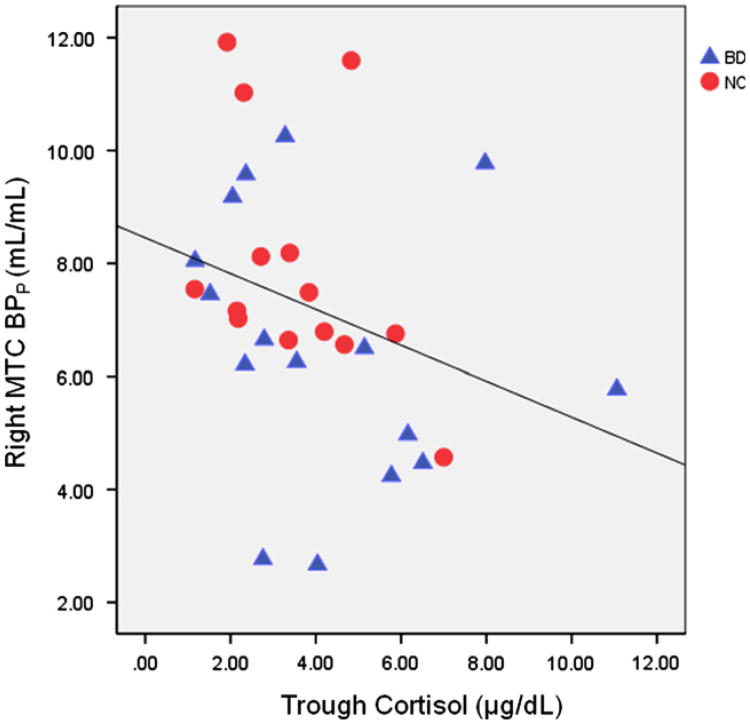
In the combined subject sample, trough cortisol values were significantly inversely correlated with right MTC BPP (rho=−0.457, p=0.011; see Figure 3) and VT (rho=−0.461, p=0.010). These findings remain significant after applying corrections for multiple comparisons for the left and right lateralized MTC regions. In addition, there were non-significant trends for inverse correlations between trough cortisol and left MTC BPP (rho=−0.338, p=0.067) and VT (rho =−0.347, p=0.060). Correlations were not significant in any other area, and did not reach statistical significance in either group alone.
Figure 3.
Right MTC BPP versus trough cortisol for healthy and BD subjects. Linear fit line is shown for illustrative purposes only.
5. Discussion
The post-synaptic 5-HT1A receptor VT and BPP were decreased significantly in the bilateral MTC, left ACC and right AI in unmedicated BD compared to healthy controls. The reduction in BPP in the MTC remained significant after applying corrections for multiple testing. Notably, the mean BPP and VT were nominally lower in the BD subjects versus the controls in every cortical ROI examined, so it remains unclear whether the abnormalities are regionally specific or reflect a widespread reduction in the 5-HT1A receptor system.
Although we did not find significant differences in BPND between patients and controls, this measure is highly sensitive to noise in the measurement of VND, which is typically an order of magnitude lower than VT in the regions of interest. Nevertheless, the difference in mean BPND values between diagnostic groups trended in the same direction as the BPP and VT findings, with model-adjusted means showing decreases in BD compared to healthy controls by 4.6% and 7% in left and right MTC, respectively. Consistent with our BPF findings, these reductions were more pronounced in female subjects alone (BPND was 5.6% and 8.5% lower in left and right MTC, respectively).
Taken together, these findings are potentially compatible with an earlier finding of reduced BPND in the MTC in subjects with BD (Drevets et al., 1999, 2007), and a report of reduced BPND in the MTC, ACC and OFC, but not the raphe, in 9 unmedicated women with post-partum depression (of whom 4 had BD) versus non-depressed post-partum controls (Moses-Kolko et al., 2007). The cortical binding of 5-HT1A is accounted for exclusively by post-synaptic 5-HT1A receptors, while binding in the raphe is attributable predominately to pre-synaptic 5-HT1A receptors, suggesting that the pathophysiology of BD particularly affects the post-synaptic system.
