Abstract
Background
Substance use disorders (SUDs) can be conceptualized as a form of risk-taking behavior with the potential for highly aversive outcomes such as health or legal problems. Risky decision-making likely draws upon several related brain processes involved in estimations of value and risk, executive control, and emotional processing. SUDs may result from a dysfunction in one or more of these cognitive processes.
Methods
We performed a systematic literature review of functional neuroimaging studies examining risk-related decision making in individuals with SUDs. A quantitative meta-analysis tool (GingerALE) and qualitative approach was used to summarize the imaging results.
Results
Meta-analysis findings indicate that individuals with SUDs exhibit differences in neural activity relative to healthy controls during risk-taking in the anterior cingulate cortex, orbitofrontal cortex, dorsolateral prefrontal cortex, striatum, insula, and somatosensory cortex. In addition, a qualitative review of the literature suggests that individuals with SUDs may have altered function in the amygdala and ventromedial prefrontal cortex.
Conclusions
The neuroimaging literature reveals that several neural substrates involved in the computation of risk may function suboptimally in SUDs. Future research is warranted to elucidate which computational processes are affected, whether dysfunctional risk-related processing recovers with sobriety, and whether different drugs of abuse have specific effects on risk-taking.
Keywords: Risk-taking, Drug Abuse, Addiction, Neuroimaging, Decision-making
1. INTRODUCTION
Substance use disorders (SUDs), which can refer to abuse or dependence, have profoundly negative impacts on society, including increased rates of morbidity and mortality, disrupted family relations, and a high cost to taxpayers (Nicosia et al., 2009). Recent research has suggested that differences in the neural processing of risk may underlie SUDs (Fishbein et al., 2005; Rogers et al., 1999), making it an important topic for improved understanding of addictive behaviors. Economists define risk as a selection among options with variably distributed outcomes (Lane and Cherek, 2000; Leland and Paulus, 2005; Slovic, 2000). Importantly, this definition of risk implies that an individual knows the probability and magnitude of the outcome associated with each option. This conceptualization differs substantially from the broader meaning of risk used by clinicians and the lay public, which incorporates experiential uncertainty but also emphasizes the potential for large (‘catastrophic’) negative consequences over positive outcomes (Schonberg et al., 2011). As a result, different experimental tasks have been used to probe risk-taking decision making depending upon whether they explore risk as defined by economists or risk more broadly. Although it is possible that common brain mechanisms may be identified in the future, current investigations of risk-taking in SUDs should attend closely to the different conceptions of risk that underlie experimental measures.
Implicit in the definition of SUD is the increased engagement in naturalistic risk-taking behavior, i.e., substance use despite uncertain adverse consequences. There is some experimental evidence that stimulant users engage in more risk-taking behaviors than non-users (Dom et al., 2006; Leland and Paulus, 2005) and that risk-taking propensity correlates with years of substance use (Rogers et al., 1999). Experimental studies also suggest that treatment for SUDs may reduce risk-taking behavior. For example, a group of 81 substance users undergoing inpatient treatment for dependence (e.g., cognitive training and a group-based 12-step program) showed significantly decreased risk taking behavior as measured by the Balloon Analog Risk Task (BART; Lejuez et al., 2002) after 30 days of inpatient treatment relative to their behavior on the BART at the beginning of treatment (Aklin et al., 2009). Furthermore, the degree to which individuals are willing to engage in risk-taking behavior may be an important factor in SUDs. For example, BART risk-taking behavior was a better predictor of drinking problems in a sample of 75 undergraduates than measures of impulsivity or delay-discounting (Fernie et al., 2010). Therefore, the degree of risk-taking may be associated with the severity and prognosis of SUDs.
This systematic literature review aims to provide a preliminary answer to the question, “Are there brain activation differences that distinguish individuals with SUDs from healthy comparison groups during risk-taking decision-making?” We propose that dysfunctions of several neural substrates may result in inappropriate computation of risk in individuals with SUDs. These dysfunctional processes could include: (1) altered valuation of options in ventromedial prefrontal cortex (VMPFC) and outcomes in orbitofrontal cortex (OFC) and striatum; (2) poor estimation of uncertainty and risk in anterior cingulate cortex (ACC) and insular cortex, (3) diminished executive control in dorsolateral prefrontal cortex (DLPFC); (4) reduced influence of emotional salience in amygdala; and (5) attenuated somatic markers in somatosensory cortex.
2. METHODS
2.1. Design
We conducted a meta-analysis of available studies to determine whether brain regions outlined in our hypotheses differed consistently across studies. An extensive literature search revealed only a small number of studies, limiting the generalizability of the present analysis. In consequence, our review should be considered an early attempt to organize the literature rather than a definitive account. To supplement the meta-analysis, we also discuss the findings of relevant studies within the context of the addiction literature.
2.2. Literature Search
A search of several databases (Medline, Google Scholar, Psych Info and Web of Science) was performed to identify potential studies up to 01/24/2013 for inclusion. Search terms included: “risk taking” <or> “decision making” <or> “Iowa Gambling” <or> “Cambridge Risk” <or> “Balloon Analog” <or> “Wheel of Fortune” <and> “substance-related disorders” <or> “drug abuse” <or> “drug dependence” <or> “alcohol” <or> “cocaine” <or> “amphetamine” <or> “heroin” <or> “opiate” <or> “stimulant” <or> “nicotine” <or> “marijuana” <and> “neuroimaging” <or> “neural” <or> “fMRI” <or> “PET.” Following the database search, the reference lists of relevant studies were explored for additional research. This process yielded 24 studies potentially eligible for meta-analysis (see Table 1).
Table 1.
