Abstract
Purpose
To evaluate the risk, risk factors, and visual impact of choroidal neovascularization (CNV) in uveitis cases.
Design
Retrospective cohort study
Methods
Standardized medical record review at five tertiary centers.
Results
Among 15,137 uveitic eyes (8,868 patients), CNV was rare in the cases of anterior or intermediate uveitis. Among the 4,041 eyes (2,307 patients) with posterior or panuveitis, 81 (2.0%) presented with CNV. Risk factors included posterior uveitis in general and specific uveitis syndromes affecting the outer retina/retinal pigment epithelium (RPE)/choroid interface. Among the 2,364 eyes (1,357 patients) with posterior or panuveitis and free of CNV at the time of cohort entry, the cumulative two-year incidence of CNV was 2.7% (95% confidence interval (95%CI): 1.8-3.5%). Risk factors for incident CNV included currently active inflammation (adjusted HR [aHR] 2.13, 95%CI: 1.26-3.60), preretinal neovascularization (aHR 3.19, 95%CI: 1.30-7.80), and prior diagnosis of CNV in the contralateral eye (aHR 5.79, 95%CI: 2.77-12.09). Among specific syndromes, the incidence was greater in Vogt-Koyanagi-Harada Syndrome (aHR 3.37, 95%CI: 1.52-7.46), and punctate inner choroiditis (aHR 8.67, 95%CI: 2.83-26.54). Incident CNV was associated with two lines’ loss of visual acuity (+0.19 logMAR units, 95%CI: 0.079–0.29) from the preceding visit.
Conclusions
CNV is an uncommon complication of uveitis associated with visual impairment, which more commonly occurs in forms affecting the outer retina/RPE/choroid interface, during periods of inflammatory activity, in association with preretinal neovascularization, and in second eyes of patients with unilateral CNV. Because CNV is treatable, a systematic approach to early detection in high-risk patients may be appropriate.
Introduction
Choroidal neovascularization (CNV) consists of pathological blood vessel growth from the choroid across Bruch’s membrane into the retina. The pathogenesis of choroidal neovascularization involves a disruption of the homeostasis between the retinal pigment epithelium (RPE) and Bruch’s membrane1. The proliferating choroidal blood vessels then extend into the subretinal space, often in the region of the macula, where they can leak fluid and lead to serous retinal detachment and scarring2, often resulting in central vision loss. CNV can arise as a complication of virtually any pathogenic process that involves the RPE and damages Bruch’s membrane3.
Although CNV is most well known as being associated with age-related macular degeneration, it also can be a severe sight-threatening complication resulting from both infectious and non-infectious uveitis1. As an important cause of vision loss among people under 50 years of age4, CNV not infrequently strikes patients during some of their most highly productive years, particularly in the setting of uveitis, which also tends to affect a working age population. If left untreated, CNV is associated with a poor prognosis4. Fortunately, recent advances in the treatment of CNV allow for the possibility of improved visual outcomes5-9, particularly in cases detected at an early stage.
Because of the association between CNV and uveitis and the potential for severe vision loss resulting from CNV, better characterization of the risk of and risk factors for CNV in this population is needed. Here, we evaluate these issues in a large retrospective multi-center cohort study of uveitis cases.
Methods
The methods of the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study previously have been described in detail10. Institutional review board (IRB) approval for the SITE Cohort Study was obtained and maintained at each center’s governing IRB prior to and throughout the period of data collection. Each institution’s IRB approved waiver of consent and HIPAA exemption for this study because it entailed retrospective chart review. The project was conducted in adherence with the principles of the Declaration of Helsinki and all federal and state laws in the United States.
This is a retrospective cohort study of patients with non-infectious ocular inflammatory diseases seen between 1978 to 2007 at five academic ocular inflammation centers in the United States. Patients with known HIV infection were excluded. Some previous SITE reports described using a random sample of about 40% of the eligible patients at one of the centers plus all eligible patients at the other four10. However, by the time of this analysis, data regarding all eligible patients with available charts at all five centers was available and were used for this analysis.
A protocol-driven structured chart review had been conducted for each patient studied, with data entered into a standardized form in a computerized database customized for the SITE Cohort Study. Data collection procedures included several quality assurance techniques and extensive cross checks, allowing correction of likely errors in real time, in addition to monitoring of data entry quality through site visiting.
For this analysis, only the cases of uveitis were studied. Data used included demographic characteristics, ocular inflammatory diagnoses, and ophthalmologic examination findings, such as visual acuity and inflammatory disease activity.
Ascertainment of Outcomes
Outcomes assessed included the presence of CNV at cohort entry, the incidence of CNV during follow-up, the incidence of CNV in second eyes of patients with initially unilateral CNV, and changes in visual acuity in relation to CNV. Choroidal neovascularization was diagnosed based on chart notes; either a clinically definite diagnosis or a diagnosis based on ancillary testing was accepted as representing CNV11. The database did notdiscriminate between cases with “active” CNV and those with evidence of prior CNV (e.g., classic disciform scar).
The incidence of CNV was calculated by following the eyes without CNV at the time of cohort entry until the first notation of CNV, until the patient ceased attending the clinic, or until study completion at the patient’s site. Potential risk factors for CNV evaluated included demographic characteristics, ocular inflammatory diagnoses, and ophthalmologic examination findings. These ophthalmological examination findings included visual acuity, time-updated inflammatory disease activity (classified as active, slightly active (reflecting minimal activity designated by descriptors such as “trace”), or inactive), and the presence and extent of anterior chamber cells, vitreous cells, and vitreous haze. Anterior chamber cells and vitreous haze were recorded using an ordinal scale in a manner as similar as possible in a retrospective study to standards promoted by an expert consensus group11; vitreous cells can be viewed as an ordinal grading.
Statistical Analysis
To evaluate risk factors associated with prevalent CNV at cohort entry, crude and adjusted odds ratios were calculated using univariate and multivariate logistic regression that incorporated generalizations of generalized estimating equations (GEE) to account for correlation between the eyes of individual patients12. Potential risk factors for incidence of CNV were evaluated on the basis of hazard ratios (HRs) and adjusted HRs (with 95% confidence intervals [CIs]), which were generated using crude and multivariate Cox proportional hazards models with a robust sandwich estimate to account for correlation between the eyes of individual patients13. Incidence of CNV at 2 years (the median follow-up time) was evaluated by calculating the cumulative incidence estimated from the crude Cox regression hazard function, which allowed incidence estimates consistent with the hazard ratios for time-varying risk factors as well as 95% CIs accounting for correlation between eyes of the same patient. The risk of developing CNV in the second eye was evaluated by including the presence of CNV in the contralateral eye as a time-updated covariate in the incidence analysis, and separately by Kaplan-Meier analysis of contralateral-eye CNV incidence among patients with bilateral uveitis and unilateral CNV in the cohort. To determine the visual acuity change associated with the development of CNV, the logMAR-equivalent visual acuity at the visit before CNV diagnosis was compared to that at the time of CNV diagnosis using a t-test. All statistical analyses were performed with SAS version 9.3 (SAS Corporation, Cary, NC).
