Abstract
Background
Left ventricular (LV) wall stress reduction is a cornerstone in treating heart failure. Large animal models and computer simulations indicate that adding non-contractile material to the damaged LV wall can potentially reduce myofiber stress. We sought to quantify the effects of a novel implantable hydrogel (Algisyl-LVR™) treatment in combination with coronary artery bypass grafting (i.e. Algisyl-LVR™+CABG) on both LV function and wall stress in heart failure patients.
Methods and results
Magnetic resonance images obtained before treatment (n=3), and at 3 months (n=3) and 6 months (n=2) afterwards were used to reconstruct the LV geometry. Cardiac function was quantified using end-diastolic volume (EDV), end-systolic volume (ESV), regional wall thickness, sphericity index and regional myofiber stress computed using validated mathematical modeling. The LV became more ellipsoidal after treatment, and both EDV and ESV decreased substantially 3 months after treatment in all patients; EDV decreased from 264±91 ml to 146±86 ml and ESV decreased from 184±85 ml to 86±76 ml. Ejection fraction increased from 32±8% to 47±18% during that period. Volumetric-averaged wall thickness increased in all patients, from 1.06±0.21 cm (baseline) to 1.3±0.26 cm (3 months). These changes were accompanied by about a 35% decrease in myofiber stress at end-of-diastole and at end-of-systole. Post-treatment myofiber stress became more uniform in the LV.
Conclusions
These results support the novel concept that Algisyl-LVR™+CABG treatment leads to decreased myofiber stress, restored LV geometry and improved function.
Keywords: Congestive heart failure, Dilated cardiomyopathy, Coronary artery bypass grafting, Left ventricular wall stress, Mathematical modeling, Magnetic resonance imaging
1. Introduction
Therapeutic options for patients with severe heart failure and who are refractory to pharmacological therapies are quite limited. While the use of circulatory support devices such as Left Ventricular Assist Devices (LVAD), and heart transplant, has increased significantly over the last two decades, these therapies offer only a modest chance at extending life and improving the poor quality of life for patients with severe heart failure. In addition, these therapies are expensive, continue to be associated with significant morbidity and mortality, and are of limited access to the vast majority of patients. For all of these reasons, alternatives to the options of LVAD and transplant are being actively pursued.
Reduction of left ventricular (LV) wall stress is considered a cornerstone in the treatment of heart failure [1]. Data obtained from clinically relevant large animal models and computer simulations indicate that the addition of non-contractile material to a damaged LV wall can potentially reduce elevated myofiber stress [2]. Algisyl-LVR™ is a medical device under clinical development and is intended to leverage this mechanism to prevent or reverse the progression of heart failure in patients who have a dilated LV. This device consists of a proprietary biopolymer gel that is injected into strategic areas of the heart muscle, where it remains as a permanent implant. Preclinical study [3] and clinical study using echocardiographic measurements [4] have demonstrated an improvement in LV size 3 months after implantation in patients who also underwent revascularization through coronary artery bypass grafting (CABG) and/or valve replacement/repair procedure.
The purpose of this paper is to debut, at a longer post-treatment period of up to 6 months, the quantitative effects of this novel treatment on both LV function and myofiber stress in a small cohort of patients who simultaneously underwent CABG. To quantify these effects as accurately as possible, we used magnetic resonance (MR) imaging and mathematical modeling. Magnetic resonance imaging is the gold standard for measurement of in-vivo ventricular volumes. Mathematical modeling makes it possible to calculate in-vivo regional myofiber stress. In-vivo regional myofiber stress is necessary to understand both normal and pathological ventricular mechanics [5] but cannot be measured directly [6]. Results from our analysis of this early clinical trial data of Algisyl-LVR™ suggest that the treatment (in combination with CABG) effectively reduces LV myofiber stress and improves cardiac function in patients with heart failure.