Methodology abbreviations are functional magnetic resonance imaging (fMRI), positron emission tomography (PET), voxel-based morphometry (VBM), single photon emission computed tomography (SPECT) and diffusion-tensor imaging (DTI). Task abbreviations are balloon analog risk task (BART), game of chicken (GOC), Iowa gambling task (IGT), Cambridge risk task (CRT) and wheel of fortune (WOF). Participants abbreviations are alcohol use disorder (AUD), cocaine use disorder (CUD), marijuana use disorder (MUD), methamphetamine use disorder (MAUD), family history of alcoholism (FHA), opiate use disorder (OUD), tobacco use disorder (TUD)
| Study | Methodology | Task | Participants (N) | Reason for exclusion |
|---|---|---|---|---|
| Eligible studies | ||||
| Bolla et al., 2003 | PET | IGT | CUD (13) | |
| Bolla et al., 2005 | PET | IGT | MUD (11) | |
| Cousijn et al., 2012 | fMRI | IGT | MUD (32) | |
| Tanabe et al., 2007 | PET | IGT | CUD, AUD and MAUD (30) | |
| Vaidya et al., 2012 | PET | IGT | MUD (46) | |
| Bjork et al., 2008 | fMRI | GOC | AUD and CUD (17) | Task |
| Crowley et al., 2010 | fMRI | BART | MUD and AUD (20) | Task |
| Ersche et al., 2005 | PET | CRT | MAUD and OUD (45) | Task |
| Fishbein et al., 2005 | PET | CRT | MAUD and OUD (13) | Task |
| Ineligible studies | ||||
| Acheson et al., 2009 | fMRI | IGT | FHA (15) | Lacks history of SUD diagnosis |
| Addicott et al., 2012 | fMRI | WOF | TUD (13) | Lacks comparison group |
| Adinoff et al., 2003 | SPECT | IGT | CUD (13) | Lacks functional imaging |
| Chiu et al., 2008 | fMRI | Investment | TUD (31) | Coordinates not reported |
| Claus and Hutchison, 2011 | fMRI | BART | AUD (79) | Lacks comparison group |
| Cservenka and Nagel, 2012 | fMRI | WOF | FHA (18) | Lacks history of SUD diagnosis |
| Ersche et al., 2006 | PET | CRT | OUD (15) | Sample overlaps with another study |
| Fein et al., 2006 | VBM | IGT | AUD (43) | Lacks functional imaging |
| Lane et al., 2010 | DTI | IGT | CUD (15) | Lacks functional imaging |
| Rogers et al., 1999 | Lesion | CRT | MAUD and OUD (31) | Lacks functional imaging |
| Schneider et al., 2012 | fMRI, VBM | CRT | Adolescents with high levels of alcohol use (33) | Lacks history of SUD diagnosis |
| Tanabe et al., 2009 | VBM | IGT | CUD, AUD and MAUD (19) | Lacks functional imaging |
| Tucker et al., 2004 | SPECT | IGT | CUD (17) | Lacks functional imaging |
| Wesley et al., 2011 | fMRI | IGT | MUD (16) | Examines outcome phase |
| Xiao et al., 2012 | fMRI | IGT | AUD (14) | Coordinates not reported |
2.3. Inclusion and Exclusion Criteria for Meta-Analysis
Studies were included if they met the following four criteria: 1) examination of a SUD group; 2) use of functional neuroimaging methods: either functional magnetic-resonance imaging (fMRI) or positron-emission tomography (PET); 3) inclusion of a risk-taking measurement; and 4) examination of activation during the decision phase of risk-taking (rather than the outcome phase). Given the limited number of studies available, all substances were grouped together, including alcohol, tobacco, marijuana, cocaine, heroin and amphetamine. SUD diagnosis could be current or in remission. The combination of fMRI and PET studies within a single meta-analysis has precedent in the literature, as both techniques observe changes in blood flow related to task performance (zu Eulenburg et al., 2012). Studies were excluded if stereotaxic coordinates were not reported or the study used connectivity techniques (e.g., diffusion-tensor imaging), structural brain imaging or perfusion studies that did not have a functional component. Studies were also excluded if: (1) they lacked a control group; (2) subjects overlapped with another study; or (3) they examined an at-risk SUD group without a specific SUD diagnosis. Since only nine studies met the above criteria, and five used the Iowa Gambling Task (IGT), the Activation likelihood estimation (ALE) was further restricted to the five studies using the IGT to control for task effects. Table 1 lists the studies and provides information on why they were included or excluded.
2.4. Activation Likelihood Estimation (ALE) meta-analysis
The meta-analysis was performed using Ginger ALE v2.1 (Eickhoff et al., 2009), a widely-used, coordinate-based technique for neuroimaging data. ALE models foci from different studies as probability distributions. Results are assessed relative to a null-distribution of random spatial association, hence random-effects modeling of the data, rather than a fixed- or mixed-effects model (Eickhoff et al., 2009, 2012). ALE utilizes a series of permutations to differentiate statistically significant clustering from random clustering (i.e., noise) of foci across multiple independent samples and provides greater spatial resolution compared to previous vote-count meta-analyses (Eickhoff et al., 2009, 2012).
To conduct the meta-analysis, the five eligible studies and their corresponding coordinates were entered into the ALE program. As recommended by Ginger ALE software, a cluster-level threshold was set at a minimum volume of 48mm3, with the false discovery rate method used to correct for multiple comparisons at p<.05 (Genovese et al., 2002). The five studies in the analysis included 253 subjects and 23 foci. Coordinates reported in Montreal-Neurological Institute space were converted to Talairach space. Results indicate the volume of the cluster, its weighted center in Talairach space (x, y, z) and a label of the region by Brodmann area and structural name.
2.5. Tasks in Review
The following paradigms have been used in neuroimaging studies to assess risk related decision-making: the Iowa Gambling Task (IGT; Bechara et al., 1994), the BART, the Wheel of Fortune task (WOF; Ernst et al., 2004), the Game of Chicken task (GOC; Bjork et al., 2008) or the Cambridge Risk Task (CRT; Rogers et al., 1999). Review of these tasks suggests that they are useful predictors of naturalistic risk-taking behavior (Schonberg et al., 2011). Each of these tasks requires subjects to choose between more certain “safe” responses and less certain “risky” responses in an attempt to maximize gains (see Table 2). The tasks vary in degree of uncertainty, number of options, and the extent to which the risky option is disadvantageous, e.g. in the BART, some risk-taking is necessary to earn points, while in the IGT minimal risk-taking provides the most points.
Table 2.
Descriptions of Risk-Taking Paradigms in Review
| Task | Description |
|---|---|
| Iowa Gambling Task (Bechara et al., 1994) | The IGT presents subjects with four decks of cards and asks them to make repeated selections from among the decks. The selected card may add or subtract points from the subject’s score. Two of the decks, “bad decks,” offer higher rewards but also even greater losses, resulting in an overall loss across time. Conversely, two “good decks” offer smaller payoffs but smaller losses, and an overall gain across trials. |
| Balloon Analog Risk Task (BART; Lejuez et al., 2002) | The BART consists of computer-simulated balloon pumping trials, where pumping the balloon to a larger size results in increased earnings, but if the balloon pops, the subject loses all the earnings for the trial. The subject decides how many pumps to administer before cashing out. |
| Wheel of Fortune (Ernst et al., 2004) | There are three conditions with varying degrees of uncertainty. In the first two conditions, subjects choose between two options, one with a higher, but less likely payout, and another with a smaller, more likely payout. Across trials, the risky options (i.e. more valuable, but less likely, gain) result in a lower overall payout. In the third condition, subjects have two options that are equally likely (50%/50%) to result in a gain. |
| Game of Chicken (Bjork et al., 2008) | Subjects begin accruing money at the start of the trial but attempt to stop before a time-meter runs out or risk one of three outcomes: 1) no-gain, 2) loss of money accrued or 3) loss of money accrued multiplied by two (double loss). As soon as the subject presses a button, the trial begins, but subjects must press the button a second time for reward accrual to stop or risk an undesired outcome. |
| Cambridge Risk Task (Rogers et al., 1999) | Subjects are told that a token is hidden in one of ten boxes displayed on a computer monitor. Some of the boxes are blue, some red, and the ratio varies across trials (e.g. 9:1, 7:3). Subjects select whether they think the token is in a red or a blue box. After the color is chosen, the subjects place a “bet” on how sure they are of their choice. An amount of points flash on the screen in an ascending sequence, and the subject presses a button to indicate how many points they wish to bet. After the bet, the outcome is revealed and the amount of the bet is either added (following a win) or subtracted (following a loss) from the subjects’ total. |
3. RESULTS
3.1. ALE Results
The meta-analysis located eleven significant clusters, including regions in OFC, DLPFC, somatosensory cortex, ACC and insula (see Table 3). Directionality of the observed differences was not measured in the meta-analysis due to sample size restrictions. However, our qualitative review of the literature addresses this issue and provides an interpretation of activation differences between SUD and control groups. Sections are organized by function in risk-taking, including estimation of value and risk, executive control, and influence of bodily state and emotions. Sections are further divided into anatomical regions.
