Abstract
Human alphaherpesviruses (αHHV) – herpes simplex virus type 1 (HSV-1), HSV-2, and varicella-zoster virus – infect mucosal epithelial cells, establish a lifelong latent infection of sensory neurons, and reactivate intermittingly to cause recrudescent disease. Although chronic αHHV infections co-exist with brisk T-cell responses, T-cell immune suppression is associated with worsened recurrent infection. Induction of αHHV-specific T-cell immunity is complex and results in poly-specific CD4 and CD8 T-cell responses in peripheral blood. Specific T-cells are localized to ganglia during the chronic phase of HSV infection and to several infected areas during recurrences, and persist long after viral clearance. These recent advances hold promise in the design of new vaccine candidates.
Introduction
Herpes simplex virus type 1 (HSV-1), HSV-2 and varicella-zoster virus (VZV) are closely related human neurotropic alphaherpesviruses (αHHV) that are endemic worldwide. Primary αHHV infections are acquired through the orofacial (HSV-1) and genital epithelia (HSV-2), albeit HSV-1 is a prominent cause of genital herpes in some settings, or via aerosols (VZV) [1,2]. Upon primary infection, αHHV establish a latent infection in sensory ganglion neurons, allowing the virus to reactivate and cause recrudescent disease [1,2]. Although frequently asymptomatic, HSV-1 and HSV-2 infections may cause herpes labialis (cold sores) and genital herpes [1]. HSV reaches the ganglia via axonal transport thereby restricting HSV infections and recurrences – that arise from neuronal reactivation and axonal transport – to the same areas [1]. VZV causes varicella (chickenpox) as a primary infection and herpes zoster (HZ; shingles) upon reactivation [2]. Memory T-cells are postulated to traffic VZV during the viremic phase of primary infection to the skin and ganglia leading to widespread cutaneous varicella lesions and neuronal latency [2]. HZ results from VZV reactivation and axonal transport to skin causing dermatomal skin rash, frequently followed by post-herpetic neuralgia (PHN) [2]. Adaptive immunity is pivotal for uncomplicated recovery from αHHV infection, illustrated by severe complications observed in immunocompromised individuals [1,2]. A live-attenuated VZV vaccine has been licensed for both preventive (varicella) and immunotherapeutic (HZ) use [2]. However, there is no vaccine for prevention or treatment of HSV-1 or HSV-2. Recent advances in the field have shed new light on the priming, specificity, and function of αHHV-specific T-cells. This review will summarize and discuss current understanding on the role of T-cell immunity in αHHV infections, with an emphasis on the virus’ natural human host.
HSV-1 and HSV-2
Most of our current understanding αHHV-specific T-cell immunity is based on HSV-1 and HSV-2, largely due to their recurrent nature and availability of small animal models that recapitulate some aspects of human HSV infection. While HSV-1 and HSV-2 are antigenically and biologically distinct and have separate disease profiles in humans, specific immune responses directed at them are generally similar and partially overlap at both the B- and T-cell level [3,4], such that data are largely interwoven and summarized rather than separated in this review.
HSV-induced priming of naïve T-cells during primary infection has only been studied in mice. During primary HSV infection, antigen is acquired by mobile, but uninfected, dendritic cells (DC) and transported from epithelial sites to draining lymph nodes [5,6]. Handoff to lymph-node resident DCs may occur prior to contact with naïve CD8 T-cells [7] and different DC may prime CD4 and CD8 T-cells [8,9]. Depending on kinetics, classic dermal CD8α+ DC or CD8α+CD103+ DC may dominate CD8 T-cell priming [8,10]. DC may mediate antigen uptake by the receptor DNGR1 and by means of toll-like receptor 3, DC may sense HSV as a danger signal to facilitate cross-priming [11,12]. Autophagy is involved in priming of CD4 T-cells [13]. DC are variably permissive for HSV infection in vitro [14], and immune evasion molecules encoded by HSV inhibit direct antigen presentation by virus-infected DC [10]. For example, the γ34.5 protein may inhibit antigen presentation via disruption of autophagy [15], and the vhs protein encoded by UL41 counteracts NFκB translocation [16]. To date, however, little evidence implicates direct infection of DC as an important component of pathogenesis or immunity in vivo. Adequate priming of naïve HSV-specific CD8 T-cells during primary infection likely depends on cognate virus-specific CD4 T-cells acting via licensure of DCs [17]. HSV-specific CD4 T-cells may also be necessary for the transition of primed CD8 T-cells into memory T-cells [17] and may prolong DC survival during CD8 T-cell priming [18].
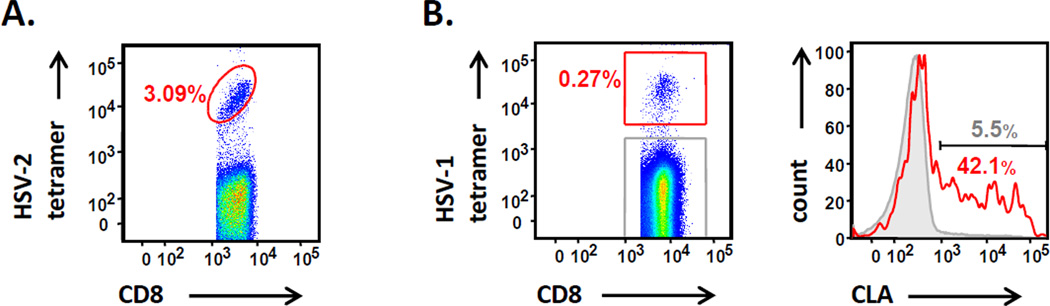
The kinetics and specificity of HSV-specific T-cells during primary infection are largely unknown in humans. After resolution of acute infection, HSV-specific memory T-cells are detected at moderate levels of 0.1 – 1% in blood of immunocompetent individuals [19,20]. Exceptionally, single HSV-2 peptide may be recognized by up to 3% of blood CD8 T-cells; the relationship of such expansions to disease severity is unknown (Figure 1A). Recently, we have defined the specificity of peripheral blood HSV-1-specific CD4 and CD8 T-cells towards 77 distinct proteins in healthy individuals [19,21]. The emerging picture is of a complex, poly-specific CD4 and CD8 T-cell response with recognition of a median of 22 and 17 distinct HSV-1 proteins, respectively. T-cell targets include HSV proteins present in large quantities in the virion (e.g., viral envelope, tegument, capsid, and regulatory proteins), as well as enzymes [19,21]. We find striking immunodominance of HSV-1 glycoprotein or regulatory protein-reactive CD4 T-cells, with rapid tapering to a large number of low-abundance responses recognizing many viral proteins. Interestingly, this HSV-specific CD4 T-cell immunodominance profile is mathematically identical to that observed for vaccinia virus (VV) [21], which causes an acute viral infection and is completely cleared from the host, in contrast to αHHV that cause chronic infections [21]. Notably, HSV proteins encoded by genes UL39 (an enzyme) and UL46 (a tegument protein) are prominent CD4 T-cell targets, equivalent to the vaccine candidates glycoprotein B (gB) and gD in terms of population prevalence [19]. For HSV-specific CD8 T-cells, typically only a few fine specificities are detectable either using direct ex vivo ELISPOT [19] or after enrichment by selection of CLA-positive T-cells [22]. Immunodominant CD8 T-cell responses occur to viral tegument and capsid proteins [4,23]. HSV-2-specific CD8 T-cells in herpetic skin lesions are predominately directed to tegument proteins and confirm the antigenicity of immediate early (IE) proteins and glycoproteins deduced from studies using blood [24,25]. CD8 T-cells recognizing tegument protein VP22 have public T-cell receptor αβ (TCRαβ) pairings [26], and HSV-specific CD8 clonotypes, defined by TCR sequencing, appear to be long-lasting and traffic from blood to cervix and skin [27]. HSV-specific CD4 T-cells are typically multifunctional for Th1/Th0-like cytokines and, after expansion, have brisk cytolytic potential [3,20]. Blood HSV-specific CD8 T-cells can express high levels of cytolytic molecules and secrete IFN-γ upon antigenic recall [4]. Counterbalancing roles for regulatory T-cells or inhibitory receptors such as PD-1 remain largely unexplored in humans [28] but can have profound effects in mice [29].
