SHORT SUMMARY
The purpose of this investigation was to define the best frequency, or frequencies, to record the cVEMP and oVEMP and to describe age-related changes in VEMP tuning. Subjects demonstrated a range of best frequencies. Compared to younger subjects, older subjects demonstrated a shift to higher best frequencies. Accordingly, 500 Hz may not be the ideal frequency to elicit VEMPs for all age groups. For this reason in cases where the VEMP response is absent at 500 Hz we recommend that attempts be made to record the VEMP for tone burst frequencies of 750 Hz and/or 1000 Hz.
INTRODUCTION
When an acoustic stimulus of sufficiently high intensity is presented to an ear, a series of reflexes are triggered that include short latency sound-evoked activations, and, sound-evoked inhibitions, of electromyographic (EMG) activity. These sound evoked muscle reflexes can be easily recorded with surface electrodes placed on either the sternocleidomastoid muscle (SCM), or, in proximity to the inferior oblique muscle (Colebatch and Halmagyi 1992; Rosengren et al. 2005; Todd et al. 2007). These evoked responses are referred to as vestibular evoked myogenic potentials (VEMP). A VEMP recorded from the SCM is commonly referred to as the cervical VEMP (cVEMP) and a VEMP recorded from surface electrodes placed beneath the eyes has been termed the ocular VEMP (oVEMP). cVEMPs and oVEMPs are used clinically to assess the function of the saccule, utricle, and the inferior and superior portions of the vestibular nerve. The end organ generator of the cVEMP is the saccule (Colebatch and Halmagyi 1992; Colebatch et al. 1994; McCue and Guinan 1994). Unlike the cVEMP, the oVEMP may depend upon utricular input as well as saccular (Curthoys 2010; Halmagyi and Carey 2010; Manzari et al. 2010; Murofushi et al. 2010; Rosengren et al. 2010; Govender et al. 2011).
Both the cVEMP and oVEMP can be recorded across the lifespan; however the responses are often reduced in amplitude, or absent, in older individuals. Age-related changes in both the cVEMP and oVEMP are well-documented and appear to be very similar for both responses (Welgampola and Colebatch 2001b; Ochi and Ohashi 2003; Su et al. 2004; Zapala and Brey 2004; Basta et al. 2007; Brantberg et al. 2007; Iwasaki et al. 2008; Nguyen et al. 2010; Tseng et al. 2010). The most consistent age-related finding is a decrease in peak-to-peak amplitude and an increase in threshold (i.e. threshold occurring at higher stimulus levels) with increasing age. Reportedly up to 40% of otologically and neurologically intact subjects over the age of 60 years do not generate a cVEMP in response to an air conduction tone burst at 500 Hz (Su et al. 2004). Absent oVEMPs in response to the same stimulus have been reported in 25% of normal subjects over 60 years of age (Piker et al. 2011). When a VEMP response is bilaterally absent in an older adult, it can be difficult to interpret. The individual may have a bilateral otolith impairment, or, the VEMP could be absent for reasons unrelated to the individual. In other words, the absence of a VEMP in an elderly patient may be due to an impairment occurring anywhere along the VEMP reflex pathway, or may be due to the recording and/or stimulus parameters used to elicit the response (e.g. stimulus level, stimulus intensity, type of stimulus such as acoustic stimulation vs a more natural vestibular stimulus such as head decelerations/accelerations).
It should be noted that 500 Hz AC oVEMPs are also absent in young normal adults a proportion of the time (Chihara et al. 2007; Cheng et al. 2009; Wang et al. 2009 Murnane et al. 2011; Rosengren et al. 2011). The absence of oVEMP responses observed in young adults has been attributed to vertical gaze elevation and/or stimulus level. The response prevalence rate increases when stimulus level increases and when the vertical gaze elevation is increased from 20° to maximum (Murnane et al. 2011; Rosengren et al. 2011). Thus, the absence of VEMP responses in normal individuals may be due to a number of factors including inadequate vertical gaze elevation, limitations in maximum stimulus levels produced by commercially available evoked potential systems, or the frequency of the stimulus used to elicit the response. One could argue that changing stimulus frequency (i.e. when the response is absent at 500 Hz) is preferable for patient comfort and safety to increasing stimulus level as the dB peak SPL levels routinely used to elicit AC VEMPs may exceed 127 pSPL. In other words, using an optimal stimulus frequency might increase the response prevalence rate in young adults as well as older adults.
Investigators have examined the effects of different stimuli on the recordability of the VEMP (Murofushi et al. 1999; Sheykholeslami et al. 2001; Welgampola and Colebatch 2001a; Akin et al. 2003; Rauch et al. 2004; Node et al. 2005; Lin et al. 2006; Timmer et al. 2006; Chihara et al. 2009; Todd et al. 2009; Todd et al. 2009; Donnellan et al. 2010; Lewis et al. 2010; Park et al. 2010; Murnane et al. 2011; Zhang et al. 2011; Winters et al. 2012). These reports have indicated that there is tuning in the vestibular system. That is, certain acoustic frequencies evoke larger amplitude VEMPs at lower threshold levels, at least in young healthy individuals. For example, Welgampola and Colebatch (2001a) measured cVEMP responses that were generated using air-conducted (AC) tone bursts at 100 Hz increments between 200 Hz and 1000 Hz. They observed the largest cVEMP amplitude in response to tone bursts occurring between 600 Hz and 1000 Hz, with a mean of ~700 Hz (Welgampola and Colebatch 2001a). Park et al. (2010) measured oVEMP responses to AC frequencies of 250, 500, 1000, and 2000 Hz in 20 normal subjects. Mean amplitudes were largest at 500 and 1000 Hz (i.e. each with a mean value of 5.7 μV), with VEMP amplitude substantially smaller at both 250 Hz (3.0 μV) and 2000 Hz (3.2 μV). Similarly, Lewis et al. (2010) reported the greatest oVEMP amplitude in response to AC tone bursts at 1000 Hz for 67% of their subjects. Other human studies have shown very similar results with the maximum cVEMP and oVEMP recorded in response to AC tone bursts between 500 and 1000 Hz (Murofushi et al. 1999; Akin et al. 2003; Rauch et al. 2004; Node et al. 2005; Lin et al. 2006; Timmer et al. 2006; Chihara et al. 2009; Murnane et al. 2011; Winters et al. 2012). The finding that mid-frequency AC tone burst stimuli yield larger VEMP responses at lower threshold levels has been offered as evidence of tuning in the vestibular system. In fact, most of the investigations examining the effects of stimulus frequency on the cVEMP and oVEMP have reported no statistically significant differences in response amplitude for stimulus frequencies between 500 and 1000 Hz. In practice, VEMPs are routinely recorded in the clinic using a 500 Hz tone burst because that frequency falls within the range where VEMP amplitude is largest and threshold is lowest.
