Abstract
Methamphetamine is a highly addictive psychostimulant drug of abuse, causing hyperthermia and neurotoxicity at high doses. Currently, there is no clinically proven pharmacotherapy to treat these effects of methamphetamine, necessitating identification of potential novel therapeutic targets. Earlier studies showed that methamphetamine binds to sigma (σ) receptors in the brain at physiologically relevant concentrations, where it acts in part as an agonist. SN79 (6-acetyl-3-(4-(4-(4-florophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one) was synthesized as a putative σ receptor antagonist with nanomolar affinity and selectivity for σ receptors over 57 other binding sites. SN79 pretreatment afforded protection against methamphetamine-induced hyperthermia and striatal dopaminergic and serotonergic neurotoxicity in male, Swiss Webster mice (measured as depletions in striatal dopamine and serotonin levels, and reductions in striatal dopamine and serotonin transporter expression levels). In contrast, di-o-tolylguanidine (DTG), a well established σ receptor agonist, increased the lethal effects of methamphetamine, although it did not further exacerbate methamphetamine-induced hyperthermia. Together, the data implicate σ receptors in the direct modulation of some effects of methamphetamine such as lethality, while having a modulatory role which can mitigate other methamphetamine-induced effects such as hyperthermia and neurotoxicity.
Keywords: Dopamine, Hyperthermia, Methamphetamine, Neurotoxicity, Serotonin, Sigma receptors
1. Introduction
High or repeated methamphetamine administration results in hyperthermia, neurotoxicity, and even mortality (Cruickshank and Dyer, 2009). In response to high doses of methamphetamine, cellular neurotoxic cascades are activated due to excessive dopamine and 5-HT release into the cytoplasm and synapse (Baldwin et al., 1993; Kuczenski et al., 1995; Gough et al., 2002). Damage to dopaminergic and serotonergic neurons, which can be measured as reductions in dopamine transporters (DAT), serotonin transporters (SERT), dopamine and 5-HT levels, is observed in several brain regions in human methamphetamine users, as well as in laboratory animals (Krasnova and Cadet, 2009).
Numerous clinical and imaging studies have indicated an association between neurotoxicity and several neuropsychiatric disorders, including psychosis (Scott et al., 2007). Motor and cognitive deficits have also been reported (Hart et al., 2012; Scott et al., 2007), including a recent study showing that long term methamphetamine abuse can increase the risk of Parkinson’s disease (Callaghan et al., 2012). Clinical cases of methamphetamine-related fatalities are also rising (Krasnova and Cadet, 2009; Hart et al., 2012), and these lethal effects have been linked to methamphetamine-induced hyperthermia (Bowyer et al., 1994).
Recent evidence has shown that methamphetamine binds to and produces some of its behavioral effects through sigma (σ) receptors, and these proteins can be targeted to mitigate the effects of methamphetamine (Kaushal and Matsumoto, 2011; Nguyen et al., 2005; Robson et al., 2012). In addition to methamphetamine, the psychostimulant cocaine also binds to σ receptors at physiologically relevant concentrations (Robson et al., 2012). With the aim of developing a medication to counteract the harmful effects of psychostimulant abuse, SN79 (6-acetyl-3-(4-(4-(4-florophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one), a putative σ receptor antagonist with druggable properties, including a long half life and good oral bioavailability, was developed (Kaushal et al., 2011a). SN79 interacts with both σ1 and σ2 receptors (Ki 27 and 7 nM, respectively; Kaushal et al., 2011a). It was also shown to attenuate the acute and subchronic effects of cocaine in mice upon intraperitoneal as well as oral administration, making it a viable preclinical drug candidate (Kaushal et al., 2011a).
In the present study, the involvement of σ receptors in methamphetamine-induced toxicity was further evaluated, with an emphasis on hyperthermia, neurotoxicity, and lethality. Modulation of methamphetamine-induced effects was assessed using the well established σ receptor agonist, di-o-tolylguanidine (DTG), and the novel σ receptor putative antagonist SN79. In the first part of the study, it was determined if DTG worsens the effects of methamphetamine. In the second part of the study, it was determined if SN79 attenuates the hyperthermia, neurotoxicity, and lethality caused by methamphetamine. Four well known markers of methamphetamine-induced neurotoxicity were measured: reductions in striatal dopamine and 5-HT levels, as well as decreases in striatal DAT and SERT expression (Krasnova and Cadet, 2009). Striatal tissue was evaluated in the present investigation because it contains the terminals of monoaminergic neurons that are primarily affected by methamphetamine-induced neurotoxicity (Brunswick et al., 1992; Kovachich et al., 1989; Ricaurte et al., 1980; Seiden et al., 1988).
Methamphetamine also causes an elevation in body temperature (Fukumura et al., 1998; Numachi et al., 2007). Earlier studies have shown that hyperthermia potentiates methamphetamine-induced dopamine and 5-HT depletions and exacerbates oxidative stress in the brain (Bowyer et al., 1994; Fukumura et al., 1998; Hirata et al., 1995), whereas hypothermia protects against these effects (Bowyer et al., 1994). Therefore, in the third part of the study, to determine if the neuroprotective effects of SN79 are associated with its ability to decrease the hyperthermic effects of methamphetamine, correlation analysis compared the body temperatures of mice in the various treatment groups with their corresponding striatal dopamine and 5-HT levels.
In addition to establishing an important involvement of σ receptors in the effects of methamphetamine, the ability of SN79 to be clinically developed as a drug candidate against methamphetamine-induced neurotoxicity was also evaluated as a post-treatment following methamphetamine exposure. Previous post-treatment studies have shown σ receptor antagonists to be effective against cocaine-induced lethality (Matsumoto et al., 2001). Therefore, in the fourth part of this study, post-treatment experiments were conducted to evaluate the effectiveness of orally administered SN79 in attenuating methamphetamineinduced striatal dopaminergic neurotoxicity.
2. Experimental procedures
2.1. Drugs and chemicals
SN79 (6-acetyl-3-(4-(4-(4-florophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2(3H)-one) was synthesized as previously described (Kaushal et al., 2011a) and provided by Dr. Christopher McCurdy (University of Mississippi, University, MS). (+)-Methamphetamine hydrochloride was obtained from Sigma (St. Louis, MO). For i.p. administrations, the drugs were dissolved in sterile saline and administered in a volume of 10 ml/g body weight. For the p.o. administrations, SN79 was dissolved in water and administered in a volume of 10 ml/g body weight. Unless specified otherwise, all other chemicals were obtained from standard commercial suppliers (Sigma-Aldrich, St. Louis, MO).
2.2. Animals
Male, Swiss Webster mice (18–28 g; Harlan, Indianapolis, IN) were housed in groups of five with a 12:12h light/dark cycle with food and water available ad libitum. Mice were randomly assigned to different treatment groups. All procedures were performed as approved by the Animal Care and Use Committee at West Virginia University.
2.3. Experimental schedule for pretreatment studies
Male, Swiss Webster mice (N = 4–10/group) were randomly assigned to four experimental groups: (1) Saline/Saline; (2) Saline/Methamphetamine (1.25, 2.5, 5, 10 mg/kg, i.p.); (3) SN79 (1, 3, 10 mg/kg, i.p.) or DTG (10 mg/kg, i.p.)/Saline; (4) SN79 (1, 3, 10 mg/kg, i.p.) or DTG (10 mg/kg, i.p.)/Methamphetamine (5 mg/kg, i.p.). The first drug (saline, DTG or SN79) was administered 15 min prior to the second drug (saline or methamphetamine), and all solutions were given intraperitoneally. Each group of mice received their assigned treatment combination a total of four times at two hour intervals.
