Abstract
Neuroplastic changes in dorsal striatum participate in the transition from casual to habitual drug use and might play a critical role in the development of methamphetamine (METH) addiction. We examined the influence of METH self-administration on gene and protein expression that may form substrates for METH-induced neuronal plasticity in the dorsal striatum. Male Sprague-Dawley rats self-administered METH (0.1 mg/kg/injection, i.v.) or received yoked saline infusions during eight 15-h sessions and were euthanized 2 h, 24 h, or 1 month after cessation of METH exposure. Changes in gene and protein expression were assessed using microarray analysis, RT-PCR and Western blots. Chromatin immunoprecipitation (ChIP) followed by PCR was used to examine epigenetic regulation of METH-induced transcription. METH self-administration caused increases in mRNA expression of the transcription factors, c-fos and fosb, the neurotrophic factor, Bdnf, and the synaptic protein, synaptophysin (Syp) in the dorsal striatum. METH also caused changes in ΔFosB, BDNF and TrkB protein levels, with increases after 2 and 24 h, but decreases after 1 month of drug abstinence. Importantly, ChIP-PCR showed that METH self-administration caused enrichment of phosphorylated CREB (pCREB), but not of histone H3 trimethylated at lysine 4 (H3K4me3), on promoters of c-fos, fosb, Bdnf and Syp at 2 h after cessation of drug intake. These findings show that METH-induced changes in gene expression are mediated, in part, by pCREB-dependent epigenetic phenomena. Thus, METH self-administration might trigger epigenetic changes that mediate alterations in expression of genes and proteins serving as substrates for addiction-related synaptic plasticity.
Keywords: methamphetamine, self-administration, dorsal striatum, ΔFosB, BDNF, pCREB
Introduction
Addiction to the illicit psychostimulant, methamphetamine (METH), is a serious public health problem worldwide because of severe medical and neuropsychiatric complications and high risks of relapse even after prolonged periods of abstinence (Callaghan et al., 2012; Sekine et al., 2008). These observations suggest that the transition from recreational drug use to addiction depends on long-lasting molecular adaptations in the brain secondary to repeated METH exposure. The adaptive changes may include alterations in gene and protein expression as well as structural modifications at dopaminergic and glutamatergic synapses (Robison and Nestler, 2011). The transition to addiction also appears to involve a shift of control over drug intake from ventral to the dorsal striatum when the drug user switches from casual to habitual and compulsive drug taking (Belin and Everitt, 2008; Everitt and Robbins, 2013; Wise, 2009).
Previous studies using chronic non-contingent amphetamine or METH administration have identified differential alterations in gene expression after repeated drug exposure in the rodent brain (Cadet et al., 2009; Konradi et al., 1996; Krasnova et al., 2011; McCoy et al., 2011; Renthal et al., 2008). They reported changes in the expression of immediate-early genes that include alterations in c-fos and fosb mRNA levels in the dorsal striatum after chronic amphetamine (Konradi et al., 1996; Renthal et al., 2008) or METH treatment (McCoy et al., 2011). Chronic METH exposure caused decreases in the expression of some immediate-early genes in animals euthanized 24 h after drug administration, with normalization of their expression after a single METH injection given to similarly treated rats (McCoy et al., 2011). However, the transcriptional alterations following METH self-administration have not been investigated. In the present study, we used METH self-administration model to decipher large-scale transcriptional changes in the dorsal striatum because of this region's role in habitual and compulsive aspects of drug addiction (Everitt and Robbins, 2013; Koob and Volkow, 2010). We also used chromatin immunoprecipitation (ChIP) followed by qPCR to identify potential epigenetic mechanisms underlying METH-induced changes in striatal gene expression. These experiments identified phosphorylated CREB (pCREB) as an important player in the regulation of changes in gene expression caused by METH self-administration.
Materials and Methods
Animals and METH Self-Administration
Male Sprague-Dawley rats (Charles River, Raleigh, NC), weighing 350–420 g, were housed individually under a reversed 12-h light/dark cycle with free access to food and water. Animal procedures were done according to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of NIDA (ASP #09-BNRB-31). The extended-access METH self-administration procedure and apparatus have been described in details previously (Krasnova et al., 2010). In brief, rats underwent eight days of 15-h METH self-administration sessions with their yoked partners receiving saline injections when METH (0.1 mg/kg/infusion) was self-administered by a rat in an active chamber. Rats were euthanized at 2 h, 24 h and 1 month after the final self-administration session and dorsal striata were isolated from the brains. Only animals with a total cumulative METH intake higher than 30 mg/kg over the eight daily sessions were used in further experiments. On the basis of these criteria, one or two rats from each group (<10% of animals) were excluded from the analyses.
RNA Isolation, Microarray Hybridization and Data Analysis
RNA extraction, RNA labeling and microarray hybridization were performed as previously described (Cadet et al., 2009). Microarray hybridization was done using RatRef-12 Expression BeadChips arrays (22 523 probes) (Illumina Inc.). A gene was identified as significantly changed if it showed increased or decreased expression according to an arbitrary cut-off at p ≤ 0.05, fold change 1.7. Functional annotation and classification analyses for significantly changed genes were performed using DAVID Annotation Tool (http: //david.abcc.ncifcrf.gov) (Huang da et al., 2009) and were supported by literature searches. To focus on changes that could be related to gene function, we included only genes classified using DAVID Annotation Tool in further analyses and excluded all undefined (LOC and RGD) genes. Microarray results were extensively validated by qRT-PCR as described below.
Quantitative RT-PCR Analyses of Gene Expression
qRT-PCR experiments were performed as previously described (Krasnova et al., 2007; 2008). Unpooled total RNA (1μg) isolated from striatal samples (n=6 per group) was reverse-transcribed with oligo dT primers using Advantage RT-for-PCR kit (Clontech). qRT-PCR experiments were done using LightCycler technology and Light Cycler FastStart DNA Master SYBR Green I kit (Roche Diagnostics). The primers were synthesized at the Synthesis and Sequencing Facility of Johns Hopkins University (Baltimore, MD). mRNA levels were normalized for each well to the clathrin mRNA levels. Purity of the qRT-PCR products was verified by melting curve analysis. The results are reported as fold changes calculated as the ratios of normalized gene expression data for each group in comparison to the control group.
Western Blot
Western blot experiments were conducted as described previously (Krasnova et al., 2011). Proteins were extracted from individual samples by sonication in lysis buffer A (10 mM HEPES, 10mM KCl, 1.5 mM MgCl2, 1% Igepal CA) that contained protease and phosphatase inhibitors (Roche Diagnostics) and centrifugation at 14 000g for 5 min at 4°C. Supernatant was collected and used as cytosolic protein fraction. The remaining pellet was resuspended in lysis buffer B (20 mM HEPES, 25% glycerol, 840 mM NaCl, 1.5 mM MgCl2, 0.4 mM EDTA) containing protease and phosphatase inhibitors and centrifuged at 15 000g for 15 min at 4°C. The supernatant was collected and used as nuclear protein fraction. 20–40 μg protein aliquots were separated by SDS-PAGE and transferred to PVDF membranes. Levels of D1 and D2 DA receptors, c-Fos, FosB, ΔFosB, BDNF and TrkB were measured in cytosolic protein fraction. Levels of CREB, pCREB and H3K4me3 were measured in nuclear protein fraction. After the transfer, membranes were blocked and incubated with antibodies against D1, D2 DA receptors (1:1000, EMD-Millipore), FosB (1:500, Cell Signaling), BDNF (1:500, Santa-Cruz Biotechnology, Inc.), TrkB (1:1000, EMD-Millipore), CREB, pCREB or H3K4me3 (1:1000, all from Cell Signaling). Immune complexes were detected with HRP-labeled second antibody and ECL+ chemiluminescence reagents (GE Healthcare). To confirm equal protein loading, blots were reprobed with anti-α-tubulin antibody. Optical densities were measured using Carestream Imaging System (Kodak). Each group contained samples from 5–9 animals that were run individually. The results are reported as % of control changes calculated as the ratios of protein expression for each METH group in comparison to control group.