Table 3.
Clusters surviving meta-analysis thresholding. These eleven clusters represent significant activation differences between groups with substance use disorders (e.g. cocaine, alcohol or marijuana) and comparison groups across five studies using the Iowa Gambling Task either using Positron Emission Tomography or functional Magnetic Resonance Imaging.
| Volume (mm3) |
x | y | z | L/R | Area | Brodmann Area |
|---|---|---|---|---|---|---|
| 152 | 18 | 28 | −10 | R | Orbitofrontal | 47 |
| 88 | 24 | 40 | 36 | R | Dorsolateral prefrontal | 9 |
| 72 | −54 | −16 | 44 | L | Somatosensory | 1 |
| 56 | 32 | −58 | −36 | R | Cerebellar tonsil | |
| 56 | −40 | 2 | −36 | L | Middle temporal gyrus | 38 |
| 56 | −38 | −6 | −18 | L | Temporal lobe | 20 |
| 56 | −2 | 24 | −8 | L | Anterior cingulate | 32 |
| 56 | −24 | −6 | 6 | L | Ventral striatum | |
| 56 | 16 | 58 | 14 | R | Dorsolateral prefrontal | 10 |
| 56 | −34 | −20 | 16 | L | Insula | 13 |
| 56 | −16 | 38 | 20 | L | Dorsolateral prefrontal | 9 |
3.2 Estimation of Value and Risk
3.2.1 Striatum
The striatum can be divided into two anatomical regions, the ventral (e.g. nucleus accumbens) and dorsal striatum (e.g., putamen and caudate), both of which have been implicated in processing reward value (Kable and Glimcher, 2009). In monkeys, single-unit recordings in the caudate (Lau and Glimcher, 2008) and putamen (Samejima et al., 2005) reveal populations of neurons that fire in proportion to the subjective value of an action, whether or not the action was chosen. Moreover, a human neuroimaging study demonstrated that subjective valuation in the striatum extends to decisions made under risk, wherein caudate activity during selection of a risky option was correlated with likelihood of choosing a risky option again in later trials (Engelmann and Tamir, 2009). These findings point to a role for the striatum in subjective valuation of risky decisions.
SUD groups have shown greater dorsal striatum activation than healthy controls in several risk-taking studies. For instance, cocaine-dependent participants who had greater putamen activation than controls during the decision-phase of the IGT also chose risky options more frequently and earned less money during the task (Bolla et al., 2003). Since level of putamen activity has been linked to the subjective value of an outcome (Samejima et al., 2005), greater activation among cocaine-dependent individuals could indicate that they find risky options subjectively more valuable, consistent with their increased selection of risky choices. In another study, stimulant (i.e., cocaine or amphetamine) users and a control group were shown a playing card from a deck containing 2–10 cards and then asked to guess if the next card would be higher or lower. When a 5, 6 or 7 was shown, the probability of the next card being higher or lower was roughly equal, so this was considered the uncertain condition. Stimulant users showed greater caudate activation relative to the control group during uncertain decisions. Since the reward was equivalent for uncertain (5–7) or more certain (2–4, 8–10) decisions, stimulant users’ greater activation during decision-making suggests they not only value risky options more highly than control subjects, but they may also value uncertainty itself more highly.
In addition, early stages of SUDs are marked by changes in reward evaluation in the ventral striatum. For example, a recent study demonstrated that adolescents with early signs of SUDs displayed greater risk-taking CRT behavior than controls, accompanied by decreased bilateral ventral striatum activation during reward anticipation (Schneider et al., 2012). Furthermore, structural MRI showed that participants who took the most risk displayed the lowest striatal volume (Schneider et al., 2012). Altered reward processing in the ventral striatum in at-risk adolescents is consistent with evidence that the development of SUDs corresponds to a transition from initial reinforcing effects of substance use in the ventral striatum to habitual drug-seeking driven by the dorsal striatum (Everitt and Robbins, 2005).
3.2.2 Ventromedial Prefrontal Cortex
VMPFC is considered to be a key region for integrating information about the current subjective value of available options (Kable and Glimcher, 2009; Paulus and Frank, 2003). For instance, Plassman et al. (2007) showed hungry participants snack foods and asked them how much they would pay for each. They found that VMPFC activation positively correlated with the subjective value of each item. Since patients with VMPFC lesions consistently perform poorly on the IGT (Bechara et al., 1994), VMPFC may be involved in evaluation of options during risk-taking, and VMPFC integrity may be essential to make advantageous decisions.
Risk-taking behavior among individuals with SUDs could reflect VMPFC impairments. Rogers et al. (1999) indicated that the performance of amphetamine abusers closely resembled VMPFC lesion patients’ CRT performance, suggesting that amphetamine dependence may be associated with similar neural deficits. Like VMPFC lesion patients, amphetamine abusers: (1) exhibited longer response times than controls when predicting which outcome they expected to occur during the CRT; and (2) were more likely to select the outcome least likely to occur, a suboptimal response strategy (Rogers et al., 1999). Moreover, several IGT studies provide evidence for altered VMPFC activation in substance abusers. Recent research demonstrated that chronic marijuana smokers exhibited greater VMPFC activation than controls during the IGT decision phase that was positively associated with lifetime marijuana use (Vaidya et al., 2012), findings consistent with prior research demonstrating heightened VMPFC activation during the IGT in cocaine-dependent participants (Bolla et al., 2003). Although findings during the IGT decision phase indicate hyperactive VMPFC in SUDs, a study examining brain activation during the IGT outcome phase determined that chronic marijuana smokers exhibited lower VMPFC activation than control subjects within this context (Wesley et al., 2011). Furthermore, whereas controls with the greatest VMPFC activation in response to outcomes during early rounds of the IGT earned the most money during remaining rounds of the task, no relationship was evident between VMPFC activity and task performance for marijuana users, suggesting a disconnect between outcome valuation and behavior in SUDs. In addition, model analysis of chronic marijuana users performance on the IGT indicates that, relative to controls, their decisions are less influenced by losses and more influenced by gains (Fridberg et al., 2010). On the whole, enhanced activation of the VMPFC during decision-making may lead individuals with SUDs to choose risky options because they subjectively regard rewards as more valuable despite potential for loss.
