Abstract
Objective
It is unclear why despite a comparable cardiometabolic risk profile, “metabolically benign” overweight/obese individuals show an elevated risk of cardiovascular disease compared to normal weight individuals.
Design and Methods
In cross-sectional analyses, we compared levels of ectopic fat (epicardial, pericardial, and hepatic fat) and adipokines (leptin, soluble leptin receptor, and high molecular weight [HMW] adiponectin) among metabolically benign (MBOO) and at-risk overweight/obese (AROO), and metabolically benign normal weight (MBNW) women, screened for the Kronos Early Estrogen Prevention Study. We defined “metabolically benign” with ≤ 1, and “at-risk” with ≥2 components of the metabolic syndrome.
Results
Compared to MBOO women, AROO women had significantly elevated odds of being in the top tertile of epicardial fat (OR:1.76, 95%CI:1.04–2.99), hepatic fat (OR:1.90, 95%CI:1.12–3.24) and leptin (OR:2.15, 95%CI:1.23–3.76), and the bottom tertile of HMW-adiponectin (OR:2.90, 95%CI:1.62–5.19). Compared to MBNW women, MBOO women had significantly higher odds of being in the top tertile of epicardial fat (OR:5.17, 95%CI:3.22–8.29), pericardial fat (OR:9.27, 95%CI:5.52–15.56) and hepatic fat (OR:2.72, 95%CI:1.77–4.19) and the bottom tertile of HMW adiponectin levels (OR:2.51, 95%CI:1.60–3.94).
Conclusions
Levels of ectopic fat and the adverse adipokine profile increase on a continuum of BMI, suggesting that the metabolically benign phenotype may be a transient state.
Keywords: Metabolically benign overweight/obesity, ectopic fat, adipokines
INTRODUCTION
Although obesity is a well-known risk factor for the development of cardiovascular disease (CVD),(1) the heterogeneity in CVD risk among obese individuals has led to the description of a metabolically benign obese phenotype that fulfills the criteria of clinical obesity by BMI or waist circumference, but has a lower burden of adiposity-associated cardiometabolic risk factors (typically fewer components of the metabolic syndrome, or alternatively, lower insulin resistance levels depending on the publication) found among those with the at-risk obese phenotype.(2–4) Initial longitudinal studies ranging in length of follow-up from 3–11 years reported a similar prevalence of CVD events between metabolically benign obese and normal weight individuals, in contrast to the elevated CVD risk found in at-risk obese individuals.(2, 5, 6) However, recent longitudinal studies extending follow-up out to 16 years or longer suggest that metabolically benign individuals are still at an increased risk of subclinical and clinical CVD compared to their normal weight peers.(7, 8) Thus it is possible that the metabolically benign obese phenotype represents a transient state, where individuals ultimately have an increased risk of incident CVD compared to normal weight individuals, but experience a delay in the onset of clinical CVD compared to their at-risk obese counterparts. The mechanisms contributing to a lower cardiometabolic burden and resulting delayed CVD risk remain largely unknown.
While several studies suggest a potential inverse link between central adiposity and the cardiometabolic health of obese individuals, (4, 9) the contribution of ectopic fat, located in lean tissues such as the heart and the liver, to cardiometabolic and CVD risk profile of the metabolically benign phenotype is unclear. Recent evidence suggests that lower levels of hepatic fat may distinguish metabolically benign from at-risk obesity.(10, 11) It remains unclear whether a similar association also exists for epicardial and pericardial fat, and how ectopic fat levels among metabolically benign obese compare to those of healthy lean individuals.
Adipose-tissue derived hormones may also play a role in both in the etiology of metabolically benign obesity and its associated CVD burden. Among individuals with similar BMI levels, adipokine expression is variable.(12, 13) The few studies that have compared the adipokine profile between at-risk and metabolically benign overweight/obese individuals show more favorable levels of adipokines in the metabolically benign group.(12) However, limited literature exists comparing the adipokine profile of metabolically benign obese and normal weight individuals. Our recent study among postmenopausal women in the Women’s Health Initiative showed that adipokine levels in metabolically benign obese were intermediate between at-risk obese and metabolically benign normal weight women. (14)
The purpose of this study was to examine if differences exist in ectopic fat levels (epicardial, pericardial, and intrahepatic fat) and adipose tissue hormones (leptin, soluble leptin receptor, and HMW adiponectin) among recently postmenopausal metabolically benign overweight/obese women, and both at-risk overweight/obese women and normal weight women screened for the Kronos Early Estrogen Prevention Study. In concordance with their intermediate CVD event rates, we hypothesized that metabolically benign overweight/obese women would demonstrate intermediate levels of ectopic fat and adipose tissue hormones, lower than the at-risk overweight/obese group, but higher than normal weight women.
MATERIALS AND METHODS
Study Design and Population
A cross-sectional analysis was performed using data from the screening and baseline visits of the Kronos Early Estrogen Prevention Study (KEEPS), a randomized, placebo-controlled, double-blind prospective trial designed to evaluate the effects of menopausal hormone treatment when initiated early in menopause on progression of subclinical atherosclerosis as defined by carotid artery intima–media thickness (c-IMT) and coronary arterial calcification (CAC). Recruitment and study procedures have been previously described.(15) In brief, a total of 1,046 women were screened between July 2005 and June 2008, with 775 of them passing earlier screening components and eventually receiving a CT scan for the purposes of confirming CAC Agatston score eligibility (women with CAC score ≥50 AU were not eligible for the study). Of those, 728 women, aged 42–58 years at baseline, 6–36 months from the last menses, with FSH values ≥35 ng/ml, estradiol levels <35 pg/ml, and in good general health were eventually enrolled into the study and subsequently randomized to one of three treatment groups: oral conjugated equine estrogen (Premarin, 0.45mg/day) with oral micronized progesterone (12days per month), transdermal 17β-estradiol (via skin patch, Climara, 50μg/day) with oral micronized progesterone (12 days per month), and one daily placebo group (inactive pill/patch) with placebo micronized progesterone (12 days per month). Women with a history of clinical CVD (myocardial infarction, angina, congestive heart failure, or thromboembolic disease) and women with a hysterectomy were excluded. Of the 775 women receiving a screening CT scan, 90 could not be included due to insufficient visible liver or spleen tissue for hepatic fat measurement, or missing cardiac fat volume measurement, leaving 685 women from the baseline and screening KEEPS visits with valid CT scans available for the current analyses.