Core body temperature was measured 1 h following each of the four treatment injections with a Thermalert TH-S monitor (Physitemp Instruments Inc., Clifton, NJ). During the temperature measurements, mice were gently held at the base of the tail and a probe (RET-3) was inserted approximately 2.5 cm past the rectum into the colon for 8–10 s until a rectal temperature was maintained for 3–4 s.
To allow sufficient time for methamphetamine-induced degeneration of nerve terminals to occur, the animals were sacrificed and their brains removed one week following the aforementioned treatments (Cappon et al., 2000). The striatum and cerebellum was dissected from each mouse and frozen in liquid nitrogen. The tissues were stored at −80 °C for later analysis of dopamine and 5-HT content.
2.3.1. Dopamine assays
Using a dopamine research enzyme immunoassay kit and protocols provided by the manufacturer (Rocky Mountain Diagnostics, Colorado Springs, CO), mouse brain striatal and cerebellar dopamine were quantified. Brain tissues were homogenized in 0.01 N HCl and dopamine was extracted and then acylated to N-acyldopamine using the buffer and reagents provided by the ELISA kit. Acylated dopamine from the tissue samples was incubated with solid phase bound dopamine, dopamine antiserum, and antiserum buffer to compete for a fixed number of antiserum binding sites. Free antigen and free antigen-antiserum complexes were removed via the wash buffer. The antibody bound to the solid phase dopamine was detected using an anti-rabbit IgG-peroxidase conjugate with 3,3’,5,5”-tetramethylbenzidine (TMB) as the substrate. The amount of antibody bound to the solid phase dopamine was measured by monitoring the reaction at 450 nm. The solid phase dopamine measured is inversely proportional to the dopamine concentration of the tissue sample and was quantified relative to a standard curve of known concentrations.
2.3.2. 5-HT assays
The protocol was the same as described above for dopamine, with the exception that brain tissues were homogenized in 0.2 M perchloric acid, followed by centrifugation at 3,000 rpm for 10 min at 4 °C. The supernatants were collected and evaluated for 5-HT levels using 5-HT research enzyme immunoassay kits (Rocky Mountain Diagnostics, Colorado Springs, CO).
2.4. DAT immunohistochemistry
The mice (N = 4/group) were randomly assigned to one of the following treatment groups: (1) Saline/Saline; (2) Saline/Methamphetamine (5 mg/kg, i.p.); (3) SN79 (3 mg/kg, i.p.)/Methamphetamine (5 mg/kg, i.p.); (4) SN79 (3 mg/kg, i.p.)/Saline. The treatments were administered a total of four times at two hour intervals. The single drug doses used for the immunohistochemical studies were selected based on their ability to produce statistically significant changes in the neurochemical measurements described above.
One week following the treatments, the mice were perfused transcardially with 0.1 M phosphate buffered saline (pH 7.4), followed by 4% paraformaldehyde. The brains were further fixed overnight in 4% paraformaldehyde. Coronal sections (50 µm) of the fixed tissue were made throughout the rostral-caudal extent of the striatum using a cryostat, and processed in a free-floating state in 0.1 M Tris-HCl buffered saline (TBS, pH 7.5).
The sections were treated with 0.3% in hydrogen peroxide (H2 O2) in TBS for 30 min at room temperature. The sections were then treated with TBS containing 0.2% Triton X-100 and 1.5% normal goat serum for 30 min at room temperature. Incubation of the sections with anti-rat DAT antibody (Chemicon International, Temecula, CA; MAB369, dilution 1:10,000) was then performed for 36 h at 4 °C. The labeled sections were washed twice in TBS and processed using Vectastain Elite ABC (Vector Laboratories, Burlingame, CA). Sections were incubated with biotinylated secondary anti-rat antisera (Abcam, Cambridge, MA; ab6844; dilution 1:200) in TBS-NBS for 60 min. This was followed by incubation of the sections with avidin-biotinylated peroxidase substrate in TBS for 60 min. The staining was visualized by reacting 3,3’-diaminobendine (DAB) containing 0.01% H2 O2 for 5 min.
The stained sections were mounted onto gelatin-coated slides and dried. The sections were dehydrated, cleared, and coverslipped. The images were captured digitally using a Leica DMIL microscope (Leica Microsystems, Bannockburn, IL) and optical density readings quantified in the rostral-caudal regions of the striatum using ImageJ software (National Institutes of Health, Bethesda, MD). To obtain the data point for a given animal, at least two sections per mouse brain were processed and the optical density readings from both the striatal regions of each section averaged.
2.5. SERT immunohistochemistry
Alternate striatal sections obtained for the DAT immunohistochemical studies were processed for SERT staining. The protocol was the same as for the DAT immunohistochemistry, with the exception of incubating with a rabbit anti-mouse SERT antibody (Millipore, Temecula, CA; AB9726, dilution 1:5,000) for 36 h at 4 °C. The labeled sections were washed thrice in TBS and processed with a Histostain-Plus kit (DAB, Broad Spectrum) (Invitrogen, Camarillo, CA). Briefly, the labeled sections were incubated in 100 µl of DAB substrate for 4 min. The sections were then washed in distilled water. The stained sections were mounted on the slides and analyzed in the same way as for DAT immunohistochemistry.
2.6. Experimental schedule for post-treatment studies
The mice (N = 10/group) were randomly assigned to one of the following treatments: (1) Saline (i.p.)/H2O (p.o.); (2) Methamphetamine (5 mg/kg, i.p.)/H2O (p.o.); (3) Saline (i.p.)/SN79 (10 mg/kg, p.o.); (4) Methamphetamine (5 mg/kg, i.p.)/SN79 (10 mg/kg, p.o.). Saline or methamphetamine (5 mg/kg) was administered i.p. a total of four times at two hour intervals; 3 h following the last injection of methamphetamine or saline, H2O or SN79 was orally administered as a post-treatment since it would be the most clinically relevant route of administration. The designated post-treatment was administered every 8 h for one week. Similarly to the above pretreatment studies, the striatum was then dissected from each treated mouse and frozen in liquid nitrogen. The tissues were stored at −80 °C until dopamine content was measured using dopamine ELISA kits (Rocky Mountain Diagnostics, Colorado Springs, CO) as described above.
The 3 h time point for initiating post-treatment with SN79 was chosen based on a time course analysis following neurotoxic dosing with methamphetamine, whereby striatal dopamine levels start declining at the 3 h time point (Kita et al., 2000). Three times per day dosing (i.e. every 8 h) was based on earlier pharmacokinetic studies with SN79, where its half life following p.o. administration was about 7–8 h (Kaushal et al., 2011a).
2.7. Statistical analysis
The data from the body temperature readings, dopamine and 5-HT assays, and DAT and SERT immunohistochemical studies were evaluated using analysis of variance (ANOVA). Post-hoc analyses were performed with Dunnett’s test for comparisons to controls or Tukey’s tests for pairwise comparisons. The body temperature of the mice from each group was correlated with the striatal dopamine and 5-HT levels obtained a week later. Fisher’s exact test was performed for lethality studies. For all statistical analyses, P<0.05 was considered statistically significant.