Figure 1.
HSV-specific CD8 T-cells in PBMC of HSV-seropositive immunocompetent individuals. (A) Cells specific for amino acids 49–57 of HSV-2 gene UL49 and restricted by HLA B*0702 can represent up to 3.09% of circulating CD8 T-cells. (B) CD8 T-cells specific for amino acids 90–99 of HSV-1 gene UL48, restricted by HLA A*0101, over-express CLA (red histogram) compared to the remaining CD8 T-cells (grey histogram).
HSV-specific T-cells profoundly localize to sites of primary and recurrent infection such as skin, cervix and eye, and chronic latent infection in TG. Recurrent HSV infections result in 10 – 40% of CD8 T-cells being HSV-specific in herpetic eye disease and skin at crust stage [24,30,31]. Oligoclonal virus-specific CD4 and CD8 T-cells, mostly recognizing tegument proteins (including the UL46 and UL47 products) were detected in the HSV-affected eye [30–33]. HSV-specific cytotoxic CD8 T-cells accumulate near sensory nerve endings in HSV-2-infected human skin biopsies [34,35]. Murine data support and extend the human observations, with HSV-specific CD8 T-cells localizing to infected skin and to latently infected ganglia [36–37]. Tissue persistence of HSV-specific CD8 T-cells in murine skin after viral clearance correlated with local protection from exogenous re-inoculation [36]. HSV-specific CD8 T-cells retained in murine skin continuously patrol the epidermis and recognize rare antigen-expressing keratinocytes [38]. In humans, CD8 T-cells show prolonged localization to the dermo-epidermal junction after HSV-2 healing and have an activated and antiviral phenotype [39,40]. Like CD8 T-cells, HSV-2-specific CD4 T-cells also display the tissue-resident memory (TRM) phenotype, persistently localizing to sites of previously infected recurrent skin infection in humans [40]. In the uterine cervix, HSV-2-specific CD4 T-cells are abundant even when the virus is suppressed with antivirals [23]. Both HSV-2-specific CD4 and CD8 T-cells preferentially express CLA, and its ligand E-selectin is up-regulated in herpetic skin [23,41]. Recent data with HSV-1- specific tetramers show that HSV-1-reactive CD8 T-cells in blood also express this homing receptor (Figure 1B). It is unclear if this adhesion molecule is important in inflammatory homing at times of overt HSV recurrence or is also involved in local T-cell retention. Taken together, these and data from other viral systems [42] are consistent with a TRM phenotype as a specialized subset of memory T-cells, and current efforts with vaccines seek to optimize the generation and guidance of these cells to the preferred anatomic site: the virus’ port d’entree [43].
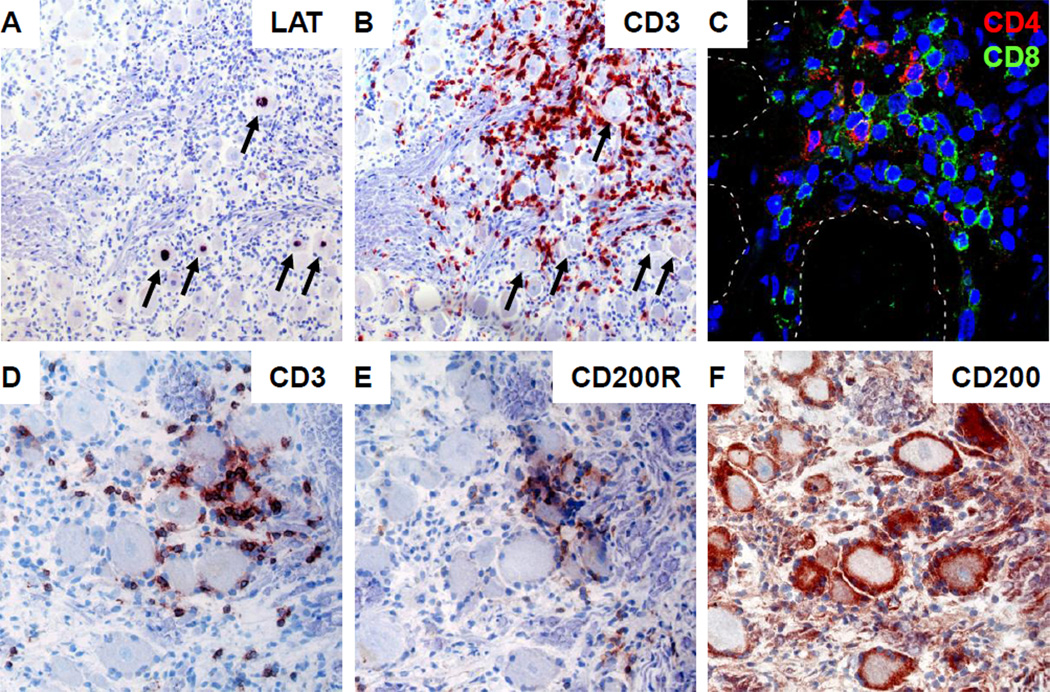
HSV-1, but not VZV, infection of human trigeminal ganglia (TG) is associated with chronic inflammation [44–45]. Ganglion-infiltrating T-cells are oligoclonal in nature and preferentially recognize HSV-1 [45–46]. These CD8 T-cells have an activated late-effector memory phenotype and form rosettes around neurons including those that express HSV-1 latency-associated transcript (Figure 2A–B). CD4 T-cells are less abundant than CD8 T-cells and appear both in neuron-surrounding T-cell coronas as well as along axons (Figure 2C) [45–46]. Neuron-surrounding satellite glial cells (SGC) function as TG-resident antigen presenting cells and express the T-cell inhibitory molecules HLA-E and PD-L1 that interact with their receptors CD94/NKG2A and PD-1 on ganglionic T-cells to control HSV latency without neuronal damage [47]. In addition, we recently demonstrated the potential role of the T-cell inhibitory CD200/CD200R axis in human TG: TG-resident SGC expressed CD200 and the interacting T-cells expressed the cognate receptor CD200R (Figure 2D–F). The human intra-TG HSV-specific T-cell response, involving both CD4 and CD8 T-cells, is directed to a limited set of viral antigens (Van Velzen et al., abstract 5.05, International Herpesvirus Workshop, Calgary, August 2012). The current data suggest that persistent local HSV-specific T-cell responses control viral latency and reactivation in ganglia and skin, respectively. Alternatively, rather than recognizing latently infected neurons, ganglionic virus-specific T-cells may be “mopping-up” an HSV reactivation that has bypassed innate immune control by SGC. Murine studies demonstrate that deposition [48] or attraction [49] of antigen-specific T-cells at anatomic sites of challenge protect from exogenous infection, but it is possible that ganglion-resident T-cells also serve as sentinels to modulate and limit endogenous reactivation or resultant centripetal spread, epithelial shedding, recrudescent lesions and transmission.