Other reasons for exclusion from the current analyses were missing values for body mass index (n=21), BMI in the underweight range (BMI<18.5 kg/m2; n=10), and missing data on key covariates (n=2), leaving 267 normal weight (BMI: 18.5–24.9 kg/m2) and 385 overweight and obese women (BMI ≥25 kg/m2). Informed consent and appropriate institutional review board approval were obtained from all participants.
Assessment of Anthropometric and Lifestyle Exposures
Baseline education (<college graduate, ≥college graduate), smoking status (current use yes/no), alcohol intake (current use yes/no), race-ethnicity (Caucasian or other), and levels of physical activity (Metabolic Equivalents, or METs, calculated as total energy expenditure from recreational physical activity in kcal/week/kg, with the questionnaire intentionally worded without reference to a specific time frame such as “last month” or “last year” to collect the “usual” patterns of physical activity) were obtained through self-reported questionnaire data. Diabetes was defined as a fasting glucose ≥126 mg/dL (7.0 mmol/L). The height (cm) and weight (kg) of participants, wearing light clothing and without shoes, was measured with a stadiometer and a calibrated balance beam scale, respectively. BMI was calculated as weight in kilograms divided by the square of height in meters. Waist circumference (cm) was measured with a non-stretchable tape after a normal expiration, at the smallest horizontal circumference between the ribs and iliac crest. Blood pressure was measured on the right arm in the seated position, after at least a 5 minute rest, and averaged across two readings.
Laboratory Measurements
All blood specimens were collected following an overnight fast, then frozen at −70°C on site until they were either utilized for lipid and glucose assays (performed locally at the clinical laboratory at each recruitment center), or sent to the Kronos Science Laboratory (Phoenix, AZ, USA) for storage or assays. C-reactive protein (CRP) and insulin were assayed by DPC Immulite 2000 (Diagnostic Products Corporation, Los Angeles, CA) at the Kronos Science Laboratories.
Leptin and soluble leptin receptor were both assayed using a sandwich ELISA (the enzyme-linked immunosorbent assay, R&D Systems, Minneapolis, MN, USA) at the Kronos Science Laboratories. The intra- and inter-assay coefficients of variations (CVs) for leptin were 1.4% and 6.2%, respectively; for soluble leptin receptor, the intra- and inter-assay CVs ranged from 6.8% to 7.4%, and 8.6% to 11.7%, respectively. High molecular weight (HMW) adiponectin was quantified using a sandwich ELISA (Linco Research, St. Charles, MO, USA), with the intra-assay and inter-assay CVs ranging from 5.0% to 6.8%, and from 12.7% to 13.5%, respectively.
Ectopic Fat Measures
Epicardial, pericardial and liver fat measurements were all performed on the same non-contrast enhanced computer tomography (CT) axial scans, which were acquired either via beam CT using C150XP or C300 electron beam tomography scanners (GE/Imatron, Inc.), or via electron multidetector CT using the General Electric helical scanner or a Siemens multi-slice scanner (minimum requirement 4 detector heads). Comparability among centers was assured by regular calibration using exchange of a standard phantom. All imaging results were read centrally by experienced readers who were blinded to participant demographics.
Hepatic attenuation values were measured using regions of interest (ROI) greater than 100 mm2 in area. As described previously,(16) one ROI was placed in the right liver lobe and one ROI in the left liver lobe. This was done on 3 consecutive slices for each region, and the average value was calculated. Whenever possible, ROIs with larger areas were used so that a greater area of the liver was included, but the regions of non-uniform parenchymal attenuation, such as hepatic vessels, were excluded. Since fat is less dense than surrounding tissues, lower levels of liver attenuation are indicative of more fat.
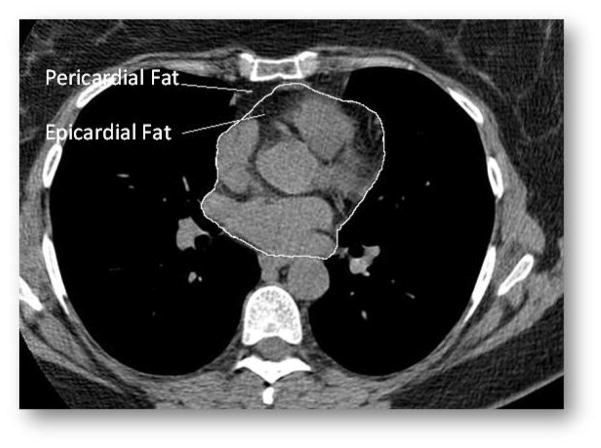
Epicardial adipose tissue was defined as fat tissue inside the pericardial sac, whereas pericardial adipose tissue was defined as fat tissue outside the pericardial sac (Figure 1), using methods similar to a previously published study.(17) Both cardiac fat measurements were performed using CT images on axial data sets from 10mm above to 30mm below the superior extent of the left main coronary artery, the heart region that includes epicardial adipose tissue located around the proximal coronary arteries (left main coronary, left anterior descending coronary, left circumflex coronary, right coronary arteries). Epicardial fat was measured by manually tracing out the pericardium every 2 to 3 slices below the start point, and then using the software to automatically trace out the segments in between these selected slices. For the total thoracic fat measurement (epicardial and pericardial fat combined), the anterior border of the volume was defined by the chest wall and posterior border by the aorta and the bronchi. Adipose tissue present in the posterior mediastinum and para-aortic adipose tissue was not included. Pericardial fat was calculated by subtracting epicardial fat from total thoracic fat volume. A threshold of −190 to −30 Hounsfield Units (HU) was used to discern fat from other tissues. Both epicardial and pericardial fat measures are volumes, with higher values indicating more fat.