3. Results
3.1. Effects of methamphetamine and the σ receptor agonist DTG on lethality
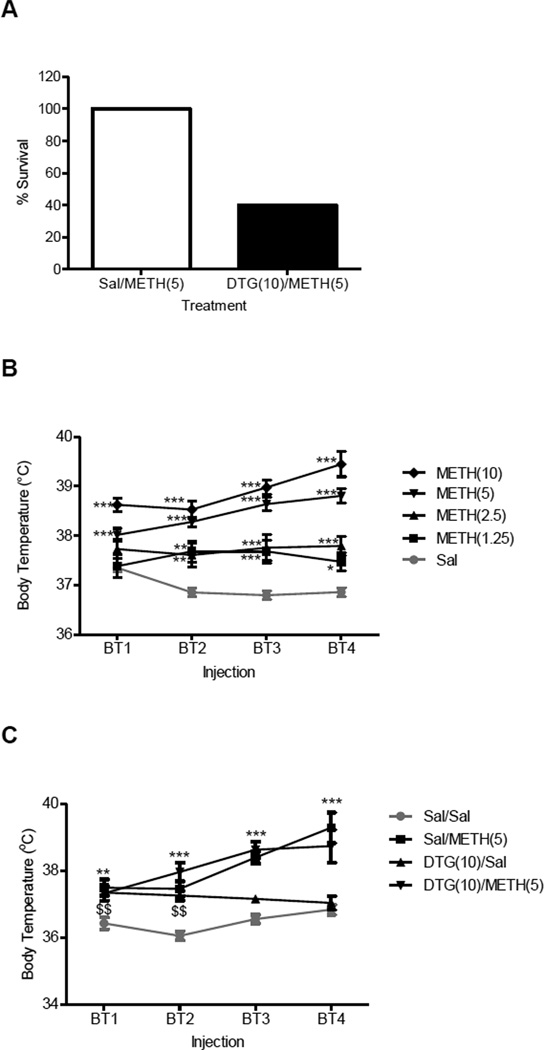
Fig. 1A summarizes the survival of the mice following neurotoxic dosing with methamphetamine in the absence and presence of DTG. There was a dose dependent increase in lethality with methamphetamine alone, although the differences were not statistically significant: 0 mg/kg methamphetamine (saline, 0/10 mice died), 5 mg/kg methamphetamine (0/10 mice died, n.s. compared to saline), 10 mg/kg methamphetamine (5/16 mice died, n.s. compared to saline). Since the 5 mg/kg dose of methamphetamine was the highest one that did not result in any deaths, it was selected for use in combination with DTG in further studies.
Figure 1.
Effects of DTG on lethality and hyperthermia in the absence and presence of methamphetamine (METH). A, male, Swiss Webster mice (N = 10/group) were pretreated i.p. with saline (Sal) or DTG (10 mg/kg) 15 min prior to receiving saline (Sal) or METH (5 mg/kg, i.p.). Dosing was repeated at two hour intervals up to a total of four times. Survival over 24 h was recorded. B, dose response of METH on core body temperature. Male, Swiss Webster mice (N = 5–10/group) were injected with saline or METH (1.25–10 mg/kg, i.p.) at two hour intervals for a total of four times. Core body temperature (BT) was measured via a rectal thermometer 1 h after each injection and data was reported as mean ± SEM. C, effect of DTG pretreatment on basal body temperature in the absence and presence of METH. Male, Swiss Webster mice (N = 5–16/group) were injected with saline or DTG (10 mg/kg, i.p.) 15 min prior to receiving saline or METH (5 mg/kg, i.p.) at two hour intervals for a total of four times. Core BT was measured via a rectal thermometer 1 h after each injection and data was reported as mean ± SEM.*P<0.05, **P<0.01, ***P<0.001 METH vs. saline; $$P<0.01 DTG vs. saline.
Fisher’s exact test confirmed that pretreatment with the σ receptor agonist DTG (10 mg/kg, i.p.) significantly increased lethality following neurotoxic dosing with methamphetamine (P<0.05; Fig. 1A). The deaths occurred within 7 h of when the mice received their first treatment combination of DTG/Methamphetamine. DTG in the absence of methamphetamine was not lethal to any of the animals tested.
3.2. Effects of methamphetamine and the σ receptor agonist DTG on body temperature
The hyperthermic effects of methamphetamine are represented in Fig. 1B. One-way ANOVA demonstrated that methamphetamine produces a dose dependent increase in core body temperature after the first injection (F(4,81) = 7.29, P<0.0001), second injection (F(4,81) = 12.70, P<0.0001), third injection (F(4,81) = 29.21, P<0.0001), and fourth injection (F(4,81) = 52.72, P<0.0001). Post-hoc Dunnett’s tests confirmed that compared to saline, the following doses of methamphetamine significantly increased body temperature under the specified conditions: after the first injection with methamphetamine 2.5 mg/kg (q = 2.53, P<0.05), 5 mg/kg (q = 3.62, P<0.01), and 10 mg/kg (q = 4.99, P<0.01); after the second injection with methamphetamine 1.25 mg/kg (q = 4.46, P<0.01), 2.5 mg/kg (q = 4.05, P<0.01), 5 mg/kg (q = 6.01, P<0.01), and 10 mg/kg (q = 5.52, P<0.01); after the third injection with methamphetamine 1.25 mg/kg (q = 4.53, P<0.01), 2.5 mg/kg (q = 4.91, P<0.01), 5 mg/kg (q = 9.01, P<0.01), and 10 mg/kg (q = 8.95, P<0.01); and after the fourth injection with methamphetamine 1.25 mg/kg (q = 3.91, P<0.01), 2.5 mg/kg (q = 5.30, P<0.01), 5 mg/kg (q = 9.46, P<0.01) and 10 mg/kg (q = 13.57, P<0.01).
Pretreatment with DTG had no significant effect on the hyperthermia produced by methamphetamine (Fig. 1C). One-way ANOVA demonstrated that there was a significant difference between the experimental groups after the first injection (F(3,36) = 6.42, P<0.005), second injection (F(3,36) = 15.73, P<0.0001), third injection (F(3,35) = 32.52, P<0.0001), and fourth injection (F(3,29) = 14.68, P<0.0001). Post-hoc Dunnett’s tests confirmed that compared to saline, methamphetamine significantly increased body temperature at all time points tested: after the first injection (q = 5.58, P<0.01), after the second injection (q = 6.86, P<0.005), after the third injection (q = 10.75, P<0.005), and after the fourth injection (q = 8.14, P<0.005). Compared to saline, DTG alone also significantly increased body temperature after the first (q = 4.80, P<0.01) and second (q = 5.88, P<0.01) injections. However, DTG in the presence of methamphetamine, did not significantly elevate body temperatures over methamphetamine alone at any of the time points (n.s.).
To determine whether the rate of increase in body temperature differed significantly between the methamphetamine-treated groups in the presence and absence of DTG, two-way analysis of variance, followed by post-hoc Bonferroni’s multiple comparison tests were conducted. Similar to the one-way analysis, the DTG/Methamphetamine group did not differ from the Saline/Methamphetamine group at any of the time points tested (n.s.).
3.3. Effects of SN79 on lethality alone and in combination with methamphetamine
Within the dose range tested (up to 10 mg/kg, i.p.), SN79 alone did not produce lethality in the animals. The combination of SN79/Methamphetamine also did not result in deaths.