Results
Among 15,137 eyes with uveitis (8,868 patients) in the SITE Cohort, an initial evaluation of the prevalence of CNV by the site of inflammation demonstrated that CNV was very rare in cases of anterior or intermediate uveitis: only four (0.06%) eyes (3 [0.07%] patients) with anterior uveitis presented with CNV, and there were no patients with intermediate uveitis who presented with CNV. Among eyes seen more than once, there were 15 cases among 4,103 eyes with anterior uveitis (2,542 patients) seen over a mean of 2.8 years, yielding a 0.13% CNV/eye-year incidence rate. There were 7 cases among 1,982 eyes with intermediate uveitis (1,085 patients) seen over a mean of 3.2 years, yielding a 0.11% CNV/eye-year incidence rate. Given that the very small number of prevalent and incident CNV events among intermediate and anterior uveitis cases provided little information on which to do a risk factor analysis, we limited the remainder of the analysis to cases of posterior or panuveitis.
Among cases of posterior or panuveitis, data were available for 4,041 eyes of 2,307 patients. The median age was 38 years (ranging from 0 to 89 years). The study population was predominantly female (64%) and Caucasian (70%). The majority (90%) of patients had bilateral uveitis at presentation.
Prevalence of Choroidal Neovascularization (CNV)
At the time of initial presentation, 81 eyes with posterior or panuveitis were found to have active CNV or sequelae of past CNV, yielding an overall prevalence of 2.0% (95% CI: 1.6%-2.4%) among cases of posterior or panuveitis presenting for tertiary uveitis care. Patients with CNV were not significantly different than those without CNV in terms of age, sex, or race (Table 1). The prevalence of CNV was significantly lower among eyes of current smokers (0.9%) and somewhat lower among past smokers (2.1%) when compared to those who had never smoked (2.6%) (overall p=0.02).
Table 1. Prevalence of choroidal neovascularization (CNV) and associated risk factors at cohort entry for patients with posterior or panuveitis.
| Characteristic | Number of Eyes |
Number of Eyes with CNV |
Prevalence | Crude Odds Ratio (95% Confidence Interval) |
Crude p-value |
Adjusted Odds Ratioa (95% Confidence Interval) |
Overall adjusted p-value |
|---|---|---|---|---|---|---|---|
|
Age at uveitis diagnosis, years |
0.81 | 0.43 | |||||
| ≤35 | 1784 | 38 | 2.1% | Reference | Reference | ||
| >35 | 2255 | 43 | 1.9% | 0.94 (0.56-1.57) | 0.81 (0.48-1.35) | ||
| Gender | 0.57 | 0.81 | |||||
| Male | 1437 | 26 | 1.8% | Reference | Reference | ||
| Female | 2604 | 55 | 2.1% | 1.17 (0.68-2.02) | 1.07 (0.62-1.87) | ||
| Race | 0.05 | 0.25 | |||||
| White | 2833 | 62 | 2.2% | Reference | Reference | ||
| Black | 534 | 9 | 1.7% | 0.76 (0.35-1.67) | 1.18 (0.54-2.56) | ||
| Hispanic | 251 | 7 | 2.8% | 1.33 (0.54-3.25) | 1.66 (0.69-4.00) | ||
| Other | 423 | 3 | 0.7% | 0.33 (0.10-1.10) | 0.48 (0.15-1.53) | ||
| Smoking | 0.02 | 0.03 | |||||
| Never | 2027 | 53 | 2.6% | Reference | Reference | ||
| Past | 528 | 11 | 2.1% | 0.74 (0.33-1.66) | 0.76 (0.34-1.71) | ||
| Current | 778 | 7 | 0.9% | 0.35 (0.14-0.84) | 0.34 (0.14-0.83) | ||
| Unknown | 708 | 10 | 1.4% | 0.51 (0.24-1.08) | 0.62 (0.29-1.33) | ||
| Uveitis Category | <0.01 | <0.01 | |||||
| Posterior Uveitis | 2512 | 69 | 2.7% | Reference | Reference | ||
| Panuveitis | 1529 | 12 | 0.8% | 0.29 (0.15-0.56) | 0.26 (0.14-0.51) | ||
|
Posterior/Panuveitis
Subtype b |
<0.01 | <0.01 | |||||
| Undifferentiated Panuveitis |
1159 | 11 | 0.9% | Reference | Reference | ||
| Multifocal Choroiditis with Panuveitis |
615 | 28 | 4.6% | 4.55 (2.04- 10.14) |
4.27 (1.94-9.39) | ||
| Multiple Evanescent White Dot Syndrome |
21 | 3 | 14.3% | 18.58 (4.60- 75.11) |
26.98 (6.25-116.40) | ||
| Birdshot Retinochoroiditis |
397 | 4 | 1.0% | 0.99 (0.31-3.19) | 1.19 (0.34-4.18) | ||
| Vogt-Koyanagi-Harada Syndrome |
320 | 1 | 0.3% | 0.30 (0.04-2.35) | 0.22 (0.03-1.73) | ||
| Retinal Vasculitis | 727 | 6 | 0.8% | 0.78 (0.23-2.63) | 0.95 (0.28-3.15) | ||
| Punctate Inner Choroiditis |
17 | 2 | 11.8% | 15.03 (5.05- 44.79) |
15.90 (5.11-49.43) | ||
| Serpiginous Choroiditis | 148 | 7 | 4.7% | 5.27 (1.82- 15.25) |
5.69 (1.93-16.82) | ||
| Otherc | 637 | 19 | 3.0% | 2.39 (0.98-5.80) | 2.43 (1.02-5.81) | ||
| Bilateral Uveitis | 0.12 | 0.15 | |||||
| No | 389 | 13 | 3.3% | Reference | Reference | ||
| Yes | 3652 | 68 | 1.9% | 0.55 (0.29-1.03) | 0.58 (0.31-1.09) | ||
| Visual Acuity | <0.01 | <0.01 | |||||
| 20/40 or better | 1695 | 17 | 1.0% | Reference | Reference | ||
| >20/40 to <20/200 | 1429 | 35 | 2.4% | 3.25 (1.60-6.60) | 3.24 (1.69-6.23) | ||
| 20/200 or worse | 907 | 29 | 3.2% | 3.88 (1.87-8.05) | 4.06 (2.06-7.99) | ||
| Missing | 10 | 0 | 0.0% | n/a | n/a |
Adjusted by race, smoking, uveitis category, and visual acuity
Posterior/panuveitis subtype was not adjusted by uveitis category as they are different ways of considering the same variable.
