Abstract
Rett Syndrome (RTT), a progressive neurological disorder characterized by developmental regression and loss of motor and language skills, is caused by mutations in the X-linked gene encoding methyl-CpG binding protein 2 (MECP2). Neurostructural phenotypes including decreased neuronal size, dendritic complexity, and spine density have been reported in postmortem RTT brain tissue and in Mecp2 animal models. How these changes in neuronal morphology are related to RTT-like phenotype and MeCP2 function, and the extent to which restoration of neuronal morphology can be used as a cellular readout in therapeutic studies, however, remain unclear. Here, we systematically examined neuronal morphology in vivo across three Mecp2 mouse models representing Mecp2 loss-of-function, partial loss-of-function, and gain-of-function mutations, at developmental time points corresponding to early- and latesymptomatic RTT-like behavioral phenotypes. We found that in Mecp2 loss-of-function mouse models, dendritic complexity is reduced in a mild, age-dependent, and brain region-specific manner, whereas soma size is reduced consistently throughout development. Neither phenotype, however, is altered in Mecp2 gain-of-function mice. Our results suggest that, in the cell types we examined, the use of dendritic morphology as a cellular readout of RTT phenotype and therapeutic efficacy should be cautioned, as it is intrinsically variable. In contrast, soma size may be a robust and reliable marker for evaluation of MeCP2 function in Mecp2 loss-of-function studies.
Keywords: Rett Syndrome, MECP2, neuronal morphology, dendritic branching, soma size, mouse model
INTRODUCTION
Rett Syndrome (RTT) is a neurological disorder that is caused by mutations in the X-linked gene encoding methyl-CpG binding protein 2 (MECP2) (Amir et al., 1999). It primarily affects young girls, with a prevalence of 1 in 10–15,000 live births. One striking feature of RTT is the time course at which clinical symptoms appear. After 6–18 months of apparently normal development, affected individuals enter a period of developmental stagnation, characterized by microcephaly, growth arrest and hypotonia. This stage is followed by a period of developmental regression, where patients display stereotypic hand wringing and social withdrawal, lose acquired motor and speech skills, and develop respiratory abnormalities (Chahrour and Zoghbi, 2007).
Phenotypic variability is present within the classic RTT profile, where affected girls differ by clinical severity (Chahrour and Zoghbi, 2007). This variability may be attributed to random X-chromosome inactivation and the variety of MECP2 mutations, including missense, nonsense, deletion, and insertion mutations, that have been identified in RTT patients (Bienvenu and Chelly, 2006). In vitro biochemical studies have demonstrated that the majority of these mutations lead to Mecp2 loss-of-function (Kriaucionis and Bird, 2003). Notably, genetic studies have also identified patients carrying duplication or triplication of MECP2 (Bienvenu and Chelly, 2006). These patients are classified under MECP2 duplication syndrome and bear a similar but distinct clinical profile to that of classical RTT (Chahrour and Zoghbi, 2007). Mice carrying two-fold levels of Mecp2 expression also show behavioral abnormalities (Collins et al., 2004; Luikenhuis et al., 2004), highlighting the importance of MeCP2 dosage and the detrimental effect of Mecp2 gain-of-function.
Histological analysis of postmortem RTT brain tissue has revealed morphological phenotypes including decreased cellular size with increased cell-packing density (Bauman et al., 1995), decreased dendritic complexity (Armstrong, 2005; Belichenko et al., 1994), and decreased spine density (Belichenko et al., 1994; Chapleau et al., 2009). Interestingly, these changes are selective, as decreased dendritic complexity has been observed in the motor, frontal, and inferior temporal cortices, but not in the visual cortex or hippocampus (Armstrong et al., 1995). Moreover, these changes appear to be cortical layer-specific, even within the same cortical area (Armstrong et al., 1995). No evidence of neuronal degeneration or atrophy has been identified (Armstrong, 2005; Jellinger et al., 1988), indicating that these morphological changes are a consequence of impaired neuronal development or structural maintenance, rather than neurodegeneration. What remains unclear, however, is how mutations in MECP2 lead to these changes in neuronal morphology and whether they contribute to RTT disease pathology or are secondary consequences of long-term illness.
To investigate the pathogenic mechanisms underlying RTT, several mouse models harboring different Mecp2 loss-of-function mutations have been developed and characterized (Brendel et al., 2011; Chen et al., 2001; Goffin et al., 2012; Guy et al., 2001; Jentarra et al., 2010; Pelka et al., 2006; Shahbazian et al., 2002). Although the mouse models recapitulate several RTT clinical features, each Mecp2 mutation confers a discrete behavioral profile, supporting the link between heterogeneity in MECP2 mutations and phenotypic variability in RTT. Similarly, underlying cellular structure in Mecp2 mutant mice differs across Mecp2 mutation (Belichenko et al., 2008; 2009b). While reduced dendritic complexity, soma size, and spine density are commonly identified in Mecp2 mutant mice (Belichenko et al., 2009a; 2009b; Fukuda et al., 2005; Kishi and Macklis, 2004; Robinson et al., 2012; Stuss et al., 2012; Tropea et al., 2009), these structural phenotypes also differ by cellular subtype and developmental time point (Chapleau et al., 2012; Fukuda et al., 2005). The extent to which these factors influence cellular structure, however, is not well understood, as the use of different techniques and Mecp2 mouse models have made direct comparisons across studies difficult (Belichenko et al., 2008; 2009a; Chapleau et al., 2009; Cohen et al., 2011; Fukuda et al., 2005; Jentarra et al., 2010; Kishi and Macklis, 2004; Metcalf et al., 2006; Moretti et al., 2006; Robinson et al., 2012; Stuss et al., 2012; Zhou et al., 2006). For example, reduced spine density has been reported in motor cortex layer II/III and V pyramidal neurons in 3-week-old Mecp2-null mice (Belichenko et al., 2009a; 2009b), and in layer V at 8 weeks (Tropea et al., 2009), while no change in spine density has been reported in the somatosensory cortex layer II/III pyramidal neurons in 8-week-old Mecp2-null mice (Kishi and Macklis, 2004). Whether these dissimilar findings are consequences of brain region-specific or age-dependent regulation of neuronal morphology is difficult to evaluate, as studies have used different imaging techniques, including Golgi staining, neuron dye labeling, and fluorescent reporters, and different Mecp2 mutant mice (Chen et al., 2001; Guy et al., 2001). Indeed, Golgi staining has revealed reduced spine density in hippocampus CA1 of 12-week-old Mecp2-null mice (Robinson et al., 2012), but DiI labeling shows decreased spine density in the same cells at 1 week, and not at 2 or 7 weeks (Chapleau et al., 2012). A comprehensive and systematic analysis using a single experimental method, therefore, is needed to clarify how age, brain region, and Mecp2 mutation influence RTT-related neuronal morphology.
Importantly, recent studies have shown that RTT-like symptoms can be ameliorated through the reintroduction of MeCP2 in mice (Giacometti et al., 2007; Guy et al., 2007; Lioy et al., 2011; Robinson et al., 2012). The reversibility of RTT phenotypes has stimulated wide interest in developing cellular assays to measure MeCP2 function. Morphological phenotypes such as spine density, dendritic outgrowth, and soma size have been used as readouts of therapeutic efficacy upon restoration of MeCP2 (Giacometti et al., 2007; Robinson et al., 2012) or IGF-1 treatment in mice and induced pluripotent stem cells (iPSCs) (Marchetto et al., 2010; Tropea et al., 2009). Thus, understanding the relationship between MeCP2 function, neuronal structure, and RTT-like phenotype, as well as identifying robust and reproducible morphological phenotypes as readouts of MeCP2 function, are imperative.
In this study, we systematically analyzed neuronal morphology in three Mecp2 mouse models, Mecp2−/y, Mecp2T158A/y, and Mecp2Tg1, that represent the spectrum of MECP2 mutations contributing to the phenotypic heterogeneity of RTT and MECP2 duplication syndrome. The Mecp2−/y mice lack MeCP2 and demonstrate the most severe behavioral phenotype, representing Mecp2 loss-of-function (Guy et al., 2001). The Mecp2T158A/ y mice, mimicking a common RTT patient missense mutation at the Threonine 158 residue, demonstrate a moderately severe behavioral phenotype, representing Mecp2 partial loss-of-function (Goffin et al., 2012). Lastly, the Mecp2Tg1 mice express two-fold levels of MeCP2 and are believed to model MECP2 duplication syndrome, representing Mecp2 gain-of-function (Collins et al., 2004). Importantly, although neuronal morphology in Mecp2 loss-of-function models has been individually studied (Belichenko et al., 2008; 2009a; Chapleau et al., 2009; Fukuda et al., 2005; Kishi and Macklis, 2004; Moretti et al., 2006; Robinson et al., 2012; Stuss et al., 2012), a direct comparison of morphological phenotypes in Mecp2 mouse models across the RTT phenotypic spectrum, including the less severe partial loss-of-function Mecp2 mutations and Mecp2 duplication, has yet to be conducted.
