Abstract
Connective tissue growth factor (CCN2/CTGF) plays an important role in extracellular matrix synthesis, especially in skeletal tissues such as cartilage, bone, and the intervertebral disc. As a result there is a growing interest in examining the function and regulation of this important molecule in the disc. This review discusses the regulation of CCN2 by TGF-β and hypoxia, two critical determinants that characterize the disc microenvironment, and discusses known functions of CCN2 in the disc. The almost ubiquitous regulation of CCN2 by TGF-β, including that seen in the disc, emphasizes the importance of the TGF-β-CCN2 relationship, especially in terms of extracellular matrix synthesis. Likewise, the unique cross-talk between CCN2 and HIF-1 in the disc highlights the tissue and niche specific mode of regulation. Taken together the current literature supports an anabolic role for CCN2 in the disc and its involvement in the maintenance of tissue homeostasis during both health and disease. Further studies of CCN2 in this tissue may reveal valuable targets for the biological therapy of disc degeneration.
Keywords: intervertebral disc, nucleus pulposus, CCN2, TGF-β, hypoxia, HIF-1α, inflammatory cytokines, disc degeneration
Introduction
Due to its important role in extracellular matrix synthesis, connective tissue growth factor (CTGF/CCN2) is the most-studied member of the CCN protein family in context of the intervertebral disc (Abbott, et al. 2012; Ali, et al. 2008; Erwin, et al. 2006; Peng, et al. 2009; Tran, et al. 2010, 2013). This matricellular protein, similar to the other 5 members of the CCN protein family, contains highly conserved modular domains that have sequence homology to known proteins. With the exception of CCN5, which lacks only the cysteine knot (CT) domain, their domain structure comprises: a secretory signal peptide (SP), insulin-like growth factor binding protein (IGFBP), vonWillebrand factor type C repeat (vWC), thrombospondin type 1 repeat (TSP), and CT domain (Fig. 1). Through these domains, CCN2 influences many biological processes by acting as a signal transducer or modifier, binding to various growth factors and extracellular proteins, and interacting with cell surface receptors (Fig. 1). The protein’s many functions are tissue specific; it regulates extracellular matrix synthesis, plays a role in angiogenesis, cell migration, adhesion, and differentiation (Gao and Brigstock 2006; Gao and Brigstock 2004; Gao and Brigstock 2006; Heng, et al. 2006; Hoshijima, et al. 2006).
Fig. 1.
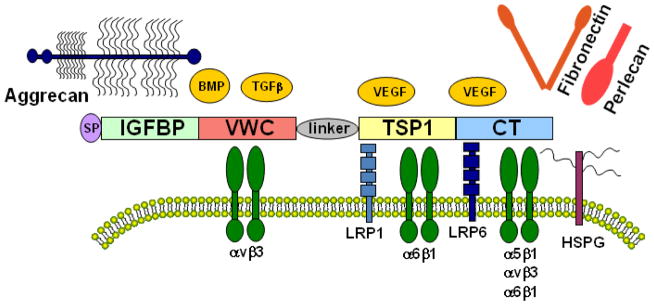
Schematic of CCN2 domain structure and several known interacting proteins. CCN2 consists of an N-terminal secretory signal peptide (SP) and four modular domains with sequence homology to insulin-like growth factor binding protein (IGFBP), vonWillebrand factor type C repeat (vWC), thrombospondin type 1 repeat (TSP), and the cysteine knot containing carboxyl domain (CT). It also contains a non-conserved, protease-susceptible linker region between the vWC and TSP domains. CCN2 signals through integrins, including α5β1, αvβ3, α6β1, and heparan sulfate proteoglycans (HSPG) on the cell surface to promote matrix synthesis, cell migration and adhesion. Interaction with lipoprotein receptor-related protein 1 (LRP1) is known to facilitate clathrin mediated internalization of CCN2. Interactions between CCN2 and growth factors including TGFβ, BMP and VEGF have been reported to differentially modulate the activity of these morphogens. Adhesion and interaction of CCN2 with extracellular matrix components such as aggrecan, fibronectin and perlecan have also been shown.
CCN2 is differentially expressed from development to maturation in vertebrates, with strong expression in skeletal tissues including growth plate and Meckel’s cartilage, bone, and the intervertebral disc (Chiou, et al. 2006; Ivkovic, et al. 2003, Shimo et al. 2004). During early embryogenesis, it is highly expressed in the somites, floorplate and notochord, and expression remains robust in several mature tissues that arise from these primitive structures (Chiou, et al. 2006; Fernando, et al. 2010; Ivkovic, et al. 2003; Mercurio, et al. 2004). As skeletogenesis occurs, strong CCN2 expression is seen in the perichondrium, adjacent chondrocytes, and later in the maturing chondrocytes of pre-hypertrophic and hypertrophic zones (Ivkovic, et al. 2003). An important role for CCN2 in promoting both chondrogenesis and osteogenesis is evident in the phenotype of CCN2 null mice which exhibit decreased extracellular matrix in the growth plate and an enlarged hypertrophic zone. This phenotype is attributed to decreased chondrocyte proliferation and defective growth plate angiogenesis that results from diminished levels of local VEGF and MMP-9 (Ivkovic, et al. 2003). Moreover, the null mice display kinked ribs and long bones and, as a consequence, die of respiratory failure soon after birth (Ivkovic, et al. 2003). In vitro studies confirm the importance of CCN2 for maintenance of the chondrocyte phenotype and in promoting chondrocyte differentiation in the growth plate (Kawaki, et al. 2008; Nishida, et al. 2007). Noteworthy, Shimo and colleagues showed that retinoid signaling, important in hypertrophy of growth plate chondrocytes, induces CCN2 expression (Shimo, et al. 2005). Similarly, role of CCN2 in promoting osteoblast differentiation and endochondral ossification, is evidenced by decreased osteoblast proliferation, collagen deposition, and mineralization in vivo and by decreased levels of alkaline phosphatase, Osteocalcin, and Col1a1 in null osteoblasts cultured in vitro (Geisinger, et al. 2012; Ivkovic, et al. 2003; Kawaki, et al. 2007; Kawaki, et al. 2011; Kubota, et al. 2007; Zhang, et al. 2010). Furthermore, investigations have clarified several mechanisms of CCN2 transcriptional regulation in chondrocytes and osteoblasts (Geisinger, et al. 2012; Huang, et al. 2010; Kawaki, et al. 2011; Shimo et al. 2005; Zhang, et al. 2010). More recently, several investigators have begun to examine the role of CCN2 in the intervertebral disc, an area where relatively less information is known about its function and regulation. Although there have been a plethora of reviews concerning CCN2 function and regulation in other tissues (Cicha and Goppelt-Struebe 2009; Hall-Glenn and Lyons 2011; Holbourn, et al. 2009; Jun and Lau 2011; Kubota and Takigawa 2007; Kubota and Takigawa 2011), the mechanistic details of its regulation in the disc have not been summarized. This mini-review focuses on the regulation of CCN2 expression in the disc by factors that are most relevant to disc health and disease and draws parallels with information available in other connective tissues.
Developmental and post-natal expression and function of CCN2 in the disc
The intervertebral disc is a complex structure that displays many of the characteristics of a polyaxial, diarthrodial joint; it separates opposing cartilage-covered vertebral bodies, permits a range of motions, and accommodates high biomechanical forces (Shapiro, et al. 2012). At the disc periphery is the fibrocartilagenous outer annulus fibrosus, which is composed of tightly packed collagen I fibrils that are inserted into contiguous superior and inferior cartilaginous endplates and vertebral bodies. The surface of the inner annulus fibrosus contains collagen II and proteoglycans, aggrecan and versican. The annulus and the cartilagenous endplates enclose the nucleus pulposus (NP), an aggrecan-rich, gel-like tissue that is sparsely populated with cells and completely avascular. Cells of the NP thus exist in a uniquely hypoxic and hyperosmolar microenvironment (Agrawal, et al. 2007; Gajghate, et al. 2009; Risbud, et al. 2006; Risbud, et al. 2010; Tsai, et al. 2007). The proper functioning of the disc is dependent on the integrity of proteoglycans and fibrilar collagens. Notably, the behavior of disc cells and their ability to maintain matrix homeostasis is sensitive to environmental stimuli and signaling factors such as CCN2.