Chromatin Immunoprecipitation (ChIP) Assay
Rats were subjected to METH self-administration as described above. Two hours after the cessation of METH self-administration animals were euthanized, dorsal striata were dissected and processed for ChIP as described by Tsankova et al. (2004). The tissue was minced and cross-linked in 1% paraformaldehyde/PBS for 15 min. Cross-linking was stopped by addition of glycine to a final concentration 0.125 M. Samples were washed in ice-cold PBS containing protease inhibitors (Roche Diagnostics), frozen on dry ice, and stored at −80°C. The fixed tissue was resuspended in SDS lysis buffer (EMD-Millipore) containing protease inhibitors and 1 mM PMSF and homogenized. Each sample was sonicated using the Bioruptor (Diagenode Inc.). The fragmentation of DNA was verified by gel analysis to confirm the sheared range of 300–600 bp. Dynabeads (Life Technologies) were incubated with 5 μg of antibodies against H3K4me3 (Cell Signaling) or pCREB (EMD-Millipore). 50 μg of chromatin lysate were diluted with ChIP dilution buffer (EMD-Millipore) to a volume of 1.5 ml. 100 μl of the preimmunoprecipitated lysate were saved as “input” for later normalization. The chromatin lysate was then immunoprecipitated with appropriate antibodies overnight at 4°C. The beads were washed with low salt, high salt, LiCl, and TE buffers (EMD-Millipore). DNA-protein complex was eluted from the beads with 500 μl NaHCO3/SDS buffer. DNA and proteins were dissociated at 65°C for 4 h under high-salt conditions, followed by RNase A and proteinase K treatments. DNA was extracted with phenol/chloroform, precipitated with ethanol, and resuspended in 10 mM Tris pH 8.0.
qPCR was performed with primers specific for c-fos, fosb, c-jun, Nr4a1-3, Syp, Bdnf-pI, Bdnf-pIV, and Bdnf-pVI promoters (Table S1). qPCR for ChIP-derived DNA samples was done using a LightCycler thermal cycler (Roche Diagnostics), with SYBR Green monitoring. qPCR specificity was verified by melting curve analysis. All PCR reactions were performed in duplicate and included negative controls (no DNA). Threshold amplification cycle numbers (Tc) and LightCycler software were used to calculate IP DNA quantities as % of input controls.
HPLC Analysis
The levels of dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), serotonin (5-HT) and 5-hidroxyindoleacetic acid (5-HIAA) were analyzed in tissue extracts using HPLC with electrochemical detector (Krasnova et al., 2007). The data were expressed as ng/mg of tissue weight and reported as % of control concentrations.
Statistical Analysis
Results were expressed as means ± S.E.M.s and were considered significant at p < 0.05. Statistical analysis of the data was done using either ANOVA for repeated measures followed by Tukey's multiple comparison test, ANOVA followed by Fisher's protected least significant difference or Student's t-test (StatView 4.02). Correlations between microarray and qRT-PCR data were assessed using linear regression.
Results
METH Self-Administration and Intake
Previous studies on the actions of METH in animals have focused mainly on the effects of non-contingent experimenter-administered drug injections on monoaminergic systems in the brain (see Carvalho et al., 2012; Krasnova and Cadet, 2009; Marshall and O'Dell, 2012, for reviews). However, self-administration models provide a better tool for studying METH addiction, because they employ patterns of exposure more typical of human drug use that include voluntary drug taking behavior and escalation of METH intake during development of METH dependence (Cho and Melega, 2002). Thus, we used an extended-access model of intravenous METH self-administration to study neuroplastic changes in the dorsal striatum. Rats self-administered METH during eight consecutive 15-h daily sessions as described previously (Krasnova et al., 2010). Control animals received yoked saline injections. Figure 1 shows a representative graph of METH intake for animals euthanized at 24 h after cessation of drug exposure. Rats self-administered an average 2.8 ± 1.0 mg/kg of METH during session 1. Access to METH self-administration caused an elevation of drug intake to 7.9 ± 0.8 mg/kg on session 2, which continued to increase and peaked at 14.2 ± 1.4 mg/kg on session 6 (Fig. 1). The escalation of METH intake was highly significant (F(7, 80) = 9.572; p < 0.001). Total cumulative drug intake over eight sessions was 86.9 ± 8.6, 85.1 ± 8.7 and 88.5 ± 8.6 mg/kg for groups of rats euthanized at 2 h, 24 h and 1 month after cessation of METH exposure, respectively, and was not significantly different between the groups. To better mimic moderate-to-high drug use by METH addicted individuals, animals with total METH intake less than 30 mg/kg (one or two rats per group, <10% of animals) were excluded from further experiments.
Figure 1.
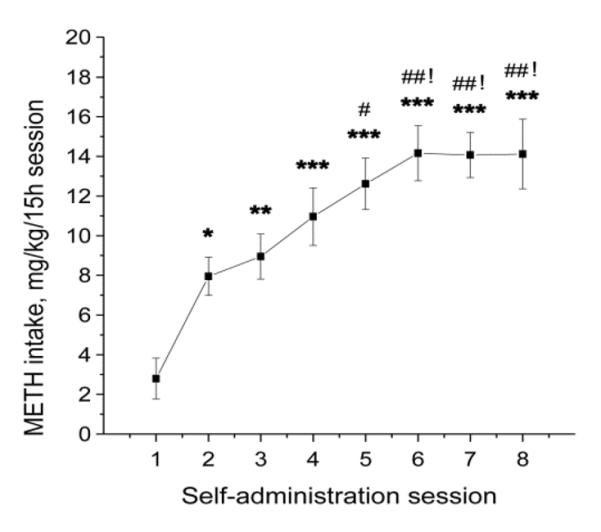
Rats escalated METH intake over the course of self-administration. Symbols show an average METH intake per 15-h session in the group of rats euthanized 24 h after cessation of self-administration. Rats increased METH intake on session 2 compared with session 1, and on session 5 compared with session 2. Animals further escalated their drug intake on session 6 in comparison to session 3 and stayed at this level until cessation of METH self-administration. METH intake by rats in the 24-h group reached 14.2 mg/kg/day. Data were analyzed by one-way ANOVA for repeated measures, followed by Tukey's multiple comparison test. N = 12. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with session 1; # p < 0.05, ## p < 0.01 compared with session2; ! p < 0.05 compared with session 3.
METH-Induced Changes in Monoamine Levels and in the Expression of D1 and D2 DA Receptors
Using similar models, we (Krasnova et al., 2010) and others (McFadden et al., 2012a,b; Schwendt et al., 2009) have been able to replicate previous biochemical observations in human METH addicts (Volkow et al., 2001b; Wilson et al., 1996). These include depletion of DA, decreases in DA transporter and reductions in tyrosine hydroxylase expression in the striatum. In agreement with our previous findings (Krasnova et al., 2010), METH self-administration also caused significant depletion in DA levels in the dorsal striatum that persisted up to 1 month (−21%) after cessation of drug exposure (F(3,32) = 7.007, p = 0.0011) (Fig. 2A). The concentrations of DOPAC were decreased at 2 (−27%) and 24 h (−23%) (F(3,32) = 3.503, p = 0.0369), but returned to control levels at 1 month post-drug (Fig. 2B). The concentrations of 5-HT were reduced at 2 and 24 h (−19% and −29%, respectively) (F(3,32) = 4.128, p = 0.0139), but normalized at 1 month withdrawal (Fig. S1). In addition, METH also caused a significant decrease in 5-HIAA levels up to 1 month post-drug (−22%) (F(3,32) = 7.978, p = 0.0004) (Fig. S1).
Figure 2.
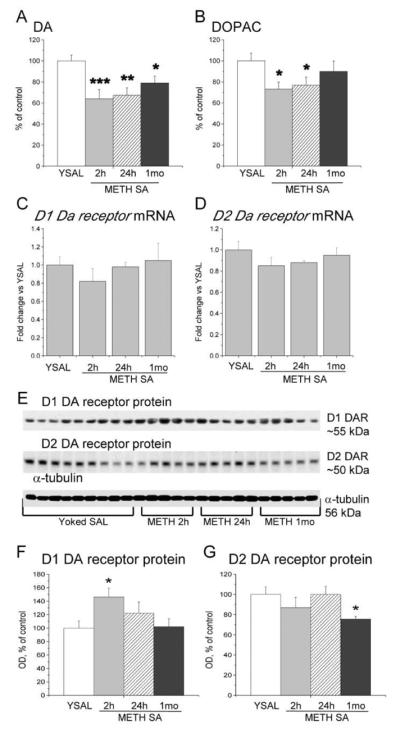
Regulation of DA levels and D1 and D2 DA receptor expression in the dorsal striatum following METH self-administration. Rats were allowed to self-administer METH for eight 15-h sessions and were euthanized at 2 h, 24 h or 1 month withdrawal. The dorsal striata were dissected and processed for HPLC, qRT-PCR and Western blot as described in the text. (A) METH caused persistent decreases in striatal DA levels up to 1 month post-drug; (B) DOPAC concentrations were decreased at 2 and 24 h, but returned to control levels after 1 month of withdrawal. (C) D1 DA receptor and (D) D2 DA receptor mRNA levels are not significantly changed at 2 h, 24 h, or 1 month after cessation of METH self-administration. (E) Representative immunoblots of membrane D1 and D2 DA receptor protein levels. (F) METH self-administration caused significant increases in D1 DA receptor protein levels. (G) D2 DA receptor protein levels are decreased after 1 month. N = 5–9 per group. Data are expressed as fold-changes (mRNA) or percent (protein, monoamine levels) of mean ± SEM values of yoked saline group. * p < 0.05, ** p < 0.01, *** p < 0.001 significantly different from yoked saline.