Given the progressive nature of RTT-like phenotypic onset, we focused on early and late developmental time points representative of none-to-mild behavioral phenotype (“early” time point) and overt behavioral phenotype (“late” time point) in each mouse model. By using the same experimental method to analyze neuronal morphology in vivo across these mouse models and developmental time points, we aimed to address the following questions in this study: 1) The effect of Mecp2 loss- or gain-of-function on the development of neuronal morphology, 2) The relationship between RTT-like behavioral symptomatic severity and underlying neuronal morphology, and 3) The brain region or cell type-specific effects of Mecp2 mutation on neuronal morphology.
We found that Mecp2 loss-of-function reduces dendritic complexity in a brain region-specific manner that correlates with the onset of behavioral phenotype. The degree of changes in dendritic complexity, however, was mild, domain-specific, and dependent on Mecp2 mutation. In contrast, a significant decrease in soma size upon Mecp2 loss-of-function persisted throughout development and across both Mecp2 loss-of-function mutations. Mecp2 gain-of-function, however, did not affect dendritic outgrowth or soma size, even after onset of gain-of-function behavioral phenotypes, suggesting that changes in cellular structure may not be effective readouts of therapeutics targeted toward MECP2 duplication syndrome. The subtlety of changes in dendritic outgrowth and the dependence on Mecp2 mutation, developmental time point, and brain region raise caution in using dendritic complexity as a cellular readout of RTT-like phenotype. Changes in soma size, however, are reproducible and robust, suggesting that soma size may be a more reliable marker in assessing MeCP2 function and therapeutic efficacy in RTT studies.
MATERIAL AND METHODS
Animal Husbandry
Experiments were conducted in accordance to the ethical guidelines of the National Institutes of Health and with an approved animal protocol from the Institutional Animal Care and Use Committee of the University of Pennsylvania. To obtain male mice carrying Mecp2 mutations and Thy1-GFP/M transgene, female mice that heterozygous for Mecp2 mutations Mecp2tm1.1Bird(Mecp2−/y, (Guy et al., 2001)), Mecp2tm1.1Joez (Mecp2T158A/y, (Goffin et al., 2012)), or Mecp2Tg1(Collins et al., 2004) were crossed with Thy1-GFP/M reporter mice (Feng et al., 2000). Mecp2−/yand Mecp2T158A/y were maintained on a C57BL/6 background (Charles River). Mecp2Tg1 were maintained on a mixed FVB/C57BL/6 background. Mice were genotyped using a PCR-based strategy for Mecp2 and Thy1-GFP/M, as detailed by the Jackson Laboratory.
Immunohistochemistry
Mice were anesthetized with 1.25% Avertin (2,2,2-Tribromoethanol) (wt/vol), transcardially perfused with 0.1 M phosphate buffered saline (PBS) for 1 min, then with 4% paraformaldehyde in 0.1 M PBS (wt/vol) for 10 min, and post-fixed in 4% paraformaldehyde for 1 hr at 4 °C. Immunohistochemistry was performed on 200-µm coronal free-floating sections sliced using a Leica VT1000S vibratome. Tissues were rinsed 2 × 5 min with 0.1 M PBS and were blocked with a solution containing 5% normal goat serum, 0.25% Triton X-100 and 0.05% sodium azide for 2 h at room temperature. Tissues were incubated with primary antibody in blocking solution (chicken antibody to GFP, Aves Labs, 1:1000) overnight at 4 °C. Fluorescence detection was performed using secondary antibody to chicken conjugated to Alexa Fluor-488 (1:1000, Invitrogen) for 2 h at room temperature. Sections were counterstained with DAPI (1:1000, USB) to visualize DNA.
Confocal microscopy
GFP-labeled cells and their entire dendritic tree were detected spanning the depth of the 200-µm slices. For examination of dendritic complexity, somatosensory cortex layer V pyramidal neurons extending their apical dendrites to the pial surface and their basal dendrites into deep layer VI and hippocampal CA1 pyramidal neurons extending their basal dendrites into the stratum oriens and their apical dendrites through the stratum radiatum and stratum lacunosummoleculare were imaged. Primary somatosensory cortex barrel field between Bregma coordinates −0.6 to −1.34 was analyzed, using the fimbria as an anatomical landmark. Rostral hippocampus between Bregma coordinates −1.34 to −1.94 was analyzed. For examination of soma size, cells located within 50µm from the surface of the slice with intact somas were imaged and analyzed. Images of apical and basal dendrites were acquired separately with a Leica confocal microscope and Leica AF6000 imaging software using an oil immersion 20x and 40x objective lens. Each image was saved as a z-stacked series of optical sections. Four slices per mouse were imaged: 5–10 neurons for layer V somatosensory cortex per mouse and 7–10 neurons for hippocampal CA1 region per mouse were analyzed. All imaging was performed blind to genotype.
Quantitative analysis of neuronal morphology
Z-projection images of individual neurons were compressed into a max projection and dendrites were traced manually using ImageJ software (National Institutes of Health) and NeuronJ, an ImageJ plugin for neurite tracing and quantification (Meijering 2004). Dendritic complexity was measured by performing Sholl analysis (Sholl 1953), counting the number of dendritic crossings through a series of concentric circles centered at the soma and spaced at 20 µm intervals. Soma size was examined in Leica AF6000 by taking an area measurement of somas outlined manually.
Statistics
Data are reported as mean ± SEM, and statistical analysis was performed using GraphPad Prism 5.0. Sholl analysis was analyzed by two-way ANOVA with Bonferroni correction, with the independent (genotype and Sholl radius) and dependent variables (number of dendritic crossings). Soma size was analyzed by Student’s unpaired t-test with Bonferonni correction where necessary.
RESULTS
Neuron imaging strategy
To minimize experimental manipulation and variation in our analysis of neuronal morphology in Mecp2 mutant mice, we took advantage of a genetic labeling approach that has been successfully utilized in other RTT morphological studies, a Thy1-GFP/M reporter line (Belichenko et al., 2009a; Cohen et al., 2011). In this line, a sparse population of neurons intrinsically expresses GFP throughout the cell body and dendritic tree in various brain regions (Figure 1a, 1b), allowing for direct visualization of individual neurons (Feng et al., 2000). To visualize neurons in Mecp2 mutant mice, we crossed male Thy1-GFP/M reporter mice to female Mecp2 heterozygotes (Mecp2−/+, Mecp2T158A/+, or Mecp2Tg1). Given the confounding effects of mosaic MeCP2 expression in females from random X-chromosome inactivation, all experiments were performed using male littermates with the following genotypes: Mecp2−/y;Thy1-GFP/M and Mecp2+/y;Thy1-GFP/M; Mecp2T158A/y;Thy1-GFP/M and Mecp2+/y;Thy1-GFP/; or Mecp2Tg1;Thy1-GFP/M and Mecp2+/y;Thy1-GFP. For simplicity, we will refer to Mecp2+/y;Thy1-GFP/M as WT and Mecp2 mutant mice expressing the Thy1-GFP/M transgene as Mecp2−/y, Mecp2T158A/y, or Mecp2Tg1.
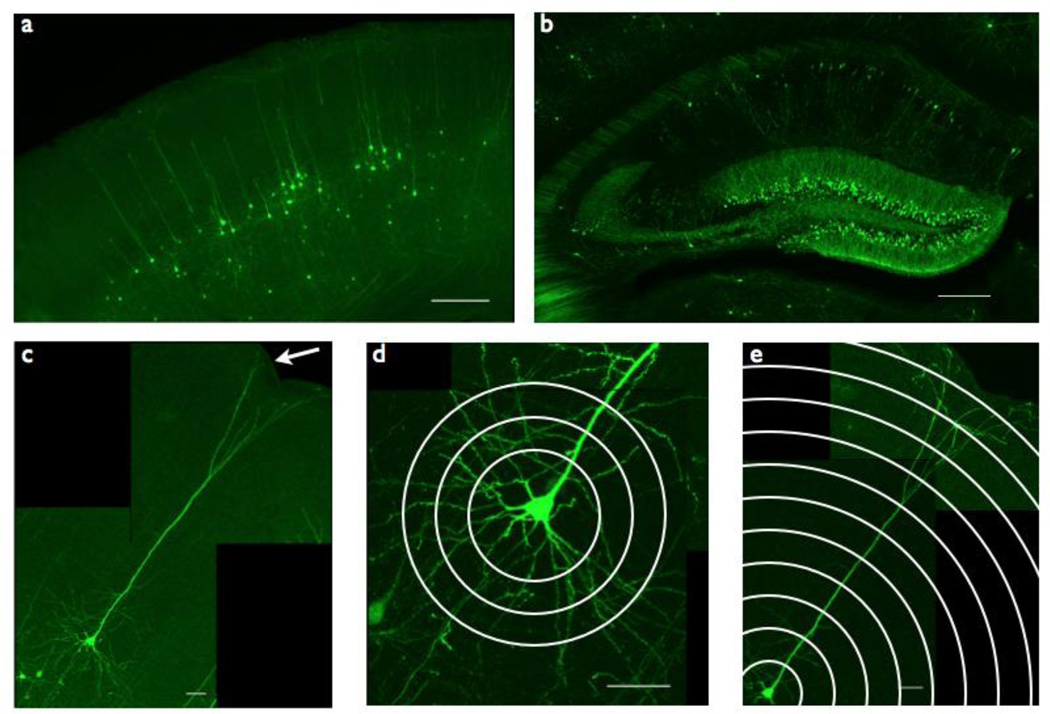
Figure 1. Thy1-GFP/Mreporter imaging strategy.