In summary, the above studies all show tone-burst frequencies yielding the largest cVEMP and oVEMP amplitude and lowest threshold in young normal adults occur at ~500 Hz. For this reason 500 Hz has been the frequency most often recommended to record both the cVEMP and oVEMP (e.g. Akin and Murnane 2008; Jacobson and McCaslin 2007). Several investigators have suggested that in vestibular systems structurally damaged by pathology, specifically Meniere’s Disease, the best frequency to record the VEMP shifts from 500 Hz in normal adults to 1000 Hz in adults with MD (Rauch et al. 2004; Node et al. 2005; Lin et al. 2006; Timmer et al. 2006; Winters et al. 2011). It is currently unknown if age-related structural changes in the vestibular system may also alter the tuning of the system. If this is true, then it might be possible to alter the stimulus parameters, specifically the stimulus frequency, to increase the likelihood of obtaining a VEMP. That is, using an age-adjusted optimal stimulus protocol for recording the VEMPs might have the effect of improving the recordability of the response, and accordingly, improve the overall sensitivity of the diagnostic test battery for the identification of vestibular impairments.
We hypothesized that age-related changes in the saccule and utricle would alter the tuning of the end organ. Thus, the purpose of the present investigation was to define for young, middle age, and older adult subjects the optimal frequency(cies) to record both the cVEMP and the oVEMP. Further, it was the objective of this study to describe age-related changes in the tuning of these VEMPs.
MATERIALS AND METHODS
Subjects
All subjects were recruited from Vanderbilt University and Vanderbilt University Medical Center. Thirty-nine subjects met inclusion criteria and participated in this investigation (i.e. mean age 46.3 ± 15.7 years; range = 22 – 78 years; 15 males). Subjects were equally divided into 3 age groups of 13 subjects each: Young Adult (18 – 39 years), Middle Age (40 – 59 years), and Old Adult (≥ 60 years). Data were obtained from one ear of the 39 participants (i.e. left ear of the odd-numbered participants and right ear of the even-numbered participants) yielding data from 20 left ears and 19 right ears. The study was approved by the Institutional Review Board at Vanderbilt University and all subjects provided full informed consent.
Screening
To assess auditory sensitivity and middle-ear status, air-conduction threshold testing was conducted at 250, 500, 1000, 2000, 4000, and 8000 Hz and bone-conduction threshold audiometry was conducted at 500, 1000, 2000, and 4000 Hz. Tympanometry and ipsilateral acoustic reflex testing at 1000 Hz was also completed. Additionally, we screened for the presence of a cVEMP and oVEMP in both ears using tone burst frequencies 500 Hz, 750 Hz, and 1000 Hz presented at 127 dB pSPL. To be enrolled in this investigation, subjects could not present with a conductive hearing loss or an audiometric asymmetry of 15 dB or greater at any frequency and had to be able to produce a VEMP at 500 Hz, 750 Hz, or 1000 Hz. One subject presented with an asymmetrical hearing loss and was excluded from the study. Three subjects did not produce a VEMP during the screening process. All three subjects were 60 years of age or older and were excluded from the study. Additional exclusion criteria for all participants included past or present complaints of dizziness or imbalance, known otologic disease, neurologic disease, or known disease affecting the cervical vertebrae or spinal cord.
Procedures
Disposable silver/silver-chloride electrodes were used to record the VEMPs. To record the cVEMP the non-inverting electrode input was placed on the ipsilateral (i.e. with reference to the ear stimulated) SCM midway between the insertion of the muscle at the mastoid and sternum. The inverting electrode was placed on the chin. The ground electrode was placed at Fpz. Individual electrode impedances were ≤ 10 kOhms. A running average of the root mean square (RMS) amplitude of the EMG from the bipolar electrode montage was monitored visually by the patient using a second evoked potential machine that contained an EMG feedback system (Interacoustics Eclipse, Denmark). This feedback system enabled subjects to maintain their tonic EMG at a level between 50 and 200 μV.
The oVEMP was recorded with a 2 channel recording system. Non-inverting electrodes were placed below the center of each lower eyelid of both the ipsilateral and contralateral eye (i.e. with reference to the ear being stimulated). The inverting electrodes were placed 2–3 cm inferior to the active electrodes. The ground electrode was placed at Fpz. Individual electrode impedances were ≤ 10 kOhms.
Subjects were placed in a semi-recumbent position in a comfortable reclining chair for recording the cVEMP. Subjects sat upright for the oVEMP recordings. To record the cVEMP, subjects were asked to lift their heads up from a headrest and turn their heads away from the ear that was being stimulated. To record the oVEMP subjects were instructed to direct their gaze to a visual target at a vertical elevation of ~30 degrees from center gaze. Subjects maintained these positions during data collection and were asked to rest while data collection was paused. Subjects were given rest periods as needed. All recordings were replicated a minimum of one time.
The stimuli for both the cVEMP and oVEMP recordings were presented monaurally through Etymotic ER-3A insert earphones. Stimuli were 125 Hz, 250 Hz, 500 Hz, 750 Hz, 1000 Hz, 1500 Hz, and 2000 Hz Blackman-gated tone bursts with a 2 ms rise/fall and 2 ms plateau (Neuroscan STIM, Herndon, VA). Each tone burst stimulus was calibrated via a Larson-Davis System 800B Precision Integrating Sound Level Meter coupled with a Larson-Davis model 2575 1 inch microphone connected to the sound source with a 2 cc coupler. The frequency spectra were analyzed using an Agilent Dynamic Signal Analyzer (model 35670A). Each stimulus block (i.e. each run) consisted of the 7 test frequencies presented in a randomized sequence at a rate of 5.1/second. Each run lasted ~30 seconds, with ~30 second rest periods between runs, and each individual frequency was presented a total of ~150 times. We believed that this unique stimulus paradigm would distribute evenly the effects of muscle fatigue. The tone bursts were presented at three different levels: 117, 122, and 127 dB pSPL. Only the responses to 127 dB pSPL stimuli will be reported for purposes of this investigation (i.e. effects of aging on VEMP tuning) since no amplitude saturation was observed and the response rate was greatest at this level for all subjects.
The bioelectrical activity was amplified and analog filtered (5 – 500 Hz) with a commercially produced multi-channel neurophysiological amplifier (Neuroscan Synamp, Herndon, VA). For each single record the electromyographic activity was digitized (i.e. at a rate of 5000 Hz) and recorded as a continuous one-channel recording on an electrophysiological recording system (Neuroscan Acquire, Herndon, VA). A second channel contained unique triggers associated with the 7 stimulus frequencies. Data were off-line epoched into segments of EMG associated with stimulus onset. The data for each frequency were signal averaged separately.
For each subject, VEMPs were derived from the 7 frequencies. All responses were repeated and the two runs were grand averaged. A present cVEMP was defined as an initial positive peak (i.e. occurring at ~15 ms) followed by a subsequent negative peak (i.e. occurring at ~25 ms). The first positive peak of the averaged run was labeled as p1 and the following negative peak labeled n1. p1 absolute latency and p1-n1 peak-to-peak amplitudes were measured and tabulated. Only the contralateral oVEMP response was used for the analysis. A present oVEMP was defined as an initial negative peak (i.e. occurring at ~10 ms) with a subsequent positive peak (i.e. occurring at ~15 ms). The first negative peak of the averaged run was labeled as n1 and the following positive peak as p1. n1 absolute latency and n1-p1 peak-to-peak amplitude was measured and tabulated. An absent response was assigned an amplitude value of 0 μV and the latency value was considered missing data.