3.2.3 Orbitofrontal Cortex
Since the OFC may be involved in the evaluation (including economic valuation) of potential outcomes (Schoenbaum and Esber, 2010), it is interesting that several studies have found that SUD groups exhibit altered OFC activation during risk-taking. A recent PET study revealed that 28-day abstinent marijuana-dependent participants displayed poorer IGT performance and displayed lower right lateral OFC activation than controls (Bolla et al., 2005)). In addition, current and former dependent opiate (e.g., heroin and methadone) users displayed increased left lateral OFC activation than controls during the CRT (Ersche et al., 2005). Moreover, heroin users who had been using the longest had the greatest left OFC activation during the CRT, suggesting duration-dependent opiate effects on OFC function (Ersche et al., 2006). Greater OFC activation reported by Ersche et al. (2005) contrasts with the decreased activation reported by Bolla et al. (2003; 2005), possibly reflecting task differences. For example, in a group of cocaine users, CRT and IGT performance were not correlated (Monterosso et al., 2001). If the OFC is involved in evaluating outcomes, altered OFC activity in SUDs may indicate disruption of an evaluative process that is critical to decisions about risk.
3.2.4 Anterior Cingulate Cortex
According to the prediction of response-outcome theory, ACC is a critical substrate for risk processing due to its role in assessing the magnitude and probability of uncertain outcomes (Alexander and Brown, 2010; Brown and Braver, 2007). Consistent with this hypothesis, ACC activation during risk-taking decision-making on the BART signaled that a person would cash out (i.e., avoid risk), whereas ACC deactivation indicated that a participant would continue inflating the balloon (i.e., seek risk; Fukunaga et al., 2012). Furthermore, in a study where participants were asked to choose between sure gains and risky ones, participants with the least ACC activation tended to be more risk-seeking for low-probability outcomes (e.g., choose a 5-percent chance for $1000 over a sure $75) and risk averse for high-probability outcomes (e.g., choose a sure $850 over a 95-percent chance for $1000; Paulus and Frank, 2006). Moreover, resting cerebral blood flow within the ACC correlated positively with performance on the IGT, pointing to a relationship between ACC activity and risk-taking (Adinoff et al., 2003; although see Tucker et al., 2004). Thus, ACC recruitment may be critical for normative risk processing.
Furthermore, ACC disruption could underlie altered risk-taking behavior in SUDs. Fishbein et al. (2005) found that recently abstinent polydrug abusers (i.e., abusing one or more of the following: marijuana, cocaine, heroin or amphetamine) displayed greater risk-taking behavior and lower rostral ACC activation than control participants during the CRT. Among substance abusers, ACC activation correlated negatively with risky choices, i.e., users with the lowest ACC activation took the greatest number of risks (Fishbein et al., 2005). Similarly, two studies showed that decreased dorsal ACC activation corresponded to heightened risk-taking in individuals with opiate, amphetamine or alcohol SUDs (Bjork et al., 2008; Ersche et al., 2005). In contrast, at-risk individuals with a family history of alcoholism exhibited greater dorsal ACC activation than controls during the IGT (Acheson et al., 2009), findings suggesting that brain mechanisms involved in the transition to SUD may not be the same as those involved in chronic SUD cases. On the whole, these studies indicate the ACC may function to avoid highly risky options and this function may be deficient among individuals with SUDs.
The relationship between diminished ACC activation and selection of unlikely outcomes in decision-making tasks may result from error-prediction failure (Bolla et al., 2004; Hester et al., 2009). Cocaine-dependent subjects showed diminished rostral ACC activation in a task that required subjects to monitor and correct their errors, and the degree of activation negatively correlated with grams of cocaine used per week (Bolla et al., 2004). Similarly, opiate-dependent subjects performed poorly on a task of response inhibition and lacked error-dependent rostral ACC activation (Forman et al., 2004). Diminished awareness of errors may prevent individuals with SUDs from learning from mistakes because mistakes do not register. Thus, decisions are made on a trial by trial basis and reflect only immediate payoff (Fishbein et al., 2005). While in controls a significant relationship has been found between risk-aversion (‘playing safe’) and rostral ACC activation following a loss, no such relationship existed in opiate dependent subjects, indicating substance abusers may not register the loss as an error (Ersche et al., 2005). Chronic marijuana smokers have also exhibited lower ACC activation than controls in response to IGT feedback (Wesley et al., 2011), and smokers with the lowest ACC in early IGT stages displayed the poorest subsequent performance. Thus, SUD individuals may not take more risks initially relative to controls, but may continue taking risks despite negative consequences due to lack of error-awareness.
3.3 Executive Control
Dorsolateral Prefrontal Cortex
DLPFC is implicated in executive control and working memory (MacDonald et al., 2000) (Champod and Petrides, 2007) and appears to be attenuated in SUDs during risky decision-making. For instance, Bolla et al. have reported decreased right DLPFC activation among cocaine-dependent (2003) and marijuana-dependent (2005) groups during the IGT. Moreover, Ersche et al. (2005) observed that SUD participants displayed lower right DLPFC activation than controls during the CRT. At-risk adolescents with a family history of alcoholism also exhibited lower DLPFC activation during the WOF than adolescents without a family history of alcoholism (Cservenka and Nagel, 2012). With respect to decision making more broadly, cocaine users also showed deficits in executive function and diminished DLPFC activation during the Stroop task (Bolla et al., 2004). Attenuated DLPFC activation accompanied by evidence for diminished executive control among various SUD groups suggests that substance abusers may possess a diminished capacity to integrate information about risk and value when making decisions.