Our finding that BPF is reduced in the MTC and ACC in unmedicated females, but not unmedicated males, with BD partly conflicts with the report of Sullivan et al. (2009), who reported increased 5-HT1A BPF in males with BD and no significant difference in females with BD relative to controls using [carbonyl-11C]WAY-100635. However, it can be inferred from the data presented in that report that the female BD subjects (N=6) had nominally (and non-significantly) lower BPF values than control females, similar in direction to our findings (Sullivan et al., 2009)
The differences between these two studies may also be explained by differences in the measurement of fp. Sullivan et al. (2009) reported that the fp of [11C]WAY-100635 was significantly higher in controls than in BD subjects (by 37%), whereas we found no difference between groups in the fp for [18F]FCWAY (p=0.551). It remains unclear why the fp of [11C]WAY-100635 would be abnormal in BD, but it is noteworthy that the fp value is technically difficult to measure for [carbonyl-11C]WAY-100635. Crucially Sullivan et al. (2009), reported that their secondary outcome measure, BPND, did not differ significantly between the BD and control groups, but was nominally lower by 5.1-13.9% across the ROIs in the bipolar group, similar in magnitude and direction to the differences we found in our sample of BD subjects versus controls.
The interpretation of the abnormal reduction in 5-HT1A receptor VT and BP we identified in depressed BD could reflect changes in either the density or the affinity of 5-HT1A receptors. The affinity of [18F]FCWAY for the 5-HT1A receptor is similar to that of [carbonyl-11C]WAY-100635, and neither radioligand demonstrates sensitivity in vivo to acute changes in endogenous serotonin concentration (Hume et al., 2001; Lang et al., 1999). Thus it is unlikely that alterations in the endogenous serotonin concentration in BD subjects account for the abnormalities in [18F]FCWAY binding observed in our study. Based upon the results of post-mortem studies, our results most likely reflect a reduction in receptor expression, since the density and mRNA concentration of 5-HT1A receptors have been reduced in depressed subjects with BD and MDD (Lopez-Figueroa et al., 2004; Lopez et al., 1998; Stockmeier et al., 2009)
The preclinical literature suggested the hypothesis that post-synaptic 5-HT1A receptor expression would be reduced in subjects who hypersecrete cortisol (Lopez et al., 1998; Meijer et al., 1997). The gene expression of post-synaptic 5-HT1A receptor levels in the rodent hippocampus is down-regulated by stimulation of mineralocorticoid and glucocorticoid receptors (Drevets et al., 2007; Lopez et al., 1998). In our previous study we found reduced 5-HT1A receptor BPND in a depressed sample composed of both MDD and BD subjects who showed significantly elevated stressed plasma cortisol levels (Drevets et al., 1999). In the current study, we targeted the circadian nadir of cortisol secretion as a sensitive indicator of hypercortisolemia, since these measures reflect the flattened profile of the diurnal rhythm of glucocorticoid secretion extant in mood disorders (Deuschle et al., 1997) which also had been associated with reduced 5-HT1A receptor function in rodent models (Meijer et al., 1997). The relationships we observed between trough cortisol levels and mesiotemporal cortical 5HT1A receptor VT and BPP also are noteworthy in light of recent evidence that treatment with mood stabilizing agents that reduce glucocorticoid signaling is associated with increases in the 5HT1A receptor binding in this region. Although our results should be considered preliminary, this study is the first to report an inverse correlation between 5-HT1A receptor VT or BPP and trough cortisol levels in humans.
In contrast, two previous studies assessing the effects of glucocorticoids on 5-HT1A receptor BPND using PET and [11C]WAY-100635 in non-depressed subjects showed no change in BPND 12 h after administering hydrocortisone 100 mg to healthy humans (Bhagwagar et al., 2003) and no reduction in 5-HT1A receptor BPND in medically ill subjects receiving prednisolone >7.5 mg (Montgomery et al., 2001). However, the first study afforded limited sensitivity for detecting an effect on 5-HT1A receptor BPND, because changes in the 5-HT1A receptor protein would not be evident until 2 to 4 days later (Lopez et al., 1998). Furthermore, the results of the second study were difficult to interpret because prednisolone predominantly stimulates glucocorticoid receptors, yet stimulation of mineralocorticoid receptors exerts the most prominent effect on 5-HT1A receptor mRNA expression (Meijer and de Kloet, 1995).