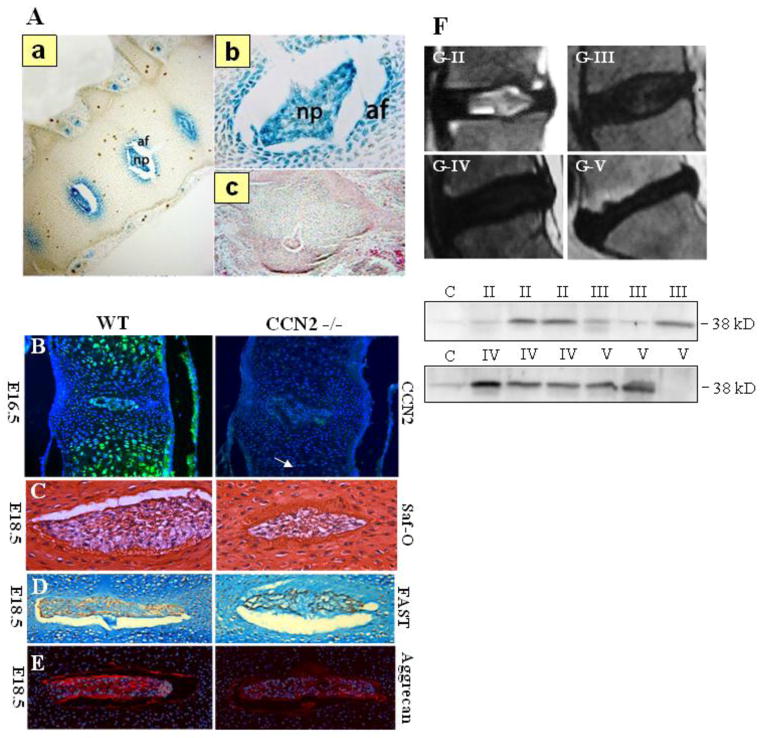
Several studies have suggested an anabolic role for CCN2 in the disc and have shown its expression in the tissue from early disc development to maturation. The notochord is the embryonic anlagen of the NP, originating from the mesoderm and serving both structural and signaling functions in the embryo (Stemple, et al. 2005). It undergoes segmentation during vertebrogenesis, during which the NP is formed from notochord while annulus fibrosus, cartilage endplates, and vertebrae are derived from the sclerotome. Work in zebrafish showed high CCN2 promoter activity in parts of the developing notochord as early as one day post fertilization and a requirement of CCN2 for the maintenance of notochord structure and integrity (Chiou, et al. 2006). Although a similar requirement for CCN2 in early notochord development was not observed in mice (Ivkovic, et al. 2003), the distinct expression of CCN2 in the developing mouse disc was observed (Huang, et al. 2010). Huang et al. showed the robust and specific activity of a 4 kb CCN2 promoter in the disc tissues of an E16.5 mouse, suggesting that CCN2 may play an important role following segmentation of the vertebrae and intervertebral disc in higher vertebrates (Huang, et al. 2010) (Fig. 2A). Very recently, we confirmed an anabolic role for CCN2 in the maintenance of matrix components in the intervertebral disc. Embryonic discs from CCN2 nulls showed decreased safranin-o and aggrecan staining compared to discs of wild type animals (Tran, et al. 2013) (Fig. 2B–E). Though the decrease in proteoglycan levels between wild type and null embryonic discs was modest, we hypothesize that, as matrix accumulation and turnover in a healthy disc is a slow process (Sivan, et al. 2006), it is possible that the cumulative effect of a small but continuous decrease in matrix production over time will have a significant impact on the structure and function of post-natal discs. Robust expression of CCN2 in post-natal discs in vivo (Hall-Glenn and Lyons, 2011, Tran, et al. 2010) and a significant decrease in the basal expression of aggrecan, collagens I and II in CCN2 silenced human NP cells (Tran, et al. 2013) supports the role of CCN2 in maintenance of matrix homeostasis. Further lending credence to this hypothesis are in vitro studies demonstrating CCN2 secretion by notochordal cells and the ability of CCN2 to stimulate aggrecan production by NP cells (Erwin, et al. 2006; Tran, et al. 2010; Tran, et al. 2011). Erwin et al. suggested that a distinct population of notochordal cells secrete CCN2 and stimulate aggrecan production by the non-notochordal NP cells (Erwin, et al. 2006). Noteworthy, as a result of fate mapping and expression studies, it is now widely accepted that all NP cells are derived from the notochord (Choi, et al. 2008; Risbud, et al. 2010; Risbud, et al. 2007; Sakai, et al. 2012). Therefore, we believe that it is important to focus, not on the ontological differences between cell populations within the NP, but instead, on how the expression and regulation of genes critical for matrix homeostasis and cell function are affected by the altered microenvironment during disc degeneration.
Fig 2.
CCN2 expression in the intervertebral disc from development to degeneration. (A) LacZ staining on spines of E16.5 transgenic mice in which lacZ expression is under the control of the 4 kb proximal CCN2 promoter a) Robust and localized LacZ staining reflective of strong CCN2 expression is evident in the NP and IAF at E16.5. b) Higher magnification image of the LacZ stained disc. (c) Non-transgenic control. (Reproduced from Huang, et al., 2010) (B) Immunofluorescence analysis of CCN2 expression in vertebral bodies from E16.5 WT (left) and CCN2−/− littermate (right). In the WT mouse, CCN2 is abundant in the hypertrophic cartilage of the vertebral body and in the nucleus pulposus. Hypertrophy of chondrocytes in the vertebral bodies appears to be delayed in mutants, but there are no obvious differences in the nucleus pulposus (arrow). (C) Sections through the spines of E16.5 WT (left) and mutant (right) littermates, stained with safranin-o. (D) Higher magnification images of the nucleus pulposus at E18.5 stained using the FAST protocol. The relative level of safranin-o-stained (Saf-O) material is decreased in mutants in the nucleus pulposus. (E) Immunofluorescence staining for aggrecan demonstrates decreased expression in the nucleus pulposus of CCN2 mutants. (Reproduced from Tran et al., 2013). (F) Expression of CCN2 in degenerated NP tissues from patients with intervertebral disc degeneration. Magnetic resonance imaging (MRI) of the spine of patients with disc disease. Representative images show Pfirrmann grade II, grade III, grade IV, and grade V changes by MRI. Western blot analysis of CCN2 expression in degenerated NP samples of MRI grades II, III, IV, and V show an increasing trend in CCN2 protein with increasing grade of degeneration (Reproduced from Tran, et al., 2010).
CCN2 expression during disc degeneration
CCN2 has been studied in the context of degenerative diseases of the skeletal system such as osteoarthritis (OA) and intervertebral disc degeneration and pathological conditions such as tissue fibrosis. During these pathologies, CCN2 expression is elevated and the role it plays is tissue specific. While CCN2 is a known, potent inducer of fibrosis in tissues including the skin, kidney, and lung; the role that it plays in degenerative diseases is less well characterized (Blaney Davidson, et al. 2006; Liu, et al. 2011; Masuko, et al. 2010; Peng, et al. 2009; Ponticos, et al. 2009; Sonnylal, et al. 2010; Tong, et al. 2009; Tran, et al. 2010). Although studies have shown the robust increase of CCN2 levels in human osteoarthritic chondrocytes (Kumar, et al. 2001; Omoto, et al. 2004; Zhang, et al. 2002), the exact role of the protein in OA pathogenesis and progression remains unknown. It has been speculated that CCN2 may have both positive and negative functions during OA. Attempting to understand its role, Blaney Davidson and colleagues overexpressed CCN2 in the synovial lining of mouse knee joints using an adenovirus, causing transient but reversible fibrosis of the synovium and depletion of proteoglycans in cartilage without apparent erosion (Blaney Davidson, et al. 2006). In a separate study, Nishida et al. injected a hydrogel containing recombinant CCN2 into OA knee joints and observed an anabolic response, characterized by increased proteoglycan content and improved joint phenotype (Nishida, et al. 2004). The discrepancy of CCN2 effects on cartilage in these studies could be due to differential microenvironmental conditions present in a normal joint versus an OA joint. An articular cartilage-specific CCN2 knockout may be required to definitively investigate the role of CCN2 in OA pathogenesis and progression.