The “other” designation includes several conditions too infrequent to analyze separately: Sympathetic Ophthalmia, acute posterior multifocal placoid pigment epitheliopathy (APMPPE), neuroretinitis, diffuse retinochoroiditis, focal retinochoroiditis, acute retinal necrosis syndrome, Eales' Disease, unspecified inflammatory chorioretinal scars, sarcoidosis, and necrotizing retinitis.
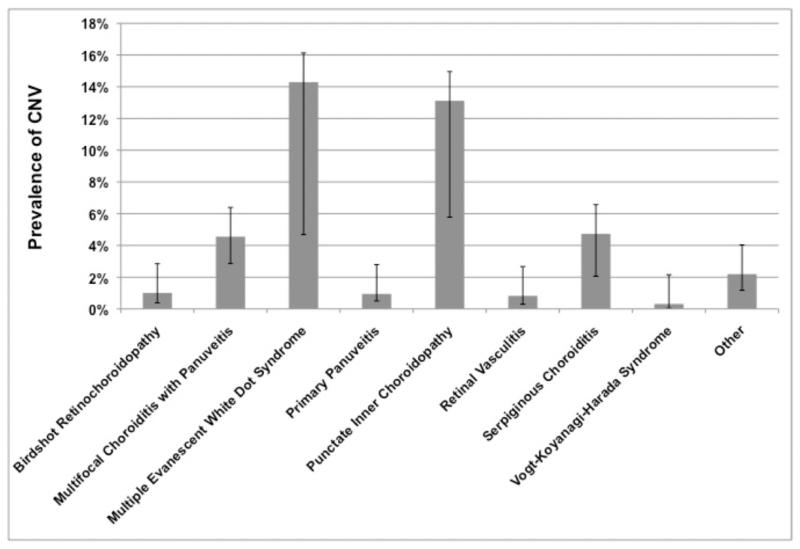
The anatomic site of inflammation14 was associated significantly with CNV at presentation, with panuveitis (0.8%) having a lower prevalence of CNV than posterior uveitis (2.7%; adjusted odds ratio [aOR] 0.26, 95% confidence interval [CI]: 0.14-0.51). Compared to eyes with panuveitis that did not fit a specific morphologic syndrome (prevalence of CNV at 0.9%), eyes that had been diagnosed with certain posterior or panuveitis syndromes that affect the region of the choroid and RPE were more likely to present with CNV (all p<0.01), including multifocal choroiditis with panuveitis (4.6%; aOR 4.27, 95% CI: 1.94-9.39), multiple evanescent white dot syndrome (14.3%; aOR 26.98, 95% CI: 6.25-116), punctate inner choroiditis (11.8%; aOR 15.90, 95% CI: 5.11-49.43), and serpiginous choroiditis (4.7%; aOR 5.69, 95% CI: 1.93-16.82); see also Figure 1.
Figure 1.
prevalence of choroidal neovascularization (CNV) among various diagnoses in patients with posterior or panuveitis, with 95% confidence intervals.
Because CNV often occurred well before presentation, we did not evaluate the inflammatory status at the time of presentation as a risk factor for prevalent CNV. Worse visual acuity was more common among eyes with CNV at presentation, which had over four times higher odds of a visual acuity of 20/200 or worse with respect to 20/40 or better than eyes without CNV at presentation (aOR 4.06, 95% CI: 2.06-7.99, p<0.001).
Incidence of CNV and Risk Factors for Developing CNV
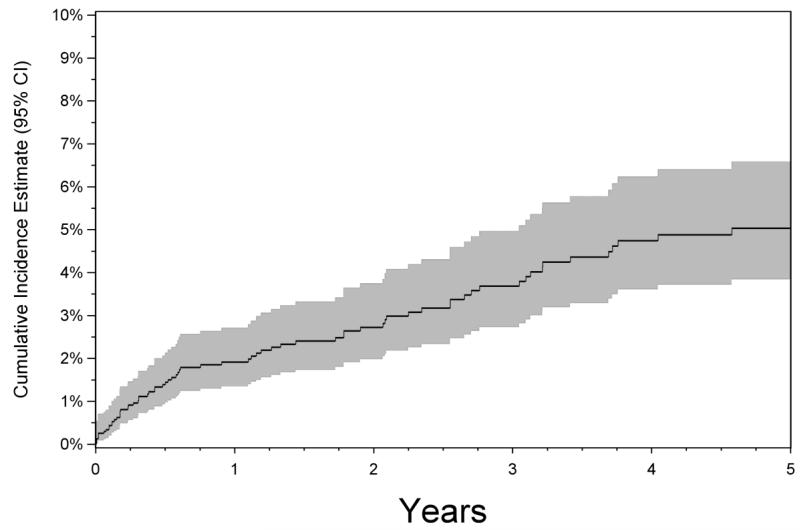
There were 2,364 eyes of 1,357 patients with posterior or panuveitis and free of CNV at the time of cohort entry for which one or more follow-up visits were available. These were followed for incidence of CNV over a median of 1.98 years (interquartile range: 0.48 to 5.24 years). The overall cumulative incidence of CNV by the two-year time point was estimated to be 2.7% (95% CI: 1.8-3.5%, see Figure 2). Inspection of the Kaplan-Meier curve indicates that the risk appeared considerably higher over the first six months after presentation, reaching nearly 2% by that point, and then increased less rapidly over time thereafter.
Figure 2.
Kaplan-Meier analysis of the risk of choroidal neovascularization (CNV) among patients with posterior or panuveitis initially free of CNV at the time of cohort entry and followed up over time. Shaded area indicates 95% confidence interval.
Several risk factors for incident CNV were identified (see Table 2). In contrast to the results of the prevalence analysis, there was no significant difference in the two-year incidence of CNV between eyes with posterior uveitis (2.9%) and eyes with undifferentiated panuveitis (2.5%; aHR 0.87, 95% CI: 0.54-1.41). Compared to eyes with panuveitis that did not fit another morphologic syndrome (which had an estimated two-year incidence of CNV of 1.5%), Vogt-Koyanagi-Harada disease (6.4%; aHR 3.37, 95% CI: 1.52-7.46) and punctate inner choroiditis (13.2%; aHR 8.67, 95% CI: 2.83-26.54) were associated with increased CNV risk. Multifocal choroiditis with panuveitis also tended to have increased risk (3.6%; aHR 2.19, 95% CI: 1.01-4.78), whereas no statistically significant increase in incidence with serpiginous retinochoroiditis was observed (2.7%; aHR 1.56, 95% CI: 0.51-4.77). Too few cases of multiple evanescent white dot syndrome were available for incidence analysis to evaluate CNV risk for that syndrome.