Because METH addiction is associated with reductions in D2 DA receptors in the human caudate (Volkow et al., 2001a; Wang et al., 2011), we tested the possibility that similar changes might also occur in the dorsal striatum of rats exposed to METH self-administration. Fig. 2 shows the effects of METH self-administration and abstinence on striatal D1 and D2 DA receptor mRNA and protein levels. There were no significant changes in the expression of D1 or D2 DA receptor mRNAs (Fig. 2C, D). However, there were transient increases in D1 DA receptor protein levels (+47%) (F(3,19) = 3.390, p = 0.0443) at 2 h that returned to control 24 h after drug cessation (Fig. 2E, F). Similar to observations in humans (Volkow et al., 2001a; Wang et al., 2011), D2 DA receptor protein levels were decreased (−24%) after 1 month of abstinence (F(3,19) = 3.401, p = 0.0389) (Fig. 2E, G). Taken together, the behavioral and biochemical similarities between this rat model and the human addiction had provided us with the confidence necessary to pursue transcriptional and epigenetic studies in rats that self-administered METH.
Transcriptional Changes in the Dorsal Striatum Induced by METH Self-Administration and Short-Term Abstinence
In order to avoid a priori theoretical biases, we used a discovery platform, Illumina 22K Rat microarray, to measure transcriptional effects of METH self-administration in the dorsal striatum. We found that 543 and 266 transcripts were differentially affected (±1.7 fold, p < 0.05) in the striatum at 2 and 24 h after the last METH self-administration session, respectively. Table 1 provides a partial list of these genes. Full lists of genes significantly changed at 2 and 24 h are shown in Tables S2 and S3.
Table 1.
Partial list of genes affected by METH self-administration and withdrawal in the rat striatum.
| Gene Name | Gene Symbol | Expression Ratio METH / Yoked SAL | p value (ANOVA) | |
|---|---|---|---|---|
| 2h | 24h | |||
| Neurotrophic factors | ||||
| Heparin-binding EGF-like growth factor | Hbegf | 4.44 | 3.45 | 0.0406; 0.0114 |
| Brain derived neurotrophic factor | Bdnf | 3.28 | 1.33 | 0.0379 |
| Nerve growth factor, gamma | Ngfg | −2.31 | 2.22 | 0.0163 |
| Synoptic proteins | ||||
| Syntaxin 1A | Stx1a | 2.30 | −1.02 | 0.0074 |
| Synapsin II | Syn2 | 2.12 | −1.11 | 0.0433 |
| Synaptophysin | Syp | 1.74 | 1.20 | 0.0044 |
| Synaptojanin 2 | Synj2 | 1.73 | 1.29 | 0.0482 |
| Neuropeptides | ||||
| Cholecystokinin | Cck | 4.93 | 1.68 | 0.0022 |
| Neuromedin S | Nms | 4.41 | −1.01 | 0.0041 |
| Neuromedin U | Nmu | 3.84 | 1.35 | 0.0001 |
| Cortistatin | Cort | 3.53 | 1.19 | 0.0047 |
| Prepronociceptin | Pnoc | 3.42 | 1.23 | 0.0079 |
| Neurotensin | Nts | 3.08 | 1.05 | 0.0069 |
| Follistatin | Fst | 2.20 | 1.24 | 0.0001 |
| Prodynorphin | Pdyn | 1.79 | 1.26 | 0.0006 |
| Neuropeptide Y | Npy | 1.74 | 1.31 | 0.0112 |
| Receptors | ||||
| Glial cell line derived neurotrophic factor receptor alpha 1 | Gfra1 | 7.76 | 1.70 | 0.0048 |
| Gastrin releasing peptide receptor | Grpr | 7.08 | −2.05 | 0.0057 |
| Cholinergic receptor, nicotinic, alpha polypeptide 4 | Chrna4 | 5.60 | 1.17 | 0.0484 |
| Neuropeptide Y receptor Y1 | Npy1r | 2.35 | −1.31 | 0.0440 |
| Angiotensin receptor 1b | Agtr1b | 2.10 | −1.12 | 0.0048 |
| Relaxin/insulin-like family peptide receptor 1 | Rxfp1 | 1.88 | 1.27 | 0.0072 |
| Cholinergic receptor, muscarinic 3, cardiac | Chrm3 | 1.82 | 1.29 | 0.0217 |
| Fibroblast growth factor receptor-like 1 | Fgfrl1 | 1.81 | 1.27 | 0.0104 |
| Fibroblast growth factor receptor 4 | Fgfr4 | 1.35 | 3.51 | 0.0132 |
| Transporters | ||||
| Solute carrier family 8, member 1 | Slc8a1 | 7.56 | 1.37 | 0.0012 |
| Vesicular glutamate transporter 2 | Vglut2 | 6.30 | 1.77 | 0.0001 |
| Solute carrier family 37, member 1 | Slc37a1 | 6.07 | −1.05 | 0.0044 |
| Solute carrier family 9, isoform 3 regulator 2 | Slc9a3r2 | 5.60 | −1.11 | 0.0035 |
| Vesicular glutamate transporter 1 | Vglut2 | 3.95 | 2.06 | 0.0138 |
| Kinases/Phosphatases | ||||
| Ca2+/calmodulin-dependent kinase kinase 2, beta | Camkk2 | −1.23 | 1.93 | 0.0342 |
| Protein kinase C, delta | Prkcd | 1.34 | 1.75 | 0.0388 |
| Transcription factors | ||||
| Neuronal pentraxin 1 | Nptx1 | 4.82 | 1.37 | 0.0027 |
| Nuclear receptor subfamily 4, group A, member 1 | Nr4a1/Nurr77 | 2.03 | 1.02 | 0.0015 |
| Nuclear receptor subfamily 4, group A, member 3 | Nr4a3 | 1.99 | −1.01 | 0.0413 |
| Nuclear receptor subfamily 4, group A, member 2 | Nr4a2/Nurr1 | 1.92 | 1.51 | 0.0409 |
| FBJ murine osteosarcoma viral oncogene homolog | c-fos | 1.87 | 1.29 | 0.0424 |
| Jun oncogene | c-jun | 1.84 | 1.13 | 0.0155 |
| Neuronal pentraxin 2 | Nptx2 | 1.77 | 1.06 | 0.0213 |
| cAMP responsive element modulator | Crem | 1.72 | 1.02 | 0.0124 |
| FBJ osteosarcoma oncogene B | fosb | 1.72 | 1.27 | 0.0452 |
2 h after cessation of METH self-administration, changes in gene expression were detected in several functional groups that included transcription factors, neurotrophic factors, neuropeptides, neurotransmitter and peptide receptors, transporters, and genes involved in neurotransmitter release (Table 1). Specifically, METH self-administration caused up-regulation of c-fos, c-jun, Crem, fosb, Nptx1, Nptx2, and Nr4a1-3 (Table 1) that play roles in transcriptional regulation. The large number of receptors and transporters affected by METH self-administration suggests that adaptive neuroplastic responses might involve multiple striatal neurotransmitter systems. In addition, METH induced increases in the expression of several neuropeptides including cholecystokinin (Cck), neurotensin (Nts) and prodynorphin (Pdyn) that are modulators of striatal DA neurotransmission (Angulo and McEwen, 1994; Loonam et al., 2003; Muschamp and Carlezon, 2013). There was also up-regulation in the expression of genes coding for synaptic proteins, syntaxin 1A (Stx1a), synapsin II (Syn2), synaptophysin (Syp) and synaptojanin 2 (Synj2), which are involved in neurotransmitter release (Cesca et al., 2010; de Wit, 2010; Liu and Ju, 2001). Moreover, METH self-administration caused substantial increases in Bdnf mRNA levels in the striatum (Table 1). BDNF is a neurotrophic factor that participates in the regulation of synaptic plasticity and is affected by exposure to several drugs of abuse (Autry and Monteggia, 2012; Russo et al., 2009).