(a) Somatosensory cortex layer V pyramidal neurons are labeled with GFP throughout the cell body and dendritic tree in Thy1-GFP/M reporter mice. Scale bar = 250 µm.
(b) Hippocampus CA1 pyramidal neurons are labeled with GFP throughout the cell body and dendritic tree in Thy1-GFP/M reporter mice. Scale bar = 250 µm.
(c) Somatosensory cortex layer V pyramidal neurons extend their apical dendrites to the pial surface (arrow) and their basal dendrites deep into layer VI. Scale bar = 50 µm.
(d) Basal dendrites of somatosensory cortex layer V pyramidal neurons, quantified by Sholl analysis, measuring number of intersections between concentric circles drawn around the cell soma (Sholl radii) and dendritic branches. Scale bar = 50 µm.
(e) Apical dendrites of somatosensory cortex layer V pyramidal neurons, quantified by Sholl analysis. Scale bar = 50 µm.
Previous studies have shown that disruption of MeCP2 results in deficits in neuronal organization, dendritic complexity and synaptic connectivity in the somatosensory cortex in both postmortem RTT tissue and mice (Cohen et al., 2011; Dani and Nelson, 2009; Kaufmann et al., 2000). We therefore analyzed neuronal morphology in layer V pyramidal neurons of the somatosensory cortex. These neurons are distinct in their cellular architecture, characterized by apical and basal dendritic trees and a pyramid-shaped soma (Spruston, 2008), and thus can be consistently identified. We measured dendritic complexity and soma size in layer V pyramidal neurons extending their apical dendrites to the pial surface and their basal dendrites into deep layer VI (Fig 1c). Given the anatomic and functional distinctions between basal and apical dendritic arbors (Spruston, 2008), we imaged and quantified basal and apical dendritic complexity separately, using Sholl analysis (Fig 1d–e), which measures dendritic crossings through a series of concentric circles with increasing radii centered around the cell soma (SHOLL, 1953).
Dendritic complexity in Mecp2 loss-of-function mice
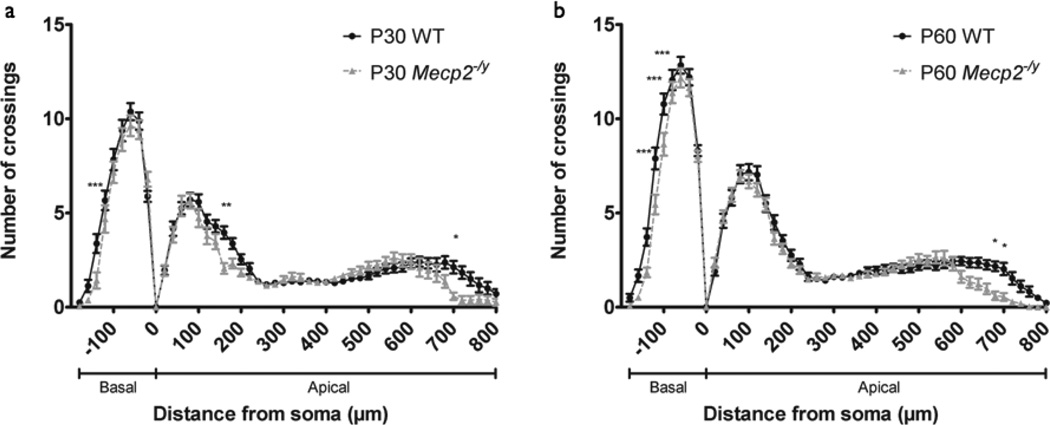
The majority of mutations in MECP2 leading to RTT are loss-of-function mutations (Chahrour and Zoghbi, 2007). Therefore, we first assessed neuronal morphology in Mecp2-null mice (Mecp2−/y) that lack both Mecp2 transcript and MeCP2 protein, and represent one of the most phenotypically severe Mecp2 mutant mice (Guy et al., 2001). Like RTT patients, Mecp2−/y mice show no initial RTT-like phenotype, but begin to develop aberrant gait and reduced mobility around 3 postnatal weeks. By 8 postnatal weeks, Mecp2−/y mice display overt RTT-like phenotypes including hindlimb clasping, tremor, and irregular breathing. We therefore chose to investigate neuronal morphology upon Mecp2 loss-of-function at P30 and P60, time points representative of early-symptomatic and late-symptomatic RTT-like phenotype, respectively. At the early-symptomatic P30 time point, Sholl analysis of somatosensory cortex layer V pyramidal neurons revealed a statistically significant decrease in dendritic complexity in Mecp2−/y mice relative to WT littermates (WT: n=24 neurons, Mecp2−/y: n=19 neurons; p<0.0001) (Fig. 2a). Post hoc tests revealed that this decrease was most pronounced in Mecp2−/y mice in both basal and proximal apical dendrites 140–160 µm from soma and in the distal apical tuft 700 µm from soma. As RTT-like behavioral phenotypes in P30 Mecp2−/y mice are mild but present, these data suggest that decreased dendritic complexity accompanies RTT-like behavioral phenotype.
Figure 2. Dendritic complexity in somatosensory cortex of Mecp2−/ymice.
(a) Sholl analysis of somatosensory cortex layer V pyramidal neurons in P30 WT (n=24 neurons from 4 mice) and Mecp2−/y mice (n=19 neurons from 4 mice) shows reduced dendritic complexity in Mecp2−/y mice relative to WT. Basal dendritic arbor is denoted b negative distance from soma, 0 urn marks soma position within cortex, and apical dendritic arbor is denoted by positive distance from soma. Two-way ANOVA with Bonferroni correction, p<0.0001 (interaction); *p<0.05, **p<0.01, ***p<0.001. Bars represent mean ± sem.
(b) Sholl analysis of somatosensory cortex layer V pyramidal neurons in P60 WT (n=36 neurons from 4 mice) and Mecp2−/y mice (n=31 neurons from 4 mice) show more widespread reduction in dendritic complexity in Mecp2−/y mice relative to WT than seen in P30 animals. Two-way ANOVA with Bonferroni correction, p<0.0001 (interaction); *p<0.05, ***p<0.001.
At the late-symptomatic P60 time point, Mecp2−/y mice also showed reduced dendritic complexity (WT: n=36 neurons; Mecp2−/y: n=31 neurons; p<0.0001) (Fig. 2b). Although mild, these decreases were more widespread than that of P30 and most pronounced in the basal arbor 100–140 µm from soma and in the distal apical arbor 680–700 µm from soma, where the significant reduction in the apical tuft is likely a reflection of reduced cortical thickness and brain size in these mice (Kishi and Macklis, 2004). Given the overt RTT-like behavioral phenotypes in P60 Mecp2−/y mice, the mild reduction in dendritic complexity is surprising but consistent with reports of normal cortical lamination and organization in 7–10 week-old Mecp2−/y mice (Belichenko et al., 2008; Guy et al., 2001; Metcalf et al., 2006) and unaffected dendritic length and complexity from in vitro studies using the same mice (Chao et al., 2007). Therefore, as excitatory input onto proximal dendrites comes primarily from local or adjacent collaterals, while input onto distal dendrites comes from more distant cortical or thalamic projections (Spruston, 2008), our data indicate that the mild but widespread reduction in dendritic complexity across domains results in altered total excitatory input onto Mecp2−/y neurons, both in early-symptomatic and late-symptomatic Mecp2−/y mice.
Dendritic complexity in MeCP2 T158A partial loss-of-function mice
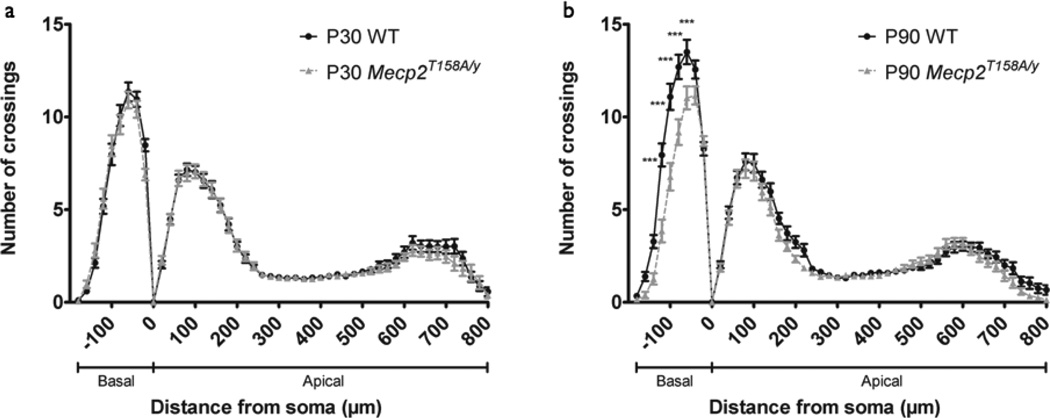
One of the most common MECP2 missense mutations occurs at Threonine 158, converting it to methionine (T158M) or alanine (T158A). We recently developed and characterized an Mecp2 T158A knockin mouse and found that Mecp2T158A/ y mice show a similar but delayed progression of RTT-like behavioral phenotypes relative to that of Mecp2−/y mice, where phenotypes begin to develop at 4–5 weeks and are overt by 13 weeks (Goffin et al., 2012). At P60, Mecp2T158A/y mice express milder locomotor, anxiety, and motor coordination phenotypes compared to age-matched Mecp2−/y mice, indicating that Mecp2 T158A is a partial loss-of-function mutation. In addition, the MeCP2 T158A protein is expressed but is less stable and shows reduced binding to methylated DNA (Goffin et al., 2012). To investigate neuronal morphology upon Mecp2 partial loss-of-function, we measured dendritic complexity at P30, prior to onset of RTT-like phenotypes, and P90, when RTT-like phenotypes are overt. Sholl analysis of layer V pyramidal neurons in the somatosensory cortex of P30 animals revealed a similar pattern of dendritic complexity in Mecp2T158A/y and WT littermates (WT: n=40 neurons; Mecp2T158A/y: n=34 neurons; p>0.05; Fig. 3a). Although both Mecp2T158A/y and Mecp2−/y mice represent Mecp2 loss-of-function mutations, their RTT-behavioral profiles at P30 are distinct, where RTT-like phenotypes are mild in Mecp2−/y mice but absent in Mecp2T158A/y mice. These data, therefore, support the hypothesis that decreased dendritic complexity accompanies RTT-like phenotype.