In terms of degenerative disc disease, several recent studies have reported elevated CCN2 levels with increasing severity of disc degeneration (Tran, et al. 2010) (Fig. 2F); increased expression has also been associated with painful discs (Peng, et al. 2009). Observing localization of CCN2 to fibroblasts, invading immunocytes and endothelial cells in degenerate and painful discs, Peng et al. suggested that elevated CCN2 is indicative of its pathological role in the disease process (Peng, et al. 2009). On the contrary, based on its role in promoting matrix synthesis in NP cells in vivo and in vitro, we propose that CCN2 functions as an anabolic factor- promoting a limited reparative response in the degenerate state, similar to the role in articular cartilage speculated by Nishida and colleagues (Nishida, et al. 2004; Tran, et al. 2010, 2011, 2013). A critical driver of degenerative disc disease progression is the changing microenvironment along with dynamically expressed inflammatory cytokines and growth factors. We hypothesize that a simultaneous increase in levels of TGF-β, a positive regulator of CCN2 in NP cells, may contribute to the elevated CCN2 levels observed in the degenerate disc (Fig. 2F). It is likely that the altered microenvironmental conditions of degeneration could change the interactions of CCN2 with other proteins and influence how the cells respond to CCN2 (Abbott, et al. 2012). Further studies of NP specific CCN2 conditional knockout mice would be required to determine its specific role in disc degeneration and controlling activities of other important signaling pathways in NP cells.
CCN2 domain structure, promoter region and conservation between species
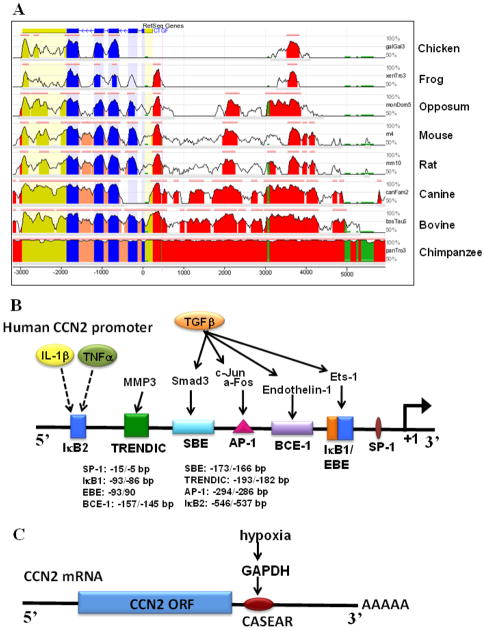
In humans, the CCN2 gene is located on chromosome 6q23.1 and comprises 5 exons, each of which encodes a highly conserved protein domain of the 38 kDa protein (Fig. 3A). Beginning at the N-terminal, the domains include: the signal peptide, IGFBP, VWC, TSP, and CT domains. The ability of these domains to bind growth factors, cell surface receptors and extracellular matrix molecules allows for the diverse, context specific functions of CCN2 and confers it the ability to modulate growth factor signaling (Fig. 1). Additionally, a non-conserved hinge region, susceptible to cleavage by proteases, separates the N and C terminal domains and adds another layer of complexity to protein function.
Fig. 3.
Conserved regulatory binding elements in the human CCN2 promoter (A) Evolutionary Conserved Regions (ECR) Genome browser shows pairwise alignment of several vertebrate CCN2 gene sequences with conservation relative to the human sequence. The x-axis represents base positions in the genome and the y-axis represents the percent identity between aligned bases at the specific position. Thus, the peaks represent regions of higher conservation with the pink line above the peaks denoting ECRs that have 80% or more identity with the human sequence. The promoter region is shown in red, coding region in blue, untranslated region in yellow, transposons and simple repeats in green, and intronic sequences are shown in peach. Note, several regions of high evolutionary conservation in the promoter region are conserved through several vertebrate species (Reproduced from Tran, et al., 2013). (B) Approximately 650 bp of human CCN2 promoter with major regulatory binding elements shown along with factors that signal through these sites and their downstream effectors. (C) Schematic of CCN2 mRNA showing the cis-acting, structure-anchored repression (CAESAR) element in the 3′ UTR, which mediates hypoxic signaling in chondrosarcoma cells (Kondo, et al., 2011).
Although the CCN proteins were first classified by some as additional IGFBPs because of the sequence similarity in the N-terminal domain, it has low affinity for IGF and little has been reported about the functions of this domain in CCN2. Aoyama et al. showed that the IGFBP domain could bind to aggrecan and along with the VWC domain could promote aggrecan production in a HCS-2/8 cells (Aoyama, et al. 2009). The VWC domain or chordin-like cysteine rich repeat is characteristic of many extracellular matrix molecules including VW factor, procollagen, TSP, and glycosylated mucins. It was shown to bind and regulate the activity of BMP and TGF-β by inhibiting the binding of BMP4 to its receptor, while enhancing receptor binding to TGF-β1 (Abreu, et al. 2002). On the other hand, the TSP domain is important for the regulation of angiogenesis by CCN2 and is shown to bind and VEGF165 from its receptors (Inoki, et al. 2002). It is also reported that CCN2 binding to LRP1, attributed to the TSP domain, is required for the endocytic uptake and transcytosis of CCN2 (Gao and Brigstock 2003; Kawata, et al. 2012). Additionally, a binding site for α6β1 integrin in this domain is important for CCN2-mediated adhesion and collagen deposition (Heng, et al. 2006; Tong and Brigstock 2006). Lastly, the CT domain, which is a critical for the biological activity, binds HSPGs, and intergrins, including αVβ3 and α5β1 to regulate adhesion, mitogenic effects and extracellular matrix production (Gao and Brigstock 2006; Gao and Brigstock 2004; Gao and Brigstock 2003; Hoshijima, et al. 2006). Interaction between the CT domain and LRP6 has been shown to modulate Wnt signaling (Mercurio, et al. 2004). Interestingly, it was recently shown that CCN2 forms homodimers and heterodimers with CCN3 through the IGFBP, VWC and CT domains that can modify the effect of these proteins on extracellular matrix production (Hoshijima, et al. 2012).
In concert with its tissue specific function, expression of CCN2 in different tissues is controlled in a unique fashion. The CCN2 promoter is highly conserved between vertebrate species, especially within the first 5 kb - a region containing many important transcription factor binding sites. Comparing CCN2 promoter alignments of several vertebrate species to the human promoter using the ECR genome database, we found specific evolutionarily conserved regions (ECRs) (Fig. 3A) (Tran, et al. 2013). Transcription factor binding sites that lie within these ECRs are likely to be the most important in governing CCN2 transcription. Figure 3B is a schematic of the most proximal ECR to the transcription start site in the human CCN2 promoter that contains many of these binding sites. Within this region lie well characterized Smad-binding element (SBE), the basal control element (BCE-1), also known as the TGF-β response element (TβRE), and an Ets factor binding element (EBE). Also located within this region are IκB sites that may be responsible for regulation of CCN2 by stimuli such as inflammatory cytokines and mechanical stress (Chaqour, et al. 2006). Further upstream in another ECR is a putative hypoxia response element (HRE) that we have recently shown not to contribute to oxemic regulation of CCN2 in NP cells (Tran, et al. 2013). In the context of NP cells and other connective tissues, the transcriptional and post- transcriptional regulation of CCN2 by soluble and microenvironmental factors is discussed below.