2. Methods
2.1. Patient selection
Eleven patients (males, age 44 to 74) were part of the safety and feasibility evaluation (www.clinicaltrials.gov, identifier NCT00847964) conducted at the German Heart Center in Munich and the Heart Center at Dresden University Hospital using Algisyl-LVR™ in combination with CABG. These patients had symptomatic heart failure with a New York Heart Association (NYHA) class of III or IV, an ejection fraction (EF)≤40% and a left ventricular end diastolic dimension indexed to body surface area of 30 to 40 mm/m2. Magnetic resonance images were obtained from three of these patients who (out of the eleven patients) had no contraindications for MR imaging. The ages of these male patients were 44 (Patient 1), 68 (Patient 2) and 57 (Patient 3). All three patients were diagnosed with NYHA class III heart failure and had ischemic cardiomyopathy, hypertension, hyperlipidemia and renal insufficiency. Of these three patients, one has a history of peripheral vascular disease, two have diabetes and two have a history of myocardial infarction. Data concerning symptoms of angina and myocardial perfusion were not collected in this study. Informed consent was obtained from each patient and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by each institution’s human research committee.
2.2. Algisyl-LVR™ injection
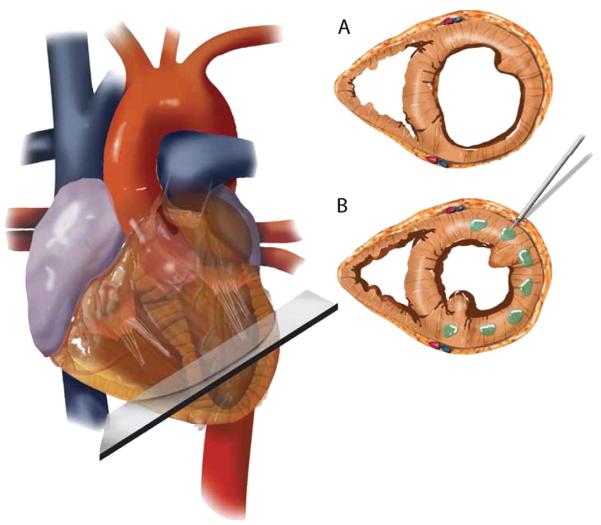
Calcium-Alginate hydrogel (Algisyl-LVR™, LoneStar Heart, Inc. Laguna Hills, CA) was used to treat these patients. This hydrogel consists of two components: an Na+-Alginate component supplied as a sterile aqueous solution with 4.6% mannitol, and a Ca2+-Alginate component consisting of water insoluble particles suspended in a sterile 4.6% mannitol solution. These two components were mixed immediately before use, and then combined in one syringe for intramyocardial injections. The hydrogel achieved its final material strength of 3–5 kPa with a halftime of 3–4 min. Algisyl-LVR™ (0.3 cc each) was then injected at 10 to 15 locations half way between the apex and the base in the LV free wall (excluding the septum) of the patients (Fig. 1).
Fig. 1.
Schematic of Algisyl-LVR™ injection in the left ventricle. (A) Short-axis view of the mid-ventricle, half way between the apex and base. (B) Algisyl-LVR™ (0.3 cc each) injected at 10 to 15 locations at the mid-ventricle free wall (excluding the septum).
2.3. Acquisition of MR Images
Magnetic resonance images were acquired using standard imaging protocols one week before treatment and at 3 months (all 3 patients) and 6 months (2 patients) after treatment (See Supplementary materials). The LV endocardial and epicardial borders were contoured or outlined from these images containing views from the long-axis and short-axis. Three-dimensional endocardial and epicardial surfaces were obtained by triangulating the resultant contour points. (Figure S1, supplemental materials). End-diastolic volume (EDV) and end-systolic volume (ESV) were determined based on these surfaces. Sphericity index (SI) was also determined based on the reconstructed LV geometry at end-of-systole. It was calculated as the ratio between the greatest short-axis dimension and the greatest long-axis dimension measured from the endocardial surface.
2.4. Mathematical modeling
Our methods have been described in detail previously [2]. Detailed methods are provided in the Online Supplement.
Although analytical equations based on Laplace’s law can estimate the LV average in-vivo wall stress [7], they cannot predict in-vivo LV myofiber stress distribution or take into account several important factors, such as myocardial tissue anisotropy [8]. To overcome these limitations, we used the acquired MR images to create patient-specific mathematical models of the LV from these patients before and after treatment (Figure S1, supplemental materials). The LV was modeled using a validated myocardial passive and active stress-strain relationship [2] with patient-specific material parameters determined from the LV cavity volumes measured by MRI. To model the LV at end-of-diastole (ED) and end-of-systole (ES), physiologically realistic pressures of 20 mm Hg and 125 mm Hg were applied respectively at the endocardial surface of the LVs. Active stress was also added to the LV after ED to simulate contraction of the LV. Myofiber stress at ED and ES were then calculated from these models.