Table 2. Risk factors for incidence of choroidal neovascularization (CNV) in patients with posterior or panuveitis.
| Characteristic | Number of Eyes at Riska |
2-Year Incidence of CNV (95% Confidence Interval) |
Crude Hazard Ratio (95% Confidence Interval) |
Crude p-value |
Adjusted Hazard Ratiob (95% Confidence Interval) |
Overall adjusted p-value |
|---|---|---|---|---|---|---|
| Overall cases | 2364 | 2.7% (1.8%-3.5%) | n/a | n/a | n/a | n/a |
| Age at uveitis diagnosis, years | 0.62 | 0.28 | ||||
| ≤35 | 1066 | 2.5% (1.5%-3.5%) | Reference | Reference | ||
| >35 | 1298 | 2.8% (1.7%-4.0%) | 1.13 (0.69-1.86) | 1.32 (0.80-2.17) | ||
| Gender | 0.61 | 0.19 | ||||
| Male | 826 | 2.9% (1.6%-4.2%) | Reference | Reference | ||
| Female | 1538 | 2.6% (1.6%-3.5%) | 0.88 (0.53-1.45) | 0.72 (0.44-1.17) | ||
| Race | 0.08 | 0.17 | ||||
| White | 1617 | 2.3% (1.3%-3.2%) | Reference | Reference | ||
| Black | 380 | 3.6% (1.7%-5.5%) | 1.61 (0.90-2.89) | 1.59 (0.89-2.84) | ||
| Hispanic | 166 | 5.4% (1.7%-9.1%) | 2.46 (1.13-5.34) | 2.02 (0.93-4.39) | ||
| Other | 201 | 2.3% (0.3%-4.2%) | 1.02 (0.40-2.57) | 0.95 (0.35-2.55) | ||
| Smoking | 0.74 | 0.88 | ||||
| Never | 1206 | 2.6% (1.6%-3.5%) | Reference | Reference | ||
| Past | 301 | 3.0% (1.1%-4.9%) | 1.18 (0.60-2.31) | 1.22 (0.62-2.42) | ||
| Current | 499 | 2.2% (0.8%-3.7%) | 0.86 (0.42-1.75) | 0.95 (0.47-1.93) | ||
| Unknown | 358 | 3.4% (1.2%-5.6%) | 1.34 (0.67-2.67) | 1.24 (0.62-2.46) | ||
| Uveitis Category c | 0.56 | 0.57 | ||||
| Posterior Uveitis | 1399 | 2.9% (1.8%-3.9%) | Reference | Reference | ||
| Panuveitis | 965 | 2.5% (1.4%-3.5%) | 0.86 (0.52-1.43) | 0.87 (0.54-1.41) | ||
| Posterior/Panuveitis Subtype | <0.01 | <0.01 | ||||
| Undifferentiated Panuveitis |
725 | 1.5% (0.6%-2.3%) | Reference | Reference | ||
| Multifocal Choroiditis with Panuveitis |
350 | 3.6% (1.5%-5.6%) | 2.47 (1.13-5.42) | 2.19 (1.01-4.78) | ||
| Multiple Evanescent White Dot Syndrome |
9 | 0% | n/a | n/a | ||
| Birdshot Retinochoroiditis | 261 | 2.6% (0.6%-4.6%) | 1.79 (0.71-4.55) | 1.67 (0.65-4.29) | ||
| Vogt-Koyanagi-Harada Syndrome |
209 | 6.4% (2.8%-9.9%) | 4.50 (2.06-9.82) | 3.37 (1.52-7.46) | ||
| Retinal Vasculitis | 429 | 1.8% (0.4%-3.2%) | 1.23 (0.49-3.08) | 1.22 (0.47-3.17) | ||
| Punctate Inner Choroiditis | 12 | 10.1% (0.0%- 25.8%) |
9.64 (3.13-29.69) | 8.67 (2.83-26.54) | ||
| Serpiginous Choroiditis | 76 | 2.7% (0.0%-5.6%) | 1.85 (0.56-6.16) | 1.56 (0.51-4.77) | ||
| Otherd | 293 | 3.2% (1.0%-5.3%) | 1.51 (0.55-4.09) J | 1.63 (0.60-4.42) | ||
| Preretinal neovascularization | 0.04 | 0.01 | ||||
| No | *a | 2.6% (1.7%-3.4%) | Reference | Reference | ||
| Yes | *a | 6.1% (1.2%- 10.8%) |
2.43 (1.04-5.69) | 3.19 (1.30-7.80) | ||
| Inflammatory Activity | 0.03 | 0.02 | ||||
| Inactive | *a | 1.9% (1.0%-2.7%) | Reference | Reference | ||
| Slightly active | *a | 3.4% (1.3%-5.6%) | 1.84 (0.91-3.74) | 1.85 (0.90-3.81) | ||
| Active | *a | 3.7% (2.2%-5.1%) | 1.96 (1.16-3.31) | 2.13 (1.26-3.60) | ||
| Missing | *a | 0% | n/a | n/a | ||
| Anterior chamber cells c | <0.01 | 0.02 | ||||
| Quiet | *a | 2.8% (1.8%-3.8%) | Reference | Reference | ||
| 0.5+ | *a | 1.6% (0.3%-2.8%) | 0.55 (0.24-1.25) | 0.57 (0.25-1.33) | ||
| 1+ | *a | 0.8% (0.0%-2.0%) | 0.30 (0.07-1.22) | 0.30 (0.07-1.28) | ||
| 2+ or worse | *a | 6.3% (2.3%- 10.2%) |
2.29 (1.16-4.55) | 2.13 (1.26-3.60) | ||
| Vitreous cells c | 0.79 | 0.89 | ||||
| Quiet | *a | 3.0% (1.9%-4.0%) | Reference | Reference | ||
| 0.5+ | *a | 2.7% (1.2%-4.1%) | 0.90 (0.49-1.64) | 0.96 (0.52-1.76) | ||
| 1 + | *a | 2.7% (0.9%-4.5%) | 0.92 (0.47-1.77) | 0.97 (0.48-1.93) | ||
| 2+ or worse | *a | 1.9% (0.4%-3.5%) | 0.65 (0.28-1.52) | 0.71 (0.31-1.64) | ||
| Missing | *a | 0% | n/a | n/a | ||
| Vitreous haze c | 0.77 | 0.93 | ||||
| Quiet | *a | 2.9% (2.0%-3.8%) | Reference | Reference | ||
| 1+ | *a | 2.2% (0.5%-3.9%) | 0.76 (0.34-1.68) | 0.83 (0.37-1.86) | ||
| 2+ or worse | *a | 1.6% (0.0%-4.0%) | 0.55 (0.13-2.42) | 0.69 (0.15-3.11) | ||
| Missing | *a | 2.2% (0.0%-4.4%) | 0.75 (0.26-2.16) | 0.84 (0.29-2.48) | ||
|
Use of any immunomodulatory
treatment e |
0.89 | 0.72 | ||||
| No | *a | 2.7% (1.6%-3.7%) | Reference | Reference | ||
| Yes | *a | 2.7% (1.7%-3.8%) | 1.04 (0.64-1.67) | 1.09 (0.68-1.75) | ||
| Bilateral Uveitis | 0.12 | 0.04 | ||||
| No | 204 | 4.4% (1.4%-7.3%) | Reference | Reference | ||
| Yes | 2160 | 2.6% (1.7%-3.4%) | 0.57 (0.28-1.16) | 0.40 (0.18-0.88) | ||
| Prior Contralateral CNV | <0.01 | <0.01 | ||||
| No | *a | 2.4% (1.7%-3.2%) | Reference | Reference | ||
| Yes | *a | 17.2% (6.6%- 26.5%) |
7.66 (4.06-14.46) | 5.79 (2.77-12.09) |
The asterisks (“*”) indicate time-varying variables, for which one eye might be included in more than one category at different points in time.