Control of CCN2 expression by TGF-β
One of the most prominent factors that regulate CCN2 expression in many cells, including NP, is TGF-β (Chen, et al. 2002; Eguchi, et al. 2001; Tran, et al. 2010; Woods, et al. 2009; Van Beek, et al. 2006). In the canonical TGF-β signaling pathway, binding of TGF-β to type II and type I (ALK) receptors result in phosphorylation of Smad3 or Smad2. These R-Smads then dimerize with Smad4 and translocate to the nucleus where they bind to SBEs in the promoter of target genes. On the other hand, non-canonical signaling of TGF-β involves activation of the MAP-kinase pathway. Grotendorst et al. identified the first binding element found to be necessary for CCN2 induction by TGF-β in fibroblasts - the TβRE or BCE-1 to more accurately reflect its role in controlling basal CCN2 promoter activity (Grotendorst, et al. 1996). In the human CCN2 promoter, this element lies at −157/−145 bp with respect to the transcription start site, falling within the most proximal ECR (Fig. 3B). This regulatory element was later shown to be involved in basal and TGF-β responsive expression of CCN2 in the HCS-2/8 chondrosarcoma cells (Eguchi, et al. 2001) and for CCN2 induction by endothelin-1, a vasoconstrictor that is released by many other cell types besides endothelial cells. Additionally, this endothelin-1-CCN2 axis was shown to be involved in TGF-β-mediated matrix synthesis (Leask 2008; Shi-wen, et al. 2007; Xu, et al. 2004).
The major cis-acting element required for TGF-β mediated induction of CCN2 is the SBE at −173/−166 bp, necessary for TGF-β induction of CCN2 in several cell types, including the NP (Arnott, et al. 2008; Chen, et al. 2002; Leask, et al. 2001; Tran, et al. 2010; Xia, et al. 2007) (Fig. 3B). An elegant mutagenesis study performed in mesangial cells showed that the mutation of the BCE-1 site did not affect TGF-β-induced CCN2 promoter activity, while mutation of the SBE ablated TGF-β responsiveness of these cells (Chen, et al. 2002). It was noted that maximal induction of CCN2 by TGF-β in mesangial cells also required Ras, MEK and ERK signaling (Chen, et al. 2002). In NP cells, our work has shown that the binding of Smad3, but not Smad2, to SBE was necessary for promoter activation. In addition to the SBE, induction by TGF-β in NP cells also required the cis-acting AP-1 site at −294/−286 bp (Tran, et al. 2010). Similar to NP cells, serum responsive expression of CCN2 in keloid fibroblasts also involves this regulatory mechanism (Xia, et al. 2007).
Another motif involved in TGF-β induction of CCN2 is a GGAA/T sequence (Ets factor binding element, EBE). Ets-1 was shown to bind a specific EBE at −94/−90 bp and is required for the induction of CCN2 transcription by TGF-β in fibroblasts (Geisinger, et al. 2012; Van Beek, et al. 2006) (Fig. 3B). These studies suggested that Ets-1 and Smad3 cooperate in a synergistic manner to increase CCN2 transcription, supporting the notion that CCN2 induction by TGF-β involves more than just canonical Smad signaling. Interestingly, in the context of scleroderma, a disease characterized by excessive fibrosis and scarring, the characteristically high basal levels of CCN2 in fibroblasts is not dependent of the SBE but on the BCE-1, suggesting that elevated CCN2 expression in some pathological conditions could derive from disregulation of normal CCN2 transcription (Holmes, et al. 2001; Leask, et al. 2001).
That TGF-β strongly induces CCN2 in most tissues provides evidence that one of the major roles of CCN2 is to promote downstream effects of TGF-β. Supporting this notion are in vivo studies that showed that only the co-injection of TGF-β and CCN2 in newborn mice produces sustained skin fibrosis (Mori, et al. 1999). Nakerakanti et al. recently showed that CCN2 was necessary for the TGF-β-induced phosphorylation of Smad1 and ERK1/2, but not Smad3 (Nakerakanti, et al. 2011). In osteoblasts and NP cells, CCN2 treatment alone has also been shown to activate ERK signaling, which may contribute to sustaining TGF-β effects (Kawaki, et al. 2011; Tran, et al., unpublished result). Moreover, in lung epithelial cells, CCN2 was shown to inhibit BMP4-induced Smad1 phosphorylation while increasing TGF-β1-induced Smad2 phosphorylation, indicating the specificity for amplifying TGF-β signaling (Abreu, et al. 2002). This relationship also seems to hold true in chondrocytes, as a combined treatment of BMP and CCN2 resulted in less proliferation than either of the factors alone (Maeda, et al. 2009). Taken together this model of TGF-β-CCN2 signaling, wherein CCN2 perpetuates some of the TGF-β signals while TGF-β induces CCN2 expression explains their synergistic and complimentary effects.
Control of CCN2 expression by hypoxia
Relevant to the hypoxic niche of the intervertebral disc, low oxygen tension is shown to regulate CCN2 in several cell types (Higgins, et al. 2004; Hong, et al. 2006; Kondo, et al. 2006; Kroening, et al. 2009; Kroening, et al. 2010; Shimo, et al. 2001). Again, CCN2 regulation by hypoxia is cell type specific and complex, with hypoxia promoting production of CCN2 in most cells, while down regulating CCN2 expression in others. In the HCS-2/8 chondrosarcoma cells, Kondo et al. showed that CCN2 levels are regulated by hypoxia post-transcriptionally through the 84 bp cis-acting element of structure-anchored repression (CAESAR) in the 3′ UTR (Kondo, et al. 2006). Recently the same group showed that GAPDH is the binding protein responsible for hypoxic stabilization of CCN2 mRNA in HCS-2/8 cells (Fig. 3C) (Kondo, et al. 2011). Concerning transcriptional regulation of CCN2 by hypoxia, Shimo et al. was the first to report induction of CCN2 in the MDA-231 breast cancer cells and suggested that HIF-1α mediated the increase; although direct binding of HIF-1 to the promoter was not demonstrated (Shimo, et al. 2001). Reinforcing the cell type specific nature of CCN2 regulation, hypoxia, through HIF-1α, suppressed CCN2 transcription in proximal tubular epithelial cells, but again direct promoter regulation was not shown (Kroening, et al. 2009). Higgins et al. was the first to demonstrate that hypoxic induction of CCN2 in tubular epithelial cells required two HREs in the murine CCN2 promoter located at −1558/−1554 and −3745/−3741 bp (Higgins, et al. 2004). It was concluded that HIF-1α induces CCN2 transcription in response to hypoxia and that both of the HRE binding sites were necessary for promoter activation. Although the latter HRE lies within an ECR, these HREs are not conserved in location in the human CCN2 promoter, begging the question, are HREs in the human promoter functional?
Recently, we analyzed the proximal 5 kb of the human CCN2 promoter with an experimentally-validated HRE matrix using the JASPAR database (http://jaspar.genereg.net/) and found three putative HREs at −640/−634, −2010/−2006 and −2264/−2258 bp, of which, the −2010/−2006 bp site lies within an ECR. In NP cells, we observe that hypoxia decreases CCN2 transcription (Tran, et al. 2013). Interestingly, mutagenesis of putative HREs shows that the suppressive effect of hypoxia may not involve direct binding of HIF-1α to these sites and suggests a complex regulation of CCN2 by hypoxia and HIF-1α in NP cells (Tran, et al. 2013). This is not surprising given the unique stabilization of HIF-1α and a similar regulation of other HIF-1α target genes in the NP (Fujita, et al. 2011; Gogate, et al. 2012). Differential regulation of CCN2 by hypoxia and HIF-1α in NP cells may derive from the fact that unlike fibroblasts or tubular epithelial cells, hypoxia is a physiological condition for these cells. Moreover, TGF-β robustly induces CCN2 and is active in the disc from development to maturation. We have shown that TGF-β can still induce CCN2 expression under hypoxia, though the magnitude of induction is decreased, supporting the idea that hypoxic suppression of CCN2 in NP cells may serve to maintain tight control of CCN2 production (Tran, et al. 2013). The fact that the location of HREs in the CCN2 promoter are not conserved in vertebrate species suggest that regulation by hypoxia is not only cell type specific, but may also be species specific.