Figure 3. Dendritic complexity in somatosensory cortex of Mecp2T158A/y mice.
(a) Sholl analysis of somatosensory cortex layer V pyramidal neurons in P30 WT (n=40 neurons from 4 animals) and Mecp2T158A/y mice (n=34 neurons from 4 animals) show no change dendritic complexity in Mecp2T158A/y mice relative to WT. Two-way ANOVA, p>0.05. Bars represent mean ± sem.
(b) Sholl analysis of somatosensory cortex layer V pyramidal neurons in P90 WT (n=36 neurons from 4 mice) and Mecp2T158A/y mice (n=39 neurons from 4 mice) show reduced dendritic complexity in Mecp2T158A/y mice relative to WT specifically in the basal dendritic arbor. Two-way ANOVA with Bonferroni correction, p<0.0001 (interaction); **p<0.01.
At the late-symptomatic P90 time point, Mecp2T158A/y mice showed decreased dendritic complexity relative to WT littermates (WT: n=36 neurons; Mecp2T158A/y: n=39; p<0.0001; Fig. 3B). Strikingly, the decrease was specific to the basal arbor 60–140 µm from soma and was not present in the apical arbor. Given that pyramidal neuron dendritic domains are believed to receive unique synaptic inputs and contain synapses with distinct properties (Spruston, 2008), the domain-specific decrease in dendritic complexity in Mecp2T158A/y mice suggests a cellular mechanism underlying a distinct behavioral profile. Although both P60 Mecp2−/y mice and P90 Mecp2T158A/y mice show similar RTT-like phenotypic severity, the selective decrease in basal dendritic complexity in Mecp2T158A/ y mice in contrast to the widespread morphological phenotype seen in Mecp2−/y mice supports an Mecp2 mutation-specific effect on neuronal architecture that may underlie RTT-like phenotypic heterogeneity.
Brain region-specific dendritic complexity in Mecp2 partial loss-of-function
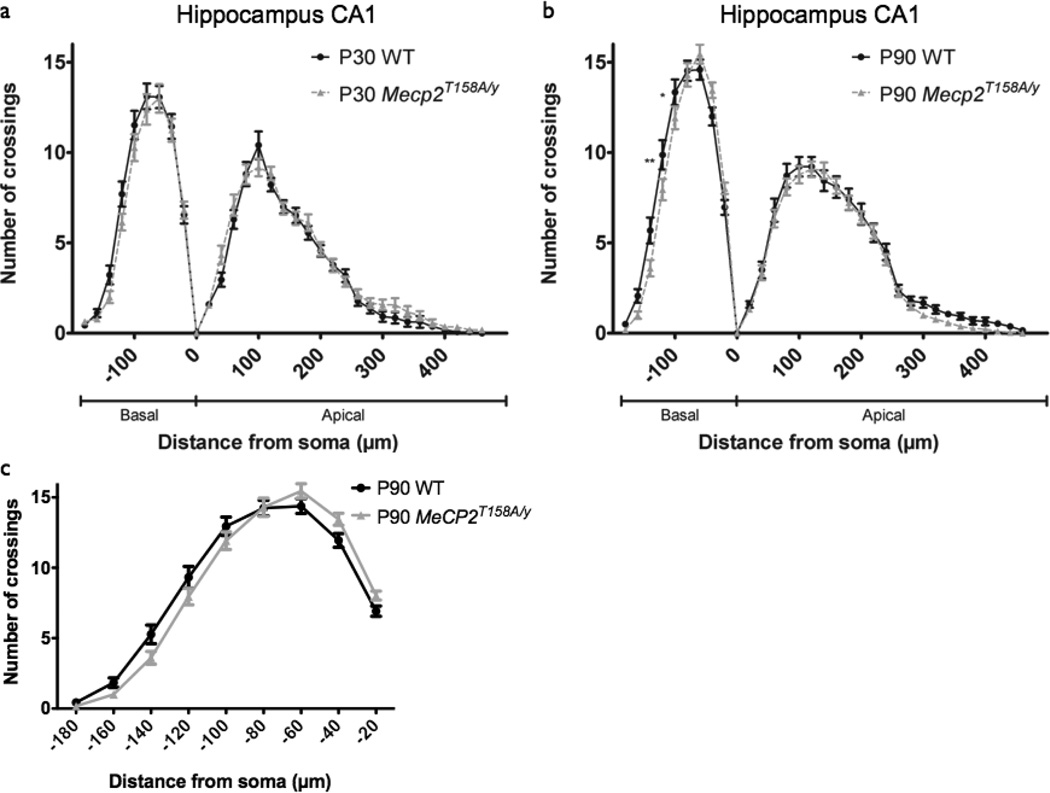
Given the brain region-specific changes in neuronal architecture in RTT post-mortem tissue (Armstrong et al., 1995) and compartment-specific changes in Mecp2 mouse models (Stuss et al., 2012), we next addressed whether the age-dependent and domain-specific decrease in dendritic complexity identified in the somatosensory cortex of Mecp2T158A/y mice would be replicated in a different brain region from the same mice. Therefore, we examined neuronal morphology in hippocampal CA1 pyramidal neurons in Mecp2T158A/y mice at the pre- and post-symptomatic ages P30 and P90. Similar to what we observed in the cortex, Mecp2 partial loss-of-function did not affect dendritic complexity in hippocampal CA1 pyramidal neurons at the early-symptomatic P30 time point (WT: n=27 neurons; Mecp2T158A/y: n=33 neurons; p>0.05; Fig. 4a). At the late-symptomatic P90 time point, however, we observed a small but significant change in dendritic complexity in Mecp2T158A/y mice (WT: n=32 neurons; Mecp2T158A/y: n=40 neurons; p<0.05; Fig. 4b). Post hoc tests revealed that this difference was specific to the basal dendritic arbor, and does not reflect a decrease in dendritic complexity, but rather, is a result of a shift towards the soma, as peak branching in Mecp2T158A/ y mice is similar to that of controls (Fig 4c). This shift toward the soma may reflect reduced hippocampal volume, which has been reported in Mecp2 loss-of-function mice (Belichenko et al., 2008). The age-dependent and domain-specific decrease in dendritic complexity in Mecp2T158A/ y mice, therefore, is specific to layer V somatosensory cortex and absent in CA1 hippocampus, consistent with RTT post-mortem studies (Armstrong et al., 1995). Together, these data support age-dependent and brain region-specific regulation of dendritic outgrowth upon Mecp2 loss-of-function.
Figure 4. Dendritic complexity in hippocampus CA1 of Mecp2T158A/y mice.
(a) Sholl analysis of hippocampal CA1 pyramidal neurons in P30 WT (27 neurons from 5 mice) and Mecp2T158A/y mice (n=33 neurons from 5 mice) show no change dendritic complexity in Mecp2T158A/y mice relative to WT. Two-way ANOVA, p>0.05. Bars represent mean ± sem.
(b) Sholl analysis of hippocampal CA1 pyramidal neurons in P90 WT (n=32 neurons from 5 mice) and Mecp2T158A/y mice (n=40 neurons from 5 mice) show altered dendritic complexity in Mecp2T158A/y mice relative to WT specifically in the basal dendritic arbor. Two-way ANOVA with Bonferroni correction, p<0.0001 (interaction); *p<0.05, **p<0.01.
(c) Sholl analysis of basal dendritic complexity of hippocampal CA1 pyramidal neurons in P90 WT and Mecp2T158A/y mice show no difference in peak number of crossings in Mecp2T158A/y mice relative to WT.