Figure 1.
Epicardial and Pericardial Fat Depots
Reproducibility measurements of hepatic attenuation and cardiac fat volume measurements were performed on 20 randomly selected scans by two readers. For intra-reader reproducibility, there were 18 scans available. Both Spearman and intra-class correlation coefficients between repeated readings of epicardial and pericardial fat and hepatic attenuation were high (0.99 each for epicardial fat; 0.86 and 0.96, respectively, for PAT; and 0.99 each for hepatic attenuation). For inter-reader reproducibility, all 20 scans were available, and again both Spearman and intra-class correlation coefficients between repeated readings of epicardial and pericardial fat and hepatic attenuation were high (0.98 each for epicardial fat; 0.96 and 0.90, respectively for pericardial fat; and 0.95 and 0.98 respectively for hepatic attenuation). Further details of reproducibility measures have been published elsewhere. (18)
Metabolically Benign and At-Risk Phenotype Definitions
Women were categorized as normal weight (BMI<25 kg/m2) or overweight/obese (BMI≥25 kg/m2). For the primary analyses, to define the metabolically benign and at-risk phenotypes, we used four components of the metabolic syndrome, as defined by the Adult Treatment Panel-III (ATP-III). Specifically, overweight/obese women (BMI≥25.0 kg/m2) were classified as metabolically benign if they had no more than 1 of the 4 ATP-III components (excluding the waist circumference criterion due to collinearity with BMI): (1) elevated blood pressure (systolic/diastolic BP ≥130/85 mmHg), (2) elevated TG (≥150 mg/dL), (3) low HDL cholesterol (<50 mg/dL), and (4) elevated fasting glucose (≥100 mg/dL).(19) Overweight/obese women who did not meet the criteria for the metabolically benign phenotype were classified as at-risk. Additionally, normal weight women (BMI <25 kg/m2)were classified as metabolically benign if they had no more than 1of the 4 ATP-III metabolic syndrome factors. Normal weight women who had 2 or more ATP-III components were excluded from these analyses (n=16).
Statistical Methods
Demographics, health history, and laboratory values in metabolically benign overweight/obese women were compared to those in at-risk overweight/obese women, and then to those in metabolically benign normal weight women, respectively, using the t-test for continuous data (or non-parametric alternative if assumptions were not met), and the chi-square statistic for categorical data. Median levels of epicardial and pericardial fat volumes, hepatic fat attenuation values, as well as median levels of adipokines in metabolically benign overweight/obese women were compared to those in at-risk overweight/obese and to those in metabolically benign normal weight women, using the Mann-Whitney rank sum test. For each fat depot and each adipokine, odds ratios (ORs) with 95% confidence intervals (CI) of being in the top tertile of epicardial fat, pericardial fat, and leptin, and bottom tertile of hepatic fat attenuation score, soluble leptin receptor, and HMW adiponectin associated with the metabolically benign overweight/obese phenotype vs. (1) the at-risk overweight/obese phenotype, and (2) the metabolically benign normal weight phenotype, were calculated using multivariate logistic regression modeling, adjusted for age, race-ethnicity, physical activity, study site, smoking, and alcohol intake. All regression models were then additionally adjusted for BMI (Model 2); the regression models comparing metabolically benign overweight/obese and at-risk overweight/obese phenotypes were also further adjusted for waist circumference (Model 3). A separate model was built for each fat depot and each adipokine. Tertile cutoffs were calculated from the whole sample of participants.
Sensitivity analyses included: (1) using an alternative definition of the metabolically benign phenotype, which required presence of ≤ 2 of the metabolic abnormalities stated in the ATP-III criteria, as seen in previous literature,(20) (n=349) (2) excluding diabetic women (n=2), (3) restricting analyses to obese women only (BMI ≥30 kg/m2) (n=149), (4) restricting analyses to women who had all three ectopic fat measures (n=642), and (5) restricting analyses to only those women who passed the initial screening and were enrolled in KEEPS and subsequently randomized (n=567). All statistical analyses were performed using STATA version 10 (Stata Corp, College Station, TX). Two-tailed values of p<0.05 were considered statistically significant.
RESULTS
Baseline Characteristics
In our sample, 296 out of 385 overweight/obese women (76.9%), and 251 out of 267 normal weight women (94.0%) were classified as metabolically benign. Baseline characteristics of study participants are presented in Table 1. In general, CVD risk factors (plasma lipids, blood pressure, and fasting blood glucose, smoking status and CRP) increased across the three categories while reported levels of physical activity decreased.
Table 1.