Adjusted by posterior/panuveitis subtype, overall inflammatory activity, preretinal neovascularization, and prior contralateral CNV.
Uveitis category was not adjusted by posterior/panuveitis subtype as they are different ways of considering the same variable. Anterior chamber cells, vitreous cells, and vitreous haze were not adjusted by overall inflammatory activity, as each is highly associated with overall activity.
The “other” designation includes several uncommon conditions too infrequent to analyze separately: Sympathetic Ophthalmia, acute posterior multifocal placoid pigment epitheliopathy (APMPPE), neuroretinitis, diffuse retinochoroiditis, focal retinochoroiditis, acute retinal necrosis syndrome, Eales’ Disease, unspecified inflammatory chorioretinal scars, sarcoidosis, and necrotizing retinitis
immunomodulatory therapy included antimetabolites, T-cell inhibitors, alkylating agents and/or biologic immune response modifiers.
In terms of physical findings, eyes diagnosed with preretinal neovascularization (simultaneous with or before the diagnosis of CNV) had an over three-fold higher risk of developing CNV (aHR 3.19, 95% CI: 1.30-7.80; p=0.01). The time-updated level of inflammatory disease activity at the time of CNV incidence was another risk factor for the development of CNV. Compared to eyes with inactive inflammation, the risk of developing CNV was significantly greater in eyes with currently active inflammation (aHR 2.13, 95% CI: 1.26-3.60). More specifically, eyes with 2+ or worse grading of their anterior chamber cells had a significantly higher two-year incidence of CNV (6.3%) compared to those whose anterior chamber cells were graded as quiet (2.8%; aHR 2.21, 95% CI: 1.07-4.57). However, increased vitreous cells and vitreous haze were not significantly associated with altered risk of incident CNV. Eyes of patients with bilateral uveitis (2.6%) were less likely to develop CNV than affected eyes of patients with unilateral uveitis (4.4%; aHR 0.40, 95% CI: 0.18-0.88). With regard to immunomodulatory therapy (antimetabolites, T-cell inhibitors, alkylating agents, or biologics), there was not a significant difference in the incidence of CNV in patients who were treated with these agents compared to patients who were not, either before (crude HR 1.04, 95% CI: 0.64-1.67, p=0.89) or after (aHR 1.09, 95% CI: 0.68-1.75, p=0.72) adjustment for activity and other variables.
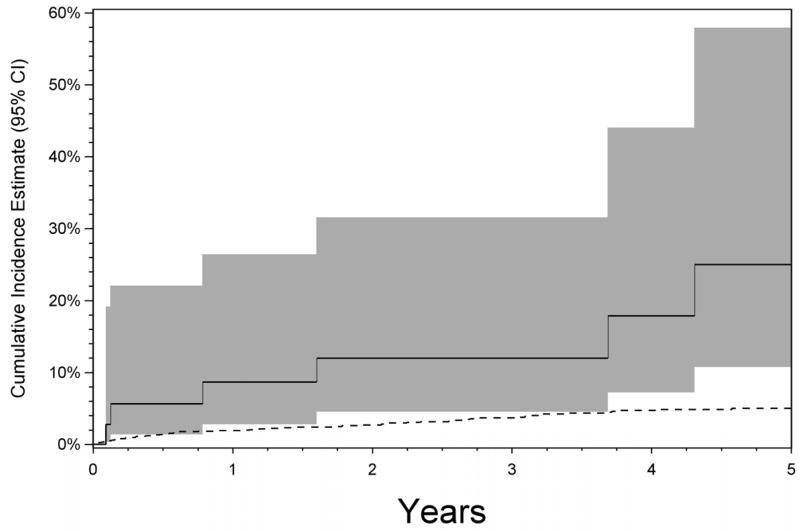
Having a prior diagnosis of CNV in the contralateral eye was associated with a several-fold higher risk of developing CNV in the second eye (aHR 5.79, 95% CI: 2.77-12.09). Kaplan-Meier analysis (Figure 3; 17.2% at 2 years, 95% CI: 6.6%-26.5%) suggests a significantly higher cumulative incidence throughout all follow-up time observed than for eyes of patients free of CNV.
Figure 3.
Kaplan-Meier analysis of the risk of choroidal neovascularization (CNV) in the second eye among patients with bilateral posterior or panuveitis and existing unilateral CNV (solid line), compared to eyes of patients with bilateral posterior or panuveitis and initially free of CNV (dashed line). Shaded area indicates 95% confidence interval.
On average, there was a nearly two line reduction in visual acuity at the visit when CNV was diagnosed compared to the last visit prior to diagnosis of CNV (change in logMAR=+0.19 units, 95% CI: 0.079 – 0.29, p=0.0009). Given that nearly all follow-up time observed in this study was in the pre-VEGF-inhibitor era, we did not evaluate outcomes of CNV after diagnosis and treatment.
Discussion
These results suggest that CNV is rare among cases of anterior uveitis and intermediate uveitis, at a level that likely primarily reflects the background risk attributable primarily to non-inflammatory diseases. Any misclassification of posterior and panuveitis as anterior or intermediate uveitis would have tended to increase the event rate, further supporting the lack of association between CNV and uveitis involving these sites.
Compared to cases of anterior and intermediate uveitis, CNV was found much more frequently (both at initial presentation and throughout follow-up) in cases of posterior and panuveitis. While still relatively uncommon, CNV is clinically important and was found to be more frequent in specific subgroups of these patients.