Executive control processes in the DLPFC may collaborate with risk monitoring regions such as the insula and ACC. Failure to distinguish risky from safe choices could result from diminished connectivity with risk-monitoring regions. In a study of healthy subjects, participants taking a higher number of risks exhibited weakened connectivity between the DLPFC and the insula (Cox et al., 2010). In addition, a diffusion tensor imaging (DTI) study by Lane et al. (2010) indicated that cocaine users possessed diminished fractional anisotropy, a measure of connectivity, in the corona radiata, a frontal span of white matter that connects DLPFC, ACC and insular regions. Further, as diminished connectivity corresponded to impaired performance on the IGT, this impairment corroborates a model of decision-making that relies on communication between the DLPFC, insula and ACC. Similarly, chronic MDMA (3,4-methylenedioxy-N-methamphetamine) users chose a higher number of disadvantageous options on the IGT than controls and DTI results showed evidence for axonal damage in the rostral corpus callosum, which connects the two halves of the prefrontal cortex (Moeller et al., 2007). SUDs may be characterized by a disruption of this functional network.
3.4 Influence of Body State and Emotion
3.4.1 Insula
Considerable evidence indicates that the insula is involved in the integration of the current state of the body with past memories to guide behavior such as risk-taking (Craig, 2002, 2009; Paulus, 2007). Several studies have indicated that the insula in conjunction with the ACC contributes to the assessment of likelihood and magnitude of consequences during risk-taking (Preuschoff et al., 2008). Preuschoff et al. (2006; 2008) asked participants to guess, after a first card was drawn from a deck, whether a second card would be higher. When compared against the probability of winning, bilateral anterior insula activation had an inverted-U shape, such that activation was greatest when risk was highest (i.e., 50 percent chance of winning) and lowest when risk was lowest (chances approaching 0 or 100 percent). Further, Paulus et al. (2003) demonstrated that insular activation during risk-taking correlates with the likelihood of avoiding risk following punishment, suggesting that insular activity may be involved in risk aversion. Insular activation may predict risk magnitude and then recruit risk-avoidance mechanisms when a critical threshold is reached, similar to other winner-takes-all decision-making regions of the brain (Kable and Glimcher, 2009). Adolescent SUDs displayed lower insular activation than controls during risky decision-making on the BART (Crowley et al., 2010). Moreover, a recent study demonstrated that alcohol dependent subjects with the lowest insula activation during decision-making took the highest number of risks on the BART (Claus and Hutchison, 2011), indicating that, as in healthy individuals (Paulus et al., 2003), anterior insula activation is associated with risk aversion in substance users. However, attenuated insular activation of substance abusing adolescents suggests that individuals with SUDs may require a greater magnitude of risk before they reach a threshold triggering risk aversion.
In contrast to BART findings, IGT research reported heightened insular activation among groups with SUDs, such as chronic marijuana users (Vaidya et al., 2012) and adolescent binge drinkers (Xiao et al., 2012). Among binge-drinkers, insular activation was positively correlated with both alcohol consumption and urgency, the tendency to act impulsively when emotionally aroused (e.g., suddenly punching a wall when angry; Whiteside and Lynam, 2001). The correlation with urgency suggests that binge-drinkers may find the high-risk option on the IGT more salient and act based on emotion, despite an eventual losing outcome. The increases in insular activation observed by Xiao et al. (2012) and Vaidya et al. (2012) contrasts with the decreased insular activation reported by Crowley et al. (2010), potentially reflecting task differences. Crowley et al. (2010) employed the BART, where risk-magnitude increases incrementally during a single trial, while Xiao et al. (2012) and Vaidya et al. (2012) used the IGT, where an option’s riskiness remains static during a single trial. Risky options may be more salient to substance abusers when compared to safe options, but when risk increases gradually these individuals may be less sensitive to changes in risk magnitude.
3.4.2 Primary Somatosensory Cortex
The identification of the primary somatosensory cortex through our meta-analysis is consistent with the somatic marker theory of SUDs, which posits that bodily states become associated with experiences, and the brain’s representations of those body states are used to guide decisions (Verdejo-Garcia and Bechara, 2009). Diminished neural representation of somatic states could prevent this information from guiding decisions, hence lesions that affect somatic-markers lead to poor decision making (Damasio et al., 1991). A study examining risk-taking among smokers on a day where they were allowed to smoke cigarettes ad libitum and again following a day of smoking abstinence found differential activation in the somatosensory cortex (Addicott et al., 2012). Specifically, during the decision-phase of the WOF, smokers exhibited greater activation in the somatosensory cortex following a day of abstinence than on a smoking day, suggesting that continued drug use may lead to diminished processing in the somatosensory cortex.
3.4.3 Amygdala
Based on evidence for the amygdala’s role in cue-outcome learning (Davis and Whalen, 2001), the somatic marker hypothesis proposes that disrupted amygdala function could lead individuals with SUDs to take more risks because they fail to appropriately link outcomes with decisions (Verdejo-Garcia and Bechara, 2009). Fein et al. (2006) showed reduced amygdalar volume among long-term abstinent alcoholics in conjunction with impaired IGT performance, suggesting that diminished amygdala integrity underlies risk-taking deficits. Crowley et al. (2010) also reported attenuated amygdalar activation during the BART among adolescents with problematic substance use. As studies suggest the amygdala is critical for cue-outcome learning (Davis and Whalen, 2001), decreased amygdalar integrity or activation may prevent the amygdala from signaling negative outcomes associated with a cue and triggering risk-avoidance mechanisms. Thus, amygdalar activation may be necessary to avoid choices linked with punishment. In contrast, however, binge-drinking adolescents exhibited greater amygdala activation and risk-taking behavior than controls during the decision-phase of the IGT (Xiao et al., 2012). These latter findings were interpreted in such a way that amygdala activation reflected an emotional cue for decision-making, but it signaled reward-seeking without consideration of negative consequences (Xiao et al., 2012). As the amygdala has been linked to reward-based and aversive learning (Davis and Whalen, 2001), it may be too early to determine if SUDs are associated with increased or decreased amygdalar activation, as the limited evidence has been equivocal. Altered amygdalar activation may bias individuals to seek rewards regardless of uncertain but possible negative consequences, or they may fail to notice negative outcomes due to lack of an emotional signal to avoid risk.
4. DISCUSSION
This review examined differences in neural processing of risk between individuals with SUDs and healthy controls. Individuals with SUDs show several processing abnormalities during risk-taking decision-making, which include altered valuation of options (VMPFC) and outcomes (OFC and striatum), poor estimation of uncertainty (ACC and insular cortex), diminished executive control (DLPFC), and an attenuated influence of emotional salience (amygdala), and reduced responsiveness to somatic markers (somatosensory cortex). These neural processing differences during risk-taking among individuals with SUDs have been linked to poorer behavioral performance on risk-taking tasks and a more extensive history of substance use.