3. Results
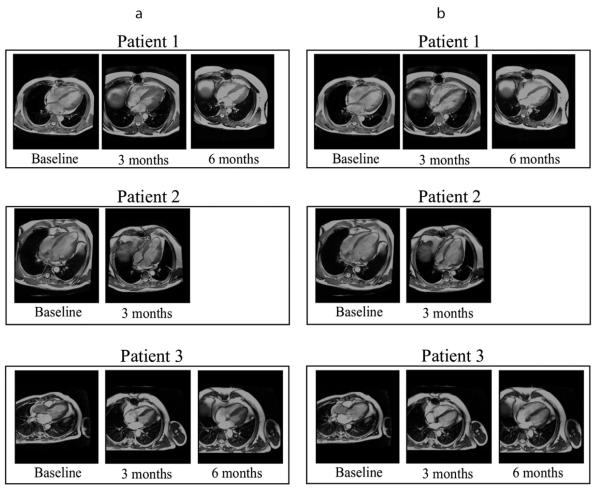
Magnetic resonance images of the LV (long-axis view) of individual patients at different time points are shown in Fig. 2. Movie clips associated with the short-axis and long-axis views of the MRI are also available as supplementary materials. Individual results from each patient are tabulated in Table 1.
Fig. 2.
Long-axis view of the MRI in 3 patients at (a) end-systole and at (b) end-diastole.
Table 1.
Summary on the effects of Algiysl-LVR™+CABG at different time points.
| Patient 1 |
Patient 2 |
Patient 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | Baseline | 3 months | 6 months | Baseline | 3 months | 6 months | |
| EDV (ml) | 197.4 | 98.2 | 98.2 | 367.6 | 244.7 | – | 228.2 | 94.6 | 93.8 |
| ESV (ml) | 122.4 | 34.0 | 23.7 | 280.4 | 173.3 | – | 148.1 | 50.1 | 35.7 |
| SV (ml) | 75.0 | 64.2 | 74.5 | 87.2 | 71.4 | – | 80.1 | 44.5 | 58.1 |
| EF (%) | 40 | 65.4 | 75.9 | 23.7 | 29.2 | – | 35.1 | 47.0 | 61.9 |
| Sphericity index | 0.74 | 0.61 | 0.56 | 0.72 | 0.7 | – | 0.77 | 0.73 | 0.67 |
| Average thickness (cm) | 1.12 | 1.52 | 1.65 | 0.83 | 1.02 | – | 1.24 | 1.35 | 1.58 |
| Average ED stress (kPa) | 5.6 | 3.1 | 3.1 | 8.7 | 6.4 | – | 5.4 | 3.6 | 2.8 |
| Average ES stress (kPa) | 29.6 | 12.7 | 9.4 | 52.4 | 37.4 | – | 29.4 | 19.3 | 12.8 |
| Peak ES stress (kPa) | 61.9 | 27.2 | 24.5 | 178.2 | 109.6 | – | 69.4 | 48.2 | 35.2 |
3.1. Patient characteristics
The NYHA classification improved from III to II in all 3 patients 3 months after treatment. In the 2 patients who had a follow-up visit 6 months after treatment, the NYHA class improved to I in one patient and remained as II in the other.
The Kansas City Cardiomyopathy Questionnaire was administered to all 3 patients. On average, the Overall Summary Score reported by the patients improved from 58.7±20.9 (mean ± standard deviation) before treatment to 78.5±18.6 and 79.2±20.4 at 3 months and at 6 months after treatment, respectively.
3.2. Wall thickness
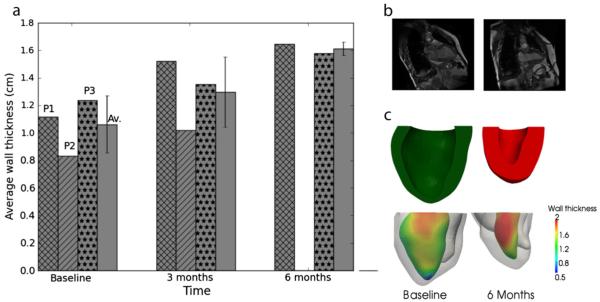
In all 3 patients, LV wall thickness increased monotonically after treatment. On average, wall thickness increased by about 20% (from 1.06±0.21 cm to 1.3±0.26 cm) 3 months after treatment (Fig. 3a). Wall thickness further increased by about 12% (average) 3 months later (Fig. 3a). The increase in wall thickness after treatment was most pronounced in regions adjacent to the injection sites on the free wall (Fig. 3b,c).