Soma size is regulated throughout development by MeCP2
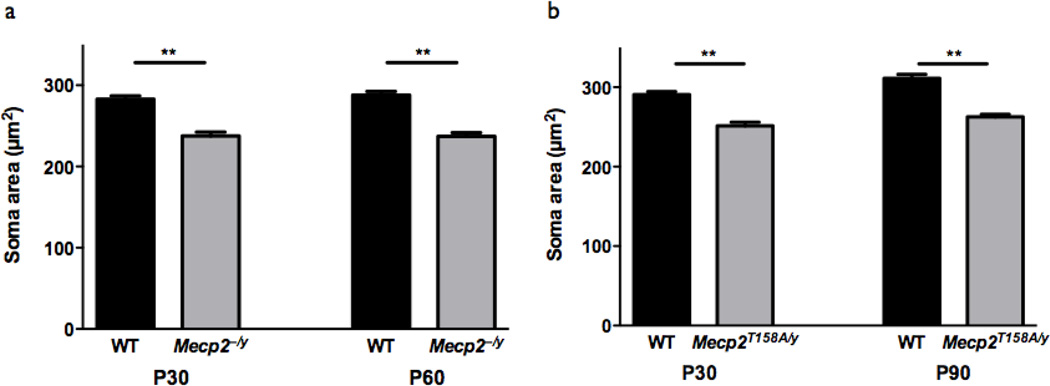
As both reduced dendritic outgrowth and soma size are believed to contribute to RTT patient microcephaly and reduced soma size has been reported in RTT post-mortem tissue (Bauman et al., 1995), we also measured soma size in our Mecp2 mutation mice at both early- and late-symptomatic time points. In striking contrast to the age-dependence and brain region-specificity of changes in dendritic complexity, we found that changes in soma size persisted throughout development and were consistent between Mecp2 loss-of-function mutations. Soma size in somatosensory cortex layer V pyramidal neurons was reduced in both P30 early-symptomatic Mecp2−/y mice (WT: 282.9 ± 4.0 µm2, n=124 neurons; Mecp2−/y: 237.6 ± 4.7µm2, n=103 neurons; p<0.01) and in P60 late-symptomatic Mecp2−/y mice (WT: 287.9 ± 4.4 µm2, n=126 neurons; Mecp2−/y: 237.2 ± 4.4 µm2, n=104 neurons; p<0.01; Fig. 5a), relative to WT littermates, indicating that Mecp2 loss-of-function leads to reduced soma size in both early and late development of RTT-like phenotypes. Similarly, soma size was reduced in both P30 pre-symptomatic Mecp2T158A/y mice (WT: 290.6 ± 3.9 µm2, n=115 neurons; Mecp2T158A/y: 251.3 ± 4.6 µm2, n=121 neurons; p < 0.01) and in P90 late-symptomatic Mecp2T158A/ y mice (WT: 311.1 ± 5.0 µm2, n=81 neurons; Mecp2T158A/ y: 262.6 ± 3.3 µm2, n=106 neurons; p<0.01) relative to WT littermates (Fig 5b), indicating that reduced soma size may even precede onset of RTT behavioral phenotypes and is a consequence of Mecp2 loss-of-function. Moreover, we previously reported that hippocampal CA1 pyramidal neuron soma size is decreased in both pre- and postsymptomatic Mecp2T158A/y mice (Goffin et al., 2012). The persistent reduction in soma size in Mecp2 loss-of-function mouse models throughout development contrasted with the agedependent and brain region-specific changes in dendritic complexity suggests that MeCP2 regulates dendritic outgrowth and soma size through distinct mechanisms and that soma size may be a more reproducible and reliable cellular marker for MeCP2 function than dendritic complexity.
Figure 5. Soma size is regulated throughout development by MeCP2 function.
(a) Somatosensory cortex layer V pyramidal neuron soma size is reduced in Mecp2−/y mice relative to WT at both P30 (WT: n=124 neurons from 4 mice; Mecp2−/y: n=103 neurons from 4 mice) and P60 (WT: n=126 neurons from 4 mice; Mecp2−/y: n=104 neurons from 4 mice). **p<0.01, unpaired two-tailed Student t-test with Bonferroni correction. Bars represent mean ± SEM.
(b) Somatosensory cortex layer V pyramidal neuron soma size is reduced in Mecp2T158A/y mice relative to WT at both P30 (WT: n=115 neurons from 4 mice; Mecp2T158A/y: n=121 neurons from 4 mice) and P90 (WT: n=81 neurons from 4 mice; Mecp2T158A/y: n=106 neurons from 4 mice). **p<0.01, unpaired two-tailed Student t-test with Bonferroni correction.
Dendritic complexity in Mecp2 gain-of-function mice
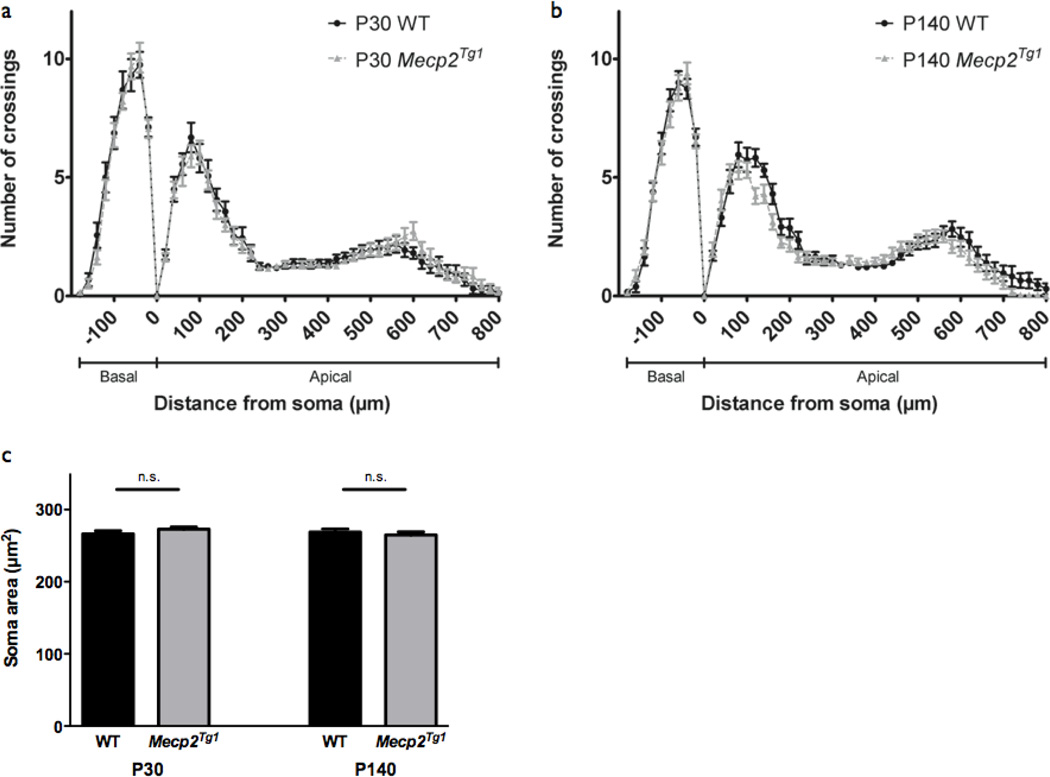
As the importance of MeCP2 dosage has been highlighted though the link between gain-of-function MECP2 mutations and neurodevelopmental disorder (Chahrour and Zoghbi, 2007), we next examined a transgenic mouse line expressing two-fold levels of MeCP2 (Mecp2Tg1) to determine how Mecp2 gain-of-function affects neuronal morphology (Collins et al., 2004). Importantly, neuronal morphology has yet to be described in Mecp2 gain-of-function mice. While these mice, similar to that of Mecp2 loss-of-function models, develop normally, they show enhanced LTP and learning behavior by 20 postnatal weeks and after 30 postnatal weeks, exhibit seizures, ataxia, and premature death (Collins et al., 2004). We therefore examined dendritic complexity and soma size in Mecp2Tg1 mice at P30 and P140, time points representative of pre- and post-development of gain-of-function phenotype, respectively. Strikingly, we found similar patterns of dendritic complexity in Mecp2Tg1 mice and WT littermates in layer V somatosensory cortex by Sholl analysis at P30 (WT: n=16 neurons; Mecp2Tg1: n=19 neurons; p>0.05) (Fig. 6a) and P140 (WT: n=23 neurons; Mecp2Tg1: n=23 neurons; p>0.05) (Fig 6b). These data are in contrast to the reduced dendritic complexity observed in late-symptomatic Mecp2 loss-of-function animals (Fig 2b, Fig 3b), and suggest a separate mechanism of dendritic outgrowth regulation in Mecp2 gain-of-function that is not coupled to behavioral phenotype.
Figure 6. Dendritic complexity in Mecp2Tg1 mice.
(a) Sholl analysis of somatosensory cortex layer V pyramidal neurons in P30 WT (n=16 neurons from 2 mice) and Mecp2Tg1 mice (n=19 neurons from 2 mice) show no change dendritic complexity in Mecp2Tg1 mice relative to WT. Two-way ANOVA, p>0.05. Bars represent mean ± sem.
(b) Sholl analysis of somatosensory cortex layer V pyramidal neurons in P140 WT (n=23 neurons from 2 mice) and Mecp2Tg1 mice (n=23 neurons from 2 mice) show no change dendritic complexity in Mecp2Tg1 mice relative to WT. Two-way ANOVA, p>0.05.