Baseline Characteristics of Normal Weight and Overweight/Obese Women
| Metabolically Benign Normal Weight (n = 251) | Metabolically Benign Overweight/Obese (n = 296) | At-Risk Overweight/Obese (n = 89) | |
|---|---|---|---|
|
|
|||
| Age, yrs | 52.9 ± 2.6 | 53.0 ± 2.5 | 53.0 ± 2.7 |
| Race-Ethnicity, % Caucasian | 82.3 | 78.3 | 74.4 |
| Current Smoking, % | 3.6 | 8.5 1 | 11.2 |
| Education, % ≥ College graduate | 74.0 | 70.2 | 64.0 |
| Systolic Blood Pressure, mmHg | 114.5 ± 15.6 | 118.4 ± 13.5 1,2 | 130.0 ± 13.7 |
| Diastolic Blood Pressure, mmHg | 72.6 ± 8.7 | 75.4 ± 8.8 1,2 | 80.1 ± 93 |
| Elevated Blood Pressure, % | 14.3 | 22.6 1,2 | 65.2 |
| Total Cholesterol, mg/dL | 215.6 ± 32.3 | 214.7 ± 31.5 | 215.8 ± 33.5 |
| HDL Cholesterol, mg/dL | 71.4 ± 16.0 | 64.5 ± 16.0 1,2 | 46.4 ± 9.0 |
| LDL Cholesterol, mg/dL | 124.8 ± 29.7 | 129.5 ± 27.6 1 | 134.2 ± 29.2 |
| Low HDL Cholesterol, % | 5.2 | 13.5 1,2 | 77.0 |
| Triglycerides, mg/dL | 70.6 ± 26.6 | 85.5 ± 36.31,2 | 165.2 ± 69.4 |
| Elevated Triglycerides, % | 1.2 | 4.1 1,2 | 59.8 |
| Glucose, mg/dL | 85.8 ± 8.7 | 89.5 ± 9.2 1,2 | 97.5 ± 10.6 |
| Elevated Glucose, % | 3.6 | 7.4 2 | 46.1 |
| Insulin, μU/mLa | 4.2 (2.9) | 5.8 (5.3) 1,2 | 9.2 (6.7) |
| BMI, kg/m2 | 22.3 ± 1.7 | 29.0 ± 2.9 1,2 | 30.6 ± 2.9 |
| Obese, n (%) BMI > 30 | 99 (33.4) | 50 (56) | |
| Waist Circumference, cm | 78.1 ± 19.7 | 92.4 ± 21.6 1,2 | 99.2 ± 25.1 |
| C-Reactive Protein, mg/dLa | 0.6 (0.9) | 1.7 (2.8) † 1 | 2.7 (3.7) |
| Physical Activity, METs per week | 25.6 ± 20.7 | 20.4 ± 19.2 1 | 19.3 ± 15.4 |
| HMW Adiponectina | 8.1 (5.6) | 6.02 (4.76)1,2 | 4.55 (3.39) |
| Leptina | 11.93 (10.65) | 27 (20.38)1,2 | 33.4 (21.9) |
| Soluble Leptin Receptora | 38.33 (10.89) | 30.98 (9.64)1,2 | 28.33 (9.46) |
Data are mean ± SD (for continuous variables) and percentages (for categorical variables).
Median (interquartile range).
P <0.05 vs. metabolically benign normal weight;
P <0.05 vs. at-risk overweight/obese
Metabolically Benign Phenotype Definition: ≤1 of: elevated blood pressure (≥130/85 mmHg), elevated TG (≥150 mg/dL), elevated fasting glucose (≥100 mg/dL), low HDL cholesterol (<50 mg/dL).
Ectopic Fat and Adipokine Distribution
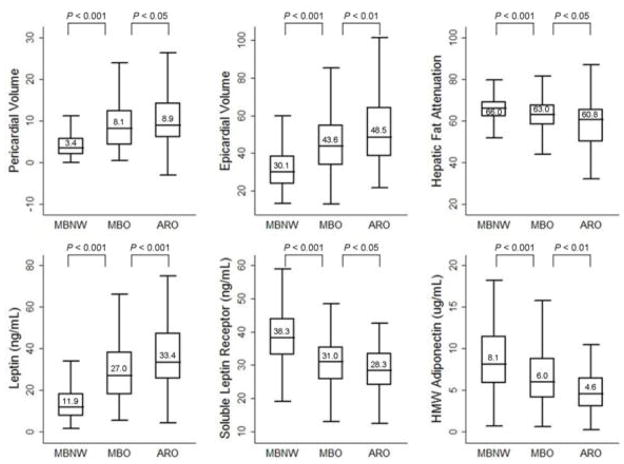
Overall, metabolically benign overweight/obese women presented with intermediate levels of epicardial, pericardial, and hepatic fat levels, evidenced by lower levels of pericardial fat and epicardial fat volumes, and lower levels of hepatic fat (evidenced by higher hepatic attenuation scores) compared to at-risk overweight/obese counterparts, but higher levels of ectopic fat depots compared to metabolically benign normal weight women (Figure 2). Likewise, the levels of leptin, soluble leptin receptor, and HMW adiponectin were intermediate among metabolically benign overweight/obese women, characterized by lower leptin and higher soluble leptin receptor and HMW adiponectin compared to at-risk overweight/obese women, and higher leptin and lower soluble leptin receptor and HMW adiponectin compared to metabolically benign normal weight women (Figure 2).
Figure 2.
Box plots representing median levels of ectopic fat and three adipokines by body size phenotypes.
Pericardial and epicardial fat measures are presented as volumes (more volume, more fat); hepatic fat measure is presented as attenuation (more attenuation, less fat). Horizontal lines represent medians, while bottom and top of the boxes represent the 25th and 75th percentiles, respectively.
MBNW = metabolically benign normal weight phenotype (n = 251). MBO = metabolically benign overweight/obese phenotype (n = 296). ARO = at-risk overweight/obese phenotype (n = 89).
Metabolically benign phenotype was defined as no more than 1 of the following 4 ATP-III components: (1) systolic/diastolic blood pressure ≥130/85mmHg, (2) fasting triglycerides ≥150 mg/dL, (3) HDL-C <50 mg/dL, and (4) fasting glucose ≥100 mg/dL.
Association of Tertiles of Ectopic Fat and Adipokines with Body Size Phenotypes
After initial multivariable adjustment, compared to metabolically benign overweight/obese women, at-risk overweight/obese women had approximately two times greater odds of having epicardial fat levels in the top tertile and hepatic fat attenuation score in the bottom tertile (i.e. high levels of hepatic fat). Likewise, at-risk overweight/obese women had approximately two times greater odds of having leptin in the top tertile, and almost three times greater odds of having HMW adiponectin in the bottom tertile (Table 2, Model 1). Further adjustment for BMI, slightly attenuated these associations, which were still elevated but no longer statistically significant, with the exception of HWM adiponectin, with at-risk overweight/obese women maintaining more than twice the odds of having HMW adiponectin in the bottom tertile compared to metabolically benign overweight/obese women(Table 2, Models 2). Adjusting for waist circumference as a surrogate for central adiposity yielded results similar to the original model (Table 2; Model 3). The odds of having high pericardial fat or low soluble leptin receptor levels were similar between benign and at-risk overweight/obese women.
Table 2.