Figure 2.
Clusters of CD4 and CD8 T-cells surround HSV-1 LAT-positive neurons in human TG. (A, B) Consecutive TG sections were stained for HSV-1 LAT by in situ hybdrization (A) and CD3 by immunohistochemistry (B), showing that T-cell clusters surround HSV-1 LAT+ neurons. Arrows indicate LAT+ neurons. (C) Human TG immunofluorescently stained for CD4 (red) and CD8 (green, showing that ganglionic T-cell clusters are composed of both CD4 and CD8 T-cells. Dashed lines indicate neurons. Nuclei were counterstained with 4’,6-diamino-2-phenylindole. (D–F) Consecutive TG sections were stained for CD3 (D), CD200R (E) and CD200 (F) by immunohistochemistry. Ganglion-infiltrating T-cells express CD200R, whereas neuron-interacting satellite glial cells express its ligand CD200. (A, B, D–F) Sections were developed with 5-bromo-4-chloro-3-indolyl-phosphate (LAT; black color) and 3-amino-9-ethylcarbazole chromogen (CD3, CD69, CD200R and CD200; red color); nuclei were counterstained with hematoxylin. Magnification: (A, B) 100X, (C) 400X, (D–F) 200X.
VZV
Compared to HSV, VZV is human-restricted pathogen. Simian varicella virus (SVV) infection of non-human primates closely resembles VZV pathogenesis in humans and is currently being used to define T-cell immunity in its natural host. VZV-specific T-cells are considered essential for recovery from varicella and for prevention of and resurgence from HZ. Varicella has an incubation period of 10–21 days [2] and VZV-specific T-cells become detectable 1 to 3 days after the appearance of skin rash [50]. The site and cells involved in priming of naïve T-cells are unknown. VZV decreases the capacity of DC to induce specific T-cell responses by reducing surface expression of both T-cell co-stimulatory molecules (i.e. CD80, CD83 and CD86) and human leukocyte antigen type I (HLA-I) and –II [51,52]. In addition, the virus reduces HLA-I expression and inhibits IFN-γ–induced HLA-II expression on VZV-infected cells to escape CD8 and CD4 T-cell recognition, respectively [53–56]. VZV hinders T-cell recognition of VZV-infected keratinocytes by inhibiting intercellular adhesion molecule 1 (ICAM-1) expression [57].
The magnitude of the VZV-specific T-cell response initiated during primary infection inversely correlates with the severity of varicella and termination of viremia [50,58]. Studies on SVV indicate that CD4 T-cell immunity plays a more critical role than antibody and CD8 T cell responses to control varicella [59]. Compared to HSV, studies on local tissue-restricted VZV-specific T-cells are scarce. Yet, both CD4 and CD8 T-cells are known to infiltrate the dermis of varicella and HZ patients [57,60–62], possibly relocating via skin-homing receptors [58]. The local VZV-specific T-cell response in patients with VZV-induced uveitis is more comprehensively characterized. Intra-ocular T-cells recognize a wide variety of VZV proteins, secrete Th1/Th0-like cytokines, have cytolytic potential, and are able to inhibit virus replication in retinal pigmented epithelial cells [63–65]. The combined data suggest a detrimental role of VZV-specific T-cells in the pathology of VZV uveitis.
Memory VZV-specific T-cells are maintained at low frequency [66], likely involving exogenous re-exposure to VZV or endogenous (subclinical) reactivation, and have a mixed central and effector memory phenotype [52,58,67,68]. Circulating VZV-specific memory CD4 T-cells are commonly CLA-negative and preferentially express CD38 and PD-1 [67,68], suggesting recent antigenic stimulation and/or exhaustion. Although similar frequencies of circulating VZV-specific CD4 and CD8 cytotoxic T-cells have been reported [69,70], recent studies indicate that VZV-specific CD8 T-cells are less abundant than CD4 T-cells [52,71,72]. T-cell reactivity to VZV antigens include structural proteins including the glycoproteins gB, gC, gE, gH, gI and the IE proteins IE4, IE62 and IE63 [67–70,72–77]. However, the latter studies were restricted to the analysis of only a limited set of virus proteins, which does not allow identification of the immunodominant T-cell antigens involved in protection and immunopathogenesis.
It is well established that the risk of HZ increases with age [78]. This is largely attributed to the decline of VZV-specific T-cell immunity, but not humoral immunity, with progressing age [79–81]. Notably, the frequency of IL-4 producing VZV-specific T-cells remains unaltered [52,73,80,82–86]. Whether waning of VZV-specific T-cell immunity reflects quantitative or qualitative changes in circulating virus-specific T-cells is unknown. Regardless, HZ induces rapid, profound and sustained T-cell responses [87,88], so that individuals typically develop zoster only once during their life. The magnitude of the VZV-specific T-cell immunity after HZ is inversely correlated with disease severity and the risk of developing PHN [88]. Despite their protective role in preventing reactivation, VZV-specific T-cells have not been detected in latently infected ganglia [45]. The data suggest that during VZV latency no viral proteins are expressed or that the proteins are not efficiently presented for T-cell recognition in situ [45,89,90]. During VZV and SVV reactivation, however, dense CD8 T-cell infiltrates have been detected in ganglia. The limited expression of granzyme B by infiltrating T-cells, combined with the overt expression of CXCL10, suggest a chemokine rather than an antigen-driven infiltration and accumulation of CD8 T-cells in reactivated ganglia [91–93].