Lastly, in an effort to account for the possibility that the differences in VEMP frequency tuning between subjects were due to differences in the transmission of the air-conduction stimuli through the middle ear, the status of the middle ear was assessed using the Mimosa Acoustics HearID middle ear power analyzer (MEPA3; Mimosa Acoustics, Champaign, IL). The MEPA3 can be used to assess the status of the middle ear and provides a measurement of how the middle ear filters the sound it receives. In other words, when sound is presented to the ear, some of the sound is absorbed by the middle ear and some of the sound is reflected from the ear drum, and this varies by frequency. Power reflectance is defined as the percentage of reflected power to incidental (total) power. Power absorption is the percentage of the absorbed power to the incident power, and mirrors the results of power reflectance. Power transmittance is the power absorption converted to a decibel scale.
Since VEMP tuning using air conduction stimuli represents the interaction between the tuning of both the vestibular and middle ear systems, MEPA measurements were completed for each subject either before or after the VEMP recordings. The MEPA3 system was calibrated prior to each recording session using a four-chamber coupler (model: CC4-V) in accordance with manufacture guidelines. A probe tip (Etymotics ER10C) was used to deliver sound into the external ear canal. An in-the-ear pressure calibration was performed for each subject, for each ear, prior to the MEPA measurement. The stimulus was a “chirp” presented at 60 dB SPL over a measurement time period of 1 second. The MEPA measurement was completed a minimum of 2 times for each ear.
Statistical Analysis
The data were analyzed using SPSS version 20.0 (SPSS, Inc., Chicago, IL). A repeated measures analysis of variance (ANOVA) using a 3 × 7 mixed design with a between-subjects factor of age group (Young, Middle Age, and Old Adult age groups) and a within-subjects factor of frequency (7 frequencies) was used to assess the effects of age group and stimulus frequency on the amplitude of the VEMP. The degrees of freedom for main effects were corrected using Greenhouse-Geisser estimates of sphericity whenever Mauchly’s test indicated that the assumption of sphericity had been violated. Pairwise comparisons using Bonferroni corrections were conducted when appropriate.
RESULTS
cVEMP
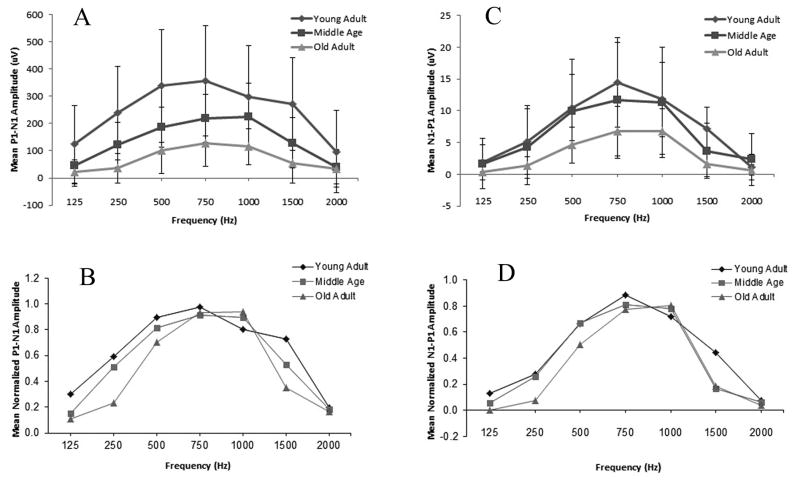
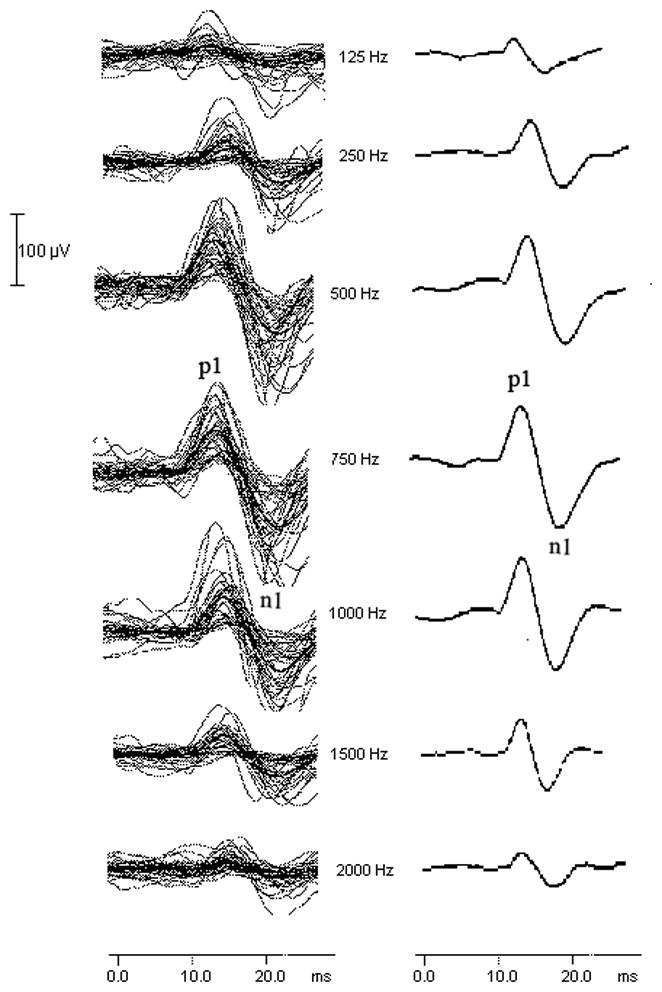
Figure 1 shows the individual (left column) and grand average (right column) cVEMP waveforms in response to 127 dB pSPL stimuli at 125, 250, 500, 750, 1000, 1500, and 2000 Hz. The first positive peak is labeled p1 and the following negative peak is labeled n1. The mean p1-n1 peak-to-peak amplitude across stimulus frequencies from each age group and for the total cohort is shown in Table 1. The largest mean p1-n1 peak-to-peak amplitude occurred at 750 Hz.
Figure 1.
The effect of stimulus frequency on the cVEMP. The individual cVEMP waveforms from all 39 subjects (including those responses classified as “absent” and entered into the database with an amplitude value of 0 μV) are in the left column and the corresponding grand average waveforms are in the right column. The first positive peak is labeled p1 and the following negative peak is labeled n1. Stimulus level was 127 dB pSPL.
Table 1.