Fig. 3.
Effects of injection on left ventricle (LV) regional wall thickness. (a) LV average wall thickness at different time points. Cross-shaded: Patient 1, diagonally-shaded: Patient 2, dot-shaded: Patient 3 and plain-shaded: average. Average is shown with standard deviation. (b) Long-axis view of magnetic resonance image (MRI) at baseline (left) and at 6 months (right) from Patient 1 at end-of-systole. (c) Top: cross-section view of LV at baseline (left) and 6 months (right). Bottom: measured regional LV wall thickness based on the MRI-reconstructed LV of Patient 1. Thickness (cm) is indicated by the color shown on LV endocardium.
3.3. Global Performance Indicators
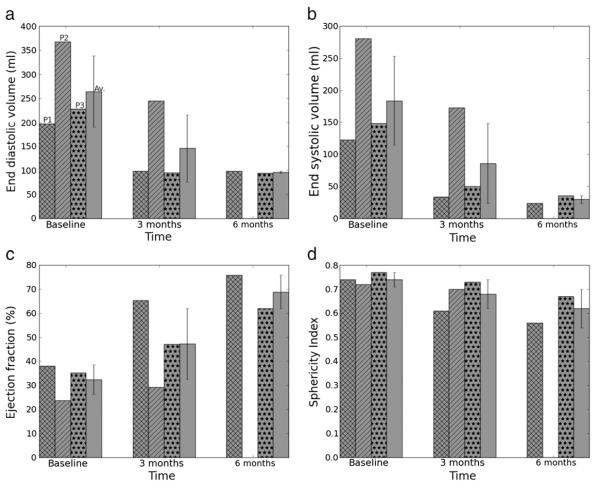
In all patients, EDV and ESV decreased substantially 3 months after treatment (EDV: 264±91 ml to 146±86 ml; ESV: 184±85 ml to 86±76 ml) (Fig. 4a, b). Between 3 and 6 months after treatment, EDV did not decrease significantly, but ESV decreased further by about 30% in the two patients who had the 6 month follow-up visit.
Fig. 4.
Global LV performance indicator. (a) End-diastolic volume, (b) End-systolic volume, (c) Ejection fraction, (d) Sphericity index at baseline, 3 and 6 months post-treatment. Cross-shaded: Patient 1, diagonally-shaded: Patient 2, dot-shaded: Patient 3 and plain-shaded: average. Average is shown with standard deviation.
Stroke volume decreased in all patients 3 months after treatment, but improved between 3 and 6 months after treatment, consistent with the continuing decrease in ESV.
Ejection fraction increased monotonically in all patients after treatment, from 32±8% at baseline to 47±18% at 3 months. It increased further in two patients 3 months later (average EF was 69% at 6 months) (Fig. 4c).
Sphericity index also improved and decreased monotonically in all patients after treatment, from 0.74±0.03 to 0.68±0.06 at 3 months. It further decreased in the two patients 3 months later and the average SI was 0.62±0.08 (Fig. 4d). The LV thus became more ellipsoidal after treatment.
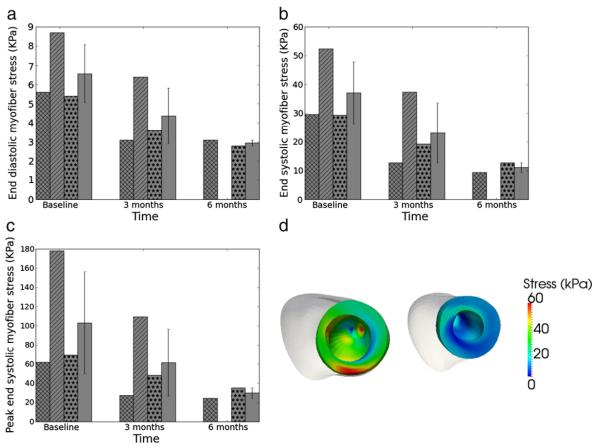
3.4. Myofiber stress
In all patients, calculated volumetric-averaged myofiber stress at ED and ES decreased after treatment (Fig. 5). Three months after treatment, volumetric-averaged ED myofiber stress decreased from 6.6±1.9 kPa to 4.4±1.8 kPa and volumetric-averaged ES myofiber stress decreased from 37.1±13.2 kPa to 23.1±12.8 kPa. Peak ES myofiber stress also decreased from 103.2±65.1 kPa to 61.7±42.8 kPa 3 months after treatment, and decreased further during the 3 to 6 month post-treatment period. Consequently, post-treatment myofiber stress became more homogeneous within the LV (Fig. 5d).