3.4. Effects of SN79 on body temperature alone and in combination with methamphetamine
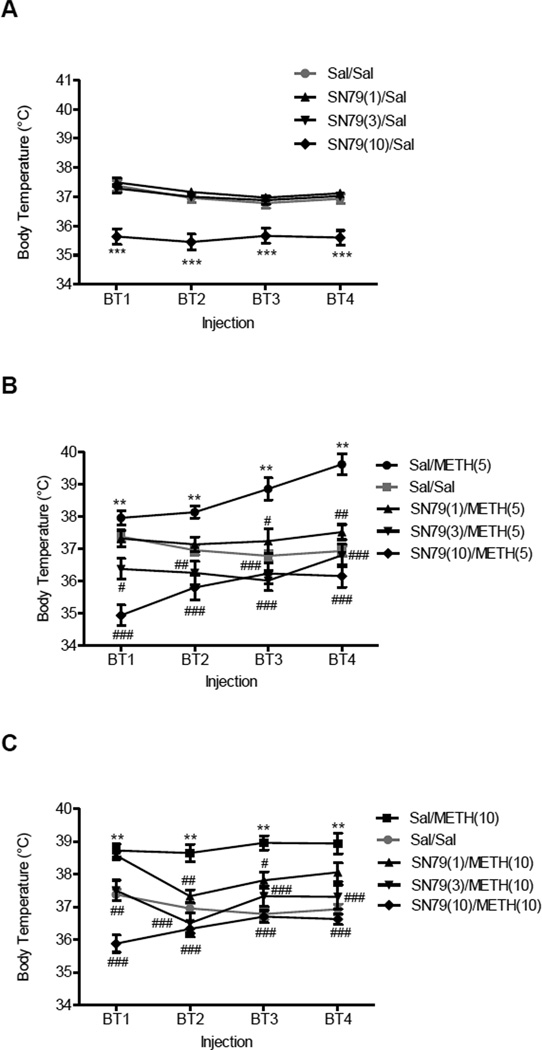
One-way ANOVA showed that SN79 significantly reduced body temperature (Fig. 2A) after the first injection (F(3,59) = 21.77, P<0.0001), second injection (F(3,59) = 20.12, P<0.0001), third injection (F(3,59) = 11.34, P<0.0001), and fourth injection (F(3,59) = 18.01, P<0.0001). Post-hoc Dunnett’s tests revealed that compared to saline, SN79 (10 mg/kg, i.p.) significantly decreased the basal body temperature after first injection (q = 6.54, P<0.001), second injection (q = 5.98, P<0.001), third injection (q = 4.36, P<0.001) and fourth injection (q = 5.58, P<0.001).
Figure 2.
Effects of SN79 on body temperature in the absence and presence of METH. Male, Swiss Webster mice (N = 5–10/group) were pretreated with saline (Sal) or SN79 (1, 3, 10 mg/kg, i.p.), and after 15 min, the mice were treated with Sal or METH (5, 10 mg/kg, i.p.). Core body temperature was measured 1 h after each injection combination. This regimen was repeated four times at 2 h intervals. A, effects of SN79 on basal body temperature. B, effects of pretreatment with SN79 on METH (5 mg/kg, i.p.)-induced hyperthermia. C, effects of pretreatment with SN79 on METH (10 mg/kg, i.p.)-induced hyperthermia. Data was reported as mean ± SEM. **P<0.01, ***P<0.001 vs. saline; #P<0.05, ##P<0.01, ###P<0.001 vs. METH.
One-way ANOVA showed that SN79 pretreatment significantly attenuated the hyperthermic effects of methamphetamine (Fig. 2B and C) after the first injection (F(11,123) = 24.04, P<0.0001), second injection (F(11,123) = 15.40, P<0.0001), third injection (F(11,123) = 18.25, P<0.0001), and fourth injection (F(11,123) = 18.21, P<0.0001). Post-hoc Tukey’s test revealed that the following doses of SN79 attenuated the hyperthermic effects of methamphetamine (5 mg/kg) after the first injection (3 mg/kg: q = 4.84, P<0.05; 10 mg/kg: q = 9.26, P<0.001), second injection (3 mg/kg: q = 5.51, P<0.01; 10 mg/kg: q = 6.86, P<0.001), third injection (1 mg/kg: q = 4.80, P<0.05; 3 mg/kg: q = 8.41, P<0.001; 10 mg/kg: q = 7.76, P<0.001), and fourth injection (1 mg/kg: q = 5.85, P<0.01; 3 mg/kg: q = 7.85, P<0.001; 10 mg/kg: q = 9.63, P<0.001). Post-hoc Tukey’s test also revealed that the following doses of SN79 attenuated the hyperthermic effects of methamphetamine (10 mg/kg) after the first injection (3 mg/kg: q = 5.71, P<0.01; 10 mg/kg: q = 13.34, P<0.001), second injection (1 mg/kg: q = 5.91, P<0.01; 3 mg/kg: q = 9.59, P<0.001; 10 mg/kg: q = 10.39, P<0.001), third injection (1 mg/kg: q = 5.18, P<0.05; 3 mg/kg: q = 7.39, P<0.001; 10 mg/kg: q = 10.20, P<0.001), and fourth injection (3 mg/kg: q = 6.94, P<0.001; 10 mg/kg: q = 9.84, P<0.001).
3.5. Effects of SN79 on dopamine levels alone and in combination with methamphetamine
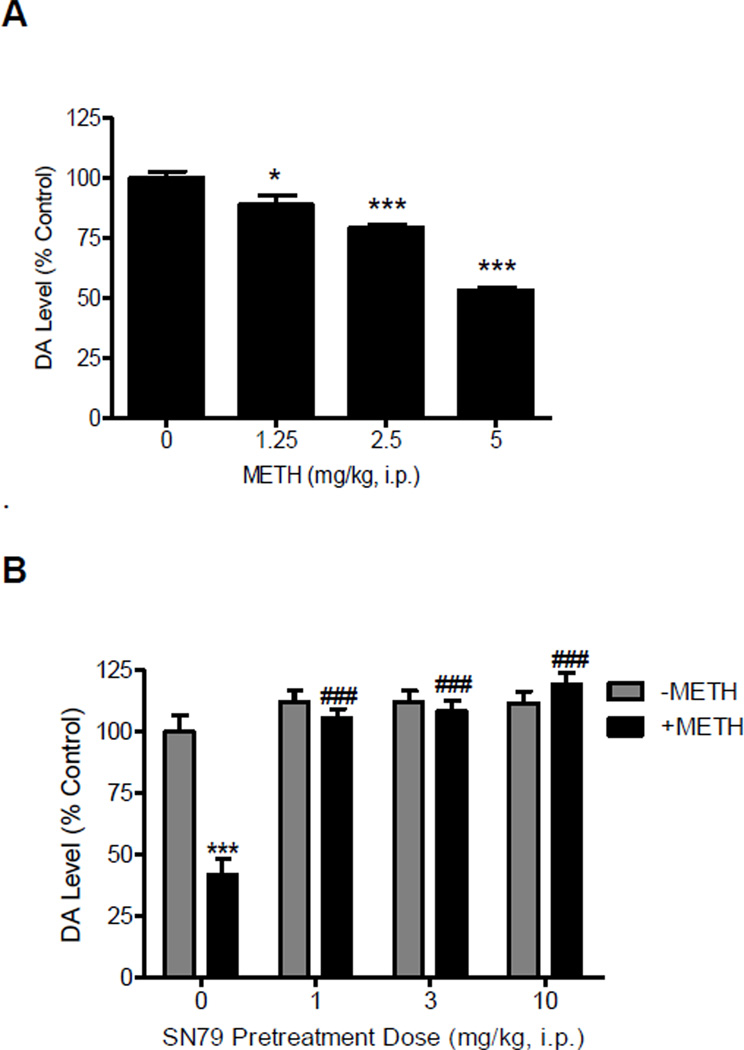
The effects of SN79 alone and in combination with methamphetamine on dopamine levels in the brain are summarized in Fig. 3. One-way ANOVA showed that methamphetamine produced a dose dependent reduction of dopamine levels in the striatum (Fig. 3A, F(3,27) = 7.26, P<0.0005). Post-hoc Dunnett’s tests revealed that the striatal changes produced by the following doses of methamphetamine differed significantly from the saline control group: 2.5 mg/kg (q = 2.55, P<0.05), 5 mg/kg (q = 4.61, P<0.01). However, no significant changes in dopamine concentration following doses of methamphetamine were observed in the cerebellum (F(3,27) = 0.20, n.s.).