Means (SD) for the p1-n1 peak-to-peak cVEMP amplitudes across frequencies measured in uV.
| 125 Hz | 250 Hz | 500 Hz | 750 Hz | 1000 Hz | 1500 Hz | 2000 Hz | |
|---|---|---|---|---|---|---|---|
| Young Adult (n = 13) | 123.6 (143.4) | 238.1 (170.5) | 338.1 (206.1) | 355.8 (202.8) | 297.1 (188.4) | 271.3 (170.5) | 96.4 (151.4) |
| Middle Age (n = 13) | 44.9 (76.8) | 120.8 (82.1) | 187.0 (74.5) | 218.3 (87.8) | 226.1 (121.3) | 129.2 (92.0) | 41.0 (61.8) |
| Old Adult (n = 13) | 21.7 (45.0) | 36.0 (55.0) | 100.9 (83.4) | 126.7 (83.9) | 114.8 (66.2) | 54.5 (72.9) | 35.3 (69.6) |
| Total (n = 39) | 63.4 (104.9) | 131.6 (139.3) | 208.6 (165.6) | 233.6 (164.1) | 212.7 (152.1) | 151.7 (148.2) | 57.6 (104.3) |
Table 2 shows the cVEMP response rate for each age group across stimulus frequencies. The cVEMP response rate was highest at 750 Hz and 1000 Hz (i.e. 39 of 39 subjects, 100%). The response rate decreased marginally at 500 Hz (i.e. 38 of 39 subjects, 97%). Response rates were poorest at the lowest and highest frequencies and tended to decrease as age increased.
Table 2.
cVEMP response rates across stimulus frequencies.
| 125 Hz | 250 Hz | 500 Hz | 750 Hz | 1000 Hz | 1500 Hz | 2000 Hz | |
|---|---|---|---|---|---|---|---|
| Young Adult (n = 13) | 69% | 92% | 100% | 100% | 100% | 100% | 46% |
| Middle Age (n = 13) | 38% | 92% | 100% | 100% | 100% | 85% | 38% |
| Old Adult (n = 13) | 23% | 46% | 92% | 100% | 100% | 53% | 31% |
The frequency resulting in the largest response amplitude (i.e. the “best frequency”) for each individual subject was always 500 Hz, 750 Hz, or 1000 Hz with the exception of a single subject who showed the largest response at 1500 Hz. The majority (i.e. 85%) of the Young Age group showed largest amplitudes at either 500 Hz or 750 Hz with 15% showing the best amplitude at 1000 Hz. In contrast to this, no one in the Old Age group demonstrated the largest amplitude at 500 Hz. However, 62% of the Old Age group showed the maximum amplitude at 750 Hz and 38% at 1000 Hz. The best frequency (i.e. frequency yielding the largest cVEMP amplitude) for the Middle Age group was evenly split at 31%, 31%, and 31% for 500 Hz, 750 Hz, and 1000 Hz, respectively, with 1 subject (7%) showing a best frequency at 1500 Hz.
The p1-n1 peak-to-peak amplitude of the cVEMP varied with age group and stimulus frequency. Main effects of frequency on cVEMP amplitude (F(3.079,110.862) = 61.055, p < .001) and age group (F(2,36) = 8.485, p = .001) were significant. Additionally, there was a statistically significant frequency by age group interaction (F(6.159,110.862) = 4.892, < .001).
Given the differences in cVEMP amplitude across age groups (as shown in Figure 2A), we re-analyzed the data using normalized amplitudes from each subject, at each frequency (f), expressed as a ratio of the largest measured amplitude (i.e. at fmax Hz). Thus, the normalized amplitude for a given frequency was equal to amplitude(f)/amplitude(fmax) and expressed on a scale from 0–1.0. Repeated measures ANOVA were completed using the normalized amplitude values. Results continued to show a significant main effect of frequency (F(4.156, 149.616) = 99.445, p < .001), age group (F(2,36) = 4.777, p = .014), and a significant frequency by age group interaction (F(8.312,149.616) = 3.080, p = .003).
Figure 2.
Diamonds represent the mean P1-N1 amplitude of the Young Adult group, squares represent the Middle Age group, and triangles represent the Old Adult group. When shown, error bars indicate ± 1 standard deviation. A. The peak-to-peak amplitude of the cVEMP as a function of stimulus frequency for each age group. B. cVEMP tuning curves from mean normalized peak amplitudes from all 3 age groups. C. The peak-to-peak amplitude of the oVEMP as a function of stimulus frequency for each age group. D. oVEMP tuning curves from mean normalized peak amplitudes from all 3 age groups.
Pairwise comparisons of the normalized amplitudes indicated that cVEMP amplitude decreased with increasing age. The mean amplitude for the Young Adult age group was significantly larger than that for both the Middle Age and Old Adult age groups. The mean amplitude for the Middle Age group was significantly larger than that of the Old Adult group. Stimulus frequencies 500 Hz, 750 Hz, and 1000 Hz produced significantly larger amplitudes than 125 Hz, 250 Hz, 1500 Hz, and 2000 Hz stimulus frequencies. However, no significant pairwise differences in mean amplitude were observed between 500 Hz, 750 Hz, and 1000 Hz.
The significant interaction effect suggests the magnitude of the frequency effect on cVEMP amplitude varied by age group. Tuning curves based on normalized amplitudes were constructed in an effort to examine more closely the effect of tone burst frequency on the cVEMP as a function of age group. The normalized tuning curves for each age group are show in Figure 2B. In general, the tuning of the cVEMP is quite broad and reaches a peak between 500 Hz and 1000 Hz. All three age groups showed similar response amplitudes at 750 Hz. The difference between the three age groups was more pronounced at the other frequencies. If we focus on the three best frequencies (i.e. 500 Hz, 750 Hz, and 1000 HZ), the Young Adult group clearly showed a larger response at 500 Hz compared to the other age groups. In contrast, the Middle Age and Old Adult group showed a larger response at 1000 Hz compared to the Young Adult group. This effect was most pronounced between the Young Adult and Old Adult groups. These findings suggest that the best frequency shifted to a slightly higher frequency for the Old Adult group.
oVEMP
Figure 3 shows the individual (left column) and grand average (right column) oVEMP waveforms in response to 127 dB pSPL stimuli at 125 Hz, 250 Hz, 500 Hz, 750 Hz, 1000 Hz, 1500 Hz, and 2000 Hz. The first negative peak is labeled n1 and the following positive peak is labeled p1. The mean n1-p1 peak-to-peak amplitude across stimulus frequencies from each age group and for the total cohort is shown in Table 3. As with the cVEMP, the largest average peak-to-peak amplitude occurred at 750 Hz.
Figure 3.
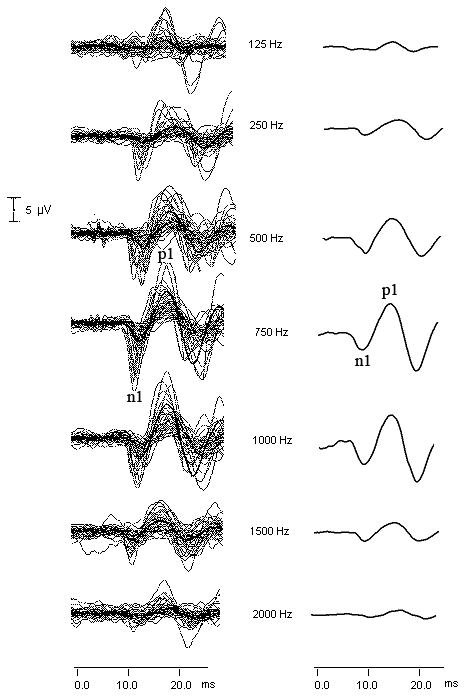
The effect of stimulus frequency on the oVEMP. The individual oVEMP waveforms from all 39 subjects (including those responses classified as “absent” and entered into the database with an amplitude value of 0 μV) are in the left column and the corresponding grand average waveforms are in the right column. The first negative peak is labeled n1 and the following positive peak is labeled p1. Stimulus level was 127 dB pSPL.