Adjusted Odds Ratios of the “risk factors”a associated with overweight/obese phenotypes
| Model 1b | Model 2c | Model 3d | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Top Tertile of Epicardial Fat Volume | ||||||
| MBO | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| ARO | 1.76 | 1.04 – 2.99 | 1.31 | 0.75 – 2.29 | 1.80 | 1.04 – 3.11 |
| Top Tertile of Pericardial Fat Volume | ||||||
| MBO | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| ARO | 1.13 | 0.68 – 1.89 | 0.74 | 0.42 – 1.29 | 1.16 | 0.68 – 1.97 |
| Bottom Tertile of Hepatic Fat Attenuation Score | ||||||
| MBO | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| ARO | 1.90 | 1.12 – 3.24 | 1.54 | 0.88 – 2.67 | 1.84 | 1.06 – 3.20 |
| Top Tertile of Leptin | ||||||
| MBO | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| ARO | 2.15 | 1.23 – 3.76 | 1.19 | 0.63 – 2.24 | 1.64 | 0.91 – 2.95 |
| Bottom Tertile of Soluble Leptin Receptor | ||||||
| MBO | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| ARO | 1.53 | 0.89 – 2.64 | 1.11 | 0.62 – 1.97 | 1.11 | 0.62 – 1.99 |
| Bottom tertile of HMW Adiponectin | ||||||
| MBO | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| ARO | 2.90 | 1.62 – 5.19 | 2.38 | 1.30 – 4.35 | 2.59 | 1.43 – 4.69 |
MBO – Metabolically Benign Obese Phenotypee
ARO – At-Risk Obese Phenotype
Risk factors include: top tertile of epicardial fat, pericardial fat, and leptin, and bottom tertile of hepatic attenuation score, soluble leptin receptor and HMW adiponectin
Model 1: adjusted for age, race, site of recruitment, education, physical activity, smoking, and alcohol
Model 2: Model 1 + BMI
Model 3: Model 1 + waist circumference
Metabolically benign phenotype definition: ≤1 of: elevated blood pressure (≥130/85 mmHg), elevated TG (≥150 mg/dL), elevated fasting glucose (≥100 mg/dL), low HDL cholesterol (<50 mg/dL).
Compared to metabolically benign normal weight women, metabolically benign overweight/obese women had statistically significantly higher multivariable-adjusted odds of all three fat measures and all three adipokines, and ORs remained strongly significant (2 to 10-fold elevated) after further adjustment for waist circumference (Table 3). This model was not further adjusted for BMI due to lack of overlapping BMI between overweight/obese and normal weight groups.
Table 3.
Adjusted Odds Ratios of the risk factors a in metabolically benign overweight/obese vs. metabolically benign normal weight body size phenotypes.b
| Model 1c | Model 2d | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Top Tertile of Epicardial Fat Volume | ||||
| MBNW | 1.00 | (reference) | 1.00 | (reference) |
| MBO | 5.17 | 3.22 – 8.29 | 4.99 | 3.04 – 8.18 |
| Top Tertile of Pericardial Fat Volume | ||||
| MBNW | 1.00 | (reference) | 1.00 | (reference) |
| MBO | 9.27 | 5.52 – 15.56 | 8.31 | 4.90 – 14.08 |
| Bottom Tertile of Hepatic Fat Attenuation Score | ||||
| MBNW | 1.00 | (reference) | 1.00 | (reference) |
| MBO | 2.72 | 1.77 – 4.19 | 2.53 | 1.62 – 3.94 |
| Top Tertile of Leptin | ||||
| MBNW | 1.00 | (reference) | 1.00 | (reference) |
| MBO | 11.07 | 6.03 – 20.32 | 9.74 | 5.19 – 18.28 |
| Bottom Tertile of Soluble Leptin Receptor | ||||
| MBNW | 1.00 | (reference) | 1.00 | (reference) |
| MBO | 4.28 | 2.69 – 6.83 | 3.49 | 2.14 – 5.70 |
| Bottom Tertile of HMW Adiponectin | ||||
| MBNW | 1.00 | (reference) | 1.00 | (reference) |
| MBO | 2.51 | 1.60–3.94 | 2.27 | 1.42 – 2.60 |
MBNW – Metabolically Benign Normal Weight Phenotype
MBO – Metabolically Benign Obese Phenotype
Risk factors include: top tertile of epicardial fat, pericardial fat, and leptin, and bottom tertile of hepatic attenuation score, soluble leptin receptor and HMW adiponectin
Metabolically benign phenotype definition: ≤1 of: elevated blood pressure (≥130/85 mmHg), elevated TG (≥150 mg/dL), elevated fasting glucose (≥100 mg/dL), low HDL cholesterol (<50 mg/dL).
Model 1: adjusted for age, race, site of recruitment, education, physical activity, smoking, and alcohol
Model 2: Model 1 + waist circumference
Sensitivity Analyses
The results were similar when the metabolically benign obese phenotype was defined by the modified ATP-III definition (n=349). Additional sensitivity analyses, which included: (1) excluding diabetic participants (n=2), (2) restricting analyses to obese women only (BMI ≥30 kg/m2) (n=149), (3) restricting analyses to women who have all three ectopic fat measures (n=596), and (4) restricting analyses to only those women who passed initial screening and were enrolled in KEEPS and subsequently randomized (n=567) also produced similar results.
DISCUSSION
In these cross-sectional analyses of recently postmenopausal women screened for KEEPS, we report that metabolically benign overweight/obese women have an intermediate ectopic fat burden and intermediate adipokine profile, characterized by lower levels of epicardial fat, hepatic fat, and leptin, and higher levels of HMW adiponectin compared to their at-risk overweight/obese counterparts, but higher ectopic fat and leptin levels and lower HMW adiponectin levels compared to metabolically benign normal weight women. These results raise the possibility that intermediate ectopic fat burden and adipokine expression may contribute to the intermediate CVD risk previously reported for metabolically benign overweight and obese women. While the associations of at-risk vs. metabolically benign overweight/obese phenotypes with high levels of epicardial fat, hepatic fat, and leptin were no longer statistically significant after further adjustment for BMI, the association with low levels of HMW adiponectin remained significant. Therefore, HMW adiponectin, specifically, may be particularly likely to contribute to the intermediate CVD risk evidenced by this phenotype, as well as to its etiology.