Figure 3.
Effects of methamphetamine (METH) and SN79 on striatal dopamine levels. A, dose response of METH on dopamine (DA) levels in the striatum. Male, Swiss Webster mice (N = 5–9/group) were injected (i.p.) with METH (1.25- 5.0 mg/kg, i.p.) or saline (0 mg/kg, i.p.) at 2 h intervals for a total of four times. DA levels in the striatum were measured one week later. B, effects of SN79 on METH-induced depletion of DA levels in striatum of mouse brain tissue. Male, Swiss Webster mice (N = 5/group) were pretreated with saline or SN79 (1, 3, 10 mg/kg, i.p.). Mice were then treated with saline (-METH 0 mg/kg, i.p.) or METH (+METH 5 mg/kg, i.p.) after 15 min. This treatment regimen was repeated at 2 h intervals for a total of four times. Tissue samples from mouse striatum were collected and DA concentration was measured one week later. Data was reported as mean ± SEM. *P<0.05, ***P <0.001 vs. saline; ###P<0.001 vs. METH.
When SN79 was administered in the absence of methamphetamine, one-way ANOVA showed that there were no significant changes in striatal and cerebellar dopamine levels (Fig. 3B, F(3,19) = 1.53, n.s.; F(3,19) = 1.30, n.s.). One-way ANOVA showed that SN79 pretreatment significantly attenuated methamphetamine-induced dopamine depletion (F(4,24) = 31.61, P<0.0001). Post-hoc Tukey’s test show that methamphetamine treatment caused a significant decrease in dopamine levels when compared with saline treatment (q = 10.68, P<0.001) and that SN79 pretreatment significantly prevented methamphetamine-induced decreases in dopamine levels (SN79 dose: 1 mg/kg, q = 11.70, P<0.001; 3 mg/kg, q = 12.24, P<0.001; 10 mg/kg q = 14.28, P<0.001).
3.6. Effects of SN79 on 5-HT levels alone and in combination with methamphetamine
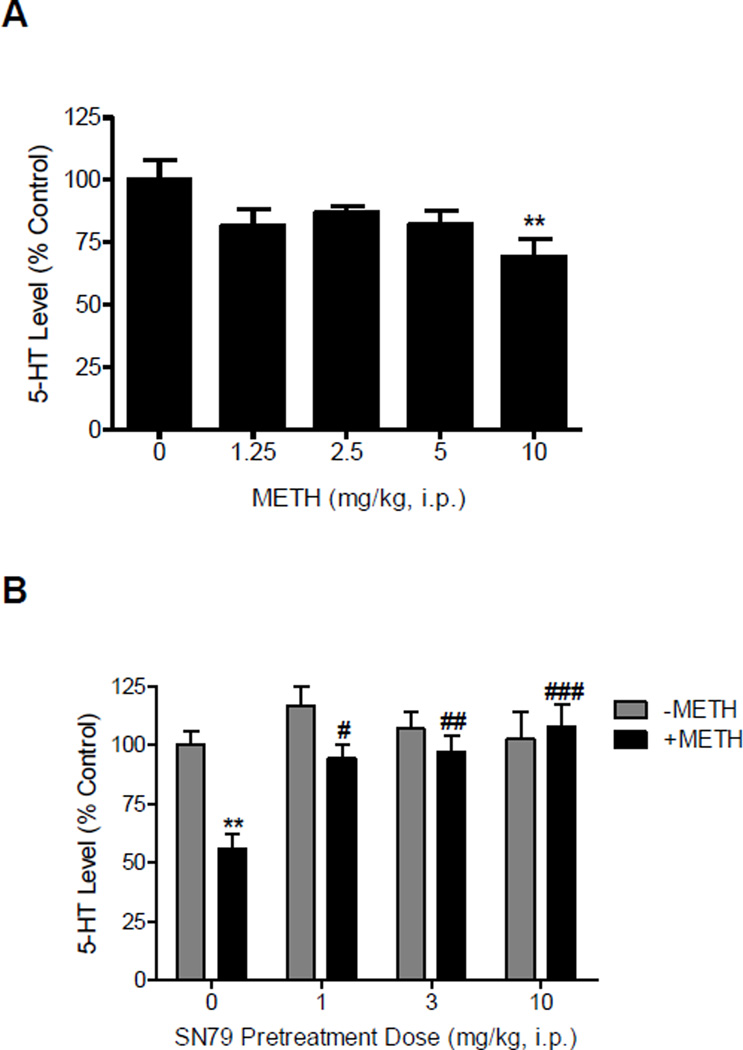
The effects of SN79 alone and in combination with methamphetamine on 5-HT levels in the brain are summarized in Fig. 4. Methamphetamine produced a significant reduction in 5-HT levels in the mouse striatum (Fig. 4A, F(4,35) = 2.93, P<0.05). Post-hoc Dunnett’s tests confirmed that striatal 5-HT levels were significantly reduced relative to the saline control following injection of 10 mg/kg, i.p. of methamphetamine (q = 3.38, P<0.01). However, in the cerebellum, methamphetamine did not produce significant 5-HT depletions (F(4,35) = 2.33, n.s.).
Figure 4.
Effects of methamphetamine (METH) and SN79 on striatal 5-HT levels. A, dose response effects of METH on 5-HT levels in the striatum. Male, Swiss Webster mice (N = 5–10/group) were injected with METH (1.25–10.0 mg/kg, i.p.) or saline (0 mg/kg, i.p.) at 2 h intervals for a total of four times. Striatal tissue samples were collected one week later and measured for 5-HT concentration. B, effect of SN79 pretreatment on METH-induced alteration of 5-HT levels in the striatum of mouse brain. Male, Swiss Webster mice (N = 5–10/group) were pretreated with saline or SN79 (1, 3, 10 mg/kg, i.p.), and 15 min later, the mice were treated with saline (-METH 0 mg/kg, i.p.) or METH (+METH 10 mg/kg, i.p.). These treatment combinations were repeated at 2 h intervals a total of four times. One week later, mouse striatum were collected and 5-HT concentration was measured. Data was reported as mean ± SEM. **P<0.01 vs. saline, #P<0.05, ##P<0.01, ###P<0.001 vs. METH.
One-way ANOVA showed that administration of SN79 alone produced no significant changes in 5-HT levels in the cerebellum and striatum (Fig. 4B, F(3,19) = 0.24, n.s. and F(3,38) = 0.72, n.s., respectively). When SN79 was given as a pretreatment prior to methamphetamine, two-way ANOVA revealed a significant effect of methamphetamine treatment (F(1,71) = 9.60, P<0.005), SN79 pretreatment (F(3,71) = 5.26, P<0.005) and SN79 pretreatment × methamphetamine treatment interaction (F(3,71) = 3.29, P<0.05). Post-hoc Tukey’s test showed that methamphetamine treatment caused a significant decrease in striatal 5-HT levels (q = 5.86, P<0.01) and that SN79 pretreatment significantly prevented methamphetamine-induced decreases in striatal 5-HT levels (SN79 dose: 1 mg/kg, q = 4.95, P<0.05; 3 mg/kg, q = 5.29, P<0.01 and 10 mg/kg, q = 6.88, P<0.001).