Table 3.
Means (SD) for the n1-p1 peak-to-peak oVEMP amplitudes across frequencies measured in uV.
| 125 Hz | 250 Hz | 500 Hz | 750 Hz | 1000 Hz | 1500 Hz | 2000 Hz | |
|---|---|---|---|---|---|---|---|
| Young Adult (n = 13) | 1.9 (2.8) | 5.1 (5.7) | 10.4 (5.2) | 14.5 (7.0) | 11.8 (5.9) | 7.2 (3.4) | 1.1 (2.0) |
| Middle Age (n = 13) | 1.7 (2.9) | 4.3 (5.0) | 9.9 (7.2) | 11.7 (8.1) | 11.3 (7.7) | 3.7 (3.4) | 2.4 (3.1) |
| Old Adult (n = 13) | 0.4 (0.0) | 1.4 (1.3) | 4.6 (2.9) | 6.8 (3.9) | 6.7 (3.6) | 1.6 (2.0) | 0.7 (0.8) |
| Total (n = 39) | 1.2 (1.8) | 3.4 (5.1) | 8.1 (5.4) | 10.8 (6.6) | 9.7 (5.7) | 4.0 (3.1) | 1.2 (1.7) |
Table 4 shows the oVEMP response prevalence rate for each age group across stimulus frequencies. The oVEMP response rate was highest at 750 Hz and 1000 Hz (i.e. 39 of 39 subjects, 100%). The response rate decreased slightly to 95% (i.e. 37 of 39 subjects) at 500 Hz and continued to fall at the lowest and highest frequencies. The response rate tended to decrease as age increased.
Table 4.
Contralateral oVEMP response rates across stimulus frequencies.
| 125 Hz | 250 Hz | 500 Hz | 750 Hz | 1000 Hz | 1500 Hz | 2000 Hz | |
|---|---|---|---|---|---|---|---|
| Young Adult (n = 13) | 46% | 69% | 92% | 100% | 100% | 69% | 31% |
| Middle Age (n = 13) | 15% | 54% | 100% | 100% | 100% | 46% | 31% |
| Old Adult (n = 13) | 0% | 23% | 92% | 100% | 100% | 31% | 8% |
As with the cVEMP, the frequency resulting in the largest response amplitude (i.e. “best” frequency) for each individual subject was always 500 Hz, 750 Hz, or 1000 Hz. The majority (i.e. 92%) of subjects in the Young Adult age group showed the best amplitudes at either 500 Hz or 750 Hz with only a single subject showing the best frequency at 1000 Hz. In contrast to this, no one in the Old Adult age group demonstrated a best frequency at 500 Hz. However, 38% of subjects in the Old Adult group showed the greatest amplitude at 750 Hz and 62% at 1000 Hz. The best frequencies for the Middle Age group were evenly split a 500 Hz (31%), 750 Hz (38%), and 1000 Hz (31%).
We conducted the same analysis on oVEMP peak-to-peak amplitude as we did with the cVEMP. Main effects of frequency on oVEMP amplitude (F(2.553, 84.261) = 58.626, p < .001) and age group (F(2,36) = 4.655, p = .017) were statistically significant. There was also a statistically significant frequency by age group interaction (F(5.107, 84.261) = 2.646, p = .028).
We re-analyzed the data using normalized amplitudes from each subject. Results showed a significant main effect of frequency on oVEMP amplitude (F(3.900, 140.391) = 94.030, p < .001; see Figure 2C). Though the mean oVEMP amplitude tended to decrease with age, an examination of the normalized amplitudes showed there was no significant main effect of age group (F(2,36) = 1.697, p = .197). The frequency by age group interaction was not significant (F(7.800,140.391) = 1.513, p = .160).
Pairwise comparisons of the normalized amplitudes indicated that stimulus frequencies 500 Hz, 750 Hz, and 1000 Hz produced significantly larger oVEMP amplitudes than 125 Hz, 250 Hz, 1500 Hz, and 2000 Hz. No significant differences were observed between oVEMP mean amplitudes obtained in response to 500 Hz, 750 Hz, and 1000 Hz tone bursts. In contrast to the cVEMP, age group did not have a significant effect on the normalized amplitudes.
oVEMP amplitude tuning curves were created based on mean normalized amplitudes (see Figure 2D). As shown in Figure 2D, the normalized tuning curves peaked between 500 and 1000 Hz and amplitude values were reduced significantly at the lower and higher frequencies. Though there was no significant interaction effect, a difference between the age groups is observed with the Young Adult group showing a clear response peak at 750 Hz and the Old Adult group showing the greatest response at 1000 Hz.
Middle Ear Power Reflectance
Qualitatively, middle ear power reflectance varied by frequency. Higher power reflectance values were observed below 1000 Hz and above 5000 Hz. At the lower and higher frequencies a larger proportion of the incident power was reflected back out through the ear canal. Results were plotted and appeared as a U-shaped curve that approximated the middle ear transfer function. These results were similar to previous investigations examining middle ear power reflectance in adults without middle ear pathology (e.g. Feeney and Sanford 2004; Allen et al. 2005) and were consistent across age groups. In other words, the tendency for the best VEMP frequency in older adults to be at a higher frequency was independent of the middle ear transfer function.
DISCUSSION
The purpose of this investigation was to characterize the tuning of the cVEMP and oVEMP and assess the effect of age on the tuning of these responses. Previous investigators have reported a best frequency for both the cVEMP and oVEMP at ~500 Hz. Our findings are consistent with others that have shown 500 Hz – 1000 Hz as the optimal stimulus frequency range. The current investigation provides additional evidence that 500 Hz is not always the best frequency. Indeed, for some subjects 750 Hz or 1000 Hz may be the best frequency.