Several limitations of the methods merit comment. We used [18F]FCWAY rather than [carbonyl-11C]WAY100635 because the latter is slow to reach equilibrium in regions with high specific binding, yet the reliability of blood metabolite data acquired during later portions of the scan is poor due to the rapid clearance of [carbonyl-11C]WAY100635 from plasma and the short half-life of 11C (20.4 min) (Gunn et al., 1998). Both radioligands have a radiolabeled metabolite that enters brain, which is addressed in the tracer kinetic model we applied for the analysis of [18F]FCWAY, but generally is not included in the model for studies of [carbonyl-11C]WAY100635. Nevertheless, [18F]FCWAY imaging has the significant limitation that the uptake of the secondary metabolite, [18F]fluoride, into the skull confounds measures of VT obtained in brain structures situated near the cortical surface. Because the MTC region in which we found significant group differences was situated more than twice the FWHM spatial resolution from the skull, it is unlikely that this result could have been influenced by any potential group difference in the spillover from skull uptake of [18F]fluoride. Additionally, in the cortical structures assessed herein, PET-[18F]FCWAY has been validated using 5-HT1A receptor antagonist pre-blocking (Carson et al., 2000) and demonstrates a rank order of regional 5-HT1A receptor BPP values that matches that of [3H]WAY-100635 binding measured autoradio-graphically in human brain tissue (Hall et al., 1997). In addition, there is the limitation of an appropriate reference region for the calculation of binding potential. An appropriate reference region would be required to have low specific binding, and similar free and non-specific binding to the target region. Cerebellar white matter has previously been shown to be devoid of 5-HT1A receptors, although differences in free and non-specific binding as well as potential lack of similarity in the K1/k2 ratio between target gray matter regions and the reference white matter region cannot fully be discounted. Limitations of this technique, in particular regarding the use of a cerebellar reference region, have been discussed in significant depth elsewhere (Shrestha et al., 2012). Nevertheless, our primary finding of reduced VT and BPP is supported by both the majority of published literature, and by our finding (in females) of reduced BPF, which has been advocated as the method of normalization by Shrestha et al. (2012).
Finally, the generalizability of our results in the BD population remains unclear. Our sample was too small to characterize the effects of clinical heterogeneity on BPP. A post-hoc analysis limited to the BDII subjects resulted in similar results as those found for the entire BD sample, albeit with lower significance values (see the Supplementary results for details). In order to address the influence of anxiety symptoms, we performed a post-hoc correlation between HAM-A anxiety score and VT and binding in the left and right MTC and found no significant relationships.
In summary, our findings indicate that, similar to the findings of post-mortem studies and of most PET studies, BD also is associated with reductions in the postsynaptic 5-HT1A receptor binding, particularly in the MTC. Nevertheless, our secondary analyses involving the BPF parameter raised the possibility that this abnormality may be more pronounced in, or possibly limited to females with BD, a hypothesis that requires testing in future studies involving larger samples of BD and control males. Finally, the demonstration that the 5-HT1A receptor binding in the MTC correlates inversely with trough cortisol levels suggests that the abnormal reduction in postsynaptic 5-HT1A receptor binding particularly affects BD subjects who hypersecrete cortisol, and thus provides an important translational bridge to the preclinical literature (Lopez et al., 1998; Meijer et al., 1997).
Supplementary Material
Acknowledgments
We appreciate the excellent assistance of Joan Williams and Michele Drevets for assistance with subject recruitment and evaluation, and the PET technicians for technical support during the PET studies. We also appreciate the assistance of the NIH Clinical Center Department of Anesthesiology for placement of arterial catheters.
Role of funding source: This research was supported by the Intramural Research Program of the NIMH, under which the first and senior authors were employed at the time of all data collection, and under which the first author is currently employed.