Our quantitative meta-analysis indicated that individuals with SUDs identified altered processing of risk in several key regions, including the ACC, insula, primary somatosensory cortex, striatum, OFC and DLPFC. Since the primary somatosensory cortex responds to sensations in the body and evidence suggests that the insula is involved in representation of bodily states (Craig, 2009), altered processing in these two regions among individuals with SUDs is consistent with the somatic marker hypothesis. This hypothesis proposes that decision-making reflects neural representations of body states, so altered activation in the insula and primary somatosensory cortex could indicate disrupted representations of body states (Verdejo-Garcia and Bechara, 2009), which may in turn affect risky decisions made by individuals with SUDs. Many studies have indicated that the striatum, OFC and VMPFC are involved in the subjective valuation of rewards (Lau and Glimcher, 2008; Padoa-Schioppa and Assad, 2006; Paulus and Frank, 2003). Since the ACC and insula contribute to risk prediction, attenuated ACC and insular processing associated with SUDs may indicate a failure to predict and monitor risk. In comparison, attenuated DLPFC activation in SUDs suggests that substance abusers may fail to recruit executive control mechanisms involved in the integration of value and risk. Research in healthy populations indicates that amygdala activation corresponds to processing of aversive outcomes (Davis and Whalen, 2001). Thus, decreased amygdala activation during risk-taking in SUDs supports the hypothesis that substance users may be less sensitive to loss. Taken together, these findings suggest altered processing of risk among individuals diagnosed with SUDs, potentially characterized by under recruitment of regions critical to risk evaluation and decision-making coupled with heightened activation of reward-processing regions (see Figure 1 for a summary model). This altered processing may dispose individuals to increased risk-taking.
Figure 1.
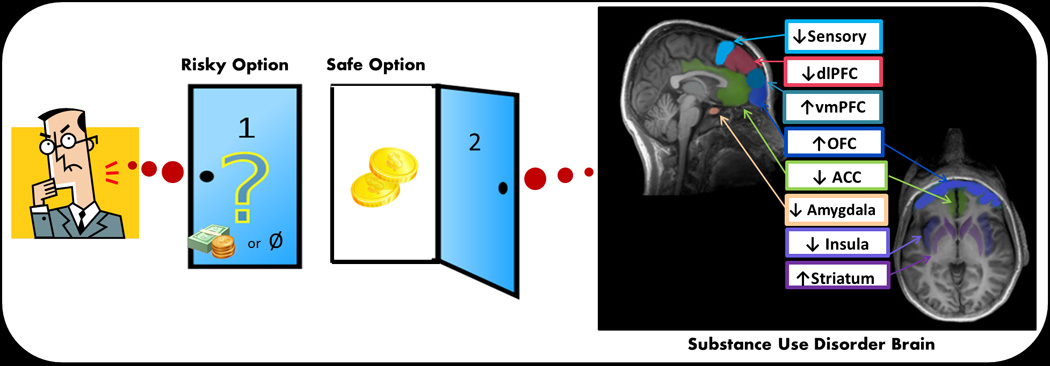
Hypothetical model of risk processing in SUD brain. Ventromedial prefrontal cortex (VMPFC), orbitofrontal cortex (OFC) and striatum contribute to the subjective evaluation of options and play an enhanced role in risk-taking decisions for individuals with SUDs. The rest of the regions in the model play a diminished role in risk-taking decisions for individuals with SUDs. Anterior cingulate cortex (ACC) and insula are involved in processing risk magnitude and probability. The insula, in conjunction with the primary somatosensory cortex, also contributes to assessment of body state. The amygdala contributes emotional evaluation of options. Finally, dorsolateral prefrontal cortex (DLPFC) is involved in executive control, possibly integrating information from risk and reward evaluation.
4.1. Future directions
Most neuroimaging studies examining risk-taking in substance users have utilized the IGT (see Table 1). Thirteen studies employed this task, relative to only five using the CRT, the second most frequently used task. However, although participants choose among options with variably distributed outcomes in the IGT, meeting the definition of risk-taking, they must also learn which decks are advantageous. Hence, IGT is not a pure risk-taking task, but is also an associative learning task (Buelow and Suhr, 2009). To better understand which regions of the brain underlie risk-taking deficits among individuals with SUDs, use of a wider variety of tasks is recommended. Furthermore, all of the studies reviewed employed paradigms in which the participant’s goal was to maximize earnings. However, risk-taking for gain is distinct behaviorally from risk-taking for loss. For example, the same person may avoid risk when choosing between definitively receiving $900 or a 90% chance to receive $1,000, but seek risk when choosing between definitely losing $900 or a 90% chance to lose $1,000 (Kahneman and Tversky, 1979). Substance use may reflect an individual’s attempt to escape aversive emotions (e.g., hopelessness) rather than attain a reward (e.g., a high), so future studies should employ tasks that assess risk-taking for loss, such as the recently developed Maryland Resource for the Behavioral Utilization of the Reinforcement of Negative Stimuli (MRBURNS) task (Macpherson et al., 2012).
It remains unclear whether altered risk-taking disposes individuals to SUDs or is a consequence of repeated substance use. There is evidence that risk-taking propensity precedes substance use disorders. A longitudinal study indicated that 3 year olds who had trouble sitting still were more likely to have alcohol use disorders at age 21 (Caspi et al., 1997). Also, adolescents with a family history of alcoholism had diminished DLPFC activation during risk-taking (Cservenka and Nagel, 2012). Both of these studies suggest pre-existing alterations in risk-processing dispose individuals to SUDs. There is also evidence that substance use increases risk-taking. For example, a study comparing rats administered chronic amphetamine doses or saline showed that amphetamine use led to decreased ACC excitability along with difficulties learning to avoid levers that produced a shock (Tse et al., 2011). Most imaging studies examined cross-sectional samples, precluding interpretation of whether risk-taking is cause or consequence of substance use, so longitudinal work is needed to understand risk-processing differences across stages of addiction. One study compared smokers’ neural activation during the WOF on a day when they smoked at will and on another day when they were forbidden to smoke (Addicott et al., 2012). Insular activation was significantly higher on the day the smokers did not smoke, indicating that neural processing may change as substance use habits change (Addicott et al., 2012). Clearly more longitudinal studies are needed to expand our understanding of the role of risk-processing in drug use initiation, transition to dependence and recovery.
4.2. Limitations
Several limiting factors must be considered when interpreting the results of the meta-analysis and the qualitative review. Due to the small number of studies available, the meta-analysis grouped together studies examining cocaine, alcohol, methamphetamine dependent participants. However, different substances could have widely varying effects on neural processing of risk. It is recommended that future research examine differential risk processing between SUD groups to determine specific effects on risk-taking. In addition, our meta-analysis did not report group differences in the amygdala or VMPFC although prior work has demonstrated differences in these structures, underscoring the limited sample size of the meta-analysis and the need for further research. Another important consideration for interpreting functional imaging differences in SUDs, which measures regional blood flow, is that substance use may lead to changes in perfusion. For example, abstinent marijuana users have persistent changes in perfusion in the anterior and middle cerebral arteries (Herning et al., 2001).