Vaccines
The only human herpesvirus vaccine available to date is the live-attenuated VZV vaccine, which induces both B- and T-cell-mediated immune responses in vaccinated children and the elderly, thereby preventing varicella and HZ, respectively [94–97]. However, the current VZV vaccine protects only 80–87% and 51% of the vaccinees against varicella and HZ, respectively [96–98], indicating an unmet need for an improved VZV vaccine with a higher efficacy. Furthermore, the vaccine contains an attenuated virus that may cause mild varicella and establishes a latent infection posing the risk of reactivation and dissemination in the population. Full VZV genome-wide screens of the VZV-specific CD4 and CD8 T-cell repertoire, such as performed recently by our groups for HSV-1 [19,21], are warranted to decipher the immunodominance of VZV proteins targeted by circulating and lesion-infiltrating virus-specific T-cells. These studies will lead to rational choice of candidate virus proteins for a safe and efficacious VZV vaccine.
There is no licensed preventive or therapeutic HSV vaccine. Although variable formulations of HSV vaccines induced protective immunity in mice [99], success in the vaccine arena has yet to translate to humans. Earlier HSV-2 glycoprotein vaccines were not active to prevent primary infection despite antibody and CD4 T-cell immunogenicity [100,101]. Three trials with truncated HSV-2 gD adjuvanted with alum and 3'-O-deacylated-monophosphoryl lipid A showed contrasting results [102,103]. Initial studies suggested the vaccine was efficacious in women with no known prior HSV-1 or HSV-2 infection, but a confirmatory study (whose endpoints included both seroconversion and disease) showed only partial efficacy against HSV-1 but not HSV-2 in this population. Provision of multiple effector T-cell populations with diverse specificity seems attractive given recent data that the CD8 and CD4 T-cell response to natural infection with HSV-1 targets 17 and 22 proteins, respectively [19,21]. Replication-incompetent whole virus formats are advancing to clinical trials [104], while replication-competent candidates – which are highly active in challenge-escalation animal models – remain interesting if safety concerns can be overcome [105]. An inherently lower immunogenicity observed with replication-incompetent viruses relative to wild-type viruses can be overcome with additional vaccine doses, at least in the case of orthopoxviruses such as VV [106]. It remains to be seen if a similar strategy can be used for HSV. Therapeutic vaccination remains a goal as an alternative to small-molecule antivirals for the treatment of recurrent lesions and the reduction of transmission of HSV. While a replication-incompetent whole virus candidate was inactive in a phase 2b therapeutic clinical trial [107, a multi-peptide approach was recently shown to increase CD4 and CD8 T-cell responses in HSV-2 infected persons [108] and a multivalent subunit candidate is under evaluation (Skoberne et al., abstract 6.05, International Herpesvirus Workshop, Calgary, August 2012).
Conclusions
The T-cell responses to HSV and VZV have interesting similarities, but also striking differences. The development of a potent αHHV-specific T-cell immunity relies on multifaceted DC priming of T-cells and CD4/CD8 T-cell cooperation at several stages and anatomic sites, thus favoring vaccines that elicit complex T-cell, and likely also B-cell responses to generate long-lasting virus-neutralizing antibodies. We do not yet know if the same T-cell antigens that are immunodominant in naturally infected humans are best for vaccines, but population prevalence at least indicates the potential for T-cell responses and is a reasonable criteria for candidate subunit approaches. While poly-specific responses such as that elicited by the live-attenuated VZV vaccine are attractive, safety concerns persist and replication-incompetent viruses, or a multivalent subunit approach based on whole proteome-covering data sets, hold promise for both HSV, and for next-generation VZV vaccines that are safe enough for immunocompromised recipients. Apart from eliciting T-cell responses to viral proteins, the emerging picture for HSV is that one must also create a virus-specific TRM population in both the ganglia and in the orofacial and anogenital mucosa. In that regard, promising results of a recent study showed that TRM can be “pulled” to the site of vaginal challenge by priming with replication competent virus at a remote site and subsequently administering CXCR3 agonist chemokines vaginally [49]. Considering therapeutic vaccination, a more thorough understanding of the T-cell response to zoster vaccine in adults with regards to specificity, T-cell levels and homing, and new priming versus re-stimulation of memory, and for HSV, detailed searches for T-cell correlates of severity, may give clues to extending and improving the success of the existing attenuated VZV vaccine and extending it to HSV.
Highlights.
HSV T-cell immunity relies on multiple DC subsets and CD4/CD8 T-cell cooperation.
Diverse CD4/CD8 responses to HSV-1 are accessible with genome-spanning tools.
HSV-specific T-cells localize to mucosal epithelia during recurrent infections.
HSV-1-specific T-cells localize to ganglia during chronic latent infection.
VZV reactivation induces systemic and ganglionic T-cell responses.
Acknowledgements
The authors would like to thank Monique van Velzen and Angelique Poot for contributing examples of in situ analyses on HSV-1 latently infected human TG, and Lichun Dong for flow cytometry data for HSV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roizman B, Knipe D, Whitley R. Herpes simplex viruses. In: Knipe DM, Howley PM, editors. Fields virology. edn 5. Lippincott Williams & Wilkins; 2007. pp. 2501–2602. [Google Scholar]
- 2.Cohen JI, Straus SE, Arvin AM. Varicella-zoster virus replication, pathogenesis, and management. In: Knipe DM, Howley PM, editors. Field virology. edn 5. Lippincott Williams and Wilkins; 2007. pp. 2773–2818. [Google Scholar]
- 3.Koelle DM, Corey L, Burke RL, Eisenberg RJ, Cohen GH, Pichyangkura R, Triezenberg SJ. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J Virol. 1994;68:2803–2810. doi: 10.1128/jvi.68.5.2803-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laing KJ, Magaret AS, Mueller DE, Zhao L, Johnston C, De Rosa SC, Koelle DM, Wald A, Corey L. Diversity in CD8(+) T cell function and epitope breadth among persons with genital herpes. J Clin Immunol. 2010;30:703–722. doi: 10.1007/s10875-010-9441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 6.Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, Heath WR. Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- 7.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 9.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nopora K, Bernhard CA, Ried C, Castello AA, Murphy KM, Marconi P, Koszinowski U, Brocker T. MHC class I cross-presentation by dendritic cells counteracts viral immune evasion. Front Immunol. 2012;3:348. doi: 10.3389/fimmu.2012.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davey GM, Wojtasiak M, Proietto AI, Carbone FR, Heath WR, Bedoui S. Cutting edge: priming of CD8 T cell immunity to herpes simplex virus type 1 requires cognate TLR3 expression in vivo. J Immunol. 2010;184:2243–2246. doi: 10.4049/jimmunol.0903013. [DOI] [PubMed] [Google Scholar]
- 12.Zelenay S, Keller AM, Whitney PG, Schraml BU, Deddouche S, Rogers NC, Schulz O, Sancho D, Reis e Sousa C. The dendritic cell receptor DNGR-1 controls endocytic handling of necrotic cell antigens to favor cross-priming of CTLs in virus-infected mice. J Clin Invest. 2012;122:1615–1627. doi: 10.1172/JCI60644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HK, Mattei LM, Steinberg BE, Alberts P, Lee YH, Chervonsky A, Mizushima N, Grinstein S, Iwasaki A. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2010;32:227–239. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosnjak L, Miranda-Saksena M, Koelle DM, Boadle RA, Jones CA, Cunningham AL. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol. 2005;174:2220–2227. doi: 10.4049/jimmunol.174.4.2220. [DOI] [PubMed] [Google Scholar]
- 15.Gobeil PAM, Leib DA. Herpes simplex virus γ34.5 interferes with autophagosome maturation and antigen presentation in dendritic cells. mBio. 2012;3:e00267-12. doi: 10.1128/mBio.00267-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotter CR, Kim WK, Nguyen ML, Yount JS, López CB, Blaho JA, Moran TM. The virion host shutoff protein of herpes simplex virus 1 blocks the replication-independent activation of NF-κB in dendritic cells in the absence of type I interferon signaling. J Virol. 2011;85:12662–12672. doi: 10.1128/JVI.05557-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith CM, Wilson NS, Waithman J, Villadangos JA, Carbone FR, Heath WR, Belz GT. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 18.Mueller SN, Jones CM, Stock AT, Suter M, Heath WR, Carbone FR. CD4+ T cells can protect APC from CTL-mediated elimination. J Immunol. 2006;176:7379–7384. doi: 10.4049/jimmunol.176.12.7379. [DOI] [PubMed] [Google Scholar]
- **. Jing L, Haas J, Chong TM, Bruckner JJ, Dann GC, Dong L, Marshak JO, McClurkan CL, Yamamoto TN, Bailer SM, et al. Cross-presentation and genome-wide screening reveal candidate T cells antigens for a herpes simplex virus type 1 vaccine. J Clin Invest. 2012;122:654–673. doi: 10.1172/JCI60556. This is the first study to address the HSV-1 proteome-wide CD4 and CD8 T-cell responses in peripheral blood of healthy HSV-1-seropositive individuals. Several HSV-1 proteins were targeted by both CD4 and CD8 T-cells in most persons.