(c) Somatosensory cortex layer V pyramidal neuron soma size is not affected in Mecp2Tg1mice relative to WT at both P30 (WT: n=101 neurons from 2 mice; Mecp2Tg1: n=110 neurons from 2 mice) and P140 (P140; n=110 neurons from 2 mice; Mecp2Tg1: n=113 neurons from 2 mice). p>0.05, unpaired two-tailed Student t-test.
Similarly, we found no changes in soma size in Mecp2Tg1 mice relative to WT littermates, both prior to (P30; WT: 266.3 ± 4.3 µm2, n=101 neurons; Mecp2Tg1: 272.8 ± 3.0 µm2, n=110 neurons) and after the development of behavioral phenotypes (P140; WT: 268.9 ± 4.2 µm2, n=110 neurons; Mecp2Tg1: 265.0 ± 4.2 µm2, n=113 neurons; p>0.05) (Fig. 6c). This finding is consistent with in vitro studies showing no effect of MeCP2 overexpression on soma size (Jugloff et al., 2005). Together, these data suggest that neuronal morphology may be affected by Mecp2 loss-of-function, but is not sensitive to two-fold MeCP2 dosage. This is not surprising, as MECP2 duplication syndrome is considered to be a separate neurodevelopmental disorder from typical RTT (Ramocki et al., 2009) and likely has a distinct pathogenic mechanism. Neuronal morphology, therefore, may be an appropriate therapeutic readout specifically for RTT patients with MECP2 loss-of-function mutations.
DISCUSSION
RTT phenotypes are believed to originate from disruptions in neuronal circuits caused by MeCP2 dysfunction in the brain (Goffin and Zhou, 2012; Katz et al., 2012), and changes in neuronal morphology provide a cellular basis for alterations in circuit function. Given the rise in the use of morphological phenotypes as readouts of therapeutic efficacy (Marchetto et al., 2010; Tropea et al., 2009) or restoration of MeCP2 function (Giacometti et al., 2007; Robinson et al., 2012), understanding how MECP2 mutations impact the maintenance of proper neuronal structure and the relationship between RTT-like phenotype and underlying cellular morphology has become vital. Robust and reproducible RTT-related morphological phenotypes have been difficult to identify, as the use of different techniques, animal models, cell types, and developmental time points across different labs have made direct comparisons between studies difficult. Therefore, we sought to eliminate the confounds of methodological bias by using the same experimental method to analyze neuronal morphology in vivo across the development of Mecp2 loss-of-function, partial loss-of-function, and gain-of-function mouse models. Given the successful utilization of Thy1-GFP/M reporter mice in other cortical and hippocampal morphological studies (Belichenko et al., 2009a; Cohen et al., 2011) and the absence of added experimental manipulations for cellular visualization, we chose to cross our Mecp2 mutant mice to a Thy1-GFP/M reporter line in which neurons are intrinsically labeled with GFP in a mosaic manner in several brain regions, including the somatosensory cortex and hippocampus CA1 (Feng et al., 2000). In our study, we aimed to address the following three questions: 1) How does Mecp2 loss- or gain-of-function affect the development of neuronal morphology? 2) What is the relationship between RTT-like behavioral phenotypic severity and underlying neuronal morphology? 3) Are these changes in neuronal morphology brain region or cell type-specific?
To address the first question, we measured dendritic complexity and soma size in Mecp2−/y and Mecp2T158A/y mice, representing Mecp2 loss-of-function and Mecp2 partial loss-of-function, respectively, and Mecp2Tg1 mice, representing Mecp2 gain-of-function. We found decreased dendritic complexity and soma size in Mecp2 loss-of-function mice (Mecp2−/y, Mecp2T158A/y) and no change in either phenotype in Mecp2 gain-of-function mice (Mecp2Tg1) relative to WT littermates. These data suggest that MeCP2 expression levels may play a role in the regulation of neuronal outgrowth, but increased MeCP2 expression has no structural effect. Our data also indicate that a greater reduction in MeCP2 may cause more severe morphological defects, as we observed a more widespread reduction in dendritic branching in Mecp2−/y mice, which lack MeCP2 protein (Guy et al., 2001), relative to Mecp2T158A/y mice, which have reduced MeCP2 protein expression (Goffin et al., 2012).
To our knowledge, neuronal morphology has not been studied in Mecp2 gain-of-function mouse models, and the effect of MeCP2 overexpression in vitro on neuronal morphology remains unclear due to reports of increased (Jugloff et al., 2005; Larimore et al., 2009), decreased (Zhou et al., 2006), and unchanged dendritic complexity (Chapleau et al., 2009) in cultured neurons and organotypic slices, and decreased dendritic outgrowth in Drosophila and Xenopus models (Marshak et al., 2012; Vonhoff et al., 2012). Our findings suggest that both dendritic outgrowth and soma size are reduced upon Mecp2 loss-of-function, whereas neither phenotype is affected by Mecp2 gain-of-function with two-fold MeCP2 expression. Notably, Mecp2Tg1 mice were maintained on a mixed FVB/C57BL/6 background, and both Mecp2 loss-of-function models were maintained on a pure C57BL/6 background. Therefore, the different genetic backgrounds may contribute to the differences seen between loss-of-function and gain-of-function models. It is possible that, although two-fold expression of MeCP2 is sufficient to impair synaptic function leading to circuit defects (Chao et al., 2007; Na et al., 2012), it is not sufficient to disrupt cellular morphology in vivo. Future study of neuronal morphology in mice expressing three-fold levels of MeCP2 may address this hypothesis, as MECP2 triplication produces more severe phenotypes both clinically and in mice (Samaco et al., 2012; Van Esch et al., 2007).
To address the second question, the relationship between RTT-like phenotypic severity and underlying neuronal morphology, we measured dendritic complexity and soma size in Mecp2−/yand Mecp2T158A/y mice prior to or at the onset of RTT-like phenotypes (P30) and after development of RTT-like phenotypes. Because RTT-like behavioral progression differs across these mouse models, the ages corresponding to late-symptomatic RTT similarly differed (P60, P90, respectively). Overall, we observed a subtle age-dependent effect of Mecp2 mutation on dendritic complexity in Mecp2 loss-of function, where progression of RTT-like behavioral phenotype correlates with underlying cellular changes. At the early-symptomatic P30 time point, only the mildly-symptomatic Mecp2−/y mice showed decreased dendritic complexity, while the pre-symptomatic Mecp2T158A/y mice did not, indicating that early in the development of RTT-like phenotypes, changes in neuronal structure vary across different Mecp2 mutations and may reflect development of RTT-like behavioral phenotypes. This hypothesis is consistent with findings from a comparative study of brain morphology in two 3-week-old Mecp2 loss-of-function mouse models, in which Mecp2−/y mice with a more severe RTT-like phenotype exhibited more widespread and marked changes in brain structure than Mecp2−/y mice with a milder RTT-like phenotype (Belichenko et al., 2008).
Similarly, we found greater changes in dendritic complexity in late-symptomatic P60 Mecp2−/yand P90 Mecp2T158A/y mice relative to that of P30. The reduction in dendritic complexity in P60 Mecp2−/y mice was slightly more widespread than that of P30 Mecp2−/y mice, and P90 Mecp2T158A/y showed changes in dendritic complexity that were absent at the pre-symptomatic time point. These data indicate that changes in dendritic outgrowth accompany the development of RTT-like phenotypes upon Mecp2 loss-of-function and are consistent with RTT post-mortem histological data showing a correlation between the degree of morphological abnormality and RTT symptom severity at time of death (Bauman et al., 1995).
The developmental changes in dendritic complexity we identified, however, were relatively mild, compared to reports from other Mecp2 loss-of-function studies (Kishi and Macklis, 2004; Robinson et al., 2012; Stuss et al., 2012), and limited to specific dendritic domains. This is surprising, as the overt behavioral phenotypes present at both late-symptomatic time points would predict underlying cellular architecture to be severely disrupted. One factor in this subtle dendritic outgrowth phenotype may be experimental bias from the Thy1-GFP/M reporter line. While an interaction between MeCP2 and a different reporter transgene Thy1-YFP/H has been reported (Stuss et al., 2012), we did not identify GFP-labeling biases in our mice. Our selection of layer V cortical neurons extending their apical tufts to the pial surface, however, could have introduced a bias for analysis of neurons with a less severe morphological phenotype within the Mecp2 mutant neuronal network. As changes in dendritic morphology have been shown to widely differ between individual neurons within a population (Belichenko et al., 2009b), we cannot exclude the possibility that our experimental method was selective for neurons with intact dendritic structures, resulting in a mild dendritic complexity phenotype.
Data from other Mecp2 mouse models, however, indicate that changes in dendritic arborization may not reflect the severity of behavioral phenotype. While MeCP2 A140V knockin mice with no obvious RTT-like behavioral phenotypes show decreased dendritic complexity (Jentarra et al., 2010), Mecp2308/y mice with motor, social, and learning deficits that model an MeCP2 early truncation show no changes in dendritic complexity or post-synaptic density in cortical and hippocampal CA1 neurons, both before and after onset of RTT-like phenotypes (Moretti et al., 2006). In addition, a developmental time window may exist in which neuronal morphology is more sensitive to MeCP2 function, as previous work has shown that newborn neurons in the hippocampus are severely affected by Mecp2 loss-of-function (Smrt et al., 2007). Together, these data suggest that while decreases in dendritic arborization may accompany the development of RTT-like behavioral phenotypes in Mecp2 loss-of-function, the degree of these changes does not necessarily match the behavioral phenotypic severity and therefore, dendritic arborization may not be a robust and consistent cellular readout of RTT-like phenotype.