4.3. Conclusions
Risk-taking decision-making involves several complex processes, which include but are not limited to the calculation of the magnitude of an outcome (i.e., how good or bad it might be), estimation of the probability associated with the outcome (i.e., how likely is the outcome) and the integration of these processes with current and anticipated bodily states. Review of the risk-taking literature supports that hypothesis that SUD individuals show brain and behavioral dysfunction in several processes. However, our understanding of these dysfunctions is incomplete and future investigations will need to better delineate which process is most sensitively affected, determine whether these dysfunctions precede the development of SUD, and which processes are amenable to intervention to improve outcomes.
Acknowledgements
This work was supported by grants from the National Institute on Drug Abuse (Grant Nos. R01-DA016663, P20-DA027834, R01-DA027797, and R01-DA018307 to Martin Paulus). The authors would like to thank Jennifer Stewart and Sonja Eberson for comments on an earlier draft of this manuscript. The authors would like to thank April May for artistic contributions to the processing model.
Role of funding source
Nothing declared.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Authors Gowin and Paulus conceived the review. Author Gowin conducted document searches and manuscript preparation. Authors Gowin, Mackey and Paulus contributed to the writing and have approved the final manuscript.
Conflict of interest
No financial disclosures or conflicts of interest were reported by the authors of this paper.
References
- Acheson A, Robinson JL, Glahn DC, Lovallo WR, Fox PT. Differential activation of the anterior cingulate cortex and caudate nucleus during a gambling simulation in persons with a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Drug Alcohol Depend. 2009;100:17–23. doi: 10.1016/j.drugalcdep.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addicott MA, Baranger DA, Kozink RV, Smoski MJ, Dichter GS, McClernon FJ. Smoking withdrawal is associated with increases in brain activation during decision making and reward anticipation: a preliminary study. Psychopharmacol. (Berl.) 2012;219:563–573. doi: 10.1007/s00213-011-2404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Devous MD, Sr, Cooper DB, Best SE, Chandler P, Harris T, Cervin CA, Cullum CM. Resting regional cerebral blood flow and gambling task performance in cocaine-dependent subjects and healthy comparison subjects. Am. J. Psychiatry. 2003;160:1892–1894. doi: 10.1176/appi.ajp.160.10.1892. [DOI] [PubMed] [Google Scholar]
- Aklin WM, Tull MT, Kahler CW, Lejuez CW. Risk-taking propensity changes throughout the course of residential substance abuse treatment. Pers. Individ. Dif. 2009;46:454–459. doi: 10.1016/j.paid.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WH, Brown JW. Computational models of performance monitoring and cognitive control. Top. Cogn. Sci. 2010;2:658–677. doi: 10.1111/j.1756-8765.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Momenan R, Smith AR, Hommer DW. Reduced posterior mesofrontal cortex activation by risky rewards in substance-dependent patients. Drug Alcohol Depend. 2008;95:115–128. doi: 10.1016/j.drugalcdep.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J. Neuropsychiatry Clin. Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26:480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Risk prediction and aversion by anterior cingulate cortex. Cogn. Affect. Behav. Neurosci. 2007;7:266–277. doi: 10.3758/cabn.7.4.266. [DOI] [PubMed] [Google Scholar]
- Buelow MT, Suhr JA. Construct validity of the Iowa Gambling Task. Neuropsychol. Rev. 2009;19:102–114. doi: 10.1007/s11065-009-9083-4. [DOI] [PubMed] [Google Scholar]
- Caspi A, Begg D, Dickson N, Harrington H, Langley J, Moffitt TE, Silva PA. Personality differences predict health-risk behaviors in young adulthood: evidence from a longitudinal study. J. Pers. Soc. Psychol. 1997;73:1052–1063. doi: 10.1037//0022-3514.73.5.1052. [DOI] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes. Proc. Natl. Acad. Sci. USA. 2007;104:14837–14842. doi: 10.1073/pnas.0607101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Hutchison KE. Neural mechanisms of risk taking and relationships with hazardous drinking. Alcohol. Clin. Exp. Res. 2011;36:932–940. doi: 10.1111/j.1530-0277.2011.01694.x. [DOI] [PubMed] [Google Scholar]
- Cox CL, Gotimer K, Roy AK, Castellanos FX, Milham MP, Kelly C. Your resting brain CAREs about your risky behavior. PLoS One. 2010;5:e12296. doi: 10.1371/journal.pone.0012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Dalwani MS, Mikulich-Gilbertson SK, Du YP, Lejuez CW, Raymond KM, Banich MT. Risky decisions and their consequences: neural processing by boys with antisocial substance disorder. PLoS One. 2010;5:e12835. doi: 10.1371/journal.pone.0012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Nagel BJ. Risky decision-making: an fMRI study of youth at high risk for alcoholism. Alcohol. Clin. Exp. Res. 2012;36:604–615. doi: 10.1111/j.1530-0277.2011.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Somatic markers and the guidance of behavior. In: Levin H, Eisenberg H, Benton A, editors. Frontal Lobe Function and Dysfunction. Vol. New York: Oxford University Press; 1991. pp. 217–228. [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dom G, De Wilde B, Hulstijn W, Van Den Brink W, Sabbe B. Decision-making deficits in alcohol-dependent patients with and without comorbid personality disorder. Alcohol. Clin. Exp. Res. 2006;30:1670–1677. doi: 10.1111/j.1530-0277.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation metaanalysis revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JB, Tamir D. Individual differences in risk preference predict neural responses during financial decision-making. Brain Res. 2009;1290:28–51. doi: 10.1016/j.brainres.2009.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Zarahn E, Leibenluft E, Zametkin A, Towbin K, Blair J, Charney D, Pine DS. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Lewis SJ, Clark L, Stocks-Gee G, London M, Deakin JB, Robbins TW, Sahakian BJ. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacol. (Berl.) 2005;180:612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Roiser JP, Fryer TD, London M, Robbins TW, Sahakian BJ. Differences in orbitofrontal activation during decision-making between methadone-maintained opiate users, heroin users and healthy volunteers. Psychopharmacol. (Berl.) 2006;188:364–373. doi: 10.1007/s00213-006-0515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fein G, Landman B, Tran H, McGillivray S, Finn P, Barakos J, Moon K. Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage. 2006;32:1465–1471. doi: 10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie G, Cole JC, Goudie AJ, Field M. Risk-taking but not response inhibition or delay discounting predict alcohol consumption in social drinkers. Drug Alcohol Depend. 2010;112:54–61. doi: 10.1016/j.drugalcdep.