Validation of Microarray Data with qRT-PCR
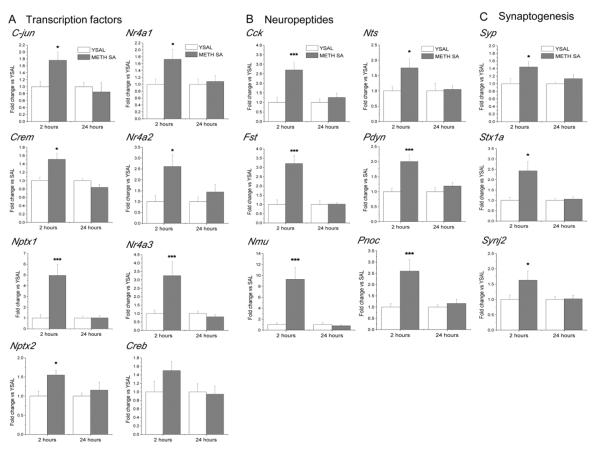
We then used qRT-PCR to validate the expression of several genes identified in the microarray experiments as significantly changed by METH self-administration. We confirmed METH-induced changes in c-jun, Crem, Nptx1, Nptx2, Nr4a1, Nr4α2, and Nr4a3 mRNA levels that were up-regulated 2 h but normalized after 24 h of drug abstinence (Fig. 3A). The expression of these genes is known to be altered by other illicit drugs, including cocaine and heroin (Graybiel et al., 1990; Horner and Keefe, 2006; McClung and Nestler, 2008).
Figure 3.
qRT-PCR validation of METH-induced changes in the expression of genes identified in microarray experiments. qRT-PCRs were performed with the same total RNA samples isolated from dorsal striata of rats that were used for microarray hybridization. Values represent means ± SEM in comparison to the respective yoked saline group. Consistent with microarray data, METH self-administration induced increases in the expression of (A) transcription factors, (B) neuropeptides, (C) genes involved in the synaptic functions at 2 h, which were normalized at 24 h after cessation of drug exposure. N = 6 per group. * p < 0.05, *** p < 0.001 in comparison to yoked saline (Student's t-test).
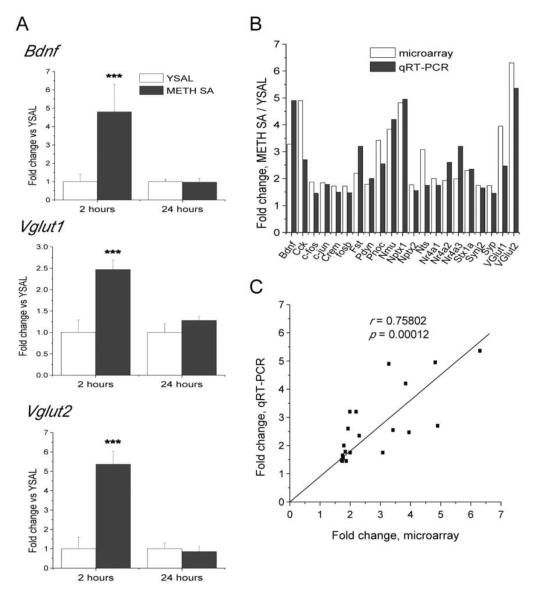
The RT-PCR data also confirmed that METH self-administration caused increases in the expression of genes coding for several neuropeptides, including Cck, Fst, Nmu, Nts, Pdyn and Pnoc (Fig. 3B). Several of these neuropeptides interact with DA systems in the brain (Angulo and McEwen, 1994; Loonam et al., 2003) and participate in the regulation of reward mechanisms (Muschamp and Carlezon, 2013). In addition, we confirmed METH-induced increases in the expression of Stx1a, Synj2 and Syp that label presynaptic terminals of functional synapses (Liu and Ju, 2001) (Fig. 3C). Finally, we verified that METH self-administration causes increases in the expression of Bdnf, Vglut1, and Vglut2 (Fig. 4A). BDNF is known to play important roles in the regulation of synaptic plasticity in the adult brain (Autry and Monteggia, 2012; Russo et al., 2009) and in several neuropsychiatric disorders including affective disorders (Autry and Monteggia, 2012).
Figure 4.
Validation of METH-induced alterations in the expression of genes identified by microarray experiments using qRT-PCR. (A) METH self-administration caused increases in the expression of Bdnf and Vgluts in the dorsal striatum. (B) The increases in the expression of 21 genes obtained using microarray were validated with qRT-PCR. All fold-changes found with qRT-PCR were significantly different (p < 0.05). (C) METH-induced changes obtained using microarray analysis significantly correlated with qRT-PCR data. N = 6 per group. *** p < 0.001 in comparison to yoked saline (Student's t-test).
In order to quantify potential relationships between the microarray and qRT-PCR data (Fig. 4B), we performed a regression analysis and found a strong positive correlation between changes in gene expression obtained using both methods (Fig. 4C). This analysis validates the inclusion criteria for choosing genes with altered expression using the discovery array platform.
Regulation of cFos, FosB and ΔFosB by METH Self-Administration in the Dorsal Striatum
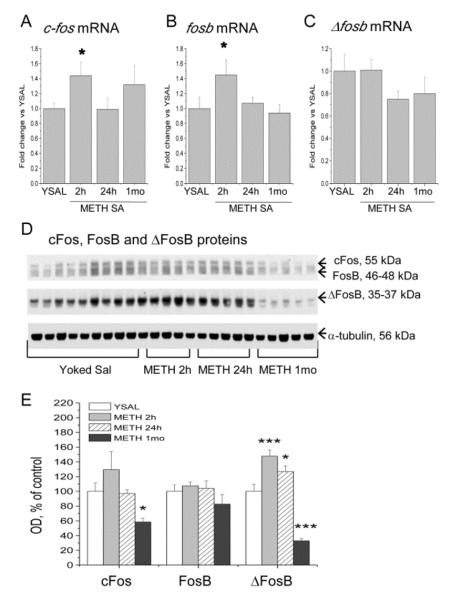
Because c-fos mRNA expression was up-regulated (+42%) at 2 h after cessation of METH self-administration (F(3,19) = 5.568; p = 0.014) (Fig. 5A), and taking into account the potential role of Fos transcription factors in the development of neuroadaptations that might underlie compulsive drug-taking (Nestler, 2012), we also measured the effects of METH self-administration and abstinence on FosB and ΔFosB mRNA and protein levels in the dorsal striatum. Similar to c-fos, METH caused increases (+45%) in fosb mRNA expression 2 h after cessation of drug exposure (F(3,19) = 3.69, p = 0.0315), with normalization after 24 h (Fig. 5B). There were no changes in Δfosb mRNA levels (Fig. 5C). In contrast to increases in c-fos mRNA expression, METH self-administration did not affect cFos protein levels after 2 or 24 h of abstinence, but caused significant decreases (−42%) at 1 month withdrawal (F(3,20) = 3.91, p = 0.0238) (Fig. 5D, E). METH did not change FosB protein levels up to 1 month after cessation of self-administration (Fig. 5D, E). Interestingly, ΔFosB protein levels were significantly increased 2 (+48%) and 24 h (+27%) after cessation of METH self-administration, but decreased expression (−67%) was detected after 1 month of abstinence (F(3,20) = 27.69, p < 0.0001) (Fig. 5D, E).
Figure 5.
Effects of METH self-administration and abstinence on c-fos, fosb and Δfosb expression in the dorsal striatum. METH induced increases in c-fos (A) and fosb (B) mRNA levels at 2 h, with normalization at 24 h and 1 month after cessation of self-administration. (C) METH self-administration did not cause significant changes in the expression of Δfosb mRNA expression. (D) Analysis of c-Fos, FosB and ΔFosB protein levels in the dorsal striatum. Representative immunoblot showing the molecular weights of the proteins used to quantify levels of c-Fos (55 kDa), FosB (46–48 kDa) and ΔFosB (35–37 kDa). Signals were normalized to α-tubulin. (E) METH self-administration did not affect the expression of FosB, but caused decreases in the levels of c-Fos protein levels at 1 month of abstinence. METH induced increases in ΔFosB protein levels at 2 and 24 h followed by decreased expression at 1 month withdrawal. N = 5–9 per group. * p < 0.05, *** p < 0.001, significantly different from yoked saline.