In contrast to the intrinsic variability of dendritic outgrowth in Mecp2 mice, we found that MeCP2 soma size regulation is persistent throughout development and dependent on MeCP2 function. Both Mecp2 loss-of-function mouse models, Mecp2−/y and Mecp2T158A/y, showed decreased soma size at early- and late-symptomatic time points relative to WT, suggesting that soma size decreases upon Mecp2 loss-of-function but does not change with increasing RTT-like symptomatic severity. These data are consistent with findings from ESC-differentiated neurons, where neurons originating from several lines of Mecp2 loss-of-function mice show decreased soma size relative to controls throughout the course of neuronal maturation (Yazdani et al., 2012), and can be rescued upon restoration of MeCP2. Together, these data indicate that soma size is regulated by MeCP2 function throughout development and can be an indicator of MeCP2 function.
To address the third question, whether changes in neuronal morphology are brain region or cell type-specific, we assessed neuronal morphology in a separate brain region by measuring dendritic complexity in hippocampal CA1 pyramidal neurons in pre-and post-symptomatic Mecp2T158A/y mice. In contrast to the age-dependent decrease in basal dendritic complexity we observed in somatosensory cortex layer V pyramidal neurons of Mecp2T158A/y mice, we found no age-dependent reduction in dendritic complexity of hippocampal CA1 pyramidal neurons. These data are consistent with region-specific morphological phenotypes identified in RTT postmortem brain tissue, where pyramidal neurons of the motor, frontal, and inferior temporal cortices show decreased dendritic complexity, but pyramidal neurons in the visual cortex and hippocampus exhibit no change with respect to age-matched controls (Armstrong et al., 1995; Belichenko et al., 1994). Specificity may even extend to cortical layer, as decreased dendritic arborization has been identified in both basal and apical dendrites of layer V motor cortex cells but only in basal dendrites of layer III motor cortex cells (Armstrong et al., 1995). A comparison of data from different studies also supports a cortical layer-specific regulation of neuronal morphology in mice, where neocortical layer II/III pyramidal neurons show decreased dendritic complexity in both proximal basal and apical dendrites and no change in spine density (Kishi and Macklis, 2004) but layer V pyramidal neurons show decreased dendritic complexity in only proximal basal and distal apical dendrites and decreased spine density (Stuss et al., 2012).
In contrast to the cell type-specific changes in dendritic outgrowth, multiple lines of evidence indicate that soma size is consistent across cell types. We previously reported decreased soma size in hippocampal CA1 pyramidal neurons in both pre- and post-symptomatic Mecp2T158A/ymice (Goffin et al., 2012), similar to what we observed in the somatosensory cortex in this study. In addition, decreased soma size has been reported in RTT post-mortem brain tissue (Armstrong, 2005) and in the locus ceruleus (Taneja et al., 2009), hippocampus CA2 (Chen et al., 2001), layer II/III somatosensory cortex (Kishi and Macklis, 2004), layer II/III motor cortex (Robinson et al., 2012), and layer V motor cortex (Stuss et al., 2012) of different Mecp2-null mouse strains. Moreover, various lines of Mecp2-deficient ESC-derived neurons (Yazdani et al., 2012) and RTT patient-derived induced pluripotent stem cells (Marchetto et al., 2010) also show reduced soma sizes, indicating that, in contrast to dendritic arborization, a reduction in soma size upon Mecp2 loss-of-function is a phenotype that is consistent across cell types and experimental systems. Similarly, our finding that soma size is not affected upon Mecp2 gain-of-function has also been observed in vitro (Jugloff et al., 2005), indicating that the absence of a change in soma size upon Mecp2 gain-of-function is also conserved across cell types.
This consistent and robust reduction in soma size across studies, Mecp2 mutations, experimental systems, cell types, and developmental time points, is in sharp contrast to changes in dendritic complexity, which varies across these parameters and can be subtle and context-dependent (Stuss et al., 2012). Therefore, in accordance with a recent study evaluating spine density throughout development in Mecp2 loss-of-function mice (Chapleau et al., 2012), we caution the use of dendritic complexity as a phenotypic endpoint for therapeutic evaluation, and suggest that soma size may be a more robust and reproducible readout of MeCP2 function. Evidence supporting soma size as a reliable morphologic phenotype comes from existing rescue experiments, where activation of a quiescent Mecp2 gene in adults results in partial restoration of dendritic complexity and length but a full restoration of soma size (Robinson et al., 2012). In addition, postnatal neuron-specific reactivation of MeCP2 sufficient to rescue hippocampal and cortical soma size in Mecp2-deficient mice (Giacometti et al., 2007) and neuronal size is restored by the re-expression of MeCP2 in several strains of MeCP2-deficient ESC-derived neurons (Yazdani et al., 2012). Future studies are needed to understand how soma size and dendritic outgrowth are differentially regulated by MeCP2. It is conceivable that dendritic arborization is more sensitive to the cellular environment, growth factors, and homeostatic regulation, whereas soma size is more directly affected by the function of nuclear proteins such as MeCP2.
Overall, our data indicates that within a population of excitatory neurons in RTT mouse models, in vivo changes in dendritic complexity upon Mecp2 loss-of-function are brain region-specific, correlated with behavioral phenotype, and mild. In contrast, soma size is regulated throughout development and may be a reliable marker for evaluating MeCP2 function and therapeutic efficacy. These phenotypes are specific to Mecp2 loss-of-function, as in vivo morphological changes upon Mecp2 gain-of-function are absent. The use of neuronal morphology as a cellular readout of RTT phenotype and restoration of neuronal circuitry, therefore, should be cautioned, as morphological phenotypes are intrinsically variable.
HIGHLIGHTS.
Dendritic complexity and soma size are uniquely affected in Mecp2 loss-of-function
Mild and variable decrease in dendritic complexity in Mecp2 loss-of-function
Persistent and robust decrease in soma size in Mecp2 loss-of-function
Unaltered dendritic branching and soma size in Mecp2 gain-of-function
ACKNOWLEDGMENTS
We thank members of the Zhou laboratory for critical readings of the manuscript. This work was supported by NIH grants R00 NS058391 (Z.Z.) and T32MH017168-29 (I-T.J.W.) and the International Rett Syndrome Foundation (Z.Z). Z.Z. is a Pew Scholar in Biomedical Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Armstrong D, Dunn JK, Antalffy B, Trivedi R. Selective dendritic alterations in the cortex of Rett syndrome. J. Neuropathol. Exp. Neurol. 1995;54:195–201. doi: 10.1097/00005072-199503000-00006. [DOI] [PubMed] [Google Scholar]
- Armstrong DD. Neuropathology of Rett syndrome. Journal of Child Neurology. 2005;20:747–753. doi: 10.1177/08830738050200090901. [DOI] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL, Arin DM. Pervasive neuroanatomic abnormalities of the brain in three cases of Rett's syndrome. Neurology. 1995;45:1581–1586. doi: 10.1212/wnl.45.8.1581. [DOI] [PubMed] [Google Scholar]
- Belichenko NP, Belichenko PV, Mobley WC. Evidence for both neuronal cell autonomous and nonautonomous effects of methyl-CpG-binding protein 2 in the cerebral cortex of female mice with Mecp2 mutation. Neurobiol. Dis. 2009a;34:71–77. doi: 10.1016/j.nbd.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Belichenko NP, Belichenko PV, Li HH, Mobley WC, Francke U. Comparative study of brain morphology in Mecp2 mutant mouse models of Rett syndrome. J. Comp. Neurol. 2008;508:184–195. doi: 10.1002/cne.21673. [DOI] [PubMed] [Google Scholar]
- Belichenko PV, Oldfors A, Hagberg B, Dahlström A. Rett syndrome: 3-D confocal microscopy of cortical pyramidal dendrites and afferents. Neuroreport. 1994;5:1509–1513. [PubMed] [Google Scholar]
- Belichenko PV, Wright EE, Belichenko NP, Masliah E, Li HH, Mobley WC, Francke U. Widespread changes in dendritic and axonal morphology in Mecp2-mutant mouse models of Rett syndrome: evidence for disruption of neuronal networks. J. Comp. Neurol. 2009b;514:240–258. doi: 10.1002/cne.22009. [DOI] [PubMed] [Google Scholar]
- Bienvenu T, Chelly J. Molecular genetics of Rett syndrome: when DNA methylation goes unrecognized. Nat. Rev. Genet. 2006;7:415–426. doi: 10.1038/nrg1878. [DOI] [PubMed] [Google Scholar]