Footnotes
Contributors: Allison C. Nugent participated in the methods development, performed the statistical analysis, participated in the interpretation of results, and wrote the manuscript. Earle E. Bain and Paul J. Carlson performed the data collection of the most subjects; Paul J. Carlson also participated in the data analysis. Alexander Neumeister and Omer Bonne performed the additional data collection for the remainder of the sample. Richard Carson designed the PET modeling methods and performed the tracer kinetic modeling and partial volume correction of the PET data. William Eckelmann developed the tracer; both Drs. Carson and Eckelmann served as advisors on the study and methods design. Peter Herscovitch aided in the study design and methods. Carlos A. Zarate Jr. provided clinical support for the patients and participated in the recruitment and evaluation of the research participants. Dennis S. Charney aided in the study design. Wayne C. Drevets designed and wrote the study protocol, oversaw all aspects of the study, and aided in the preparation of the manuscript.
Conflict of interest: Dr. Zarate is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression. Dr. Zarate has assigned his rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government. Dr. Drevets is named as co-inventors on the use patent application, “Scopolamine in the Treatment of Depression” that is pending. Dr. Drevets has assigned his rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government. Dr. Drevets is currently a full-time employee of Johnson and Johnson. The authors have no other conflicts of interest to report.
Appendix A. Supplementary materials: Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.euroneuro.2012.11.005.
References
- Bhagwagar Z, Montgomery AJ, Grasby PM, Cowen PJ. Lack of effect of a single dose of hydrocortisone on serotonin(1A) receptors in recovered depressed patients measured by positron emission tomography with [11C]way-100635. Biol Psychiatry. 2003;54(9):890–895. doi: 10.1016/s0006-3223(03)00466-9. [DOI] [PubMed] [Google Scholar]
- Blier P, Bergeron R, de Montigny C. Selective activation of postsynaptic 5-HT1A receptors induces rapid antidepressant response. Neuropsychopharmacology. 1997;16(5):333–338. doi: 10.1016/S0893-133X(96)00242-4. [DOI] [PubMed] [Google Scholar]
- Carson RE, Lang L, Watabe H, Der MG, Adams HR, Jagoda E, Herscovitch P, Eckelman WC. PET evaluation of [(18)F]FCWAY, an analog of the 5-HT(1A) receptor antagonist, WAY-100635. Nucl Med Biol. 2000;27(5):493–497. doi: 10.1016/s0969-8051(00)00118-9. [DOI] [PubMed] [Google Scholar]
- Deuschle M, Schweiger U, Weber B, Gotthardt U, Korner A, Schmider J, Standhardt H, Lammers CH, Heuser I. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab. 1997;82(1):234–238. doi: 10.1210/jcem.82.1.3689. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. Pet imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46(10):1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, Mathis C. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34(7):865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York State Psychiatric Institute, Biometrics Research; New York, NY: 2002. [Google Scholar]
- Gray L, Scarr E, Dean B. Serotonin 1A receptor and associated g-protein activation in schizophrenia and bipolar disorder. Psychiatry Res. 2006;143(2-3):111–120. doi: 10.1016/j.psychres.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Sargent PA, Bench CJ, Rabiner EA, Osman S, Pike VW, Hume SP, Grasby PM, Lammertsma AA. Tracer kinetic modeling of the 5-HT1A receptor ligand [carbonyl-11C]WAY-100635 for PET. Neuroimage. 1998;8(4):426–440. doi: 10.1006/nimg.1998.0379. [DOI] [PubMed] [Google Scholar]
- Hall H, Lundkvist C, Halldin C, Farde L, Pike VW, McCarron JA, Fletcher A, Cliffe IA, Barf T, Wikstrom H, Sedvall G. Autoradiographic localization of 5-HT1A receptors in the postmortem human brain using [3H]WAY-100635 and [11C]WAY-100635. Brain Res. 1997;745(1-2):96–108. doi: 10.1016/s0006-8993(96)01131-6. [DOI] [PubMed] [Google Scholar]