Our finding of an increased risk of developing CNV in the setting of current inflammatory activity is consistent with the concept that CNV is driven at least in part by inflammatory processes. While the global assessment of inflammatory activity and anterior chamber cells were associated with increased risk of incident CNV, vitreous cells and vitreous haze were not. This may be because diseases with a high risk of CNV (e.g. the posterior uveitides) typically have few vitreous cells or almost never have substantial haze, whereas those uveitides with a low risk of CNV often do have cells and haze. Therefore, the interpretation of how cells and haze relate to risk of CNV is not straightforward because of the distinct characteristics of the underlying uveitic entities. However, in general, there seems to be convincing evidence that inflammation plays a crucial role in the pathogenesis of CNV. A review by Dhingra et al. concerning the formation of choroidal neovascular membranes in posterior segment intraocular inflammation identified leukocytes and chronic chorioretinal inflammation as being critical for the development of CNV15. Activated macrophages and other inflammatory cells secrete enzymes that damage cells and cause degradation in Bruch’s membrane, and the cytokines released by these inflammatory cells may promote the growth of CNV through the break in Bruch’s membrane and into the sub-RPE space, potentially leading to edema, exudation, hemorrhages, and fibrosis which result in profound central vision loss15-17. The salutary effects of anti-inflammatory and immunosuppressive agents for the treatment of CNV provides further support for an inflammatory contribution to pathogenesis. For example, in the pre-vascular endothelial growth factor (VEGF) inhibitor era, corticosteroids had been proposed as a first-line approach18 based on their inhibitory action on inflammatory cells and subsequently the suppression of pro-angiogenic mediators, as well as their ability to decrease vascular permeability and prevent vascular budding15, 18. In addition, there has been some evidence suggesting that adding corticosteroids to photodynamic therapy (PDT) is more effective than PDT alone in inflammatory CNV15. These considerations and our results suggest that controlling inflammation is not only indicated for treating the uveitis itself, but also for preventing and/or treating CNV.
Given the pivotal role of inflammation in CNV, it is not surprising that CNV emerged as being more common among those with posterior uveitis or panuveitis than anterior and intermediate uveitis, because a higher likelihood of damage to the RPE and/or Bruch’s membrane is expected when there is adjacent chorioretinal inflammation. Forooghian et al. specifically associated a history of CNV with posterior uveitis in one cross-sectional retrospective case series19.
Despite the role of inflammation in the pathogenesis of CNV and the aforementioned evidence for effectiveness of corticosteroid treatment, treatment with immunomodulatory therapy (e.g. immunosuppressants and biologics) was not significantly associated with a different risk of CNV in our analysis. However, because more severe cases probably were both more likely to develop CNV and to go on to receive immunosuppressive treatment, any potential protective effect of immunodulatory therapy on CNV may have been masked. Therefore, the data available here cannot answer the question of whether immunomodulatory therapy significantly mitigates the risk of CNV, other than by inference in that control of inflammation appeared to be associated with lower CNV risk, and use of immunosuppression can lead to control of inflammation.
Among patients with posterior and panuveitis, the cases at highest risk appear to be those with bilateral uveitis who already have unilateral CNV. The increased risk of CNV in patients with pre-existing unilateral CNV suggests that there could be systemic (e.g., genetic) or disease-specific factors that make a particular individual prone to damage of the RPE or Bruch’s membrane or otherwise particularly susceptible to CNV. Such cases should be carefully managed to ensure inflammation is controlled as well as possible, and to detect second eye CNV promptly to facilitate timely treatment.
We also observed that, among cases of posterior or panuveitis, those cases with disease affecting a site adjacent to or including Bruch’s membrane had increased risk of CNV. Others have reported a high risk of CNV for cases of punctate inner choroiditis and multifocal choroiditis with panuveitis (in a group of patients overlapping with those reported here)20 and Vogt-Koyanagi-Harada disease21, consistent with our results. CNV may be especially frequent in multifocal choroiditis (MFC) due to widespread involvement of the choriocapillaris1. Examining the risk of CNV in MFC in the future may be influenced by the definition of multifocal choroiditis, punctate inner choroidopathy (PIC) and related conditions. Some have posited that MFC and PIC should be categorized as the same entity22, while others have found that they are distinguishable based on the morphology of chorioretinal lesions and present with distinct clinical characteristics – specifically, that patients with MFCPU tend to be older, have a higher frequency of structural complications, and are more likely to have bilateral visual impairment of 20/50 or worse, while patients with PIC tend to be younger and have a higher frequency of CNV at presentation23. In this analysis, we have reported multifocal choroiditis with panuveitis and punctate inner choroiditis separately given that it is not universally agreed upon whether multifocal choroiditis with panuveitis and punctate inner choroidopathy are the same or different conditions and because their risk patterns seemed potentially different.
We found a broader range of conditions affecting a site adjacent to or including Bruch’s membrane to be associated with increased CNV risk in the prevalence analysis than in the incidence analysis, suggesting that these conditions may tend to cause CNV early in the clinical course rather than later, which also is suggested by the higher incidence of CNV during the first six months of follow-up than subsequently. Little previous information is available to evaluate this concept of early higher risk, although it has been suggested that “idiopathic” choroidal neovascularization may be a herald of subsequently manifest posterior uveitis24, which may lend support to the concept. Nevertheless, patients with unilateral CNV should be followed carefully over time, as their risk of CNV remains substantial (see Figure 3) even after the early period.
Our data also suggest that vasculopathy may be associated with CNV. Preretinal neovascularization—which is commonly associated with retinal ischemia and mediated by VEGF25—was associated with an over three-fold risk of developing CNV in this cohort of uveitis patients. Although the percentage of cases with CNV that also exhibited preretinal neovascularization was relatively low, it was still more than expected based on chance alone. The tendency for both to occur may reflect shared pathogenetic mechanisms, such as ischemia and/or inflammation-induced production of VEGF, leading to the association between the two phenomena. The importance of VEGF in the pathogenesis of CNV is underscored by the effectiveness of anti-VEGF agents in the treatment of CNV not only in uveitis, but also in the context of AMD, myopia,16, 17, 26-28 and other conditions.
Eyes of patients with bilateral uveitis had a lower risk of incident CNV and tended to be less likely to have CNV at the time of presentation for tertiary care than affected eyes of patients with unilateral uveitis. Perhaps unilateral cases of uveitis were less likely to be referred for tertiary care unless they had had a major complication, such as CNV. Also, we speculate that the lower risk of CNV in bilateral cases may have been because of more aggressive control of inflammation among patients subsequent to development of unilateral CNV. The association between current smoking and a lower risk of prevalent CNV at the time of presentation for tertiary uveitis care was not confirmed in the incidence analysis, and is of uncertain significance.