Ectopic fat may contribute to CVD risk among obese individuals. Fat deposition in the liver has been associated with both insulin resistance and coronary heart disease.(21, 22) Several cross-sectional studies suggest that greater amounts of epicardial and pericardial fat are associated with the presence of coronary artery disease and atherosclerosis.(10, 23, 24) Epicardial fat is thought to have direct local toxic effects on vascular inflammation and calcification of the coronary arteries,(25) and pericardial fat, located superficial to the pericardium, is thought to have a negative impact on cardiac muscle cells due to its close proximity to the pericardium.(26) While several endocrine properties differentiate epicardial fat vs. pericardial fat,(27) it remains unknown whether these differences result in differential CVD risk for epicardial vs. pericardial fat. A limited literature directly comparing the two depots suggests stronger associations of epicardial vs. pericardial fat with coronary artery calcification, (28–31) while a recent study suggests that pericardial rather than epicardial fat is more strongly associated with the components of the metabolic syndrome, and therefore is a better cardiometabolic risk marker.(32) In the present study, high levels of epicardial fat were more strongly associated with the at-risk vs. metabolically benign overweight/obese phenotypes than pericardial fat. After adjustment for BMI, the trend towards elevated odds ratios remained (OR: 1.31, 95% CI 0.75–2.39 for epicardial fat), but fell short of statistical significance, which although could be attributed to the relatively small number (n=81) of at-risk overweight/obese women, shows a stronger association between epicardial fat and obesity.
In the present study, high levels of hepatic fat were strongly associated with the at-risk vs. metabolically benign overweight/obese phenotypes. Even after adjustment for BMI, the trend towards elevated odds ratios remained (OR: 1.54, 95% CI: 0.88–2.67 for hepatic fat) and was significant when the model was adjusted for waist circumference (OR: 1.84; 95% CI: 1.06–3.20) in the at-risk overweight/obese women. Hepatic fat is strongly associated with insulin resistance,(33) and studies with relatively small sample sizes demonstrate lower hepatic fat and hepatic enzyme levels among metabolically benign vs. at-risk individuals.(10, 11) The present study fills this important gap in literature by directly evaluating the levels of hepatic and cardiac fat among a large sample of postmenopausal women defined as metabolically benign obese individuals. Although the odds of having ectopic fat were significantly higher in metabolically benign overweight/obese women as compared to healthy lean women, the attenuation between the two obese phenotypes after adjustment for BMI suggests that ectopic fat deposition likely does not make a substantial contribution to BMI-independent variability in CVD risk between metabolically benign and at-risk obese individuals.
In addition to increased ectopic fat burden, the altered profile of adipose tissue hormone secretion could contribute to development and progression of CVD. Leptin has been shown to increase skeletal muscle glucose uptake and oxidation, and may be independently involved in insulin secretion and inflammation.(34) High leptin levels observed among obese individuals likely indicate leptin resistance, (35) and have frequently been associated with CVD.(36) While free leptin in the blood is presumed to be the biologically active form,(19) soluble leptin receptor, the main leptin binding protein, plays an important role in determining levels of free leptin by controlling the leptin clearance rate. Unlike other adipokines, adiponectin has been shown to have anti-inflammatory and anti-atherosclerotic effects by increasing fatty acid oxidation and glucose uptake in skeletal muscles and reducing hepatic glucose production, which are associated with improved insulin sensitivity.(37) While some studies find that metabolically benign obese individuals are characterized by more favorable levels of adipokines compared to at-risk obese individuals,(12) other studies find no significant differences.(13, 38) Our recent examination of adipokine profiles among postmenopausal women enrolled in the Women’s Health Initiative ancillary study revealed intermediate total adiponectin and leptin levels in metabolically benign obese women (results for adiponectin remained statistically significant after further adjustment for BMI).(39) The current study provides additional support through comparable findings in a younger, recently postmenopausal KEEPS population, again finding intermediate adipokine levels in metabolically benign overweight/obese women. Specifically, both overweight/obese subgroups had significantly higher levels of leptin and significantly lower levels of soluble leptin receptor compared to normal weight women. However, compared to metabolically benign overweight/obese women, the at-risk obese subgroup had higher leptin, but no difference in soluble leptin receptor levels, likely indicating higher levels of free leptin, and therefore, higher levels of leptin resistance in this subgroup. Our finding of a significant association between low levels of adiponectin and metabolically benign vs. at-risk obesity, even after adjusting for BMI, suggests a possible role for this adipokine in defining metabolic health, independent of obesity.
Several limitations of this study must be taken into consideration. The design of the present study was cross-sectional, limiting conclusions regarding the contributions of ectopic fat and adipokines to the etiology vs. health outcomes of metabolically benign obesity. Also, our study sample included only recently postmenopausal women in good general health, so the results may not be generalizable to other populations. In addition, although women on antidiabetic and lipid lowering medications were excluded from KEEPS, we did not have data available on the use of antihypertensive medications. However, a subgroup sensitivity analysis on women who were subsequently enrolled in KEEPS and randomized did not show any differences in findings, thus it is unlikely that medication use significantly biased our findings. Despite these limitations, this is the first study published to date to have compared cardiac fat volumes among these phenotypes, and the first to have also simultaneously measured hepatic fat and several adipokines.