- Brendel C, Belakhov V, Werner H, Wegener E, Gärtner J, Nudelman I, Baasov T, Huppke P. Readthrough of nonsense mutations in Rett syndrome: evaluation of novel aminoglycosides and generation of a new mouse model. J. Mol. Med. 2011;89:389–398. doi: 10.1007/s00109-010-0704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY. The Story of Rett Syndrome: From Clinic to Neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chao H-T, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron. 2007;56:58–65. doi: 10.1016/j.neuron.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapleau CA, Boggio EM, Calfa G, Percy AK, Giustetto M, Pozzo-Miller L. Hippocampal CA1 Pyramidal Neurons of Mecp2 Mutant Mice Show a Dendritic Spine Phenotype Only in the Presymptomatic Stage. Neural Plast. 2012;2012:1–9. doi: 10.1155/2012/976164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapleau CA, Calfa GD, Lane MC, Albertson AJ, Larimore JL, Kudo S, Armstrong DL, Percy AK, Pozzo-Miller L. Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations. Neurobiol. Dis. 2009;35:219–233. doi: 10.1016/j.nbd.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Cohen S, Gabel HW, Hemberg M, Hutchinson AN, Sadacca LA, Ebert DH, Harmin DA, Greenberg RS, Verdine VK, Zhou Z, et al. Genome-Wide Activity-Dependent MeCP2 Phosphorylation Regulates Nervous System Development and Function. Neuron. 2011;72:72–85. doi: 10.1016/j.neuron.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AL, Levenson JM, Vilaythong AP, Richman R, Armstrong DL, Noebels JL, David Sweatt J, Zoghbi HY. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Human Molecular Genetics. 2004;13:2679–2689. doi: 10.1093/hmg/ddh282. [DOI] [PubMed] [Google Scholar]
- Dani VS, Nelson SB. Intact long-term potentiation but reduced connectivity between neocortical layer 5 pyramidal neurons in a mouse model of Rett syndrome. Journal of Neuroscience. 2009;29:11263–11270. doi: 10.1523/JNEUROSCI.1019-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Itoh M, Ichikawa T, Washiyama K, Goto Y-I. Delayed maturation of neuronal architecture and synaptogenesis in cerebral cortex of Mecp2-deficient mice. J. Neuropathol. Exp. Neurol. 2005;64:537–544. doi: 10.1093/jnen/64.6.537. [DOI] [PubMed] [Google Scholar]
- Giacometti E, Luikenhuis S, Beard C, Jaenisch R. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1931–1936. doi: 10.1073/pnas.0610593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin D, Zhou Z. The neural circuit basis of Rett syndrome. Front. Biol. 2012;7:428–435. doi: 10.1007/s11515-012-1248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin D, Allen M, Zhang L, Amorim M, Wang I-TJ, Reyes A-RS, Mercado-Berton A, Ong C, Cohen S, Hu L, et al. Rett syndrome mutation MeCP2 T158A disrupts DNA binding, protein stability and ERP responses. Nat. Neurosci. 2012;15:274–283. doi: 10.1038/nn.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger K, Armstrong D, Zoghbi HY, Percy AK. Neuropathology of Rett syndrome. Acta Neuropathol. 1988;76:142–158. doi: 10.1007/BF00688098. [DOI] [PubMed] [Google Scholar]
- Jentarra GM, Olfers SL, Rice SG, Srivastava N, Homanics GE, Blue M, Naidu S, Narayanan V. Abnormalities of cell packing density and dendritic complexity in the MeCP2 A140V mouse model of Rett syndrome/X-linked mental retardation. BMC. Neurosci. 2010;11:19. doi: 10.1186/1471-2202-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugloff DGM, Jung BP, Purushotham D, Logan R, Eubanks JH. Increased dendritic complexity and axonal length in cultured mouse cortical neurons overexpressing methyl-CpG-binding protein MeCP2. Neurobiol. Dis. 2005;19:18–27. doi: 10.1016/j.nbd.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Katz DM, Berger-Sweeney JE, Eubanks JH, Justice MJ, Neul JL, Pozzo-Miller L, Blue ME, Christian D, Crawley JN, Giustetto M, et al. Preclinical research in Rett syndrome: setting the foundation for translational success. Dis Model Mech. 2012;5:733–745. doi: 10.1242/dmm.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, MacDonald SM, Altamura CR. Dendritic cytoskeletal protein expression in mental retardation: an immunohistochemical study of the neocortex in Rett syndrome. Cereb. Cortex. 2000;10:992–1004. doi: 10.1093/cercor/10.10.992. [DOI] [PubMed] [Google Scholar]
- Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol. Cell. Neurosci. 2004;27:306–321. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kriaucionis S, Bird A. DNA methylation and Rett syndrome. Human Molecular Genetics. 2003;12(Spec No 2):R221–R227. doi: 10.1093/hmg/ddg286. [DOI] [PubMed] [Google Scholar]
- Larimore JL, Chapleau CA, Kudo S, Theibert A, Percy AK, Pozzo-Miller L. Bdnf overexpression in hippocampal neurons prevents dendritic atrophy caused by Rettassociated MECP2 mutations. Neurobiol. Dis. 2009;34:199–211. doi: 10.1016/j.nbd.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N, et al. A role for glia in the progression of Rett's syndrome. Nature. 2011;475:497–500. doi: 10.1038/nature10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikenhuis S, Giacometti E, Beard CF, Jaenisch R. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6033–6038. doi: 10.1073/pnas.0401626101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MCN, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak S, Meynard MM, De Vries YA, Kidane AH, Cohen-Cory S. Cell-Autonomous Alterations in Dendritic Arbor Morphology and Connectivity Induced by Overexpression of MeCP2 in Xenopus Central Neurons In Vivo. PLoS ONE. 2012;7:e33153. doi: 10.1371/journal.pone.0033153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf BM, Mullaney BC, Johnston MV, Blue ME. Temporal shift in methyl-CpG binding protein 2 expression in a mouse model of Rett syndrome. Neuroscience. 2006;139:1449–1460. doi: 10.1016/j.neuroscience.2006.01.060. [DOI] [PubMed] [Google Scholar]
- Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Armstrong D, Arancio O, Sweatt JD, Zoghbi HY. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. Journal of Neuroscience. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na ES, Nelson ED, Adachi M, Autry AE, Mahgoub MA, Kavalali ET, Monteggia LM. A Mouse Model for MeCP2 Duplication Syndrome: MeCP2 Overexpression Impairs Learning and Memory and Synaptic Transmission. Journal of Neuroscience. 2012;32:3109–3117. doi: 10.1523/JNEUROSCI.6000-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelka GJ, Watson CM, Radziewic T, Hayward M, Lahooti H, Christodoulou J, Tam PPL. Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain. 2006;129:887–898. doi: 10.1093/brain/awl022. [DOI] [PubMed] [Google Scholar]
- Ramocki MB, Peters SU, Tavyev YJ, Zhang F, Carvalho CMB, Schaaf CP, Richman R, Fang P, Glaze DG, Lupski JR, et al. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann. Neurol. 2009;66:771–782. doi: 10.1002/ana.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L, Guy J, McKay L, Brockett E, Spike RC, Selfridge J, De Sousa D, Merusi C, Riedel G, Bird A, et al. Morphological and functional reversal of phenotypes in a mouse model of Rett syndrome. Brain. 2012;135:2699–2710. doi: 10.1093/brain/aws096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Mandel-Brehm C, McGraw CM, Shaw CA, McGill BE, Zoghbi HY. Crh and Oprm1 mediate anxiety-related behavior and social approach in a mouse model of MECP2 duplication syndrome. Nat. Genet. 2012;44:206–211. doi: 10.1038/ng.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, Armstrong D, Paylor R, Zoghbi H. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- SHOLL DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Smrt RD, Eaves-Egenes J, Barkho BZ, Santistevan NJ, Zhao C, Aimone JB, Gage FH, Zhao X. Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons. Neurobiol. Dis. 2007;27:77–89. doi: 10.1016/j.nbd.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat. Rev. Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Stuss DP, Boyd JD, Levin DB, Delaney KR. MeCP2. Mutation Results in Compartment-Specific Reductions in Dendritic Branching and Spine Density in Layer 5 Motor Cortical Neurons of YFP-H Mice. PLoS ONE. 2012;7:e31896. doi: 10.1371/journal.pone.0031896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja P, Ogier M, Brooks-Harris G, Schmid DA, Katz DM, Nelson SB. Pathophysiology of locus ceruleus neurons in a mouse model of Rett syndrome. Journal of Neuroscience. 2009;29:12187–12195. doi: 10.1523/JNEUROSCI.3156-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Giacometti E, Wilson NR, Beard C, McCurry C, Fu DD, Flannery R, Jaenisch R, Sur M. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2029–2034. doi: 10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Esch H, Jansen A, Bauters M, Froyen G, Fryns J-P. Encephalopathy and bilateral cataract in a boy with an interstitial deletion of Xp22 comprising the CDKL5 and NHS genes. Am. J. Med. Genet. A. 2007;143:364–369. doi: 10.1002/ajmg.a.31572. [DOI] [PubMed] [Google Scholar]
- Vonhoff F, Williams A, Ryglewski S, Duch C. Drosophila as a model for MECP2 gain of function in neurons. PLoS ONE. 2012;7:e31835. doi: 10.1371/journal.pone.0031835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani M, Deogracias R, Guy J, Poot RA, Bird A, Barde Y-A. Disease modeling using embryonic stem cells: MeCP2 regulates nuclear size and RNA synthesis in neurons. Stem Cells. 2012;30:2128–2139. doi: 10.1002/stem.1180. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hong EJ, Cohen S, Zhao W-N, Ho H-YH, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]