- 20.Moss NJ, Magaret A, Laing KJ, Kask AS, Wang M, Mark KE, Schiffer JT, Wald A, Koelle DM. Peripheral blood CD4 T-cell and plasmacytoid dendritic cell (pDC) reactivity to herpes simplex virus 2 and pDC number do not correlate with the clinical or virologic severity of recurrent genital herpes. J Virol. 2012;86:9952–9963. doi: 10.1128/JVI.00829-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jing L, Schiffer JT, Chong TM, Bruckner JJ, Davies DH, Felgner PL, Haas J, Wald A, Verjans GM, Koelle DM. CD4 T-cell memory to viral infections of humans shows pronounced immunodominance independent of duration or viral persistence. J Virol. 2013;87:2617–2627. doi: 10.1128/JVI.03047-12. The authors demonstrated that there are HSV antigens that drive numerically dominant CD4 T-cell specificities.
- 22.Koelle DM, Liu Z, McClurkan CL, Cevallos RC, Vieira J, Hosken NA, Meseda CA, Snow DC, Wald A, Corey L. Immunodominance among herpes simplex virus-specific CD8 T cells expressing a tissue-specific homing receptor. Proc Natl Acad Sci U S A. 2003;100:12899–12904. doi: 10.1073/pnas.2131705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koelle DM, Liu Z, McClurkan CM, Topp MS, Riddell SR, Pamer EG, Johnson AS, Wald A, Corey L. Expression of cutaneous lymphocyte-associated antigen by CD8(+) T cells specific for a skin-tropic virus. J Clin Invest. 2002;110:537–548. doi: 10.1172/JCI15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koelle DM, Chen HB, Gavin MA, Wald A, Kwok WW, Corey L. CD8 CTL from genital herpes simplex lesions: recognition of viral tegument and immediate early proteins and lysis of infected cutaneous cells. J Immunol. 2001;166:4049–4058. doi: 10.4049/jimmunol.166.6.4049. [DOI] [PubMed] [Google Scholar]
- 25.Mikloska Z, Ruckholdt M, Ghadiminejad I, Dunckley H, Denis M, Cunningham AL. Monophosphoryl lipid A and QS21 increase CD8 T lymphocyte cytotoxicity to herpes simplex virus-2 infected cell proteins 4 and 27 through IFN-gamma and IL-12 production. J Immunol. 2000;164:5167–5176. doi: 10.4049/jimmunol.164.10.5167. [DOI] [PubMed] [Google Scholar]
- 26.Dong L, Li P, Oenema T, McClurkan CL, Koelle DM. Public TCR use by herpes simplex virus-2-specific human CD8 CTLs. J Immunol. 2010;184:3063–3071. doi: 10.4049/jimmunol.0903622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Posavad CM, Huang ML, Barcy S, Koelle DM, Corey L. Long term persistence of herpes simplex virus-specific CD8+ CTL in persons with frequently recurring genital herpes. J Immunol. 2000;165:1146–1152. doi: 10.4049/jimmunol.165.2.1146. [DOI] [PubMed] [Google Scholar]
- 28.Diaz GA, Koelle DM. Human CD4+ CD25 high cells suppress proliferative memory lymphocyte responses to herpes simplex virus type 2. J Virol. 2006;80:8271–8273. doi: 10.1128/JVI.00656-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verjans GM, Feron EJ, Dings ME, Cornelissen JG, Van der Lelij A, Baarsma GS, Osterhaus AD. T cells specific for the triggering virus infiltrate the eye in patients with herpes simplex virus-mediated acute retinal necrosis. J Infect Dis. 1998;178:27–34. doi: 10.1086/515586. [DOI] [PubMed] [Google Scholar]
- 31.Verjans GM, Remeijer L, van Binnendijk RS, Cornelissen JG, Volker-Dieben HJ, Baarsma SG, Osterhaus AD. Identification and characterization of herpes simplex virus-specific CD4+ T cells in corneas of herpetic stromal keratitis patients. J Infect Dis. 1998;177:484–488. doi: 10.1086/517382. [DOI] [PubMed] [Google Scholar]
- 32.Koelle DM, Reymond SN, Chen H, Kwok WW, McClurkan C, Gyaltsong T, Petersdorf EW, Rotkis W, Talley AR, Harrison DA. Tegument-specific, virus-reactive CD4 T cells localize to the cornea in herpes simplex virus interstitial keratitis in humans. J Virol. 2000;74:10930–10938. doi: 10.1128/jvi.74.23.10930-10938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verjans GM, Dings ME, McLauchlan J, van Der Kooi A, Hoogerhout P, Brugghe HF, Timmermans HA, Baarsma GS, Osterhaus AD. Intraocular T cells of patients with herpes simplex virus (HSV)-induced acute retinal necrosis recognize HSV tegument proteins VP11/12 and VP13/14. J Infect Dis. 2000;182:923–927. doi: 10.1086/315759. [DOI] [PubMed] [Google Scholar]
- 34.Koelle DM, Schomogyi M, Corey L. Antigen-specific T cells localize to the uterine cervix in women with genital herpes simplex virus type 2 infection. J Infect Dis. 2000;182:662–670. doi: 10.1086/315749. [DOI] [PubMed] [Google Scholar]
- 35.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. The authors showed that upon HSV skin infection CD8 T-cells are largely sequestered to the site of infection, whereas CD4 T-cells traffic throughout the dermis and enter the circulation. This study implicates a migratory T-cell subset specialization.