3.7. DAT immunohistochemistry
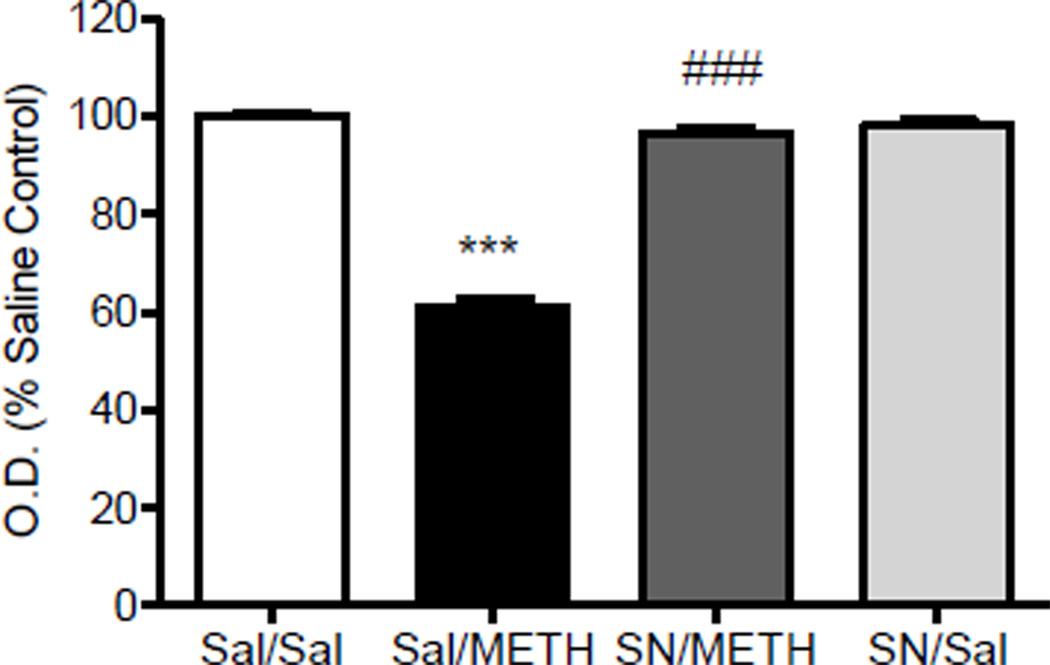
Fig. 5 illustrates the effects of methamphetamine and SN79 on DAT immunoreactivity in the mouse striatum. One-way ANOVA revealed a significant difference between the treatment groups (F(3,71) = 162.90, P<0.0001). Post-hoc Tukey’s multiple comparison tests confirmed that methamphetamine caused a significant reduction in DAT immunoreactivity compared to the saline controls (q = 28.66, P<0.001). Pretreatment with SN79 significantly attenuated the methamphetamine-induced neurotoxicity (q = 24.66, P<0.001), whereas treatment with SN79 alone had no significant effect on DAT expression (q = 1.81, n.s.).
Figure 5.
Dopamine transporter (DAT) Immunohistochemistry: Effect of SN79 pretreatment on methamphetamine (METH)-induced decrease in striatal DAT levels. Male, Swiss Webster mice (N = 4/group) were pretreated with saline (Sal) or SN79 (SN, 3 mg/kg, i.p.). After 15 min, the mice were then treated with Sal or METH (5 mg/kg, i.p.). This treatment schedule was repeated four times at 2 h intervals. One week later, the brains were removed and stained for DAT immunoreactivity. Average optical density readings (mean ± SEM) are shown. ***P<0.001 vs. saline, ###P<0001 vs. METH.
3.8. SERT immunohistochemistry
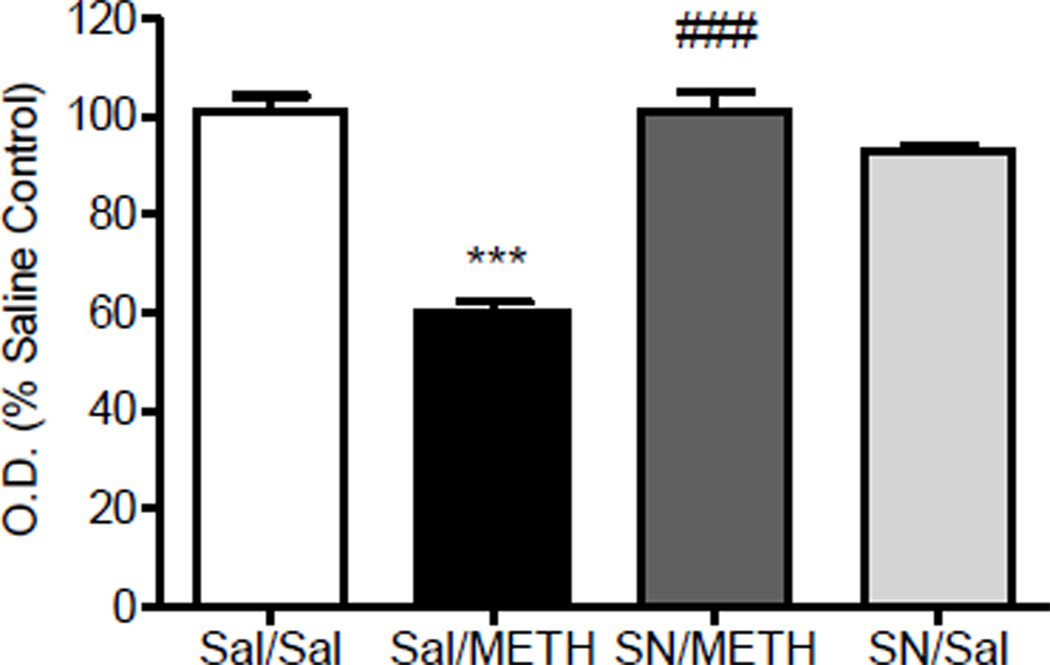
Fig. 6 illustrates the effects of methamphetamine and SN79 on SERT immunoreactivity in the mouse striatum. One-way ANOVA revealed a significant difference between the treatment groups (F(3,54) = 53.73, P<0.0001). Post-hoc Tukey’s multiple comparison tests confirmed that methamphetamine caused a significant reduction in SERT immunoreactivity compared to control animals (q = 15.07, P<0.001). Pretreatment with SN79 significantly attenuated the methamphetamine-induced neurotoxicity (q = 14.81, P<0.001), whereas treatment with SN79 alone had no significant effects on SERT expression (q = 2.91, n.s.).
Figure 6.
5-HT transporter (SERT) Immunohistochemistry: Effect of SN79 pretreatment on methamphetamine (METH)-induced decrease in striatal SERT levels. Male, Swiss Webster mice (N = 4/group) were pretreated with saline (Sal) or SN79 (SN, 3 mg/kg, i.p.). After 15 min, the mice were then treated with Sal or METH (5 mg/kg, i.p.). This treatment schedule was repeated four times at 2 h intervals. One week later, the brains were removed and stained for SERT immunoreactivity. Average optical density readings (mean ± SEM) are shown. ***P<0.001 vs. saline, ###P<0001 vs. METH.