Interestingly, in many cell types there are reports of CCN2 controlling the expression and activity of HIF-1α. This regulatory feedback is also cell type specific with CCN2 increasing HIF activity in some cells while decreasing it in others. For example, in the HCS-2/8 chondrosarcoma cells, CCN2 induced HIF-1α mRNA and protein as well as elevated VEGF levels, suggesting positive feedback between these factors, which may be required for tumor survival and metastasis (Nishida, et al. 2009). Whereas, in lung adenocarcinoma cells, CCN2 was shown to promote HIF-1α degradation and strongly suppress VEGF levels (Chang, et al. 2006). In NP cells treatment with rCCN2 decreases HIF-1α transcriptional as well as transactivation domain (TAD) activity, suggesting that CCN2 may interfere with HIF-1α interaction with co-activators (Tran, et al. 2013). Supporting this possibility, CCN2 was shown in human mesangial cells to be internalized, phosphorylated by protein kinase C, and translocated to the nucleus where it may directly bind to the promoter or interact with other factors that regulate gene transcription (Wahab, et al. 2001).
CCN2 regulation by factors relevant to intervertebral disc physiology and pathophysiology
In chondrocytic and chondrosarcoma cells, CCN2 transcription is regulated by several factors in a more complex fashion than other cell types studied. In HCS-2/8 cells, Eguchi et al. showed that MMP3 can act as a transcriptional inducer through the TRENDIC binding element at −193/−182 bp (Eguchi, et al. 2008) (Fig. 3B). This finding was surprising as it revealed that a protein previously known only as a proteolytic enzyme has transcriptional activity. It also suggests that CCN2 is closely involved in maintaining matrix homeostasis, perhaps serving as a negative feedback on the matrix degradation cascade. Additionally, a recent study revealed that proliferative and hypertrophic growth plate chondrocytes, regulate CCN2 expression through distinct mechanisms mediated by Sox-9 and β-catenin, respectively (Huang, et al. 2010). Interestingly, during both of these stages of chondrocyte differentiation, CCN2 regulation occurs through the identical TCF/LEF motif that is conserved in both mouse and human. In proliferating chondrocytes, where Sox9 is an important transcription factor to maintain cell phenotype, it binds to the TCF/LEF motif and causes suppression of CCN2. As the chondrocyte differentiates into a hypertrophic phenotype, β-catenin binds to the same site and instead causes induction of CCN2. It is of interest to examine the biological relevance of the TCF/LEF motif in NP cells, as the regulation of matrix homeostasis in the disc is also regulated by Sox9 and β-catenin (Cheng, et al. 2009; Hiyama, et al. 2010; Lee, et al. 2008).
Summary and Future Perspectives
Over a decade after CCN2 was first discovered, much has been reported about its regulation, expression, and functions. More recently it has been shown that both its expression and function are regulated in a tissue and cell type specific manner. Relevant to discussion of the disc, CCN2 seems to modulate the effects of stimuli such as TGF-β and hypoxia. Mounting evidence suggests that the TGF-β-CCN2 axis is important for matrix production and that TGF-β regulates CCN2 expression, mainly through the SBE with the necessity of cell type specific co-activators such as AP-1 and Ets-1. Hypoxia is another important regulator of CCN2 that controls expression in a cell type and context dependent manner. While aberrant hypoxic induction of CCN2 in fibroblasts drives pathological tissue fibrosis; in the disc, where hypoxia is a physiological niche condition, hypoxic suppression of CCN2 may be required to maintain its physiological levels (Fig. 4). During degeneration, the oxemic status of the NP is thought to change due to blood vessel infiltration (Gruber, et al. 2005; Roberts, et al. 2006). It is thus plausible that the resultant decrease in HIF-1α activity and known increase in TGF-β contributes to the raised CCN2 levels in the degenerate discs. Further study of CCN2 effects in the degenerative disc and surrounding tissues are needed to determine its ultimate role in this process.
Fig. 4.
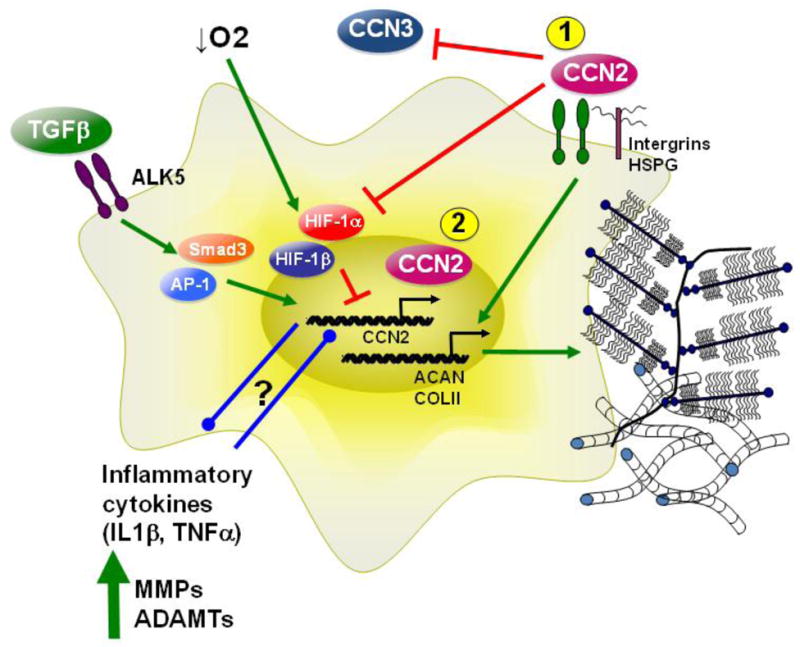
Schematic summarizing CCN2 regulation and function in NP cells by TGF-β and hypoxia- two major determinants of disc microenvironment. Upon binding of TGF-β to the TGF-β type II and type I receptor (ALK5), Smad3 is phosphorylated and binds along with Smad4 to SBEs on the CCN2 promoter to increase CCN2 levels. AP-1 binding to the CCN2 promoter is also required for promoter induction by TGF-β. In NP cells, hypoxia decreases CCN2 through a HIF-1α dependent mechanism. In the degenerate disc, an increase in inflammatory cytokines, such as IL-1β and TNF-α, results in increased expression of matrix degrading enzymes like MMPs and ADAMTs. The relationship between inflammatory cytokines, and CCN2 is an active area of interest. Known and possible functions of CCN2 in NP cells: 1) Secreted CCN2 signals through integrins and HSPGs on the cell surface to cause an increase in aggrecan and collagen type II expression. 2) Nuclear localization of CCN2 in NP cells and reports of CCN2 endocytosis in other cells raise the possibility of its role as a transcriptional co-activator/repressor.
Recent in vivo and in vitro studies have clearly shown the anabolic role of CCN2 in stimulating matrix production by NP cells (Tran, et al. 2010, 2013) and it may be possible to exploit this protein as part of a regenerative cocktail to deliver to the degenerate disc. In fact the delivery of CCN2 into the disc using an injectable hydrogel can be envisioned based on promising results seen in articular cartilage in an experimentally induced OA model (Nishida, et al. 2004). However, for this therapy to be successful for treating disc disease, it will be important to understand the relationship between CCN2 and inflammation in tissue (Fig. 4).
Highlights.