- 37.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, van Beek AE, Gomez-Eerland R, Ritsma L, van Rheenen J, Maree AF, Zal T, et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci U S A. 2012;109:19739–19744. doi: 10.1073/pnas.1208927109. This study showed that skin-resident CD8 T-cells induced by cutaneous HSV infection continuously migrate between epidermal keratinocytes, allowing them to detect rare antigen-expressing cells and thereby potentially contribute to protective local immunity.
- 39.Peng T, Zhu J, Phasouk K, Koelle DM, Wald A, Corey L. An effector phenotype of CD8+ T cells at the junction epithelium during clinical quiescence of herpes simplex virus 2 infection. J Virol. 2012;86:10587–10596. doi: 10.1128/JVI.01237-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, Remington M, Magaret A, Koelle DM, Wald A, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15:886–892. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez JC, Kwok WW, Wald A, McClurkan CL, Huang J, Koelle DM. Expression of cutaneous lymphocyte-associated antigen and E-selectin ligand by circulating human memory CD4+ T lymphocytes specific for herpes simplex virus type 2. J Infect Dis. 2005;191:243–254. doi: 10.1086/426944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. This study demonstrates that vaccinia virus skin infection causes accumulation of tissue-resident CD8 T-cells in the entire skin surface, which remain present for months after infection. Further, these tissue-resident CD8 T-cells provide superior protection against re-infection compared to central memory T-cells.
- 43.Cuburu N, Graham BS, Buck CB, Kines RC, Pang YY, Day PM, Lowy DR, Schiller JT. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J Clin Invest. 2012;122:4606–4620. doi: 10.1172/JCI63287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hufner K, Derfuss T, Herberger S, Sunami K, Russell S, Sinicina I, Arbusow V, Strupp M, Brandt T, Theil D. Latency of alpha-herpes viruses is accompanied by a chronic inflammation in human trigeminal ganglia but not in dorsal root ganglia. J Neuropathol Exp Neurol. 2006;65:1022–1030. doi: 10.1097/01.jnen.0000235852.92963.bf. [DOI] [PubMed] [Google Scholar]
- 45.Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, Langerak AW, Kinchington PR, Osterhaus AD. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A. 2007;104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Derfuss T, Segerer S, Herberger S, Sinicina I, Hufner K, Ebelt K, Knaus HG, Steiner I, Meinl E, Dornmair K, et al. Presence of HSV-1 immediate early genes and clonally expanded T-cells with a memory effector phenotype in human trigeminal ganglia. Brain Pathol. 2007;17:389–398. doi: 10.1111/j.1750-3639.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Velzen M, Laman JD, Kleinjan A, Poot A, Osterhaus AD, Verjans GM. Neuron-interacting satellite glial cells in human trigeminal ganglia have an APC phenotype. J Immunol. 2009;183:2456–2461. doi: 10.4049/jimmunol.0900890. [DOI] [PubMed] [Google Scholar]
- 48.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 49. Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–467. doi: 10.1038/nature11522. This study illustrates that a vaccine-induced memory CD8 T-cells can be"pulled" to mucosal surfaces by chemokines, resulting in a tissue-resident pathogen-specific memory CD8 T-cell population.
- 50.Arvin AM, Koropchak CM, Williams BR, Grumet FC, Foung SK. Early immune response in healthy and immunocompromised subjects with primary varicella-zoster virus infection. J Infect Dis. 1986;154:422–429. doi: 10.1093/infdis/154.3.422. [DOI] [PubMed] [Google Scholar]
- 51.Morrow G, Slobedman B, Cunningham AL, Abendroth A. Varicella-zoster virus productively infects mature dendritic cells and alters their immune function. J Virol. 2003;77:4950–4959. doi: 10.1128/JVI.77.8.4950-4959.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Besouw NM, Verjans GM, Zuijderwijk JM, Litjens NH, Osterhaus AD, Weimar W. Systemic varicella zoster virus reactive effector memory T-cells impaired in the elderly and in kidney transplant recipients. J Med Virol. 2012;84:2018–2025. doi: 10.1002/jmv.23427. [DOI] [PubMed] [Google Scholar]
- 53.Abendroth A, Lin I, Slobedman B, Ploegh H, Arvin AM. Varicella-zoster virus retains major histocompatibility complex class I proteins in the Golgi compartment of infected cells. J Virol. 2001;75:4878–4888. doi: 10.1128/JVI.75.10.4878-4888.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abendroth A, Slobedman B, Lee E, Mellins E, Wallace M, Arvin AM. Modulation of major histocompatibility class II protein expression by varicella-zoster virus. J Virol. 2000;74:1900–1907. doi: 10.1128/jvi.74.4.1900-1907.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen JI. Infection of cells with varicella-zoster virus down-regulates surface expression of class I major histocompatibility complex antigens. J Infect Dis. 1998;177:1390–1393. doi: 10.1086/517821. [DOI] [PubMed] [Google Scholar]
- 56.Eisfeld AJ, Yee MB, Erazo A, Abendroth A, Kinchington PR. Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J Virol. 2007;81:9034–9049. doi: 10.1128/JVI.00711-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nikkels AF, Sadzot-Delvaux C, Pierard GE. Absence of intercellular adhesion molecule 1 expression in varicella zoster virus-infected keratinocytes during herpes zoster: another immune evasion strategy? Am J Dermatopathol. 2004;26:27–32. doi: 10.1097/00000372-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Malavige GN, Jones L, Kamaladasa SD, Wijewickrama A, Seneviratne SL, Black AP, Ogg GS. Viral load, clinical disease severity and cellular immune responses in primary varicella zoster virus infection in Sri Lanka. PLoS One. 2008;3:e3789. doi: 10.1371/journal.pone.0003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Haberthur K, Engelmann F, Park B, Barron A, Legasse A, Dewane J, Fischer M, Kerns A, Brown M, Messaoudi I. CD4 T cell immunity is critical for the control of simian varicella virus infection in a nonhuman primate model of VZV infection. PLoS Pathog. 2011;7:e1002367. doi: 10.1371/journal.ppat.1002367. By using simian varicella virus infection of rhesus macaques as a model for human VZV infection, the authors depleted B-cells, CD4 and CD8 T-cells prior to primary infection. This study showed a major role for CD4 T-cell immunity in controlling varicella.