Fig. 5.
Effects of treatment on myofiber stress. (a) Volumetric-averaged end-diastolic stress. (b) Volumetric-averaged end-systolic stress. (c) Peak end-systolic stress. (d) End-systolic regional myofiber stress distribution of Patient 1 LV at baseline (left) and 6 months after surgery (right). LV epicardium is displayed in white.
4. Discussion
The overall result from this first study to quantify the effects of Algisyl-LVR™ injection+CABG in humans is promising. This treatment resulted in a decrease in SI (i.e. the LV became more ellipsoidal) and an increase in LV wall thickness that was accompanied by a substantial reduction of EDV and ESV. The two treatments given, CABG and Algisyl-LVR™, have different mechanisms of action, with CABG rescuing hibernating viable myocardium while the injection of Algisyl-LVR™ should, in principle, produce local stress reduction in the myocardial wall. Both of these should lead to reverse remodeling and long-term LV geometry and function changes.
While surgical revascularization probably contributed to the observed decrease in the LV chamber volume through the rescue of hibernating myocardium and subsequent remodeling, it is unlikely that the dramatic and rapid changes reported here can fully be attributed to revascularization alone, based on reports from previous clinical studies. The Algisyl-LVR™ treatment is responsible for at least a portion of the dramatic changes we observed. For example, in three retrospective analyses on patients with ischemic cardiomyopathy, EF was found to have increased by 3.2 percentage points (25.8% to 29%) at 7–35 days after surgery [9], by 12 percentage points (33% to 45%) at 8±3 months after surgery [10] and by 7.8 percentage points (23.7% to 31.5%) at 9.1±12.7 months after surgery [11]. End diastolic volume and end-systolic volume index were found to have decreased after CABG by only about 10% (194±24 ml to 174±39 ml) [9] and by 6% at 4 months in the STICH trial [12], respectively. In a prospective study by Carluccio et al. [10], the average end-diastolic and end-systolic volume index were found to have decreased by 16% (118±26 ml/m2 to 99±26 ml/m2) and 28% (78±23 ml/m2 to 56±23 ml/m2) respectively, at 8±3 months after surgery. In comparison, our results indicate that just 3 months after treatment, EF increased by 15 percentage points and both EDV and ESV decreased by about 50%. Over a longer time period of 6 months, EF increased by an average of 31.4 percentage points in our 2 patients, whereas both the average EDV and ESV (from these 2 patients) decreased by 55% and 78%, respectively. In addition, the 6-month changes in both of these patients are outside the range of response Carluccio et al. observed in the 40+ patients in their study, suggesting that it is very unlikely that the improvement in our patients came solely from revascularization.
Due to the positive effects on ventricular geometry, calculated myofiber stress became more homogeneous in the LV and was reduced substantially in the patients. Left ventricular myocardial oxygen consumption MVO2 would, therefore, also be predicted to have reduced substantially [13]. Because an increase in wall stress is strongly correlated with subsequent LV remodeling, and the reduction of left ventricular wall stress is known to attenuate LV remodeling [14], we postulate that the injection of Algisyl-LVR™ into the LV free wall may stimulate reverse remodeling over a sustained period of time.
In all 3 patients, performance indicators of LV cardiac function that were analyzed improved monotonically and remarkably 6 months after treatment, with the exception of stroke volume. Although stroke volume decreased 3 months after treatment in all patients, it increased between the 3 to 6 month period in the two patients who underwent MRI at the 6-month follow-up visit. In Patient 1, stroke volume reverted back to its baseline value 6 months after treatment. This transient decrease in stroke volume is consistent with the results from a mathematical model of Algisyl-LVR™ injection in a dog with a failing LV [15]. In that study, the immediate effects of injections were a decrease in both myofiber stress and stroke volume. The optimal number of implants producing the best compromise between lowering the myofiber stress and stroke volume was found to be 7, and the best position for the implants was found to be at the LV mid wall (half-way between the apex and base) excluding the septum. Because the LV is larger in humans than in dogs, the number of implants (of similar size) was increased from 7 to 10–15 when treating patients.