3.9. Correlation between body temperature and striatal monoamine levels
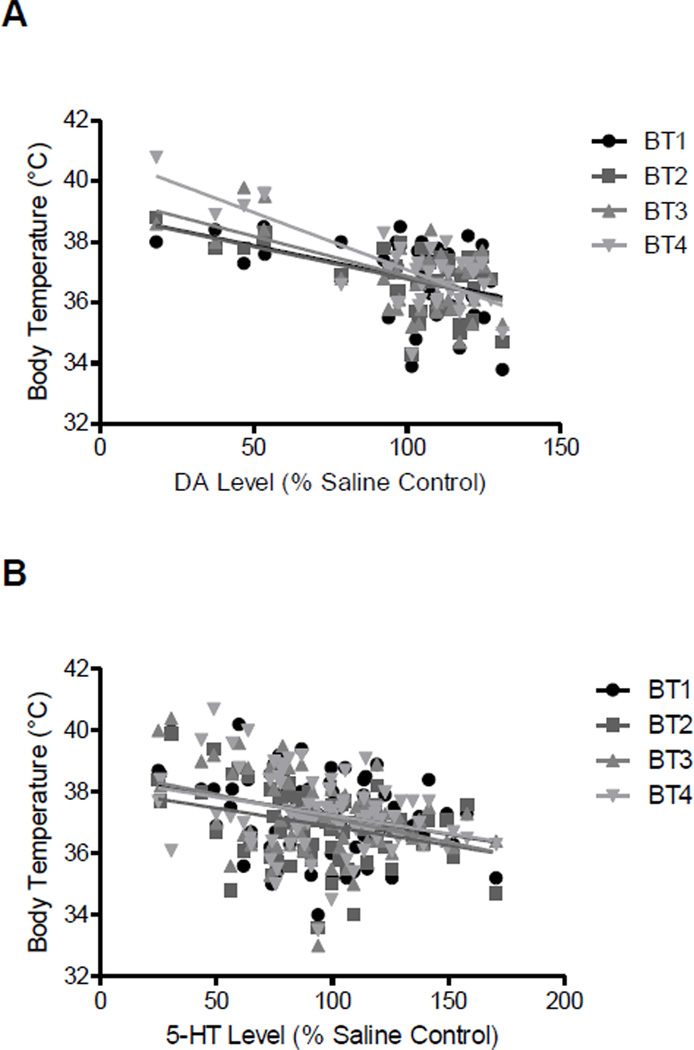
Table 1 summarizes the mean ± S.E.M. body temperatures and corresponding striatal dopamine and 5-HT levels for each of the experimental groups, with the statistical results from the correlation shown at the bottom of the table. There was a significant correlation between body temperature at all four time points and striatal dopamine levels one week later (r2 0.17 to 0.57, P<0.01 to P<0.001). There was also a significant correlation between body temperature at all four time points and striatal 5-HT levels one week later (r2 0.08 to 0.14, P<0.05 to P<0.001). The correlation plot for each of the comparisons is shown in Fig. 7, with the body temperatures and corresponding striatal dopamine (panel A) and 5-HT (panel B) levels measured one week later for each animal graphed.
Table 1. Correlation of body temperature and striatal (A) dopamine and (B) 5-HT levels of mice pretreated with saline or SN79 (1–10 mg/kg) prior to saline or METH (5 and 10 mg/kg).
The values from individual mice were used to calculate the correlation between core body temperature (BT) at each of the four time points and striatal (A) dopamine (DA) and (B) 5-HT levels measured one week later. The table summarizes the mean ± S.E.M. for each of the groups, with the statistical results shown at the bottom. The numbers in the parentheses of the treatment group column represent doses (in mg/kg, i.p.) of SN79 or methamphetamine (METH).
| A. | |||||
|---|---|---|---|---|---|
| Treatment Group | DA (% Saline Control) |
BT1 | BT2 | BT3 | BT4 |
| Sal/Sal | 100 ± 6.9 | 37.86 ± 0.24 | 37.24 ± 0.29 | 37.06 ± 0.29 | 36.8 ± 0.30 |
| SN79(1)/Saline | 112.39 ± 4.55 | 37.76 ± 0.17 | 37.16 ± 0.14 | 36.92 ± 0.19 | 36.9 ± 0.26 |
| SN79(3)/Saline | 112.04 ± 4.75 | 37.06 ± 0.33 | 36.78 ± 0.31 | 36.78 ± 0.41 | 36.8 ± 0.26 |
| SN79(10)/Saline | 111.87 ± 4.7 | 35.2 ± 0.48 | 35.42 ± 0.41 | 35.54 ± 0.35 | 35.6 ± 0.33 |
| Saline/METH(5) | 41.95 ± 6.59 | 37.96 ± 0.23 | 38.14 ± 0.19 | 38.86 ± 0.34 | 39.62 ±0.32 |
| SN79(1)/METH(5) | 105.58 ± 3.70 | 37.32 ± 0.26 | 37.14 ± 0.23 | 37.24 ± 0.39 | 37.52 ± 0.24 |
| SN79(3)/METH(5) | 108.48 ± 4.53 | 36.38 ± 0.36 | 36.26 ± 0.36 | 36.02 ± 0.32 | 36.8 ± 0.35 |
| SN79(10)/METH(5) | 119.58 ± 4.76 | 34.94 ± 0.32 | 35.8 ± 0.38 | 36.24 ± 0.32 | 36.16 ± 0.35 |
| P | <0.01 | <0.0005 | <0.0001 | <0.0001 | |
| Pearson r | −0.4164 | −0.5291 | −0.5819 | −0.7555 | |
| r2 | 0.1734 | 0.28 | 0.3386 | 0.5708 | |
| B. | |||||
|---|---|---|---|---|---|
| Treatment Group | 5-HT (% Saline Control) |
BT1 | BT2 | BT3 | BT4 |
| Sal/Sal | 100 ± 6.86 | 37.14 ± 0.19 | 36.82 ± 0.15 | 36.65 ± 0.20 | 37 ± 0.19 |
| SN79(1)/Saline | 115.59 ± 7.95 | 37.32 ± 0.20 | 37.12 ± 0.20 | 36.99 ± 0.16 | 37.24 ± 0.12 |
| SN79(3)/Saline | 107.58 ± 6.70 | 37.4 ± 0.15 | 37.11 ± 0.13 | 36.94 ± 0.14 | 37.14 ± 0.13 |
| SN79(10)/Saline | 99.51 ± 11.80 | 35.85 ± 0.31 | 35.46 ± 0.36 | 35.72 ± 0.36 | 35.6 ± 0.34 |
| Saline/METH(10) | 55.76 ± 6.14 | 38.78 ± 0.22 | 38.45 ± 0.20 | 39.17 ± 0.18 | 38.83 ± 0.34 |
| SN79(1)/METH(10) | 93.18 ± 5.63 | 38.56 ± 0.14 | 37.37 ± 0.22 | 37.9 ± 0.27 | 38.16 ± 0.31 |
| SN79(3)/METH(10) | 95.81 ± 6.91 | 37.31 ± 0.27 | 36.43 ± 0.34 | 37.27 ± 0.34 | 37.53 ± 0.33 |
| SN79(10)/METH(10) | 108.08 ± 8.83 | 35.88 ± 0.26 | 36.33 ± 0.24 | 36.71 ± 0.18 | 36.63 ± 0.17 |
| P | <0.01 | <0.005 | <0.001 | <0.05 | |
| Pearson r | −0.3025 | −0.3208 | −0.3735 | −0.2875 | |
| r2 | 0.09152 | 0.1029 | 0.1395 | 0.08267 | |
Figure 7.