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Fishbein DH, Eldreth DL, Hyde C, Matochik JA, London ED, Contoreggi C, Kurian V, Kimes AS, Breeden A, Grant S. Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Brain Res. Cogn. Brain Res. 2005;23:119–136. doi: 10.1016/j.cogbrainres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, Barch DM, Stenger VA, Wick-Hull C, Pisarov LA, Lorensen E. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biol. Psychiatry. 2004;55:531–537. doi: 10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Fridberg DJ, Queller S, Ahn W-Y, Kim W, Bishara AJ, Busemeyer JR, Porrino L, Stout JC. Cognitive mechanisms underlying risky decision-making in chronic cannabis users. J. Math Psychol. 2010;54:28–38. doi: 10.1016/j.jmp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga R, Brown J, Bogg T. Decision making in the Balloon Analogue Risk Task (BART): anterior cingulate cortex signals loss aversion but not the infrequency of risky choices. Cogn. Affect. Behav. Neurosci. 2012;12:479–490. doi: 10.3758/s13415-012-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Herning RI, Better WE, Tate K, Cadet JL. Marijuana abusers are at increased risk for stroke. Preliminary evidence from cerebrovascular perfusion data. Ann. N. Y. Acad. Sci. 2001;939:413–415. doi: 10.1111/j.1749-6632.2001.tb03652.x. [DOI] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63:733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47:263–291. [Google Scholar]
- Lane SD, Cherek DR. Analysis of risk taking in adults with a history of high risk behavior. Drug Alcohol Depend. 2000;60:179–187. doi: 10.1016/s0376-8716(99)00155-6. [DOI] [PubMed] [Google Scholar]
- Lane SD, Steinberg JL, Ma L, Hasan KM, Kramer LA, Zuniga EA, Narayana PA, Moeller FG. Diffusion tensor imaging and decision making in cocaine dependence. PLoS One. 2010;5:e11591. doi: 10.1371/journal.pone.0011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B, Glimcher PW. Value representations in the primate striatum during matching behavior. Neuron. 2008;58:451–463. doi: 10.1016/j.neuron.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J. Exp. Psychol. Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Leland DS, Paulus MP. Increased risk-taking decision-making but not altered response to punishment in stimulant-using young adults. Drug Alcohol Depend. 2005;78:83–90. doi: 10.1016/j.drugalcdep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Macpherson L, Calvin NT, Richards JM, Guller L, Mayes LC, Crowley MJ, Daughters SB, Lejuez CW. Development and preliminary validation of a behavioral task of negative reinforcement underlying risk-taking and its relation to problem alcohol use in college freshmen. Alcohol. Clin. Exp. Res. 2012;36:950–957. doi: 10.1111/j.1530-0277.2011.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Lane SD, Buzby M, Swann AC, Hasan KM, Kramer LA, Narayana PA. Diffusion tensor imaging in MDMA users and controls: association with decision making. Am. J. Drug Alcohol Abuse. 2007;33:777–789. doi: 10.1080/00952990701651564. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ehrman R, Napier KL, O'Brien CP, Childress AR. Three decision-making tasks in cocaine-dependent patients: do they measure the same construct? Addiction. 2001;96:1825–1837. doi: 10.1046/j.1360-0443.2001.9612182512.x. [DOI] [PubMed] [Google Scholar]
- Nicosia N, Pacula R, Kilmer B, Lundberg R, Chiesa J. The Economic Cost of Methamphetamine Use in the United States, 2005. RAND Cooperation; Santa Monica: 2009. pp. 1–171. [Google Scholar]
- Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Frank LR. Ventromedial prefrontal cortex activation is critical for preference judgments. Neuroreport. 2003;14:1311–1315. doi: 10.1097/01.wnr.0000078543.07662.02. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Frank LR. Anterior cingulate activity modulates nonlinear decision weight function of uncertain prospects. Neuroimage. 2006;30:668–677. doi: 10.1016/j.neuroimage.2005.09.061. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J. Neurosci. 2007;27:9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff K, Bossaerts P, Quartz SR. Neural differentiation of expected reward and risk in human subcortical structures. Neuron. 2006;51:381–390. doi: 10.1016/j.neuron.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J. Neurosci. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science. 2005;310:1337–1340. doi: 10.1126/science.1115270. [DOI] [PubMed] [Google Scholar]
- Schneider S, Peters J, Bromberg U, Brassen S, Miedl SF, Banaschewski T, Barker GJ, Conrod P, Flor H, Garavan H, Heinz A, Ittermann B, Lathrop M, Loth E, Mann K, Martinot JL, Nees F, Paus T, Rietschel M, Robbins TW, Smolka MN, Spanagel R, Strohle A, Struve M, Schumann G, Buchel C. Risk taking and the adolescent reward system: a potential common link to substance abuse. Am. J. Psychiatry. 2012;169:39–46. doi: 10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Esber GR. How do you (estimate you will) like them apples? Integration as a defining trait of orbitofrontal function. Curr. Opin. Neurobiol. 2010;20:205–211. doi: 10.1016/j.conb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg T, Fox CR, Poldrack RA. Mind the gap: bridging economic and naturalistic risktaking with cognitive neuroscience. Trends Cogn. Sci. 2011;15:11–19. doi: 10.1016/j.tics.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovic PE. Perception of Risk. Vol. London: Earthscan Publications Ltd; 2000. [Google Scholar]
- Tse MT, Cantor A, Floresco SB. Repeated amphetamine exposure disrupts dopaminergic modulation of amygdala-prefrontal circuitry and cognitive/emotional functioning. J. Neurosci. 2011;31:11282–11294. doi: 10.1523/JNEUROSCI.1810-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KA, Potenza MN, Beauvais JE, Browndyke JN, Gottschalk PC, Kosten TR. Perfusion abnormalities and decision making in cocaine dependence. Biol. Psychiatry. 2004;56:527–530. doi: 10.1016/j.biopsych.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Vaidya JG, Block RI, O'Leary DS, Ponto LB, Ghoneim MM, Bechara A. Effects of chronic marijuana use on brain activity during monetary decision-making. Neuropsychopharmacology. 2012;37:618–629. doi: 10.1038/npp.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2009;56(Suppl. 1):48–62. doi: 10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res. 2011;191:51–59. doi: 10.1016/j.pscychresns.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Pers. Individ. Dif. 2001;30:669–689. [Google Scholar]
- Xiao L, Bechara A, Gong Q, Huang X, Li X, Xue G, Wong S, Lu ZL, Palmer P, Wei Y, Jia Y, Johnson CA. Abnormal affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychol. Addict Behav. 2012 doi: 10.1037/a0027892. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- zu Eulenburg P, Caspers S, Roski C, Eickhoff SB. Meta-analytical definition and functional connectivity of the human vestibular cortex. Neuroimage. 2012;60:162–169. doi: 10.1016/j.neuroimage.2011.12.032. [DOI] [PubMed] [Google Scholar]