Our study augments and extends the previous study by Retzlaff et al. [16], who used echocardiography to assess the 3-month effects of Algisyl-LVR™ in 6 patients with ischemic or non-ischemic dilated cardiomyopathy who had undergone the injection treatment in combination with either CABG or valve repair. In that study, sustained improvements in the LV size, function and in quality of life were observed as early as 3 days after surgery in all patients. Our results show that these improvements extend beyond 3 months and were still observed even in the 3 to 6 month period, although improvements occurred at a slower rate.
The injection therapy studied here differs fundamentally from other developing treatments for heart failure such as surgical ventricular restoration (SVR) [12] and passive restraint devices (i.e. Acorn CorCap Device [17] and Myocor Coapsys [18]), even though they all share the same primary aim of reducing ventricular wall stress. While injection therapy sought to achieve this aim by increasing the LV wall thickness by adding biocompatible material into the ventricular wall, the strategy of the other treatments is to alter the shape of the LV (Myocor Coapsys) and to decrease the LV chamber size either through surgery (SVR) or by mechanically restraining the LV (Acorn CorCap). Results from clinical trials have shown that LV size decreased after these treatments. Left ventricular end systolic volume index, end-diastolic dimension and EDV were found to have decreased by 19%, 10% and by about 20 ml after treatment with SVR+CABG [12], Coapsys+CABG [18] and Acorn+mitral valve repair [17], respectively. Mathematical models studying the effects of these treatments have also shown that the ventricular wall stress decreased after these treatments [19,20]. However, the recently completed STICH Trial [12] concluded that SVR did not add any benefit when performed with CABG even though the size of the LV was reduced significantly after treatment.
Compared to these treatments, our results show that the combination of Algisyl-LVR™ injection into the LV wall and CABG resulted in a much greater decrease in LV chamber size (45% decrease in EDV and 53% decrease in ESV at only 3 months). Thus, the strategy of thickening the LV wall to promote reverse remodeling apparently has a greater effect in restoring the LV size than in directly altering its size or shape.
However, a decrease in LV chamber size is, of course, not a sole indicator of the overall improvement in LV function, as was exemplified by the above-mentioned STICH trial. In addition to a decrease in LV chamber size, our results show that this favorable outcome was accompanied by other indicators of improvement in LV function, such as an increase in LV wall thickness and ejection fraction, as well as a reduction in calculated myofiber stress. Moreover, besides decreasing LV size, injection of Algyisl-LVR™ with CABG also decreased the SI of the LV, making it more ellipsoidal than it was before treatment. An improvement in LV SI was also observed in the Acorn study [18]. Studies have shown that an increase in LV sphericity is associated with the development of LV dysfunction [21] and it is widely believed that the LV functions better when it is more ellipsoidal. By contrast, the LV after SVR has been found to be typically more spherical than it was before surgery [22].
5. Study limitations
While very promising, our study has four main limitations. First, there is no control group of patients who received only CABG. As a result, the effects of Algiysl-LVR™ shown here were confounded with the effects of CABG. To overcome this limitation and to assess the efficacy of Algisyl-LVR™, we have relied on the results from past clinical studies to estimate the magnitude of the effects of CABG alone on patients who had ischemic cardiomyopathy. The improvements in LV function after treatment by CABG reported in past clinical studies suggest that CABG should produce a significantly smaller change and a less rapid improvement than what is observed here. Second, given that the original intent of this study was to evaluate structural changes to the heart and clinical outcomes after treatment by Algisyl-LVR™+CABG, the extent of scarred myocardium was not acquired by delayed hyperenhancement MRI before and after treatment. Consequently, the effect of Algisyl-LVR™+CABG treatment on the extent and function of viable myocardium is not known in this study, which also diminishes the ability to delineate the effects of CABG versus Algisyl-LVR™ injection. Third, stroke volume was calculated from the difference between EDV and ESV. Consequently, we did not account for the impact of mitral regurgitation on true forward stroke volume. While none of the patients have undergone mitral valve replacement, the reduction in LV volume and restoration of LV shape might be expected to lessen the degree of mitral regurgitation. Last, we were forced to assume physiologically reasonable values of 20 mm Hg as ED pressure and 125 mm Hg as ES pressure for all patients, since patient-specific LV pressure data, which requires invasive measurements using micromanometer-tipped catheters, was not available. Our experience has, however, indicated that a variation in pressure within physiological limits will not affect the conclusion drawn here. Based on a previous study, we found that a variation in ES pressure by about ±10 mm Hg resulted in roughly a ±10% change in the peak ES myofiber stress (Figure S2, supplemental materials). That variation is small compared to the 40% decrease in peak ES myofiber stress post-treatment (3-months) that we observed in the current study.