In conclusion, our results suggest that metabolically benign overweight/obese postmenopausal women are characterized by an intermediate ectopic fat burden and intermediate adipokine profile – more favorable compared to their at-risk overweight/obese counterparts but less favorable compared to metabolically benign normal weight women. Intermediate levels of ectopic fat, leptin and soluble leptin receptor were not independent of BMI, while adiponectin was. Therefore, our current findings highlight the potential importance of adiponectin, especially, as a contributor to heterogeneity in cardiometabolic profile and CVD risk among obese individuals. Studies suggest that rather than being fully protected from adiposity-related complications, the metabolically benign overweight/obese subgroup seem to present with delayed manifestation of cardiometabolic abnormalities and CVD events,(7, 8, 40) leading to speculation that the metabolically benign phenotype represents a transient obese state on the pathway to the at-risk phenotype. Our findings of intermediate ectopic fat and adipokine levels among metabolically benign overweight/obese women, rather than levels similar to healthy normal weight women, support this perspective.
Acknowledgments
Funding/Support: KEEPS was funded by grants from the Aurora Foundation to the Kronos Longevity Research Institute, NIH HL90639 to VMM, 1 UL1 RR024150, and the Mayo Foundation. Study medications were supplied in part by Bayer Health Care and by Abbott Pharmaceuticals. Premarin® was purchased through standard commercial channels. Ectopic fat measures reported herein were funded by NIH HL094581 (to Dr. Wildman). This study was also supported by grant # 10PRE3410007 from the American Heart Association (to Dr. Ogorodnikova). This publication was also made possible by CTSA Grants UL1 RR025750 and KL2 RR025749 and TL1 RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Role of the Sponsors: The Aurora Foundation did not have input into the design or conduct of the study or the review or approval of this article.
Footnotes
ClinicalTrials.gov number is NCT00154180.
KEEPs: Investigators and Staff:
Albert Einstein College of Medicine: Genevieve Neal-Perry, Ruth Freeman, Hussein Amin, Nanette Santoro
Brigham and Women’s Hospital/Harvard Medical School: JoAnn Manson, Maria Bueche, Marie Gerhard-Herman, Kate Kalan, Jan Lieson, Kathryn M. Rexrode, Barbara Richmond, Frank Rybicki, Brian Walsh
Columbia College of Physicians and Surgeons: Rogerio Lobo, Luz Sanabria, Maria Soto, Michelle P. Warren, Ralf C. Zimmerman
Kronos Longevity Research Institute: S. Mitchell Harman, Mary Dunn, Panayiotis D. Tsitouras, Viola Zepeda
Mayo Clinic: Virginia M. Miller, Philip A. Araoz, Rebecca Beck, Dalene Bott-Kitslaar, Sharon L. Mulvagh, Lynne T. Shuster, Teresa G. Zais
University of California, Los Angeles, CAC Reading Center: Matthew Budoff, Chris Dailing, Yanlin Gao, Angel Solano
University of California, San Francisco: Marcelle Cedars, Nancy Jancar, Grechen Good; Statistical Reading Center: Lisa Palermo
University of Southern California, Atherosclerosis Research Unit/Core Imaging and Reading Center: Howard N. Hodis/Yanjie Li
University of Utah School of Medicine: Eliot Brinton, Paul N. Hopkins, M. Nazeem Nanjee, Kirtly Jones, Timothy Beals, Stacey Larrinaga-Shum
VA Puget Sound Health Care System and University of Washington School of Medicine: George R. Merriam, Pamela Asberry, SueAnn Brickle, Colleen Carney, Molly Carr, Monica Kletke, Lynna C. Smith
Yale University, School of Medicine: Hugh Taylor, Kathryn Czarkowski, Lubna Pal, Linda MacDonald, Mary Jane Minkin, Diane Wall.
Others: Frederick Naftolin (New York University), Nanette Santoro (University of Colorado)
CONFLICT OF INTEREST
No potential conflicts of interest relevant to this article were reported.
Dr. Brinton was a consultant to Abbott Laboratories for products not related to menopausal hormone therapy.
Additional Contributions: We gratefully acknowledge the dedicated efforts of all the investigators and staff at the KEEPS clinical centers, the KEEPS Data Coordinating Center at KLRI, and the NIH Institutes supporting ancillary studies. Above all, we recognize and thank the KEEPS participants for their dedication and commitment to the KEEPS research program.
This publication was also made possible by CTSA Grants UL1 RR025750 and KL2 RR025749 and TL1 RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessary represent the official view of the NCRR or NIH. The sponsors had no role in the design, conduct or reporting of the study.
References
- 1.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–72. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 2.Song Y, Manson JE, Meigs JB, et al. Comparison of usefulness of body mass index versus metabolic risk factors in predicting 10-year risk of cardiovascular events in women. American Journal of Cardiology. 2007;100:1654–8. doi: 10.1016/j.amjcard.2007.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marini MA, Succurro E, Frontoni S, et al. Metabolically healthy but obese women have an intermediate cardiovascular risk profile between healthy nonobese women and obese insulin-resistant women. Diabetes Care. 2007;30:2145–7. doi: 10.2337/dc07-0419. [DOI] [PubMed] [Google Scholar]
- 4.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 5.Kip KE, Marroquin OC, Kelley DE, et al. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women’s Ischemia Syndrome Evaluation (WISE) study. Circulation. 2004;109:706–13. doi: 10.1161/01.CIR.0000115514.44135.A8. [DOI] [PubMed] [Google Scholar]
- 6.Katzmarzyk PT, Janssen I, Ross R, et al. The importance of waist circumference in the definition of metabolic syndrome: prospective analyses of mortality in men. Diabetes Care. 2006;29:404–9. doi: 10.2337/diacare.29.02.06.dc05-1636. [DOI] [PubMed] [Google Scholar]
- 7.Flint AJ, Hu FB, Glynn RJ, et al. Excess weight and the risk of incident coronary heart disease among men and women. Obesity (Silver Spring) 2010;18:377–83. doi: 10.1038/oby.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogorodnikova AD, Kim M, McGinn AP, Muntner P, Khan U, Wildman RP. Incident cardiovascular disease events in metabolically benign obese individuals. Obesity (Silver Spring) 2012;20:651–9. doi: 10.1038/oby.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brochu M, Tchernof A, Dionne IJ, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86:1020–5. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 10.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–16. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 11.Messier V, Karelis AD, Robillard ME, et al. Metabolically healthy but obese individuals: relationship with hepatic enzymes. Metabolism. 2010;59:20–4. doi: 10.1016/j.metabol.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Aguilar-Salinas CA, Garcia EG, Robles L, et al. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab. 2008;93:4075–9. doi: 10.1210/jc.2007-2724. [DOI] [PubMed] [Google Scholar]