- 60.Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30:50–58. doi: 10.1097/01.pas.0000176427.99004.d7. [DOI] [PubMed] [Google Scholar]
- 61.Morizane S, Suzuki D, Tsuji K, Oono T, Iwatsuki K. The role of CD4 and CD8 cytotoxic T lymphocytes in the formation of viral vesicles. Br J Dermatol. 2005;153:981–986. doi: 10.1111/j.1365-2133.2005.06849.x. [DOI] [PubMed] [Google Scholar]
- 62.Zak-Prelich M, McKenzie RC, Sysa-Jedrzejowska A, Norval M. Local immune responses and systemic cytokine responses in zoster: relationship to the development of postherpetic neuralgia. Clin Exp Immunol. 2003;131:318–323. doi: 10.1046/j.1365-2249.2003.02061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milikan JC, Baarsma GS, Kuijpers RW, Osterhaus AD, Verjans GM. Human ocular-derived virus-specific CD4+ T cells control varicella zoster virus replication in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:743–751. doi: 10.1167/iovs.08-2611. [DOI] [PubMed] [Google Scholar]
- 64.Milikan JC, Kinchington PR, Baarsma GS, Kuijpers RW, Osterhaus AD, Verjans GM. Identification of viral antigens recognized by ocular infiltrating T cells from patients with varicella zoster virus-induced uveitis. Invest Ophthalmol Vis Sci. 2007;48:3689–3697. doi: 10.1167/iovs.07-0020. [DOI] [PubMed] [Google Scholar]
- 65.Milikan JC, Kuijpers RW, Baarsma GS, Osterhaus AD, Verjans GM. Characterization of the varicella zoster virus (VZV)-specific intra-ocular T-cell response in patients with VZV-induced uveitis. Exp Eye Res. 2006;83:69–75. doi: 10.1016/j.exer.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 66.Asanuma H, Sharp M, Maecker HT, Maino VC, Arvin AM. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus, and cytomegalovirus by intracellular detection of cytokine expression. J Infect Dis. 2000;181:859–866. doi: 10.1086/315347. [DOI] [PubMed] [Google Scholar]
- 67.Jones L, Black AP, Malavige GN, Ogg GS. Phenotypic analysis of human CD4+ T cells specific for immediate-early 63 protein of varicella-zoster virus. Eur J Immunol. 2007;37:3393–3403. doi: 10.1002/eji.200737648. [DOI] [PubMed] [Google Scholar]
- 68.Malavige GN, Jones L, Black AP, Ogg GS. Varicella zoster virus glycoprotein E-specific CD4+ T cells show evidence of recent activation and effector differentiation, consistent with frequent exposure to replicative cycle antigens in healthy immune donors. Clin Exp Immunol. 2008;152:522–531. doi: 10.1111/j.1365-2249.2008.03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arvin AM, Sharp M, Smith S, Koropchak CM, Diaz PS, Kinchington P, Ruyechan W, Hay J. Equivalent recognition of a varicella-zoster virus immediate early protein (IE62) and glycoprotein I by cytotoxic T lymphocytes of either CD4+ or CD8+ phenotype. J Immunol. 1991;146:257–264. [PubMed] [Google Scholar]
- 70.Sadzot-Delvaux C, Arvin AM, Rentier B. Varicella-zoster virus IE63, a virion component expressed during latency and acute infection, elicits humoral and cellular immunity. J Infect Dis. 1998;178(Suppl 1):S43–S47. doi: 10.1086/514259. [DOI] [PubMed] [Google Scholar]
- 71.Frey CR, Sharp MA, Min AS, Schmid DS, Loparev V, Arvin AM. Identification of CD8+ T cell epitopes in the immediate early 62 protein (IE62) of varicella-zoster virus, and evaluation of frequency of CD8+ T cell response to IE62, by use of IE62 peptides after varicella vaccination. J Infect Dis. 2003;188:40–52. doi: 10.1086/375828. [DOI] [PubMed] [Google Scholar]
- 72.Kleemann P, Distler E, Wagner EM, Thomas S, Klobuch S, Aue S, Schnurer E, Schild H, Theobald M, Plachter B, et al. Varicella-zoster virus glycoproteins B and E are major targets of CD4+ and CD8+ T cells reconstituting during zoster after allogeneic transplantation. Haematologica. 2012;97:874–882. doi: 10.3324/haematol.2011.052597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arvin AM, Sharp M, Moir M, Kinchington PR, Sadeghi-Zadeh M, Ruyechan WT, Hay J. Memory cytotoxic T cell responses to viral tegument and regulatory proteins encoded by open reading frames 4, 10, 29, and 62 of varicella-zoster virus. Viral Immunol. 2002;15:507–516. doi: 10.1089/088282402760312377. [DOI] [PubMed] [Google Scholar]
- 74.Huang Z, Vafai A, Lee J, Mahalingam R, Hayward AR. Specific lysis of targets expressing varicella-zoster virus gpI or gpIV by CD4+ human T-cell clones. J Virol. 1992;66:2664–2669. doi: 10.1128/jvi.66.5.2664-2669.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jones L, Black AP, Malavige GN, Ogg GS. Persistent high frequencies of varicella-zoster virus ORF4 protein-specific CD4+ T cells after primary infection. J Virol. 2006;80:9772–9778. doi: 10.1128/JVI.00564-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sadzot-Delvaux C, Kinchington PR, Debrus S, Rentier B, Arvin AM. Recognition of the latency-associated immediate early protein IE63 of varicella-zoster virus by human memory T lymphocytes. J Immunol. 1997;159:2802–2806. [PubMed] [Google Scholar]
- 77.van der Heiden PL, de Boer R, van der Steen DM, Kester MG, van der Hoorn MW, Haarman WM, Barnby-Porritt HE, Fry JW, Napper CE, Marijt EW, et al. Identification of varicella-zoster virus-specific CD8 T cells in patients after T-cell-depleted allogeneic stem cell transplantation. J Virol. 2009;83:7361–7364. doi: 10.1128/JVI.02662-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hope-Simpson RE. The Nature of Herpes Zoster: A Long-Term Study and a New Hypothesis. Proc R Soc Med. 1965;58:9–20. doi: 10.1177/003591576505800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burke BL, Steele RW, Beard OW, Wood JS, Cain TD, Marmer DJ. Immune responses to varicella-zoster in the aged. Arch Intern Med. 1982;142:291–293. [PubMed] [Google Scholar]
- 80.Levin MJ, Smith JG, Kaufhold RM, Barber D, Hayward AR, Chan CY, Chan IS, Li DJ, Wang W, Keller PM, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis. 2003;188:1336–1344. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]
- 81.Miller AE. Selective decline in cellular immune response to varicella-zoster in the elderly. Neurology. 1980;30:582–587. doi: 10.1212/wnl.30.6.582. [DOI] [PubMed] [Google Scholar]
- 82.Jenkins DE, Redman RL, Lam EM, Liu C, Lin I, Arvin AM. Interleukin (IL)-10, IL-12, and interferon-gamma production in primary and memory immune responses to varicella-zoster virus. J Infect Dis. 1998;178:940–948. doi: 10.1086/515702. [DOI] [PubMed] [Google Scholar]
- 83.Tyring SK, Stek JE, Smith JG, Xu J, Pagnoni M, Chan IS, Silber JL, Parrino J, Levin MJ. Varicella-zoster virus-specific enzyme-linked immunospot assay responses and zoster-associated pain in herpes zoster subjects. Clin Vaccine Immunol. 2012;19:1411–1415. doi: 10.1128/CVI.00095-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Weinberg A, Lazar AA, Zerbe GO, Hayward AR, Chan IS, Vessey R, Silber JL, MacGregor RR, Chan K, Gershon AA, et al. Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. J Infect Dis. 2010;201:1024–1030. doi: 10.1086/651199. This study is the first to demonstrate that VZV-specific immunity in naturally infected individuals peaks in mid-adulthood at 34 years of age, i.e. decades after primary infection.