6. Conclusion
Our results support the novel concept that the injection of material into the LV free-wall with CABG is an effective strategy for inducing LV reverse remodeling that improves the LV cardiac function and results in a decreased myofiber stress. Although the power of this finding is limited by the small sample size, the effects of this treatment are interesting and remarkable. The results of a recently initiated, randomized, controlled study to evaluate Algisyl-LVR™ solely as a method of LV augmentation for patients with severe heart failure (AUGMENT-HF) should provide definitive conclusions about the clinical benefits of Algisyl-LVR™ therapy.
Supplementary Material
Acknowledgment
We would like to thank Dr. Robert Bauernschmitt for sharing his patient MRI data, Nina Khoshnevisrad for digitizing that data, Pamela Derish in Department of Surgery at UCSF for proofing the manuscript and Paul Schiffmacher for helping with the figure illustration.
The author(s) of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [23].
Footnotes
Acknowledgment of grant support: This study was supported by National Heart, Lung, and Blood Institute Grants R01-HL-77921, R01-HL-86400 (to J. M. Guccione), and R01-HL-63954 (to R. C. Gorman).
Potential conflict of interest: Mr. Hinson and Dr. Randall Lee are, respectively, an employee and a consultant of LoneStar Heart, Inc.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ijcard.2013.01.003.
References
- [1].Guccione JM, Salahieh A, Moonly SM, Kortsmit J, Wallace AW, Ratcliffe MB. Myosplint decreases wall stress without depressing function in the failing heart: a finite element model study. Ann Thorac Surg. 2003;76:1171–80. doi: 10.1016/s0003-4975(03)00731-8. [DOI] [PubMed] [Google Scholar]
- [2].Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation. 2006;114:2627–35. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- [3].Sabbah HN, Wang M, Jiang A, et al. Circumferential mid-ventricular intramyocardial injections of alginate hydrogel improve left ventricular function and prevent progressive remodeling in dogs with chronic heart failure. Circulation. 2009;120:S912. [Google Scholar]
- [4].Lee RJ, Hinson A, Helgerson S, Bauernschmitt R, Sabbah HN. Polymer-based restoration of left ventricular mechanics. Cell Transplant. 2012 Mar 28; doi: 10.3727/096368911X637461. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [5].Yin FC. Ventricular wall stress. Circ Res. 1981;49(4):829–42. doi: 10.1161/01.res.49.4.829. [DOI] [PubMed] [Google Scholar]
- [6].Huisman RM, Elzinga G, Westerhof N, Sipkema P. Measurement of left ventricular wall stress. Cardiovasc Res. 1980;14(3):142–53. doi: 10.1093/cvr/14.3.142. [DOI] [PubMed] [Google Scholar]
- [7].Janz RF. Estimation of local myocardial stress. Am J Physiol. 1982;242(5):H875–81. doi: 10.1152/ajpheart.1982.242.5.H875. [DOI] [PubMed] [Google Scholar]
- [8].Zhang Z, Tendulkar A, Sun K, et al. Comparison of the Young–Laplace law and finite element based calculation of ventricular wall stress: implications for postinfarct and surgical ventricular remodeling. Ann Thorac Surg. 2011;91(1):150–6. doi: 10.1016/j.athoracsur.2010.06.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Maxey TS, Reece TB, Ellman PI, et al. Coronary artery bypass with ventricular restoration is superior to coronary artery bypass alone in patients with ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2004;127(2):428–34. doi: 10.1016/j.jtcvs.2003.09.024. [DOI] [PubMed] [Google Scholar]
- [10].Carluccio E, Biagioli P, Alunni G, et al. Patients with hibernating myocardium show altered left ventricular volumes and shape, which revert after revascularization. J Am Coll Cardiol. 2006;47(5):969–77. doi: 10.1016/j.jacc.2005.09.064. [DOI] [PubMed] [Google Scholar]