CCN2 is expressed during disc development and levels increase during disc disease
CCN2 is an important regulator of matrix synthesis by nucleus pulposus cells
CCN2 expression is sensitive to TGF-β and hypoxia in the nucleus pulposus
The relationship between CCN2 and HIF-1α in NP cells is unique
Acknowledgments
This study was supported by NIH grants AR050087, AR055655, F31AG038125 (CT)
Abbreviations
- CCN2
connective tissue growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott RD, Purmessur D, Monsey RD, Brigstock DR, Laudier DM, Iatridis JC. Degenerative grade affects the responses of human nucleus pulposus cells to Link-N, CTGF, and TGFβ3. J Spinal Disord Tech. 2012 Aug 18; doi: 10.1097/BSD.0b013e31826e0ca4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Guttapalli A, Narayan S, Albert TJ, Shapiro IM, Risbud MV. Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physio Cell Physiol. 2007;293:C621–631. doi: 10.1152/ajpcell.00538.2006. [DOI] [PubMed] [Google Scholar]
- Ali R, Le Maitre CL, Richardson SM, Hoyland JA, Freemont AJ. Connective tissue growth factor expression in human intervertebral disc: implications for angiogenesis in intervertebral disc degeneration. Biotech Histochem. 2008;83:239–245. doi: 10.1080/10520290802539186. [DOI] [PubMed] [Google Scholar]
- Aoyama E, Hattori T, Hoshijima M, Araki D, Nishida T, Kubota S, Takigawa M. N-terminal domains of CCN family 2/connective tissue growth factor bind to aggrecan. Biochem J. 2009;420:413–420. doi: 10.1042/BJ20081991. [DOI] [PubMed] [Google Scholar]
- Arnott JA, Zhang X, Sanjay A, Owen TA, Smock SL, Rehman S, DeLong WG, Safadi FF, Popoff SN. Molecular requirements for induction of CTGF expression by TGF-beta1 in primary osteoblasts. Bone. 2008;42:871–885. doi: 10.1016/j.bone.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney Davidson EN, Vitters EL, Mooren FM, Oliver N, Berg WBV, van der Kraan PM. Connective tissue growth factor/CCN2 overexpression in mouse synovial lining results in transient fibrosis and cartilage damage. Arthritis Rheum. 2006;54:1653–1661. doi: 10.1002/art.21795. [DOI] [PubMed] [Google Scholar]
- Chang C, Lin M, Lin B, Jeng Y, Chen S, Chu C, Chen RJ, Chang K, Yang P, Kuo M. Effect of connective tissue growth factor on hypoxia-inducible factor 1alpha degradation and tumor angiogenesis. J Natl Cancer Inst. 2006;98:984–995. doi: 10.1093/jnci/djj242. [DOI] [PubMed] [Google Scholar]
- Chaqour B, Yang R, Sha Q. Mechanical stretch modulates the promoter activity of the profibrotic factor CCN2 through increased actin polymerization and NF-kappaB activation. J Biol Chem. 2006;281:20608–20622. doi: 10.1074/jbc.M600214200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Blom IE, Sa S, Goldschmeding R, Abraham DJ, Leask A. CTGF expression in mesangial cells: involvement of SMADs, MAP kinase, and PKC. Kidney Int. 2002;62:1149–1159. doi: 10.1111/j.1523-1755.2002.kid567.x. [DOI] [PubMed] [Google Scholar]
- Cheng C, Uchiyama Y, Hiyama A, Gajghate S, Shapiro IM, Risbud MV. PI3K/AKT regulates aggrecan gene expression by modulating Sox9 expression and activity in nucleus pulposus cells of the intervertebral disc. J Cell Physiol. 2009;221:668–676. doi: 10.1002/jcp.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou M, Chao T, Wu J, Kuo C, Chen J. The physiological role of CTGF/CCN2 in zebrafish notochond development and biological analysis of the proximal promoter region. Biochem Biophys Res Commun. 2006;349:750–758. doi: 10.1016/j.bbrc.2006.08.095. [DOI] [PubMed] [Google Scholar]
- Choi KS, Cohn MJ, Harfe BD. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn. 2008;237:3953–3958. doi: 10.1002/dvdy.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicha I, Goppelt-Struebe M. Connective tissue growth factor: context-dependent functions and mechanisms of regulation. Biofactors. 2009;35:200–208. doi: 10.1002/biof.30. [DOI] [PubMed] [Google Scholar]
- Eguchi T, Kubota S, Kondo S, Shimo T, Hattori T, Nakanishi T, Kuboki T, Yatani H, Takigawa M. Regulatory mechanism of human connective tissue growth factor (CTGF/Hcs24) gene expression in a human chondrocytic cell line, HCS-2/8. J Biochem. 2001;130:79–87. doi: 10.1093/oxfordjournals.jbchem.a002965. [DOI] [PubMed] [Google Scholar]
- Eguchi T, Kubota S, Kawata K, Mukudai Y, Uehara J, Ohgawara T, Ibaragi S, Sasaki A, Kuboki T, Takigawa M. Novel transcription-factor-like function of human matrix metalloproteinase 3 regulating the CTGF/CCN2 gene. Mol Cell Biol. 2008;28:2391–2413. doi: 10.1128/MCB.01288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin WM, Ashman K, O’Donnel P, Inman RD. Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum. 2006;54:3859–3867. doi: 10.1002/art.22258. [DOI] [PubMed] [Google Scholar]
- Fernando CA, Conrad PA, Bartels CF, Marques T, To M, Balow SA, Nakamura Y, Warman ML. Temporal and spatial expression of CCN genes in zebrafish. Dev Dyn. 2010;239:1755–1767. doi: 10.1002/dvdy.22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Chiba K, Shapiro IM, Risbud MV. HIF-1α and HIF-2α degradation is differentially regulated in nucleus pulposus cells of the intervertebral disc. J Bone Miner Res. 2011;9999:1–12. doi: 10.1002/jbmr.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajghate S, Hiyama A, Shah M, Sakai D, Anderson DG, Shapiro IM, Risbud MV. Osmolarity and Intracellular Calcium Regulate Aquaporin2 Expression Through TonEBP in Nucleus Pulposus Cells of the Intervertebral Disc. J Bone Miner Res. 2009;24:992–1001. doi: 10.1359/JBMR.090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Brigstock DR. A novel integrin alpha5beta1 binding domain in module 4 of connective tissue growth factor (CCN2/CTGF) promotes adhesion and migration of activated pancreatic stellate cells. Gut. 2006;55:856–862. doi: 10.1136/gut.2005.079178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Brigstock DR. Low density lipoprotein receptor-related protein (LRP) is a heparin-dependent adhesion receptor for connective tissue growth factor (CTGF) in rat activated hepatic stellate cells. Hepatol Res. 2003;27:214–220. doi: 10.1016/s1386-6346(03)00241-9. [DOI] [PubMed] [Google Scholar]
- Gao R, Brigstock DR. Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C-terminal domain to integrin alpha(v)beta(3) and heparan sulfate proteoglycan. J Biol Chem. 2004;279:8848–8855. doi: 10.1074/jbc.M313204200. [DOI] [PubMed] [Google Scholar]
- Geisinger MT, Astaiza R, Butler T, Popoff SN, Planey SL, Arnott JA. Ets-1 is essential for connective tissue growth factor (CTGF/CCN2) induction by TGF-beta1 in osteoblasts. PLoS One. 2012;7:e35258. doi: 10.1371/journal.pone.0035258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogate SS, Fujita N, Skubutyte R, Shapiro IM, Risbud MV. Tonicity enhancer binding protein (TonEBP) and hypoxia-inducible factor (HIF) coordinate heat shock protein 70 (Hsp70) expression in hypoxic nucleus pulposus cells: role of Hsp70 in HIF-1alpha degradation. J Bone Miner Res. 2012;27:1106–1117. doi: 10.1002/jbmr.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotendorst GR. Growth Transforming Growth Factor J Response the Expression of the Connective Tissue Factor Gene1 Element. Cell Growth Differ. 1996;7:469–480. [PubMed] [Google Scholar]
- Gruber HE, Ashraf N, Kilburn J, Williams C, Norton HJ, Gordon BE, Hanley EN., Jr Vertebral endplate architecture and vascularization: application of micro-computerized tomography, a vascular tracer, and immunocytochemistry in analyses of disc degeneration in the aging sand rat. Spine (Phila Pa 1976) 2005;30:2593–2600. doi: 10.1097/01.brs.0000187877.30149.83. [DOI] [PubMed] [Google Scholar]