Because of the effects of CNV in the subretinal space, it is not surprising that both prevalent and incident CNV were associated with decreased visual acuity. In the incidence analysis, there was only a two line reduction in visual acuity between the pre-CNV visit and the first visit with CNV, suggesting that an opportunity exists in uveitis practices for treatment to prevent further visual loss and possibly restore vision at least partially by recognizing CNV and administering appropriate treatment in a timely manner. For situations of particularly high risk, such as cases with unilateral CNV and patients with PIC, clinicians should consider counseling patients regarding the potential symptoms of CNV, and should consider deploying screening tests for CNV such as Amsler grids.
Limitations of the study are those of a retrospective cohort study, such as information bias arising from missing data that may not have been documented in medical records and heterogeneity in methods for documentation of CNV, and less complete follow-up than a prospective study might offer, if it could be implemented. However, the median follow-up of two years for this large cohort of patients was relatively favorable, and CNV can be reliably diagnosed clinically as well as via ancillary testing. There was also potential referral bias, given that the included centers may have encountered more severe cases and simply more cases overall than in non-tertiary settings. As in all studies, unrecognized confounding also cannot be excluded. Strengths of the study include optimization of data quality by several quality assurance techniques, and a higher level of statistical precision in estimating rates and power in detecting associations than in previous reports due to the study’s relatively large sample size.
In conclusion, in a tertiary setting, choroidal neovascularization is rare in anterior and intermediate uveitis cases, and is an uncommon but clinically important complication of posterior and panuveitis. Cases where the inflammation involves the region of the outer retina/retinal pigment epithelium(RPE)/choroid interface tend to have higher risk, with punctate inner choroiditis having the highest risk. Current inflammatory activity is a modifiable risk factor for CNV. Clinical findings of preretinal neovascularization and especially the presence of CNV in the contralateral eye in patients with bilateral uveitis also were strong risk factors. Because CNV has the potential to inflict severe vision loss, which now can be mitigated by a range of treatments, clinicians should consider educating high-risk patients regarding the potential symptoms of CNV and—when appropriate—deploy screening tests such as Amsler grids in order to facilitate early detection and treatment. The risk of CNV is highest in the early period after presentation for tertiary care, but close follow-up is advisable as long as risk is high, which could be for many years in high risk situations like punctuate inner choroiditis or cases with unilateral CNV and bilateral uveitis.
Acknowledgements
a. Funding/Support: This study was supported primarily by National Eye Institute Grant EY014943 (JHK). Additional support was provided by Research to Prevent Blindness and the Paul and Evanina Mackall Foundation. JHK was an RPB James S Adams Special Scholar Award recipient, JET was an RPB Harrington Special Scholar Award recipient, and DAJ and JTR were Research to Prevent Blindness Senior Scientific Investigator Award recipients during the course of the study. GAL-C was previously supported by and RBN continues to be supported by intramural funds of the National Eye Institute. EBS receives support from the Department of Veterans’ Affairs. None of the sponsors had any role in the design and conduct of the report; collection, management, analysis, and interpretation of the data; or in the preparation, review, and approval of this manuscript.
d. Other Acknowledgments: None
Biographies

Sally Baxter is a medical student and Gamble Scholar at the Perelman School of Medicine at the University of Pennsylvania. She attended Duke University as an Angier B. Duke Scholar and graduated summa cum laude. A Marshall Scholar, she received her MSc in Public Health from the London School of Hygiene and Tropical Medicine. Her research interests are in clinical epidemiology, public health, and international ophthalmology. She is currently applying for residency training in ophthalmology.

Dr. John H. Kempen is Associate Professor of Ophthalmology and Epidemiology at the University of Pennsylvania School of Medicine, and Director of both the Ocular Inflammation Service and Ophthalmic Epidemiology/International Health. His research centers on the effects of treatment for patients with ocular inflammatory and infectious diseases. He has published widely on these topics. Dr. Kempen serves as Chairman of the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study, and as Vice-Chairman of the Multicenter Uveitis Steroid Treatment (MUST) Trial, the first two NIH-sponsored multicenter research activities in the field of non-infectious uveitis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
b. Financial Disclosures: Sally L. Baxter, none; Maxwell Pistilli, none; Siddharth S. Pujari, none; Teresa L. Liesegang, none; Eric B. Suhler, none; Jennifer E. Thorne serves a consultant for Allergan, XOMA; C. Stephen Foster serves as a lecturer for Alcon, Inspire, Ista and Centocor; as a Consultant for Sirion; as a consultant and lecturer for Allergan and Bausch & Lomb; and as an equity owner for EyeGate; Douglas A. Jabs serves a consultant for Abbott Laboratories, Alcon Laboratories, Allergan Pharmaceutical Corporation, Corcept Therapeutics, Genentech, Genzyme Corporation, GlaxoSmith Kline, Roche Pharmaceuticals, and is on the Data and Safety Monitoring Board for Applied Genetic Technologies Corporation and Novartis Pharmaceutical Corporation; Grace A. Levy-Clarke is employed by Johnson & Johnson Vision Care; Robert B. Nussenblatt, none; James T. Rosenbaum serves or has served as a consultant for Abbott Laboratories, Amgen, Allergan, Genentech, Novartis, and UCB, and has been involved in Clinical Trials for Abbott Laboratories, Genentech, Lux Biosciences, and Eyegate Pharma; John H. Kempen serves or has served as a consultant for Alcon, Allergan, Can-Fite, Clearside, Lux Biosciences, Sanofi-Pasteur, and Xoma.
c. Contributions of Authors in each of these areas: Design and conduct of the study (SLB, MP, JHK); collection, management, analysis, and interpretation of the data (all authors); and preparation (SLB, MP, JHK) and/or review and approval of the manuscript (all authors).