- [11].Pruz RB, Weiss ES, Patel ND, Nwakanma LU, Baumgartner WA, Conte JV. Coronary artery bypass grafting with or without surgical ventricular restoration: a comparison. Ann Thorac Surg. 2008;86(3):806–14. doi: 10.1016/j.athoracsur.2008.05.009. [DOI] [PubMed] [Google Scholar]
- [12].Jones RH, Velazquez EJ, Michler RE, et al. For the STICH hypothesis 2 investigators. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705–17. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Strauer BE, Beer K, Heitlinger K, Höfling B. Left ventricular systolic wall stress as a primary determinant of myocardial oxygen consumption: comparative studies in patient with normal left ventricular function, with pressure and volume overload and with coronary heart disease. Basic Res Cardiol. 1977;72:306–13. doi: 10.1007/BF01906378. [DOI] [PubMed] [Google Scholar]
- [14].Aikawa Y, Rohde L, Plehn J, et al. Regional wall stress predicts ventricular remodeling after anteroseptal myocardial infarction in the Healing and Early Afterload Reducing Trial (HEART): an echocardiography-based structural analysis. Am Heart J. 2001;141(2):234–42. doi: 10.1067/mhj.2001.112237. [DOI] [PubMed] [Google Scholar]
- [15].Wenk JF, Wall ST, Peterson RC, et al. A method for automatically optimizing medical devices for treating heart failure: designing polymeric injection patterns. J Biomech Eng. 2009;131:121011. doi: 10.1115/1.4000165. [DOI] [PubMed] [Google Scholar]
- [16].Retzlaff B, Voss B, Will A, Rüdiger L, Bauernschmitt R. First in man experience with left ventricular reconstruction in patients with systolic heart failure using a novel approach of biopolymer hydrogel implantation. Circulation. 2010:122–A19753. [Google Scholar]
- [17].Mann DL, Acker MA, Jessup M, Sabbah HN, Starling RC, Kubo SH. Clinical evaluation of the CorCap cardiac support device in patients with dilated cardiomyopathy. Ann Thorac Surg. 2007;84:1226–35. doi: 10.1016/j.athoracsur.2007.03.095. [DOI] [PubMed] [Google Scholar]
- [18].Grossi EA, Patel N, Woo JY, et al. For the RESTOR-MV study group Outcomes of the RESTOR-MV trial (randomized evaluation of a surgical treatment for off-pump repair of the mitral valve) J Am Coll Cardiol. 2010;56:1984–93. doi: 10.1016/j.jacc.2010.06.051. [DOI] [PubMed] [Google Scholar]
- [19].Jhun C-S, Wenk JF, Zhang Z, et al. Effects of adjustable passive constraint on the failing left ventricle: a finite-element model study. Ann Thorac Surg. 2010;89:132–7. doi: 10.1016/j.athoracsur.2009.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sun K, Zhang Z, Suzuki T, et al. Dor procedure for dyskinetic anteroapical myocardial infarction fails to improve contractility in the borderzone. J Thorac Cardiovasc Surg. 2010;140(1):233–9. doi: 10.1016/j.jtcvs.2009.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sabbah HN, Konno T, Stein PD, Mancini GB, Goldstein S. Left ventricular shape changes during the course of evolving heart failure. Am J Physiol. 1992;263(1):H266–70. doi: 10.1152/ajpheart.1992.263.1.H266. [DOI] [PubMed] [Google Scholar]
- [22].Zhong L, Sola S, Tan RS, et al. Effects of surgical ventricular restoration on left ventricular contractility assessed by a novel contractility index in patients with ischemic cardiomyopathy. Am J Cardiol. 2009;103(5):674–9. doi: 10.1016/j.amjcard.2008.10.031. [DOI] [PubMed] [Google Scholar]
- [23].Shewan LG, Coats AJ. Ethics in the authorship and publishing of scientific articles. Int J Cardiol. 2011;153:239–40. doi: 10.1016/j.ijcard.2011.10.119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.