- Hall-Glenn F, Lyons KM. Roles for CCN2 in normal physiological processes. Cell Mole Life Sci. 2011;68:3209–3217. doi: 10.1007/s00018-011-0782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng EC, Huang Y, Black SA, Jr, Trackman PC. CCN2, connective tissue growth factor, stimulates collagen deposition by gingival fibroblasts via module 3 and alpha6- and beta1 integrins. J Cell Biochem. 2006;98:409–420. doi: 10.1002/jcb.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins DF, Biju MP, Akai Y, Wutz A, Johnson RS, Haase VH. Hypoxic induction of Ctgf is directly mediated by Hif-1. Am J Physiol Renal Physiol. 2004;287:F1223–1232. doi: 10.1152/ajprenal.00245.2004. [DOI] [PubMed] [Google Scholar]
- Hiyama A, Sakai D, Risbud MV, Tanaka M, Arai F, Abe K, Mochida J. Enhancement of intervertebral disc cell senescence by WNT/β-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 2010;62:3036–3047. doi: 10.1002/art.27599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbourn KP, Perbal B, Ravi Acharya K. Proteins on the catwalk: modelling the structural domains of the CCN family of proteins. J Cell Commun Signal. 2009;3:25–41. doi: 10.1007/s12079-009-0048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Abraham DJ, Sa S, Shiwen X, Black CM, Leask A. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem. 2001;276:10594–10601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- Hong K, Yoo S, Kang S, Choi J, Kim W, Cho C. Hypoxia induces expression of connective tissue growth factor in scleroderma skin fibroblasts. Clin Exp Immunol. 2006;146:362–370. doi: 10.1111/j.1365-2249.2006.03199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshijima M, Hattori T, Aoyama E, Nishida T, Yamashiro T, Takigawa M. Roles of heterotypic CCN2/CTGF-CCN3/NOV and homotypic CCN2-CCN2 interactions in expression of the differentiated phenotype of chondrocytes. FEBS J. 2012;279:3584–3597. doi: 10.1111/j.1742-4658.2012.08717.x. [DOI] [PubMed] [Google Scholar]
- Hoshijima M, Hattori T, Inoue M, Araki D, Hanagata H, Miyauchi A, Takigawa M. CT domain of CCN2/CTGF directly interacts with fibronectin and enhances cell adhesion of chondrocytes through integrin alpha5beta1. FEBS Lett. 2006;580:1376–1382. doi: 10.1016/j.febslet.2006.01.061. [DOI] [PubMed] [Google Scholar]
- Huang B, Brugger SM, Lyons KM. Stage-specific control of connective tissue growth factor (CTGF/CCN2) expression in chondrocytes by Sox9 and beta-catenin. T J Biol Chem. 2010;285:27702–27712. doi: 10.1074/jbc.M110.108498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, Ikeda E, Takata S, Kobayashi K, Okada Y. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2002;16:219–221. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaki H, Kubota S, Suzuki A, Yamada T, Matsumura T, Mandai T, Yao M, Maeda T, Lyons KM, Takigawa M. Functional requirement of CCN2 for intramembranous bone formation in embryonic mice. Biochem Biophys Res Commun. 2007;366:450–456. doi: 10.1016/j.bbrc.2007.11.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaki H, Kubota S, Suzuki A, Lazar N, Yamada T, Matsumura T, Ohgawara T, Maeda T, Perbal B, Lyons KM, Takigawa M. Cooperative regulation of chondrocyte differentiation by CCN2 and CCN3 shown by a comprehensive analysis of the CCN family of proteins in cartilage. J Bone Miner Res. 2008;23:1751–1764. doi: 10.1359/jbmr.080615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaki H, Kubota S, Suzuki A, Suzuki M, Kohsaka K, Hoshi K, Fujii T, Lazar N, Ohgawara T, Maeda T, Perbal B, Takano-Yamamoto T, Takigawa M. Differential roles of CCN family proteins during osteoblast differentiation: Involvement of Smad and MAPK signaling pathways. Bone. 2011;49:975–989. doi: 10.1016/j.bone.2011.06.033. [DOI] [PubMed] [Google Scholar]
- Kawata K, Kubota S, Eguchi T, Aoyama E, Moritani NH, Kondo S, Nishida T, Takigawa M. Role of LRP1 in transport of CCN2 protein in chondrocytes. J Cell Sci. 2012;125:2965–2972. doi: 10.1242/jcs.101956. [DOI] [PubMed] [Google Scholar]
- Kondo S, Kubota S, Mukudai Y, Moritani N, Nishida T, Matsushita H, Matsumoto S, Sugahara T, Takigawa M. Hypoxic regulation of stability of connective tissue growth factor/CCN2 mRNA by 3′-untranslated region interacting with a cellular protein in human chondrosarcoma cells. Oncogene. 2006;25:1099–1110. doi: 10.1038/sj.onc.1209129. [DOI] [PubMed] [Google Scholar]
- Kondo S, Kubota S, Mukudai Y, Nishida T, Yoshihama Y, Shirota T, Shintani S, Takigawa M. Binding of glyceraldehyde-3-phosphate dehydrogenase to the cis-acting element of structure-anchored repression in ccn2 mRNA. Biochem Biophys Res Commun. 2011;405:382–387. doi: 10.1016/j.bbrc.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Kroening S, Neubauer E, Wessel J, Wiesener M, Goppelt-Struebe M. Hypoxia interferes with connective tissue growth factor (CTGF) gene expression in human proximal tubular cell lines. Nephrol Dial Transplant. 2009;24:3319–3325. doi: 10.1093/ndt/gfp305. [DOI] [PubMed] [Google Scholar]
- Kroening S, Neubauer E, Wullich B, Aten J, Goppelt-Struebe M. Characterization of connective tissue growth factor expression in primary cultures of human tubular epithelial cells: modulation by hypoxia. Am J Physiol Renal Physiol. 2010;298:F796–806. doi: 10.1152/ajprenal.00528.2009. [DOI] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. Role of CCN2/CTGF/Hcs24 in bone growth. Int Rev Cytol. 2007;10:1–11. doi: 10.1016/S0074-7696(07)57001-4. [DOI] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. CCN family proteins and angiogenesis: from embryo to adulthood. Angiogenesis. 2007;10:1–11. doi: 10.1007/s10456-006-9058-5. [DOI] [PubMed] [Google Scholar]
- Kubota S, Takigawa M. The role of CCN2 in cartilage and bone development. J Cell Commun Signal. 2011;5:209–217. doi: 10.1007/s12079-011-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A, Sa S, Holmes A, Shiwen X, Black CM, Abraham DJ. The control of ccn2 (ctgf) gene expression in normal and scleroderma fibroblasts. Mol Pathol. 2001;54:180–183. doi: 10.1136/mp.54.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A. Targeting the TGFbeta, endothelin-1 and CCN2 axis to combat fibrosis in scleroderma. Cell Signal. 2008;20:1409–1414. doi: 10.1016/j.cellsig.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kong MH, Song KY, Lee KH, Heo SH. The Relation Between Sox9, TGF-beta1, and Proteoglycan in Human Intervertebral Disc Cells. J Korean Neurosurg Soc. 2008;43:149–154. doi: 10.3340/jkns.2008.43.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Abraham DJ, Leask A. CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum. 2011;63:239–246. doi: 10.1002/art.30074. [DOI] [PubMed] [Google Scholar]
- Maeda A, Nishida T, Aoyama E, Kubota S, Lyons KM, Kuboki T, Takigawa M. CCN family 2/connective tissue growth factor modulates BMP signalling as a signal conductor, which action regulates the proliferation and differentiation of chondrocytes. J Biochem. 2009;145:207–216. doi: 10.1093/jb/mvn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuko K, Murata M, Yudoh K, Shimizu H, Beppu M, Nakamura H, Kato T. Prostaglandin E2 regulates the expression of connective tissue growth factor (CTGF/CCN2) in human osteoarthritic chondrocytes via the EP4 receptor. BMC Res Notes. 2010;3:5. doi: 10.1186/1756-0500-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signaling and interacts with the WNT receptor complex. Development. 2004;131:2137–2147. doi: 10.1242/dev.01045. [DOI] [PubMed] [Google Scholar]
- Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, Takigawa M, Nakanishi T, Takehara K. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: A mouse fibrosis model. J Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Nakerakanti SS, Bujor AM, Trojanowska M. CCN2 is required for the TGF-β induced activation of Smad1-Erk1/2 signaling network. PLoS one. 2011;6:e21911–e21911. doi: 10.1371/journal.pone.0021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T, Kondo S, Maeda A, Kubota S, Lyons KM, Takigawa M. CCN family 2/connective tissue growth factor (CCN2/CTGF) regulates the expression of Vegf through Hif-1alpha expression in a chondrocytic cell line, HCS-2/8, under hypoxic condition. Bone. 2009;44:24–31. doi: 10.1016/j.bone.2008.08.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T, Kawaki H, Baxter RM, Deyoung RA, Takigawa M, Lyons KM. CCN2 (Connective Tissue Growth Factor) is essential for extracellular matrix production and integrin signaling in chondrocytes. J Cell Commun Signal. 2007;1:45–58. doi: 10.1007/s12079-007-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T, Kubota S, Kojima S, Kuboki T, Nakao K, Kushibiki T, Tabata Y, Takigawa M. Regeneration of defects in articular cartilage in rat knee joints by CCN2 (connective tissue growth factor) J Bone Miner Res. 2004;19:1308–1319. doi: 10.1359/JBMR.040322. [DOI] [PubMed] [Google Scholar]
- Peng B, Chen J, Kuang Z, Li D, Pang X, Zhang X. Expression and role of connective tissue growth factor in painful disc fibrosis and degeneration. Spine. 2009;34:E178–182. doi: 10.1097/BRS.0b013e3181908ab3. [DOI] [PubMed] [Google Scholar]
- Ponticos M, Holmes AM, Shi-wen X, Leoni P, Khan K, Rajkumar VS, Hoyles RK, Bou-Gharios G, Black CM, Denton CP, Abraham DJ, Leask A, Lindahl GE. Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen. Arthritis Rheum. 2009;60:2142–2155. doi: 10.1002/art.24620. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Guttapalli A, Stokes DG, Hawkins D, Danielson KG, Schaer TP, Albert TJ, Shapiro IM. Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98:152–159. doi: 10.1002/jcb.20765. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Schaer TP, Shapiro IM. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. Dev Dyn. 2010;239:2141–2148. doi: 10.1002/dvdy.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbud MV, Guttapalli A, Tsai T, Lee JY, Danielson KG, Vaccaro AR, Albert TJ, Gazit Z, Gazit D, Shapiro IM. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine. 2007;32:2537–2544. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Schipani E, Shapiro IM. Hypoxic regulation of nucleus pulposus cell survival: from niche to notch. Am J Pathol. 2010;176:1577–1583. doi: 10.2353/ajpath.2010.090734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10–14. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- Sakai D, Nakamura Y, Nakai T, Mishima T, Kato S, Grad S, Alini M, Risbud MV, Chan D, Cheah KS, Yamamura K, Masuda K, Okano H, Ando K, Mochida J. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro IM, Vresilovic EJ, Risbud MV. Is the spinal motion segment a diarthrodial polyaxial joint: what a nice nucleus like you doing in a joint like this? Bone. 2012;50:771–776. doi: 10.1016/j.bone.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimo T, Kubota S, Kondo S, Nakanishi T, Sasaki a, Mese H, Matsumura T, Takigawa M. Connective tissue growth factor as a major angiogenic agent that is induced by hypoxia in a human breast cancer cell line. Cancer Lett. 2001;174:57–64. doi: 10.1016/s0304-3835(01)00683-8. [DOI] [PubMed] [Google Scholar]
- Shimo T, Kanyama M, Wu C, Sugito H, Billings PC, Abrams WR, Rosenbloom J, Iwamoto M, Pacifici M, Koyama E. Expression and roles of connective tissue growth factor in Meckel’s cartilage development. Dev Dyn. 2004;231:136–147. doi: 10.1002/dvdy.20109. [DOI] [PubMed] [Google Scholar]
- Shimo T, Koyama E, Sugito H, Wu C, Shimo S, Pacifici M. Retinoid signaling regulates CTGF expression in hypertrophic chondrocytes with differential involvement of MAP kinases. J Bone Miner Res. 2005;20:867–877. doi: 10.1359/JBMR.041235. [DOI] [PubMed] [Google Scholar]
- Shi-wen X, Kennedy L, Renzoni Ea, Bou-Gharios G, du Bois RM, Black CM, Denton CP, Abraham DJ, Leask A. Endothelin is a downstream mediator of profibrotic responses to transforming growth factor beta in human lung fibroblasts. Arthritis Rheum. 2007;56:4189–4194. doi: 10.1002/art.23134. [DOI] [PubMed] [Google Scholar]
- Sivan SS, Tsitron E, Wachtel E, Roughley PJ, Sakkee N, van der Ham F, DeGroot J, Roberts S, Maroudas A. Aggrecan turnover in human intervertebral disc as determined by the racemization of aspartic acid. J Biol Chem. 2006;281:13009–13014. doi: 10.1074/jbc.M600296200. [DOI] [PubMed] [Google Scholar]
- Sonnylal S, Leoni P, Naff K, Van Pelt CS, Nakamura H, Leask A, Abraham D, Bou-Gharios GdCB. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010;62:1523–1532. doi: 10.1002/art.27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–2512. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- Tong Z, Chen R, Alt DS, Kemper S, Perbal B, Brigstock DR. Susceptibility to liver fibrosis in mice expressing a connective tissue growth factor transgene in hepatocytes. Hepatology. 2009;50:939–947. doi: 10.1002/hep.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong ZY, Brigstock DR. Intrinsic biological activity of the thrombospondin structural homology repeat in connective tissue growth factor. J Endocrinol. 2006;188:R1–8. doi: 10.1677/joe.1.06719. [DOI] [PubMed] [Google Scholar]
- Tran CM, Markova D, Smith HE, Susarla B, Ponnappan RK, Anderson DG, Symes A, Shapiro IM, Risbud MV. Regulation of CCN2/CTGF expression in the nucleus pulposus of the intervertebral disc: Role of smad and AP1 signaling. Arthritis Rheum. 2010;62:1983–1992. doi: 10.1002/art.27445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran CM, Smith HE, Symes A, Rittie L, Perbal B, Shapiro IM, Risbud MV. Transforming growth factor beta controls CCN3 expression in nucleus pulposus cells of the intervertebral disc. Arthritis Rheum. 2011;63:3022–3031. doi: 10.1002/art.30468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran CM, Fujita N, Huang BL, Ong JR, Lyons KM, Shapiro IM, Risbud MV. HIF-1α and CCN2 form a Regulatory Circuit in Hypoxic Nucleus Pulposus Cells: CCN2 Suppresses HIF-1α Level and Transcriptional Activity. J Biol Chem. 2013 Mar 24; doi: 10.1074/jbc.M112.448860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T, Guttapalli A, Agrawal A, Albert TJ, Shapiro IM, Risbud MV. MEK/ERK Signaling Controls Osmoregulation of Nucleus Pulposus Cells of the Intervertebral Disc by Transactivation of TonEBP/OREBP. J Bone Miner Res. 2007;22:965–974. doi: 10.1359/jbmr.070322. [DOI] [PubMed] [Google Scholar]
- Van Beek JP, Kennedy L, Rockel JS, Bernier SM, Leask A. The induction of CCN2 by TGFbeta1 involves Ets-1. Arthritis Res Ther. 2006;8:R36. doi: 10.1186/ar1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab NA, Brinkman H, Mason RM. Uptake and intracellular transport of the connective tissue growth factor: a potential mode of action. Biochem J. 2001;359:89–97. doi: 10.1042/0264-6021:3590089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Pala D, Kennedy L, McLean S, Rockel JS, Wang G, Leask A, Beier F. Rac1 signaling regulates CTGF/CCN2 gene expression via TGFbeta/Smad signaling in chondrocytes. Osteoarthr Cartil. 2009;17:406–413. doi: 10.1016/j.joca.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Xia W, Kong W, Wang Z, Phan T, Lim IJ, Longaker MT, Yang GP. Increased CCN2 transcription in keloid fibroblasts requires cooperativity between AP-1 and SMAD binding sites. Ann Surg. 2007;246:886–895. doi: 10.1097/SLA.0b013e318070d54f. [DOI] [PubMed] [Google Scholar]
- Xu S, Howat SL, Renzoni Ea, Holmes A, Pearson JD, Dashwood MR, Bou-Gharios G, Denton CP, du Bois RM, Black CM, Leask A, Abraham DJ. Endothelin-1 induces expression of matrix-associated genes in lung fibroblasts through MEK/ERK. J Biol Chem. 2004;279:23098–23103. doi: 10.1074/jbc.M311430200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Arnott JA, Rehman S, Delong WG, Sanjay A, Safadi FF, Popoff SN. Src is a major signaling component for CTGF induction by TGF-beta1 in osteoblasts. J Cell Physiol. 2010;224:691–701. doi: 10.1002/jcp.22173. [DOI] [PMC free article] [PubMed] [Google Scholar]