Regulation of BDNF and TrkB Receptor by METH Self-Administration
To get a better picture of METH effects on Bdnf mRNA expression, we measured the time-course of METH-induced changes in the mRNAs for Bdnf and its receptor, TrkB. METH induced significant increases (+380%) in Bdnf exon I mRNA expression after 2 h, with normalization after 24 h and 1 month of abstinence (F(3,19) = 5.41, p = 0.0068) (Fig. 6A). Trkb mRNA expression showed no significant changes at any time point (Fig. 6B). At 2 and 24 h after cessation of METH self-administration, BDNF protein levels were increased by 89% and 63%, respectively, compared to yoked saline controls (Fig. 6C, D). In contrast, after 1 month of abstinence, BDNF protein levels were significantly decreased (−59%) (F(3,20) = 29.71, p < 0.0001) (Fig. 6C, D). We also measured the levels of TrkB protein after cessation of METH self-administration and found significant increases at 2 (+45%) and 24 h (+33%) (Fig. 6C, E). Similar to BDNF, TrkB protein levels were decreased (−33%) after 1 month of abstinence (F(3,20) = 22.13, p <0.0001) (Fig. 6C, E).
Figure 6.
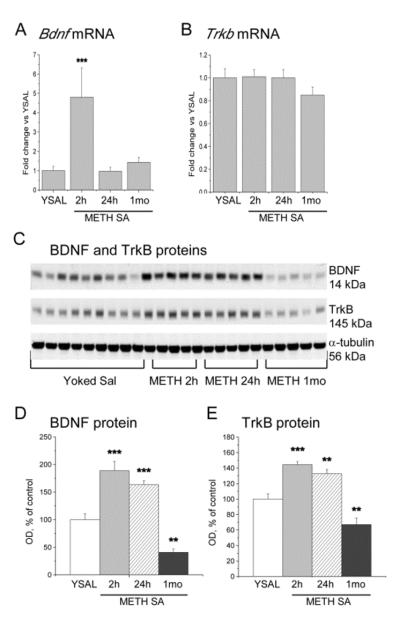
METH-induced changes in the expression of BDNF and its receptor, TrkB, in the dorsal striatum. (A) Bdnf mRNA expression (exon I) was increased 2 h after cessation of METH self-administration but returned to control level at 24 h and 1 month. (B) METH self-administration did not cause any changes in the expression of Trkb mRNA up to 1 month after cessation of METH self-administration. (C) Immunoblot analysis of BDNF and TrkB protein levels in the dorsal striatum. METH self-administration induced increases in (D) BDNF and (E) TrkB protein levels at 2 and 24 h but decreases at 1 month abstinence. Data are presented as means ± SEM. N = 5–9 per group. * p < 0.05, ** p < 0.01, *** p < 0.001, significantly different from yoked saline control.
METH Self-Administration Caused Enrichment of pCREB, but not of H3K4me3, on c-fos, fosb, Bdnf and Syp Promoters
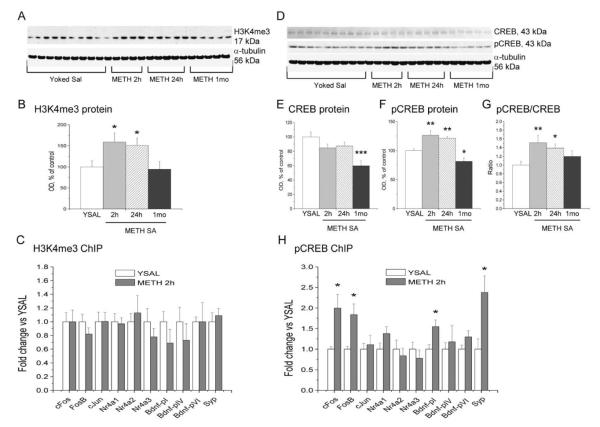
Gene expression is regulated, in part, by epigenetic modifications that include histone acetylation, methylation, and phosphorylation (Nelson and Monteggia, 2011; Wang et al., 2008). In order to investigate potential epigenetic mechanisms that might be involved in METH-induced changes in gene expression, we initially focused on the expression of histone 3 trimethylated at lysine 4 (H3K4me3) because this histone modification has been linked to activation of gene expression (Ng et al., 2009). We found that METH self-administration caused significant increases (F(3,20) = 3.565, p = 0.0363) in H3K4me3 protein levels after 2 h (+60%) and 24 h (+51%), with normal levels measured after 1 month of abstinence (Fig. 7A, B). Next, we used ChIP-PCR to test if there might be increased enrichment of H3K4me3 on the promoters of some METH-upregulated genes of interest. These included c-fos, fosb, c-jun, Nr4a1-3, and Syp, which are involved in synaptic plasticity or regulated by synaptic activity (Fig. 7C). We also measured the binding of H3K4me3 on Bdnf promoters pI (Bdnf-pI), pIV (Bdnf-pIV), and pVI (Bdnf-pVI). There were no significant METH-induced changes in the enrichment of H3K4me3 on promoters of any of these genes (Fig. 7C).
Figure 7.
Transcriptional responses in the dorsal striatum caused by METH self-administration are associated with increased enrichment of pCREB, but not H3K4me3, on promoters of genes related to synaptic plasticity. (A) Immunoblot analysis of H3K4me3 protein levels following cessation of METH self-administration. (B) METH caused increased H3K4me3 levels after 2 and 24 h abstinence from METH self-administration. (C) There were no changes in H3K4me3 enrichment on the promoters of METH-regulated genes. (D) Western blots of CREB and pCREB levels in METH self-administration and yoked saline rats. (E) Analysis of optical density showed decreases in CREB levels at 1 month abstinence. (F) METH induced increases in pCREB levels at 2 and 24 h, followed by decreases at 1 month after cessation of drug exposure. (G) pCREB/CREB ratios were increased at 2 and 24 h, but normalized at 1 month post-drug. (H) There was a significant increase in the levels of pCREB on promoters of c-fos, fosb,Syp and BdnfpI. Data presented as means ± SEM. N = 5–9 per group. * p < 0.05, ** p < 0.01, *** p < 0.001, significantly different from yoked saline control.
In addition to histone modifications, inducible gene expression is regulated by a number of factors that include phosphorylation of CREB and subsequent activation of gene expression (Nestler, 2012; Robison and Nestler, 2011). Thus, we measured the expression of CREB and pCREB protein levels at various time-points following the cessation of METH self-administration. There were significant decreases in CREB protein levels (−40%) at 1 month (F(3,20) = 6.17, p = 0.0038) (Fig. 7D, E), but increases in pCREB levels at 2 (+27%) and 24 h (+21%), followed by decreased levels (−19%) after 1 month of METH abstinence (F(3,20) = 15.232, p < 0.0001) (Fig. 7D, F). pCREB/CREB ratios were significantly increased at 2 and 24 h (+51% and +39%, respectively), but returned to basal levels at 1 month after cessation of drug exposure (F(3,20) = 4.074, p = 0.0226) (Fig. 7G). To examine the role of CREB-regulated transcription in METH-induced gene expression, we used ChIP-PCR to measure the enrichment of pCREB on the promoters of the same genes listed above. These genes are known to contain CRE motifs (Fass et al., 2003; Tao et al., 1998). Four of these genes (c-fos, fosb, Bdnf-pI, and Syp) showed significantly increased enrichment of pCREB on their promoters 2 h after cessation of METH self-administration (Fig. 7H), results that are consistent with upregulation of their transcripts by METH.