- 85.Zhang Y, Cosyns M, Levin MJ, Hayward AR. Cytokine production in varicella zoster virus-stimulated limiting dilution lymphocyte cultures. Clin Exp Immunol. 1994;98:128–133. doi: 10.1111/j.1365-2249.1994.tb06618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y, White CJ, Levin M, Hayward A. Cytokine production in varicella-zoster virus-stimulated lymphocyte cultures. Neurology. 1995;45:S38–S40. doi: 10.1212/wnl.45.12_suppl_8.s38. [DOI] [PubMed] [Google Scholar]
- 87.Hayward A, Levin M, Wolf W, Angelova G, Gilden D. Varicella-zoster virus-specific immunity after herpes zoster. J Infect Dis. 1991;163:873–875. doi: 10.1093/infdis/163.4.873. [DOI] [PubMed] [Google Scholar]
- 88.Weinberg A, Zhang JH, Oxman MN, Johnson GR, Hayward AR, Caulfield MJ, Irwin MR, Clair J, Smith JG, Stanley H, et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200:1068–1077. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ouwendijk WJ, Flowerdew SE, Wick D, Horn AK, Sinicina I, Strupp M, Osterhaus AD, Verjans GM, Hufner K. Immunohistochemical detection of intra-neuronal VZV proteins in snap-frozen human ganglia is confounded by antibodies directed against blood group A1-associated antigens. J Neurovirol. 2012;18:172–180. doi: 10.1007/s13365-012-0095-0. [DOI] [PubMed] [Google Scholar]
- 90.Zerboni L, Sobel RA, Lai M, Triglia R, Steain M, Abendroth A, Arvin A. Apparent expression of varicella-zoster virus proteins in latency resulting from reactivity of murine and rabbit antibodies with human blood group a determinants in sensory neurons. J Virol. 2012;86:578–583. doi: 10.1128/JVI.05950-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gowrishankar K, Steain M, Cunningham AL, Rodriguez M, Blumbergs P, Slobedman B, Abendroth A. Characterization of the host immune response in human Ganglia after herpes zoster. J Virol. 2010;84:8861–8870. doi: 10.1128/JVI.01020-10. This is the first study to analyze immune cell infiltrates in human ganglia obtained months after herpes zoster, showing a predominant CD8 T-cell infiltrate.
- 92. Ouwendijk WJ, Abendroth A, Traina-Dorge V, Getu S, Steain M, Wellish M, Andeweg AC, Osterhaus AD, Gilden D, Verjans GM, et al. T-cell infiltration correlates with CXCL10 expression in ganglia of cynomolgus macaques with reactivated simian varicella virus. J Virol. 2013;87:2979–2982. doi: 10.1128/JVI.03181-12. The authors used reactivation of latent simian varicella virus in rhesus macaques, as a model for VZV reactivation in humans, to study T-cell immunity in ganglia at variable times after zoster. The dynamics of CD8 T-cell infiltration correlated with CXCL10, but not viral antigen expression.
- 93.Steain M, Gowrishankar K, Rodriguez M, Slobedman B, Abendroth A. Upregulation of CXCL10 in human dorsal root ganglia during experimental and natural varicella-zoster virus infection. J Virol. 2011;85:626–631. doi: 10.1128/JVI.01816-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Asano Y, Nagai T, Miyata T, Yazaki T, Ito S, Yamanishi K, Takahashi M. Long-term protective immunity of recipients of the OKA strain of live varicella vaccine. Pediatrics. 1985;75:667–671. [PubMed] [Google Scholar]
- 95.Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825–835. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 97.Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;2:1288–1290. doi: 10.1016/s0140-6736(74)90144-5. [DOI] [PubMed] [Google Scholar]
- 98.Vazquez M, LaRussa PS, Gershon AA, Steinberg SP, Freudigman K, Shapiro ED. The effectiveness of the varicella vaccine in clinical practice. N Engl J Med. 2001;344:955–960. doi: 10.1056/NEJM200103293441302. [DOI] [PubMed] [Google Scholar]
- 99.Johnston C, Koelle DM, Wald A. HSV-2: in pursuit of a vaccine. J Clin Invest. 2011;121:4600–4609. doi: 10.1172/JCI57148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM, Jr, Handsfield HH, Warren T, Marr L, Tyring S, et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA. 1999;282:331–340. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- 101.Langenberg AG, Corey L, Ashley RL, Leong WP, Straus SE. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. N Engl J Med. 1999;341:1432–1438. doi: 10.1056/NEJM199911043411904. [DOI] [PubMed] [Google Scholar]
- 102.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 104.Reszka NJ, Dudek T, Knipe DM. Construction and properties of a herpes simplex virus 2 dl5-29 vaccine candidate strain encoding an HSV-1 virion host shutoff protein. Vaccine. 2010;28:2754–2762. doi: 10.1016/j.vaccine.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Halford WP, Puschel R, Gershburg E, Wilber A, Gershburg S, Rakowski B. A live-attenuated HSV-2 ICP0 virus elicits 10 to 100 times greater protection against genital herpes than a glycoprotein D subunit vaccine. PLoS One. 2011;6:e17748. doi: 10.1371/journal.pone.0017748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Earl PL, Americo JL, Wyatt LS, Eller LA, Whitbeck JC, Cohen GH, Eisenberg RJ, Hartmann CJ, Jackson DL, Kulesh DA, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 107.de Bruyn G, Vargas-Cortez M, Warren T, Tyring SK, Fife KH, Lalezari J, Brady RC, Shahmanesh M, Kinghorn G, Beutner KR, et al. A randomized controlled trial of a replication defective (gH deletion) herpes simplex virus vaccine for the treatment of recurrent genital herpes among immunocompetent subjects. Vaccine. 2006;24:914–920. doi: 10.1016/j.vaccine.2005.08.088. [DOI] [PubMed] [Google Scholar]
- 108.Wald A, Koelle DM, Fife K, Warren T, Leclair K, Chicz RM, Monks S, Levey DL, Musselli C, Srivastava PK. Safety and immunogenicity of long HSV-2 peptides complexed with rhHsc70 in HSV-2 seropositive persons. Vaccine. 2011;29:8520–8529. doi: 10.1016/j.vaccine.2011.09.046. [DOI] [PubMed] [Google Scholar]