References
- 1.Neri P, Lettieri M, Fortuna C, Manoni M, Giovaninni A. Inflammatory choroidal neovascularization. Middle East Afr J Ophthalmol. 2009;16(4):245–251. doi: 10.4103/0974-9233.58422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penn J, editor. Retinal and Choroidal Angiogenesis. Springer; Dordrecht, The Netherlands: 2008. [Google Scholar]
- 3.Campa C, Costagliola C, Incorvaia C, et al. Inflammatory mediators and angiogenic factors in choroidal neovascularization: pathogenetic interactions and therapeutic implications. Mediators Inflamm. doi: 10.1155/2010/546826. Epub 2010 Aug 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller DG, Singerman LJ. Vision loss in younger patients: a review of choroidal neovascularization. Optom Vis Sci. 2006;83(5):316–325. doi: 10.1097/01.opx.0000216019.88256.eb. [DOI] [PubMed] [Google Scholar]
- 5.Julian K, Terrada C, Fardeau C, et al. Intravitreal bevacizumab as first local treatment for uveitis-related choroidal neovascularization: long-term results. Acta Ophthalmol. 2011;89(2):179–184. doi: 10.1111/j.1755-3768.2010.02046.x. [DOI] [PubMed] [Google Scholar]
- 6.Mansour AM, Arevalo JF, Ziemssen F, et al. Long-term visual outcomes of intravitreal bevacizumab in inflammatory ocular neovascularization. Am J Ophthalmol. 2009;148(2):310–316 e312. doi: 10.1016/j.ajo.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Tran TH, Fardeau C, Terrada C, Ducos De, Lahitte G, Bodaghi B, Lehoang P. Intravitreal bevacizumab for refractory choroidal neovascularization (CNV) secondary to uveitis. Graefes Arch Clin Exp Ophthalmol. 2008;246(12):1685–1692. doi: 10.1007/s00417-008-0906-4. [DOI] [PubMed] [Google Scholar]
- 8.Arevalo JF, Adan A, Berrocal MH, et al. Intravitreal bevacizumab for inflammatory choroidal neovascularization: results from the Pan-American Collaborative Retina Study Group at 24 months. Retina. 2011;31(2):353–363. doi: 10.1097/IAE.0b013e3181ed8cec. [DOI] [PubMed] [Google Scholar]
- 9.Cornish KS, Williams GJ, Gavin MP, Imrie FR. Visual and optical coherence tomography outcomes of intravitreal bevacizumab and ranibizumab in inflammatory choroidal neovascularization secondary to punctate inner choroidopathy. Eur J Ophthalmol. 2011;21(4):440–445. doi: 10.5301/EJO.2010.6117. [DOI] [PubMed] [Google Scholar]
- 10.Kempen JH, Daniel E, Gangaputra S, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study. Ophthalmic Epidemiol. 2008;15(1):47–55. doi: 10.1080/09286580701585892. [DOI] [PubMed] [Google Scholar]
- 11.Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang K-Y, Zeger SL. Regression analysis for correlated data. Annu Rev Public Health. 1993;14:43–68. doi: 10.1146/annurev.pu.14.050193.000355. [DOI] [PubMed] [Google Scholar]
- 13.Wei L, Lin D, Weissfeld L. Regression analysis of multivariate incomplete failure time data by using the marginal distribution. J Am Stat Assoc. 1989;84:1065–1073. [Google Scholar]
- 14.International Uveitis Study Group (IUSG): clinical classification of uveitis. Ocul Immunol Inflamm. 2008;16(1):1–2. doi: 10.1080/09273940801899822. [DOI] [PubMed] [Google Scholar]
- 15.Dhingra N, Kelly S, Majid MA, Bailey CB, Dick AD. Inflammatory choroidal neovascular membrane in posterior uveitis-pathogenesis and treatment. Indian J Ophthalmol. 2010;58(1):3–10. doi: 10.4103/0301-4738.58467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinnunen K, Yla-Herttuala S. Vascular endothelial growth factors in retinal and choroidal neovascular diseases. Ann Med. 2012;44(1):1–17. doi: 10.3109/07853890.2010.532150. [DOI] [PubMed] [Google Scholar]
- 17.van Wijngaarden P, Qureshi SH. Inhibitors of vascular endothelial growth factor (VEGF) in the management of neovascular age-related macular degeneration: a review of current practice. Clin Exp Optom. 2008;91(5):427–437. doi: 10.1111/j.1444-0938.2008.00305.x. [DOI] [PubMed] [Google Scholar]
- 18.O’Toole L, Tufail A, Pavesio C. Management of choroidal neovascularization in uveitis. Int Ophthalmol Clin. 2005;45(2):157–177. doi: 10.1097/01.iio.0000155902.49562.4a. [DOI] [PubMed] [Google Scholar]
- 19.Forooghian F, Yeh S, Faia L, Nussenblatt RB. Uveitic foveal atrophy: clinical features and associations. Arch Ophthalmol. 2009;127(2):179–186. doi: 10.1001/archophthalmol.2008.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorne JE, Wittenberg S, Jabs DA, et al. Multifocal choroiditis with panuveitis incidence of ocular complications and of loss of visual acuity. Ophthalmology. 2006;113(12):2310–2316. doi: 10.1016/j.ophtha.2006.05.067. [DOI] [PubMed] [Google Scholar]
- 21.Read RWRA, Butani N, Johnston R, Labree L, Smith R, Rao N. Complications and Prognostic Factors in Vogt-Koyanagi-Harada Disease. Am J Ophthalmol. 2001;131(5):599–606. doi: 10.1016/s0002-9394(01)00937-0. [DOI] [PubMed] [Google Scholar]
- 22.Essex RW, Wong J, Jampol LM, Dowler J, Bird AC. Idiopathic multifocal choroiditis: a comment on present and past nomenclature. Retina. 2013;33(1):1–4. doi: 10.1097/IAE.0b013e3182641860. [DOI] [PubMed] [Google Scholar]
- 23.Kedhar SR, Thorne JE, Wittenberg S, Dunn JP, Jabs DA. Multifocal choroiditis with panuveitis and punctate inner choroidopathy: comparison of clinical characteristics at presentation. Retina. 2007 Nov-Dec;27(9):1174–1179. doi: 10.1097/IAE.0b013e318068de72. [DOI] [PubMed] [Google Scholar]
- 24.Machida S, Fujiwara T, Murai K, Kubo M, Kurosaka D. Idiopathic choroidal neovascularization as an early manifestation of inflammatory chorioretinal diseases. Retina. 2008;28(5):703–710. doi: 10.1097/IAE.0b013e318160798f. [DOI] [PubMed] [Google Scholar]
- 25.D’Amore J. Mechanisms of retinal and choroidal neovascularization. Invest Ophthalmol Vis Sci. 1994;35(12):3974–3978. [PubMed] [Google Scholar]
- 26.Cohen SY. Anti-VEGF drugs as the 2009 first-line therapy for choroidal neovascularization in pathologic myopia. Retina. 2009;29(8):1062–1066. doi: 10.1097/IAE.0b013e3181b1bb1a. [DOI] [PubMed] [Google Scholar]
- 27.Tolentino M. Systemic and Ocular Safety of Intravitreal Anti-VEGF Therapies for Ocular Neovascular Disease. Surv Ophthalmol. 2011;56(2):95–113. doi: 10.1016/j.survophthal.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Ng D, Kwok A, Chan C. Anti-vascular endothelial growth factor for myopic choroidal neovascularization. Clin Experiment Ophthalmol. 2011;40:e98–e110. doi: 10.1111/j.1442-9071.2011.02684.x. [DOI] [PubMed] [Google Scholar]