Given that there was no single “best” stimulus frequency but instead a range of best frequencies, it is clear that, unlike the auditory system, the vestibular system is not sharply tuned. In this regard, it is not clear what benefit sharp tuning would provide the vestibular system. Tuning of the otolith end organs has been attributed to the elastic and inertial properties of the end organs, the mechanical resonance of individual stereocilia, and/or the electrical tuning of the hair cells (Fernandez and Goldberg 1976; Young et al. 1977; Crawford and Fettiplace 1981; Holton and Hudspeth 1983; Fettiplace and Fuchs 1999; Welgampola and Colebatch 2001a). The physical mass of the saccule and utricle and the way they are attached to the temporal bone contribute to their inertial properties and passive resonance. In other words, a smaller (i.e. less massive), and more rigidly attached (i.e. stiffer) end organ should resonate at a higher frequency than a larger (i.e. more massive) and more mobile (i.e. more compliant) end organ. Also, stereocilia behave as mechanical resonators with the resonant frequency being a function of the stereocilia’s stiffness and mass. Thus shorter and stiffer stereocilia should resonate at a higher frequency (Fettiplace and Fuchs 1999). The electrical tuning of a hair cell is determined by the number of potassium (K+) channels (Fettiplace and Fuchs 1999; Steinacker 2004). The cell’s resonant frequency is correlated with the number of K+ channels, with more channels producing a higher resonant frequency (Fettiplace and Fuchs 1999; Steinacker 2004). Thus, the term “resonant frequency” or perhaps “best frequency” may be the most accurate term to use when describing the effects of stimulus frequency on the vestibular system.
In the auditory system, the basilar membrane is sharply tuned in young healthy subjects. The better the physiological condition of the cochlea, the sharper the tuning (Moore 1998). Accordingly, we hypothesized that the better the physiological condition of the saccule and utricle, the sharper would be vestibular tuning. In other words, we hypothesized that damage to the saccule or utricle, as a result of aging, would alter the resonant frequency of the end organ thus altering the tuning characteristics of the vestibular system. Consistent with the findings of previous investigators, we observed an effect of age on the amplitude of both the cVEMP and oVEMP. Remember that the tonic EMG level of the SCM was maintained within a range of 50 and 200 uV during the recording of the cVEMP. Accordingly it is possible that if older subjects maintained lower EMG values during data collection that the lower EMG level contributed, at least in part, to the age-related cVEMP amplitude reduction observed in older subject groups (Akin et al. 2011). Still, this does not explain the reduced oVEMP in older subjects. The effect of age was more pronounced for the cVEMP where amplitudes from both the middle age and older adult groups were significantly smaller compared to the young adult group. In comparison, oVEMP amplitudes were significantly smaller for the older adult group only. What was particularly interesting, however, was the interaction between age group and stimulus frequency. The effect of stimulus frequency on the amplitude of the cVEMP differed by age group with the Young Adult group showing a response peak at 750 Hz and the Old Adult group showing a peak at 1000 Hz. In contrast, the effect of stimulus frequency on the amplitude of the oVEMP was not significantly different across age groups. However, qualitatively older adults demonstrated a slightly higher “best” frequency compared to young adults. Overall, aging does appear to affect the tuning of both VEMPs, and the effect is greater for the cVEMP than the oVEMP. It has been suggested that the utricle makes a greater contribution to the AC oVEMP than to the AC cVEMP. It is possible that the changes we have shown may be reflective of the greater age-related changes in the structure of the saccule compared to the utricle reported in the literature (Schuknecht et al. 1965; Johnsson 1971; Rosenhall 1973; Ross et al. 1976; Richter 1980; Igarashi et al. 1993).
Several investigators have proposed that the frequency tuning characteristics of the oVEMP and cVEMP may provide insight concerning the peripheral origin of the two responses. Specifically, Zhang et al. (2011) observed the greatest AC oVEMP amplitude at ~600 Hz (n1-p1 amplitude = 3.5 uV), but noted that a second peak in the tuning curve was noted at 100 Hz (n1-p1 amplitude = 1.9 uV). They hypothesized that the 600 Hz peak was mediated by the saccule and 100 Hz by the utricle. We did not observe a second lower frequency peak, but it is possible that our 125 Hz stimulus was distorted by the 6ms fixed duration or, conversely, our response rate at 125 Hz was too low to observe any changes in the VEMP tuning curves. Todd et al. (2009) used a biomechanical model to estimate the resonances of the utricle and the saccule and related those resonances to the results of their VEMP tuning experiments. They reported that the resonant frequency of the saccule end organ was higher than the resonant frequency of the utricle but showed that both cVEMPs and oVEMPs were tuned to AC tone bursts around 400–800 Hz and to vibration around 100 Hz. They concluded that the resonance they observed was the result of the end organ, electrical resonance of the hair cell ionic currents, and the afferent fibers projecting from the end organ to the muscle. In other words, the tuning of the VEMP response is not solely due to the resonance of the end organ, but to multiple factors including central projections. All of these possible contributing factors can be affected by age.
CONCLUSION
The results of this investigation have shown that a range of frequencies evoke the largest cVEMP and oVEMP. The best frequency for evoking a VEMP, on average, increases for older adult patients. Accordingly, for older patients 500 Hz may not be the ideal frequency to elicit VEMPs. In fact, for some older subjects in this investigation the VEMP was only present in response to tone-burst stimuli of 750 Hz and 1000 Hz. These changes may represent the accumulated effects of age on the electrical resonance of the hair cells, or, age-related changes in the mechanical properties of the otolith end organs. In cases where the VEMP response is absent at 500 Hz we recommend that attempts be made to evoke the VEMP with a tone burst of 750 Hz or 1000 Hz.
Acknowledgments
This investigation was submitted in partial fulfillment of the requirements for Ph.D. in Hearing and Speech Sciences at Vanderbilt University.
This investigation was supported in part by the Vanderbilt CTSA grant UL1 RR024975-01 from NCRR/NIH.
Footnotes
Conflicts of Interest and Source of Funding:
Authors GJ and DM are consultants for Interacoustics. For the remaining authors none were declared.