- 13.Labruna G, Pasanisi F, Nardelli C, et al. High leptin/adiponectin ratio and serum triglycerides are associated with an “at-risk” phenotype in young severely obese patients. Obesity (Silver Spring) 2011;19:1492–6. doi: 10.1038/oby.2010.309. [DOI] [PubMed] [Google Scholar]
- 14.Khan UI, Ogorodnikova AD, Xu L, et al. The Adipokine Profile of Metabolically Benign Obese and At-Risk Normal Weight Postmenopausal Women: The Women’s Health Initiative Observational Study. Obesity (Silver Spring) 2012 doi: 10.1002/oby.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller VM, Black DM, Brinton EA, et al. Using basic science to design a clinical trial: baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) J Cardiovasc Transl Res. 2009;2:228–39. doi: 10.1007/s12265-009-9104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeb I, Li D, Nasir K, Katz R, Larijani VN, Budoff MJ. Computed tomography scans in the evaluation of Fatty liver disease in a population based study: the multi-ethnic study of atherosclerosis. Academic radiology. 2012;19:811–8. doi: 10.1016/j.acra.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–33. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 18.Huang G, Wang D, Zeb I, et al. Intra-thoracic fat, cardiometabolic risk factors, and subclinical cardiovascular disease in healthy, recently menopausal women screened for the Kronos Early Estrogen Prevention Study (KEEPS) Atherosclerosis. 2012;221:198–205. doi: 10.1016/j.atherosclerosis.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan JL, Bluher S, Yiannakouris N, Suchard MA, Kratzsch J, Mantzoros CS. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes. 2002;51:2105–12. doi: 10.2337/diabetes.51.7.2105. [DOI] [PubMed] [Google Scholar]
- 20.Song Y, Manson JE, Meigs JB, Ridker PM, Buring JE, Liu S. Comparison of usefulness of body mass index versus metabolic risk factors in predicting 10-year risk of cardiovascular events in women. Am J Cardiol. 2007;100:1654–8. doi: 10.1016/j.amjcard.2007.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamaguchi M, Kojima T, Takeda N, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–84. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Targher G, Bertolini L, Poli F, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–6. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 23.Iacobellis G, Sharma AM, Pellicelli AM, Grisorio B, Barbarini G, Barbaro G. Epicardial adipose tissue is related to carotid intima-media thickness and visceral adiposity in HIV-infected patients with highly active antiretroviral therapy-associated metabolic syndrome. Curr HIV Res. 2007;5:275–9. doi: 10.2174/157016207780077084. [DOI] [PubMed] [Google Scholar]
- 24.Taguchi R, Takasu J, Itani Y, et al. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis. 2001;157:203–9. doi: 10.1016/s0021-9150(00)00709-7. [DOI] [PubMed] [Google Scholar]
- 25.Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365:1817–20. doi: 10.1016/S0140-6736(05)66585-3. [DOI] [PubMed] [Google Scholar]
- 26.Ding J, Kritchevsky SB, Harris TB, et al. The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 2008;16:1914–9. doi: 10.1038/oby.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev. 2007;8:253–61. doi: 10.1111/j.1467-789X.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- 28.Ahmadi N, Nabavi V, Yang E, et al. Increased epicardial, pericardial, and subcutaneous adipose tissue is associated with the presence and severity of coronary artery calcium. Acad Radiol. 2010;17:1518–24. doi: 10.1016/j.acra.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–6. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–13. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 31.Huang G, Wang D, Zeb I, et al. Intra-thoracic fat, cardiometabolic risk factors, and subclinical cardiovascular disease in healthy, recently menopausal women screened for the Kronos Early Estrogen Prevention Study (KEEPS) Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sicari R, Sironi AM, Petz R, et al. Pericardial Rather Than Epicardial Fat is a Cardiometabolic Risk Marker: An MRI vs Echo Study. J Am Soc Echocardiogr. 2011;24:1156–62. doi: 10.1016/j.echo.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Bugianesi E, Gastaldelli A, Vanni E, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–42. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 34.Ceddia RB, William WN, Jr, Curi R. Comparing effects of leptin and insulin on glucose metabolism in skeletal muscle: evidence for an effect of leptin on glucose uptake and decarboxylation. Int J Obes Relat Metab Disord. 1999;23:75–82. doi: 10.1038/sj.ijo.0800762. [DOI] [PubMed] [Google Scholar]
- 35.Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50:1511–25. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 36.Li WC, Hsiao KY, Chen IC, Chang YC, Wang SH, Wu KH. Serum leptin is associated with cardiometabolic risk and predicts metabolic syndrome in Taiwanese adults. Cardiovasc Diabetol. 2011;10:36. doi: 10.1186/1475-2840-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein BJ, Scalia R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89:2563–8. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 38.You T, Ryan AS, Nicklas BJ. The metabolic syndrome in obese postmenopausal women: relationship to body composition, visceral fat, and inflammation. J Clin Endocrinol Metab. 2004;89:5517–22. doi: 10.1210/jc.2004-0480. [DOI] [PubMed] [Google Scholar]
- 39.Ogorodnikova AD, Xu L, Wassertheil-Smoller S, et al. The Adipokine Profile of Metabolically Benign Obese and At-Risk Normal Weight Postmenopausal Women: The Women’s Health Initiative Observational Study. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan UI, Wang D, Thurston RC, et al. Burden of subclinical cardiovascular disease in “metabolically benign” and “at-risk” overweight and obese women: The Study of Women’s Health Across the Nation (SWAN) Atherosclerosis. 2011;217:179–86. doi: 10.1016/j.atherosclerosis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]