Discussion
The transition from recreational drug use to addiction and recurrent relapse is hypothesized to involve a progressive shift from ventral to dorsal striatal control over drug intake as a result of long-term molecular and structural adaptations in mesostriatal dopaminergic pathways (Belin and Everitt, 2008; Everitt and Robbins, 2013; Volkow et al., 2011). These adaptative changes are secondary, in part, to drug-induced transcriptional and epigenetic alterations in the brain (Robison and Nestler, 2011). Indeed, we have found that METH self-administration was associated with transcriptional changes in genes that regulate transcription, synaptic transmission, and synaptic plasticity in the dorsal striatum. Specifically, there was increased ΔFosB protein expression 2 and 24 h after cessation of METH self-administration followed by a decrease after 1 month of abstinence. Our observations are consistent with recent findings of increased ΔFosB expression in the caudate-putamen of rats allowed to chronically self-administer METH (Cornish et al., 2012). A role for ΔFosB in METH self-administration is supported by a report that ΔFosB increases the motivation for cocaine self-administration (Colby et al., 2003) and by the finding that induction of ΔFosB is sufficient to increase locomotor and rewarding effects of cocaine (Kelz et al., 1999; Muschamp et al., 2012). The present data obtained at early stages of abstinence (↑ΔFosB) are consistent with the notion that the protein might regulate the transition from occasional drug use to increased drug-seeking and drug-taking behaviors and, possibly, the maintenance of addiction (Robison and Nestler, 2011). However, it is unlikely that ΔFosB is directly involved in relapse susceptibility because there were significant decreases in ΔFosB expression after 1 month of abstinence, a time at which animals might be more prone to reinstatement of drug-taking behavior (Shaham et al., 2003). Nevertheless, the protein could have had secondary effects by inducing the transcription of neurotransmitter receptors and other synaptic proteins, including ion channels and cytoskeletal proteins that might be involved in triggering relapse (McClung and Nestler, 2008; Renthal et al., 2008; Robison and Nestler, 2011). Indeed, given the consistency of the effects caused by various drugs on ΔFosB expression, it is very likely that this protein might be an important node that connects various networks involved in the early manifestations, maintenance of, and, perhaps, relapse to addiction.
In addition to changes in ΔFosB, we found significant increases in BDNF and TrkB 2 at 24 h after cessation of METH self-administration but decreases after 1 month of METH abstinence. It is of interest that METH increased TrkB protein, but not TrkB mRNA levels in the dorsal striatum. A similar dissociation between TrkB mRNA and protein expression has been previously reported in the rat nucleus accumbens after cocaine self-administration (Fumagalli et al., 2009; Graham et al., 2009). Together, these observations suggest that METH-induced increase in striatal TrkB protein levels is not due to up-regulated TrkB mRNA expression but is secondary to non-transcriptional mechanisms including increased translation and protein stability, and/or reduced protein degradation.
Although METH exposure increased both striatal BDNF mRNA and protein levels at 2 h, BDNF protein levels were increased at 24 h in the absence of changes in mRNA expression. Dissociations between the effects of psychostimulants on BDNF mRNA and protein levels have also been reported by other investigators (Graham et al., 2007; Fumagalli et al., 2007, 2013; Li et al., 2013). As with TrkB expression and at first glance, mechanisms other than increased mRNA levels appear to be responsible for METH-induced increased BDNF protein levels. These mechanisms might include increased stabilization of BDNF protein secondary to the early increases in BDNF mRNA and protein levels observed at 2 h after cessation of METH self-administration. Processing of pro-BDNF into mature BDNF has also been reported to increase in a Ca2+-dependent manner via elevated secretion of tissue plasminogen activator (Gualandris et al., 1996). Another possible mechanism is METH-induced anterograde transport of BDNF protein from cortical neurons that project to the striatum and that also express high BDNF levels (Altar et al., 1997; Conner et al., 1997; Guillin et al., 2001; Lessmann et al., 2003). In support of this hypothesis, increases in BDNF protein levels have been shown in the prefrontal cortical target areas such as nucleus accumbens, basolateral amygdala and central nucleus of the amygdala after infusion of BDNF into rat dorsomedial prefrotal cortex (Berglind et al., 2007, McGinty et al., 2010). Finally, our observations of dissociations between the mRNA and protein levels for DA receptors, BDNF, and TrkB are consistent with the findings that correlations among individual mRNA and protein concentrations vary widely in the same organism as well as across species and are often surprisingly low (Anderson and Seilhamer, 1997; Unwin and Whetton, 2005; De Souse Abreu et al., 2009; Evguenieva-Hackenberg and Klug, 2011).
Our results showing increases in BDNF protein levels at 2 and 24 h after METH self-administration but decreases after 1 month of withdrawal are in agreement with clinical studies reporting elevated BDNF levels in the plasma of chronic METH users (Kim et al., 2005), with BDNF concentrations in METH addicts dropping with increased duration of abstinence (Hilburn et al., 2011). Our results are also consistent with the idea that BDNF signaling participates in the development of addiction (Russo et al., 2009). In this regard, BDNF can enhance the expression of a marker of functional synapses Syp (Liu and Ju, 2001); an effect that is blocked by BDNF and TrkB antibodies (Li and Keifer, 2008, 2012). Thus, our finding that METH also caused increase in Syp mRNA levels suggests that the drug might enhance the functionality of striatal synapses (Liu and Ju, 2001) and cause sustained changes in neurotransmission in METH-addicted rats. Increased BDNF expression is likely to enhance the activation of TrkB downstream pathways (Ji et al., 2010), possibly involving a cascade of events that include MAPK, phosphatidylinositol and/or PLC-γ pathways (Park and Poo, 2013). These signaling events might be responsible, in part, for METH-induced long-lasting morphological changes in the brain. This idea is consistent with observations of METH-induced dendritic spine formation on dorsal striatal neurons (Jedynak et al., 2007), an effect that is indeed dependent on BDNF-TrkB signaling (Rauskolb et al., 2010). Thus, it is possible that sustained BDNF accumulation is not necessary for its long-lasting impact on the density of dendritic spines, but only for the triggering of multiple cellular processes that facilitate persistent striatal reorganization of synaptic connectivity.
Note, however, that, after 1 month of abstinence, there were significant decreases in BDNF expression, suggesting the possibility that low BDNF levels might be conducive to relapse, as previously reported for ethanol drinking (Jeanblanc et al., 2009). These observations are in contrast to those reported for cocaine withdrawal, which is associated with progressive increases in BDNF levels in the nucleus accumbens up to 90 days of abstinence (Grimm et al., 2003). It is to be noted that Grimm et al. (2003) had measured BDNF in the nucleus accumbens and VTA, whereas we measured the expression in the dorsal striatum. It is also of interest to discuss the observations that there were non-significant decreases in Ngf and Camkk2 mRNA expression at 2 h but increases in their mRNAs at 24 h. Together with BDNF, NGF belongs to secreted neurotrophins that regulate neuronal circuits and synaptic plasticity (Park and Poo, 2013). Therefore, increase in the expression of NGF could, in addition to BDNF, contribute to synaptic remodeling and neuroplastic changes caused by METH self-administration in the dorsal striatum. Similarly, CaMKK2 is highly expressed in the brain where it plays important role in activating intracellular responses to elevated Ca2+. Up-regulated expression of CaMKK2 could contribute to increased phosphorylation of transcription factors, including CREB (Kokubo et al., 2009). Our observation of increased CREB phosphorylation in the present study suggests that CaMKK2 might also be involved in the regulation of gene expression observed after cessation of METH self-administration.
In eukaryotic cells, DNA exists as chromatin, whose basic unit is the nucleosome (Kornberg and Lorch, 1999). The nucleosome consists of 147 bp of DNA wrapped around a histone octomer that contains 2 of H2A, H2B, H3, and H4 histones. The histone tails protrude through that complex and form a scaffold for modifications such as acetylation, methylation, and phosphorylation that play very important roles in modulating gene expression (Nelson and Monteggia, 2011; Wang et al., 2008). As a first step towards testing the idea that METH-induced gene expression might be regulated by epigenetic modifications, we measured the levels of H3K4me3, a histone modification linked to activation of gene expression (Ng et al., 2009) and found METH-induced increases in H3K4me3 protein expression. Unexpectedly, none of the chosen genes of interest showed increased H3K4me3 enrichment on their promoters.