Contributor Information
Erin G. Piker, Division of Otolaryngology-Head and Neck Surgery, Department of Surgery, Duke University Medical Center
Gary P. Jacobson, Division of Vestibular Sciences, Department of Hearing and Speech Sciences, Vanderbilt University Medical Center
Robert F. Burkard, Department of Rehabilitation Science, University of Buffalo
Devin L. McCaslin, Division of Vestibular Sciences, Department of Hearing and Speech Sciences, Vanderbilt University Medical Center
Linda J. Hood, Department of Hearing and Speech Sciences, Vanderbilt University Medical Center
References
- Akin F, Murnane O. Vestibular Evoked Myogenic Potentials. In: Jacobson G, Shepard N, editors. Balance Function Assessment and Management. San Diego: Plural Publishing; 2008. pp. 405–434. [Google Scholar]
- Akin FW, Murnane OD, Proffitt TM. The effects of click and tone-burst stimulus parameters on the vestibular evoked myogenic potential (VEMP) J Am Acad Audiol. 2003;14(9):500–509. doi: 10.3766/jaaa.14.9.5. [DOI] [PubMed] [Google Scholar]
- Akin FW, Murnane OD, Tampas JW, Clinard CG. The effect of age on the vestibular evoked myogenic potential and sternocleidomastoid muscle tonic electromyogram level. Ear Hear. 2011;32(5):617–622. doi: 10.1097/AUD.0b013e318213488e. [DOI] [PubMed] [Google Scholar]
- Allen JB, et al. Evaluation of human middle ear function via an acoustic power assessment. J Rehabil Res Dev. 2005;42(4 Suppl 2):63–78. doi: 10.1682/jrrd.2005.04.0064. [DOI] [PubMed] [Google Scholar]
- Basta D, Todt I, Ernst A. Characterization of age-related changes in vestibular evoked myogenic potentials. J Vestib Res. 2007;17(2–3):93–98. [PubMed] [Google Scholar]
- Brantberg K, Granath K, Schart N. Age-related changes in vestibular evoked myogenic potentials. Audiol Neurootol. 2007;12(4):247–253. doi: 10.1159/000101332. [DOI] [PubMed] [Google Scholar]
- Cheng PW, Chen CC, Wang SJ, Young YH. Acoustic, mechanical and galvanic stimulation modes elicit ocular vestibular-evoked myogenic potentials. Clin Neurophysiol. 2009;120(10):1841–1844. doi: 10.1016/j.clinph.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Chihara Y, Iwasaki S, Fujimoto C, Ushio M, Yamasoba T, Murofushi T. Frequency tuning properties of ocular vestibular evoked myogenic potentials. Neuroreport. 2009;20(16):1491–1495. doi: 10.1097/WNR.0b013e3283329b4a. [DOI] [PubMed] [Google Scholar]
- Chihara Y, Iwasaki S, Ushio M, Murofushi T. Vestibular-evoked extraocular potentials by air-conducted sound: another clinical test for vestibular function. Clin Neurophysiol. 2007;118(12):2745–2751. doi: 10.1016/j.clinph.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Halmagyi GM. Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology. 1992;42(8):1635–1636. doi: 10.1212/wnl.42.8.1635. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry. 1994;57(2):190–197. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Fettiplace R. An electrical tuning mechanism in turtle cochlear hair cells. J Physiol. 1981;312:377–412. doi: 10.1113/jphysiol.1981.sp013634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys IS. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin Neurophysiol. 2010;121(2):132–144. doi: 10.1016/j.clinph.2009.09.027. [DOI] [PubMed] [Google Scholar]
- Donnellan K, Wei W, Jeffcoat B, Mustain W, Xu Y, Eby T, Phd WZ. Frequency tuning of bone-conducted tone burst-evoked myogenic potentials recorded from extraocular muscles (BOVEMP) in normal human subjects. Laryngoscope. 2010;120(12):2555–2560. doi: 10.1002/lary.21100. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Sanford CA. Age effects in the human middle ear: wideband acoustical measures. J Acoust Soc Am. 2004;116(6):3546–3558. doi: 10.1121/1.1808221. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. III. Response dynamics. J Neurophysiol. 1976;39(5):996–1008. doi: 10.1152/jn.1976.39.5.996. [DOI] [PubMed] [Google Scholar]
- Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annu Rev Physiol. 1999;61:809–834. doi: 10.1146/annurev.physiol.61.1.809. [DOI] [PubMed] [Google Scholar]
- Govender S, Rosengren SM, Colebatch JG. Vestibular neuritis has selective effects on air- and bone-conducted cervical and ocular vestibular evoked myogenic potentials. Clin Neurophysiol. 2011 doi: 10.1016/j.clinph.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Carey JP. Vestibular evoked myogenic potentials - we live in interesting times. Clin Neurophysiol. 2010;121(5):631–633. doi: 10.1016/j.clinph.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Holton T, Hudspeth AJ. A micromechanical contribution to cochlear tuning and tonotopic organization. Science. 1983;222(4623):508–510. doi: 10.1126/science.6623089. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Saito R, et al. Otoconia in young and elderly persons: a temporal bone study. Acta Otolaryngol Suppl. 1993;504:26–29. doi: 10.3109/00016489309128117. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Smulders YE, Burgess AM, McGarvie LA, Macdougall HG, Halmagyi GM, Curthoys IS. Ocular vestibular evoked myogenic potentials to bone conducted vibration of the midline forehead at Fz in healthy subjects. Clin Neurophysiol. 2008;119(9):2135–2147. doi: 10.1016/j.clinph.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Jacobson G, McCaslin D. The Vestibular Evoked Myogenic Potential and other Sonomotor Evoked Potentials. In: Burkard R, Don M, Eggermont J, editors. Auditory Evoked Potentials: Basic Principles and Clinical Application. Baltimore: Lippincott Williams & Wilkins; 2007. pp. 572–598. [Google Scholar]
- Johnsson LG. Degenerative changes and anomalies of the vestibular system in man. Laryngoscope. 1971;81(10):1682–1694. doi: 10.1288/00005537-197110000-00016. [DOI] [PubMed] [Google Scholar]
- Lewis A, Mustain W, Xu Y, Eby T, Zhou W. Frequency tuning in the tone burst-evoked myogenic potentials in extraocular muscles in normal human subjects. J Otolaryngol Head Neck Surg. 2010;39(5):491–497. [PubMed] [Google Scholar]
- Lin MY, et al. Vestibular evoked myogenic potentials (VEMP) can detect asymptomatic saccular hydrops. Laryngoscope. 2006;116(6):987–992. doi: 10.1097/01.mlg.0000216815.75512.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzari L, Burgess AM, Curthoys IS. Dissociation between cVEMP and oVEMP responses: different vestibular origins of each VEMP? Eur Arch Otorhinolaryngol. 2010;267(9):1487–1489. doi: 10.1007/s00405-010-1317-9. [DOI] [PubMed] [Google Scholar]
- McCue MP, Guinan JJ., Jr Acoustically responsive fibers in the vestibular nerve of the cat. J Neurosci. 1994;14(10):6058–6070. doi: 10.1523/JNEUROSCI.14-10-06058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue MP, Guinan JJ., Jr Spontaneous activity and frequency selectivity of acoustically responsive vestibular afferents in the cat. J Neurophysiol. 1995;74(4):1563–1572. doi: 10.1152/jn.1995.74.4.1563. [DOI] [PubMed] [Google Scholar]
- Moore B. Cochlear Hearing Loss. London: Whurr Publishers Ltd; 1998. [Google Scholar]