- Hume S, Hirani E, Opacka-Juffry J, Myers R, Townsend C, Pike V, Grasby P. Effect of 5-ht on binding of [(11)C] WAY 100635 to 5-HT(iA) receptors in rat brain, assessed using in vivo microdialysis nd pet after fenfluramine. Synapse. 2001;41(2):150–159. doi: 10.1002/syn.1069. [DOI] [PubMed] [Google Scholar]
- Lang L, Jagoda E, Schmall B, Vuong BK, Adams HR, Nelson DL, Carson RE, Eckelman WC. Development of fluorine-18-labeled 5-HT1A antagonists. J Med Chem. 1999;42(9):1576–1586. doi: 10.1021/jm980456f. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, Ou XM, Albert PR. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23(25):8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Figueroa AL, Norton CS, Lopez-Figueroa MO, Armellini-Dodel D, Burke S, Akil H, Lopez JF, Watson SJ. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mrna expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol Psychiatry. 2004;55(3):225–233. doi: 10.1016/j.biopsych.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Little KY, Watson SJ. A.E. Bennett research award. Regulation of serotonin1a, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry. 1998;43(8):547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Meijer OC, de Kloet ER. A role for the mineralocorticoid receptor in a rapid and transient suppression of hippocampal 5-HT1A receptor mRNA by corticosterone. J Neuroendocrinol. 1995;7(8):653–657. doi: 10.1111/j.1365-2826.1995.tb00804.x. [DOI] [PubMed] [Google Scholar]
- Meijer OC, Van Oosten RV, De Kloet ER. Elevated basal trough levels of corticosterone suppress hippocampal 5-hydroxytryptamine(1A) receptor expression in adrenally intact rats: implication for the pathogenesis of depression. Neuroscience. 1997;80(2):419–426. doi: 10.1016/s0306-4522(97)00008-0. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, Bench CJ, Young AH, Hammers A, Gunn RN, Bhagwagar Z, Grasby PM. PET measurement of the influence of corticosteroids on serotonin-1A receptor number. Biol Psychiatry. 2001;50(9):668–676. doi: 10.1016/s0006-3223(01)01205-7. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Price JC, Thase ME, Meltzer CC, Kupfer DJ, Mathis CA, Bogers WD, Berman SR, Houck PR, Schneider TN, Drevets WC. Measurement of 5-HT1A receptor binding in depressed adults before and after antidepressant drug treatment using positron emission tomography and [C-11]WAY-100635. Synapse. 2007;61(7):523–530. doi: 10.1002/syn.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, Eckelman W, Herscovitch P, Charney DS, Drevets WC. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24(3):589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Arango V, Olvet DM, Oquendo MA, Van Heertum RL, John Mann J. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 2005;25(7):785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57(2):174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Rabiner EA, Bhagwagar Z, Clark L, Cowen P, Goodwin GM, Grasby PM. 5-HT(1A) receptor binding in euthymic bipolar patients using positron emission tomography with [carbonyl-(11)C]WAY-100635. J Affect Disord. 2010;123(1-3):77–80. doi: 10.1016/j.jad.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 2009;88(1):17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, Hirvonen J, Hines CS, Henter ID, Svenningsson P, Pike VW, Innis RB. Serotonin-1A receptors in major depression quantified using pet: controversies, confounds, and recommendations. Neuroimage. 2012;59(4):3243–3251. doi: 10.1016/j.neuroimage.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA, Howley E, Shi XC, Sobanska A, Clarke G, Friedman L, Rajkowska G. Antagonist but not agonist labeling of serotonin-1A receptors is decreased in major depressive disorder. J Psychiatr Res. 2009;43(10):887–894. doi: 10.1016/j.jpsychires.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Ogden RT, Oquendo MA, Kumar JS, Simpson N, Huang YY, Mann JJ, Parsey RV. Positron emission tomography quantification of serotonin-1A receptor binding in medication-free bipolar depression. Biol Psychiatry. 2009;66(3):223–230. doi: 10.1016/j.biopsych.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden markov random field model and the expectation maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.