Gene expression is also dependent on combinatorial effects of transcription factors that bind on specific DNA sequences (Qiu and Ghosh, 2008). In the case of psychostimulants, they appear to exert their transcriptional and other long-term biochemical effects, in part, via stimulation of striatal D1 DA receptor subtypes, followed by increases in protein kinase A activity, phosphorylation of CREB, and consequent CREB-mediated transcription (Ares-Santos et al., 2012; Cadet et al., 2010; Granado et al., 2011; Muschamp and Carlezon, 2013). Specifically, it has been shown that D1 DA receptor antagonists (Beauvais et al., 2010; Jayanthi et al., 2009) or deletion of D1 DA receptors (Moratalla et al., 1996) can interfere with amphetamine- or METH-induced increases in c-Fos, FosB, and CREM gene and protein levels in the dorsal striatum, indicating that this receptor subtype might also play a key role in the control of gene and protein expression observed in the present study. We thus investigated the possibility that this cascade might also be involved in METH self-administration by testing whether pCREB might regulate the expression of some genes of interest and found, for the first time, that METH self-administration is accompanied by increased recruitment of pCREB on the promoters of c-fos, fosB,Bdnf, and Syp, all of which show METH-induced increases in mRNA expression in the dorsal striatum. Our findings, therefore, indicate that these genes are co-regulated at both epigenetic and transcriptional levels and that they might work in concert to maintain METH-addicted state.
In conclusion, our study shows that METH self-administration causes long-term changes of ΔFosB, BDNF and TrkB expression in the dorsal striatum after various lengths of abstinence. Increased ΔFosB, BDNF, and TrkB levels were prominent during early stages of abstinence from METH self-administration whereas decreased levels were observed 1 month after cessation of drug exposure. The latter time interval might be associated with increased chance of reinstatement of drug taking (Shaham et al., 2003). These observations suggest that, in the case of METH, animals might reinstate because of ΔFosB, BDNF, and TrkB deficits. Our demonstration of increased pCREB binding on c-fos, fosb, and Bdnf promoters suggests that CREB is indeed an important player in regulating METH-induced gene expression and synaptic plasticity. Future studies using epigenetic approaches such as ChIP followed by large-scale DNA sequencing (ChIP-Seq) should help to decipher the roles of pCREB, H3K4me3 and other epigenetic modifications in the global effects of METH on gene transcription in the dorsal striatum.
Supplementary Material
Highlights
Rats underwent a 15-hour extended access to METH self-administration for 8 days
Changes in gene and protein expression were studied in the dorsal striatum
METH caused changes in ΔFosB, BDNF and TrkB expression up to 1 month of withdrawal
METH also produced increased pCREB binding on c-fos, fosb, and Bdnf promoters
METH self-administration-induced transcription is mediated, in part, by pCREB
Acknowledgements
This work is supported by Intramural Research Program of NIDA, NIH, DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures. All authors declare that they have no conflicts of interest.
References
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Anderson L, Seilhamer J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis. 1997;18:533–537. doi: 10.1002/elps.1150180333. [DOI] [PubMed] [Google Scholar]
- Angulo JA, McEwen BS. Molecular aspects of neuropeptide regulation and function in the corpus striatum and nucleus accumbens. Brain Res. Brain Res. Rev. 1994;19:1–28. doi: 10.1016/0165-0173(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Ares-Santos S, Granado N, Oliva I, O'Shea E, Martin ED, Colado MI, et al. Dopamine D(1) receptor deletion strongly reduces neurotoxic effects of methamphetamine. Neurobiol Dis. 2012;45:810–820. doi: 10.1016/j.nbd.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais G, Jayanthi S, McCoy MT, Ladenheim B, Cadet JL. Differential effects of methamphetamine and SCH23390 on the expression of members of IEG families of transcription factors in the rat striatum. Brain Res. 2010;1318:1–10. doi: 10.1016/j.brainres.2009.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr., Miller SW, et al. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur. J. Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, McCoy MT, Beauvais G, Cai NS. Dopamine D1 receptors, regulation of gene expression in the brain, and neurodegeneration. CNS Neurol. Disord. Drug Targets. 2010;9:526–538. doi: 10.2174/187152710793361496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, McCoy MT, Cai NS, Krasnova IN, Ladenheim B, Beauvais G, et al. Methamphetamine preconditioning alters midbrain transcriptional responses to methamphetamine-induced injury in the rat striatum. PLoS One. 2009;4:e7812. doi: 10.1371/journal.pone.0007812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Verdichevski M, Sykes J, Jaffer SR, Kish SJ. All-cause mortality among individuals with disorders related to the use of methamphetamine: A comparative cohort study. Drug Alcohol Depend. 2012;125:290–294. doi: 10.1016/j.drugalcdep.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Carvalho M, Carmo H, Costa VM, Capela JP, Pontes H, Remiao F, et al. Toxicity of amphetamines: an update. Arch. Toxicol. 2012;86:1167–1231. doi: 10.1007/s00204-012-0815-5. [DOI] [PubMed] [Google Scholar]
- Cesca F, Baldelli P, Valtorta F, Benfenati F. The synapsins, key actors of synapse function and plasticity. Prog. Neurobiol. 2010;91:313–348. doi: 10.1016/j.pneurobio.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Cho AK, Melega WP. Patterns of methamphetamine abuse and their consequences. J Addict. Dis. 2002;21:21–34. doi: 10.1300/j069v21n01_03. [DOI] [PubMed] [Google Scholar]
- Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine. J. Neurosci. 2003;23:2488–2493. doi: 10.1523/JNEUROSCI.23-06-02488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J. Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Hunt GE, Robins L, McGregor IS. Regional c-Fos and FosB/DeltaFosB expression associated with chronic methamphetamine self-administration and methamphetamine-seeking behavior in rats. Neuroscience. 2012;206:100–114. doi: 10.1016/j.neuroscience.2012.01.004. [DOI] [PubMed] [Google Scholar]
- de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol. Biosyst. 2009;5:1512–1526. doi: 10.1039/b908315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Molecular mechanism of secretory vesicle docking. Biochem. Soc. Trans. 2010;38:192–198. doi: 10.1042/BST0380192. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: Devolving views of their roles in drug addiction. Neurosci. Biobehav. Rev. 2013 doi: 10.1016/j.neubiorev.2013.02.010. DOI: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Evguenieva-Hackenberg E, Klug G. New aspects of RNA processing in prokaryotes. Curr. Opin. Microbiol. 2011;14:587–592. doi: 10.1016/j.mib.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Fass DM, Butler JE, Goodman RH. Deacetylase activity is required for cAMP activation of a subset of CREB target genes. J. Biol. Chem. 2003;278:43014–43019. doi: 10.1074/jbc.M305905200. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Caffino L, Racagni G, Riva MA. Repeated stress prevents cocaine-induced activation of BDNF signaling in rat prefrontal cortex. Eur. Neuropsychopharmacol. 2009;19:402–408. doi: 10.1016/j.euroneuro.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Di Pasquale L, Caffino L, Racagni G, Riva MA. Repeated exposure to cocaine differently modulates BDNF mRNA and protein levels in rat striatum and prefrontal cortex. Eur. J. Neurosci. 2007;26:2756–2763. doi: 10.1111/j.1460-9568.2007.05918.x. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Moro F, Caffino L, Orrù A, Cassina C, Giannotti G, et al. Region-specific effects on BDNF expression after contingent or non-contingent cocaine i.v. self-administration in rats. Int. J. Neuropsychopharmacol. 2013;16:913–918. doi: 10.1017/S146114571200096X. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat. Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Graham DL, Krishnan V, Larson EB, Graham A, Edwards S, Bachtell RK, et al. Tropomyosin-related kinase B in the mesolimbic dopamine system: region-specific effects on cocaine reward. Biol. Psychiatry. 2009;65:696–701. doi: 10.1016/j.biopsych.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, Oliva I, O'Shea E, Martin ED, Colado MI, et al. Dopamine D2-receptor knockout mice are protected against dopaminergic neurotoxicity induced by methamphetamine or MDMA. Neurobiol Dis. 2011;42:391–403. doi: 10.1016/j.nbd.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc. Natl. Acad. Sci. USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine, implications for incubation of cocaine craving. J. Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualandris A, Jones TE, Strickland S, Tsirka SE. Membrane depolarization induces calcium-dependent secretion of tissue plasminogen activator. J. Neurosci. 1996;16:2220–2225. doi: 10.1523/JNEUROSCI.16-07-02220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411:86–89. doi: 10.1038/35075076. [DOI] [PubMed] [Google Scholar]