- Murnane OD, Akin FW, Kelly KJ, Byrd S. Effects of stimulus and recording parameters on the air conduction ocular vestibular evoked myogenic potential. J Am Acad Audiol. 2011;22(7):469–480. doi: 10.3766/jaaa.22.7.7. [DOI] [PubMed] [Google Scholar]
- Murofushi T, Matsuzaki M, Wu CH. Short tone burst-evoked myogenic potentials on the sternocleidomastoid muscle: are these potentials also of vestibular origin? Arch Otolaryngol Head Neck Surg. 1999;125(6):660–664. doi: 10.1001/archotol.125.6.660. [DOI] [PubMed] [Google Scholar]
- Murofushi T, Wakayama K, Chihara Y. oVEMP to air-conducted tones reflects functions of different vestibular populations from cVEMP? Eur Arch Otorhinolaryngol. 2010;267(6):995–996. doi: 10.1007/s00405-010-1246-7. [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Welgampola MS, Carey JP. Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol. 2010;31(5):793–802. doi: 10.1097/MAO.0b013e3181e3d60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node M, Seo T, Miyamoto A, Adachi A, Hashimoto M, Sakagami M. Frequency dynamics shift of vestibular evoked myogenic potentials in patients with endolymphatic hydrops. Otol Neurotol. 2005;26(6):1208–1213. doi: 10.1097/01.mao.0000176172.87141.5d. [DOI] [PubMed] [Google Scholar]
- Ochi K, Ohashi T. Age-related changes in the vestibular-evoked myogenic potentials. Otolaryngol Head Neck Surg. 2003;129(6):655–659. doi: 10.1016/s0194-5998(03)01578-x. [DOI] [PubMed] [Google Scholar]
- Park HJ, Lee IS, Shin JE, Lee YJ, Park MS. Frequency-tuning characteristics of cervical and ocular vestibular evoked myogenic potentials induced by air-conducted tone bursts. Clin Neurophysiol. 2010;121(1):85–89. doi: 10.1016/j.clinph.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Piker EG, Jacobson GP, McCaslin DL, Hood LJ. Normal characteristics of the ocular vestibular evoked myogenic potential. J Am Acad Audiol. 2011;22(4):222–230. doi: 10.3766/jaaa.22.4.5. [DOI] [PubMed] [Google Scholar]
- Rauch SD, Zhou G, Kujawa SG, Guinan JJ, Herrmann BS. Vestibular evoked myogenic potentials show altered tuning in patients with Meniere’s disease. Otol Neurotol. 2004;25(3):333–338. doi: 10.1097/00129492-200405000-00022. [DOI] [PubMed] [Google Scholar]
- Rosengren SM, Govender S, Colebatch JG. Ocular and cervical vestibular evoked myogenic potentials produced by air- and bone-conducted stimuli: comparative properties and effects of age. Clin Neurophysiol. 2011;122(11):2282–2289. doi: 10.1016/j.clinph.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Rosengren SM, McAngus Todd NP, Colebatch JG. Vestibular-evoked extraocular potentials produced by stimulation with bone-conducted sound. Clin Neurophysiol. 2005;116(8):1938–1948. doi: 10.1016/j.clinph.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Rosengren SM, Welgampola MS, Colebatch JG. Vestibular evoked myogenic potentials: past, present and future. Clin Neurophysiol. 2010;121(5):636–651. doi: 10.1016/j.clinph.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Rosenhall U. Degenerative patterns in the aging human vestibular neuro-epithelia. Acta Otolaryngol. 1973;76(2):208–220. doi: 10.3109/00016487309121501. [DOI] [PubMed] [Google Scholar]
- Ross MD, Peacor D, et al. Observations on normal and degenerating human otoconia. Ann Otol Rhinol Laryngol. 1976;85(3 pt 1):310–326. doi: 10.1177/000348947608500302. [DOI] [PubMed] [Google Scholar]
- Schuknecht, et al. The Pathological Types of Cochleo-Saccular Degeneration. Acta Otolaryngol. 1965;59:154– 170. [Google Scholar]
- Sheykholeslami K, Habiby Kermany M, Kaga K. Frequency sensitivity range of the saccule to bone-conducted stimuli measured by vestibular evoked myogenic potentials. Hear Res. 2001;160(1–2):58–62. doi: 10.1016/s0378-5955(01)00333-1. [DOI] [PubMed] [Google Scholar]
- Steinacker A. Sensory Processing and Ionic Currents in Vestibular Hair Cells. In: Highstein S, Fay R, Popper A, editors. The Vestibular System. Vol. 19. Springer; New York: 2004. pp. 202–234. [Google Scholar]
- Su HC, Huang TW, Young YH, Cheng PW. Aging effect on vestibular evoked myogenic potential. Otol Neurotol. 2004;25(6):977–980. doi: 10.1097/00129492-200411000-00019. [DOI] [PubMed] [Google Scholar]
- Timmer FC, Zhou G, Guinan JJ, Kujawa SG, Herrmann BS, Rauch SD. Vestibular evoked myogenic potential (VEMP) in patients with Meniere’s disease with drop attacks. Laryngoscope. 2006;116(5):776–779. doi: 10.1097/01.mlg.0000205129.78600.27. [DOI] [PubMed] [Google Scholar]
- Todd NP, Rosengren SM, Aw ST, Colebatch JG. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air- and bone-conducted sound. Clin Neurophysiol. 2007;118(2):381–390. doi: 10.1016/j.clinph.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Todd NP, Rosengren SM, Colebatch JG. A utricular origin of frequency tuning to low-frequency vibration in the human vestibular system? Neurosci Lett. 2009;451(3):175–180. doi: 10.1016/j.neulet.2008.12.055. [DOI] [PubMed] [Google Scholar]
- Todd NP, Rosengren SM, Govender S, Colebatch JG. Low-frequency tuning in the human vestibular-ocular projection is determined by both peripheral and central mechanisms. Neurosci Lett. 2009;458(1):43–47. doi: 10.1016/j.neulet.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Tseng CL, Chou CH, Young YH. Aging effect on the ocular vestibular-evoked myogenic potentials. Otol Neurotol. 2010;31(6):959–963. doi: 10.1097/MAO.0b013e3181e8fb1a. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Jaw FS, Young YH. Ocular vestibular-evoked myogenic potentials elicited from monaural versus binaural acoustic stimulations. Clin Neurophysiol. 2009;120(2):420–423. doi: 10.1016/j.clinph.2008.10.157. [DOI] [PubMed] [Google Scholar]
- Welgampola MS, Colebatch JG. Characteristics of tone burst-evoked myogenic potentials in the sternocleidomastoid muscles. Otol Neurotol. 2001a;22(6):796–802. doi: 10.1097/00129492-200111000-00014. [DOI] [PubMed] [Google Scholar]
- Welgampola MS, Colebatch JG. Vestibulocollic reflexes: normal values and the effect of age. Clin Neurophysiol. 2001b;112(11):1971–1979. doi: 10.1016/s1388-2457(01)00645-9. [DOI] [PubMed] [Google Scholar]
- Winters SM, Berg IT, Grolman W, Klis SF. Ocular vestibular evoked myogenic potentials: frequency tuning to air-conducted acoustic stimuli in healthy subjects and Meniere’s disease. Audiol Neurootol. 2012;17(1):12–19. doi: 10.1159/000324858. [DOI] [PubMed] [Google Scholar]
- Young ED, Fernandez C, Goldberg JM. Responses of squirrel monkey vestibular neurons to audio-frequency sound and head vibration. Acta Otolaryngol. 1977;84(5–6):352–360. doi: 10.3109/00016487709123977. [DOI] [PubMed] [Google Scholar]
- Zapala DA, Brey RH. Clinical experience with the vestibular evoked myogenic potential. J Am Acad Audiol. 2004;15(3):198–215. doi: 10.3766/jaaa.15.3.3. [DOI] [PubMed] [Google Scholar]
- Zhang AS, Govender S, Colebatch JG. Tuning of the ocular vestibular evoked myogenic potential (oVEMP) to AC sound shows two separate peaks. Exp Brain Res. 2011;213(1):111–116. doi: 10.1007/s00221-011-2783-z. [DOI] [PubMed] [Google Scholar]