- Hilburn C, Nejtek V, A., Underwood WA, Singh M, Patel G, Gangwani P, et al. Is serum brain-derived neurotrophic factor related to craving for or use of alcohol, cocaine, or methamphetamine? Neuropsychiatr. Dis. Treat. 2011;7:357–364. doi: 10.2147/NDT.S18953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner KA, Keefe KA. Regulation of psychostimulant-induced preprodynorphin, c-fos and zif/268 messenger RNA expression in the rat dorsal striatum by mu opioid receptor blockade. Eur. J. Pharmacol. 2006;532:61–73. doi: 10.1016/j.ejphar.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Huang da W., Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, McCoy MT, Beauvais G, Ladenheim B, Gilmore K, Wood W, 3rd, et al. Methamphetamine induces dopamine D1 receptor-dependent endoplasmic reticulum stress-related molecular events in the rat striatum. PLoS One. 2009;4:e6092. doi: 10.1371/journal.pone.0006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, Carnicella S, Kharazia V, Janak PH, Ron D. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J. Neurosci. 2009;29:13494–13502. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak JP, Uslaner JM, Esteban JA, Robinson TE. Methamphetamine-induced structural plasticity in the dorsal striatum. Eur. J. Neurosci. 2007;25:847–853. doi: 10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- Ji Y, Lu Y, Yang F, Shen W, Tang TT, Feng L, et al. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nature Neurosci. 2010;13:302–309. doi: 10.1038/nn.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr., Whisler K, Gilden L, Beckmann AM, et al. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Roh S, Kim Y, Yoon SJ, Lee HK, Han CS, et al. High concentrations of plasma brain-derived neurotrophic factor in methamphetamine users. Neurosci. Lett. 2005;388:112–115. doi: 10.1016/j.neulet.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Kokubo M, Nishio M, Ribar TJ, Anderson KA, West AE, Means AR. BDNF-mediated cerebellar granule cell development is impaired in mice null for CaMKK2 or CaMKIV. J. Neurosci. 2009;29:8901–8913. doi: 10.1523/JNEUROSCI.0040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Leveque JC, Hyman SE. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J. Neurosci. 1996;16:4231–4239. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg RD, Lorch Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell. 1999;98:285–294. doi: 10.1016/s0092-8674(00)81958-3. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res. Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Ladenheim B, Hodges AB, Volkow ND, Cadet JL. Chronic methamphetamine administration causes differential regulation of transcription factors in the rat midbrain. PLoS One. 2011;6:e19179. doi: 10.1371/journal.pone.0019179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Betts ES, Dada A, Jefferson A, Ladenheim B, Becker KG, et al. Neonatal dopamine depletion induces changes in morphogenesis and gene expression in the developing cortex. Neurotox. Res. 2007;11:107–130. doi: 10.1007/BF03033390. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Li SM, Wood WH, McCoy MT, Prabhu VV, Becker KG, et al. Transcriptional responses to reinforcing effects of cocaine in the rat hippocampus and cortex. Genes Brain Behav. 2008;7:193–202. doi: 10.1111/j.1601-183X.2007.00338.x. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, et al. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS One. 2010;5:e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion, current facts and future prospects. Prog. Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Li W, Keifer J. Coordinate action of pre- and postsynaptic brain-derived neurotrophic factor is required for AMPAR trafficking and acquisition of in vitro classical conditioning. Neuroscience. 2008;155:686–697. doi: 10.1016/j.neuroscience.2008.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Keifer J. Rapid enrichment of presynaptic protein in boutons undergoing classical conditioning is mediated by brain-derived neurotrophic factor. Neuroscience. 2012;203:50–58. doi: 10.1016/j.neuroscience.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, DeJoseph MR, Urban JH, Bahi A, Dreyer JL, Meredith GE, et al. Different roles of BDNF in nucleus accumbens core versus shell during the incubation of cue-induced cocaine craving and its long-term maintenance. J. Neurosci. 2013;33:1130–1142. doi: 10.1523/JNEUROSCI.3082-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Ju G. Quantitative evaluation of synaptophysin-like immunoreactive nerve terminals or varicosities in anterior pituitary of normal and adrenalectomized rats. J. Neuroendocrinol. 2001;13:967–974. doi: 10.1046/j.1365-2826.2001.00720.x. [DOI] [PubMed] [Google Scholar]
- Loonam TM, Noailles PA, Yu J, Zhu JP, Angulo JA. Substance P and cholecystokinin regulate neurochemical responses to cocaine and methamphetamine in the striatum. Life Sci. 2003;73:727–739. doi: 10.1016/s0024-3205(03)00393-x. [DOI] [PubMed] [Google Scholar]
- Marshall JF, O'Dell SJ. Methamphetamine influences on brain and behavior, unsafe at any speed? Trends Neurosci. 2012;35:536–545. doi: 10.1016/j.tins.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology. 2008;33:3–17. doi: 10.1038/sj.npp.1301544. [DOI] [PubMed] [Google Scholar]
- McCoy MT, Jayanthi S, Wulu JA, Beauvais G, Ladenheim B, Martin TA, et al. Chronic methamphetamine exposure suppresses the striatal expression of members of multiple families of immediate early genes (IEGs) in the rat, normalization by an acute methamphetamine injection. Psychopharmacology (Berl) 2011;215:353–365. doi: 10.1007/s00213-010-2146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Stout KA, Vieira-Brock PL, Allen SC, Nielsen SM, Wilkins G, et al. Methamphetamine self-administration acutely decreases monoaminergic transporter function. Synapse. 2012a;66:240–245. doi: 10.1002/syn.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Hadlock GC, Allen SC, Vieira-Brock PL, Stout KA, Ellis JD, et al. Methamphetamine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by a subsequent methamphetamine exposure. J. Pharmacol. Exp. Ther. 2012b;340:295–303. doi: 10.1124/jpet.111.188433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Jr., Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratalla R, Xu M, Tonegawa S, Graybiel AM. Cellular responses to psychomotor stimulant and neuroleptic drugs are abnormal in mice lacking the D1 dopamine receptor. Proc. Natl. Acad. Sci. USA. 1996;93:14928–14933. doi: 10.1073/pnas.93.25.14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Carlezon WA., Jr. Roles of Nucleus Accumbens CREB and Dynorphin in Dysregulation of Motivation. Cold Spring Harbor Perspec. Med. 2013 doi: 10.1101/cshperspect.a012005. DOI, 10.1101/cshperspect.a012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Nemeth CL, Robison AJ, Nestler EJ, Carlezon WA., Jr. DeltaFosB enhances the rewarding effects of cocaine while reducing the pro-depressive effects of the kappa-opioid receptor agonist U50488. Biol. Psychiatry. 2012;71:44–50. doi: 10.1016/j.biopsych.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ED, Monteggia LM. Epigenetics in the mature mammalian brain, effects on behavior and synaptic transmission. Neurobiol. Learn. Mem. 2011;96:53–60. doi: 10.1016/j.nlm.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Transcriptional mechanisms of drug addiction. Clin. Psychopharmacol. Neurosci. 2012;10:136–143. doi: 10.9758/cpn.2012.10.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SS, Yue WW, Oppermann U, Klose RJ. Dynamic protein methylation in chromatin biology. Cell Mol. Life Sci. 2009;66:407–422. doi: 10.1007/s00018-008-8303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nature Rev. Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Ghosh A. A brief history of neuronal gene expression, regulatory mechanisms and cellular consequences. Neuron. 2008;60:449–455. doi: 10.1016/j.neuron.2008.10.039. [DOI] [PubMed] [Google Scholar]
- Rauskolb S, Zagrebelsky M, Dreznjak A, Deogracias R, Matsumoto T, Wiese S, et al. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J. Neurosci. 2010;30:1739–1749. doi: 10.1523/JNEUROSCI.5100-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Carle TL, Maze I, Covington HE, 3rd, Truong HT, Alibhai I, et al. Delta FosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J. Neurosci. 2008;28:7344–7349. doi: 10.1523/JNEUROSCI.1043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nature Rev. Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56(Suppl 1):73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J. Pharmacol. Exp. Ther. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, et al. Methamphetamine causes microglial activation in the brains of human abusers. J. Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse, history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J. Neurosci. 2004;24:5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin RD, Whetton AD. Systematic proteome and transcriptome analysis of stem cell populations. Cell Cycle. 2006;5:1587–1591. doi: 10.4161/cc.5.15.3101. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Ann. Rev. Pharmacol. Toxicol. 2011;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am. J. Psychiatry. 2001a;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers, association with metabolism in the orbitofrontal cortex. Am. J. Psychiatry. 2001b;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol. Psychiatry. 2012;17:918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat. Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.