Relationship between core body temperature taken after each of the four drug administrations (BT1-4) and A, striatal dopamine (DA) levels or B, striatal 5-HT levels measured one week later. The graphs depict the relationship for individual animals corresponding to the data summarized in Table 1.
3.10. Post-treatment studies
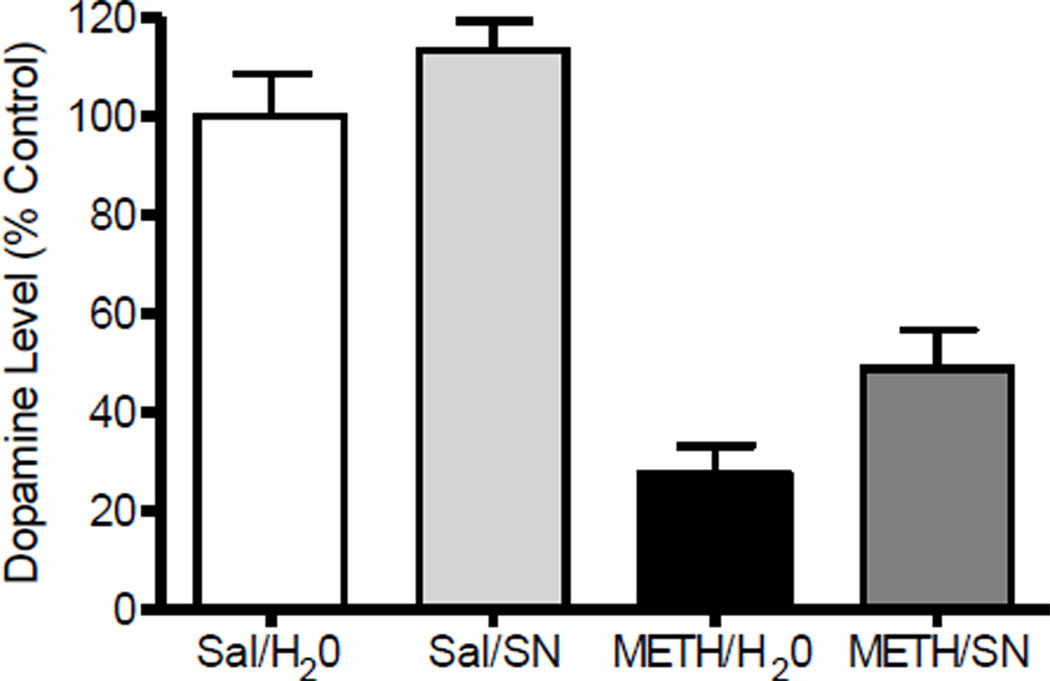
Fig. 8 shows the effects of oral administration of SN79 when administered following neurotoxic dosing with methamphetamine or control injections of saline. One-way ANOVA indicated a significant difference between the experimental groups (F(3,34) = 32.46, P<0.001). Post-hoc Tukey’s test confirmed that the difference between the control and methamphetamine groups differed significantly (q = 10.10, P<0.005). Post-treatment with SN79 caused a 25% improvement in dopamine levels relative to animals exposed to methamphetamine alone (post- treatment with distilled water), but the change was not statistically significant using post-hoc Tukey’s test (q = 2.89, n.s.).
Figure 8.
Effects of post-treatment with SN79 on methamphetamine (METH)-induced striatal dopamine (DA) depletion. Male, Swiss Webster mice (N = 10/group) were treated (i.p.) with saline (Sal) or METH (5 mg/kg) at 2 h intervals a total of four times. Beginning 3 h after the last injection, mice were orally administered distilled water (H2O) or SN79 (SN, 10 mg/kg) every 8 h for one week. The striata were dissected from each mouse and DA levels measured. Data was reported as mean ± SEM. ***P<0.005 vs. Sal/H2O.
4. Discussion
The current study demonstrates that pretreatment with SN79 can mitigate methamphetamine-induced lethality, hyperthermia, and striatal neurotoxicity (reductions in dopamine and 5-HT levels and DAT and SERT expression levels in the striatum). When administered as a post-treatment under oral dosing conditions, SN79 elicited partial recovery in striatal dopamine depletions caused by methamphetamine. These data thus suggest that σ receptors can be targeted to reduce the effects of methamphetamine.
In contrast to the protective effects of SN79 and other σ receptor antagonists, pretreatment with the well established σ receptor agonist, DTG, increased the lethal effects of methamphetamine, but had no significant effects on methamphetamine-induced hyperthermia. This suggests that the lethal effects of methamphetamine may involve, at least in part, direct activation of σ receptors. However, the observed deaths are unlikely to be the result of σ receptor activated hyperthermia since DTG had no significant effects on this endpoint. Nevertheless, downstream intervention with putative σ receptor antagonists, such as SN79, can attenuate both the lethal and hyperthermic effects of methamphetamine.
In this study, SN79 pretreatment protected against four markers of methamphetamine-induced striatal dopamine and 5-HT nerve terminal degeneration (dopamine and 5-HT depletion and reductions in DAT and SERT expression levels). This neuroprotective effect is consistent with that observed with other σ receptor putative antagonists such as AC927 and CM156 (Matsumoto et al., 2008; Kaushal et al., 2011b), suggesting that similarly to these earlier characterized compounds, potential mechanisms targeted by SN79 to convey neuroprotection may include reductions in reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation, and caspase activation (Kaushal et al., 2012).
SN79 pretreatment also attenuated methamphetamine-induced hyperthermia, which is consistent with the effects of other σ receptor antagonists, including AC927 and CM156 (Matsumoto et al., 2008; Kaushal et al., 2011b). On its own, the putative σ receptor antagonist SN79 at the highest dose tested (10 mg/kg, i.p.) caused hypothermia, whereas the σ receptor agonist DTG showed a trend towards hyperthermia. The localization of σ receptors in the hypothalamus (McLean and Weber, 1988), a part of the brain known to have an important role in thermoregulation, is consistent with the ability of σ ligands to modulate body temperature.
The effects of SN79 alone on body temperature suggest that at higher doses, SN79 may act as an inverse agonist at σ receptors or block basal tone. This would be consistent with the observation that at the same dose (10 mg/kg), SN79 on its own caused sedative effects in mice (Kaushal et al., 2011a). σ Receptors are densely located in the motor areas of the brain, and affect motor function (McLean and Weber, 1988; Walker et al., 1990). The sedative and hypothermic effects of SN79 at 10 mg/kg may thus be explained by either inverse agonist activity at σ receptors or antagonism of tonic activation of endogenous systems.
Although the mechanisms involved in methamphetamine-induced neurotoxicity is not very clearly understood, hyperthermia is considered an important factor (Bowyer et al., 1994). Elevated temperature can exacerbate the neurotoxic effects of methamphetamine by increasing ROS/RNS generation and DAT activity, and also by disrupting the blood brain barrier (Fleckenstein et al., 1997; Kiyatkin et al., 2007; Numachi et al., 2007; Sharma et al., 2007; Xie et al., 2000). Previous pharmacological and ambient temperature controlled studies have suggested that attenuation of methamphetamine-induced hyperthermia can provide protection against its neurotoxic effects (Bowyer et al., 1994). Correlation analysis of the temperature and dopamine and 5-HT depletion data in the present study also suggested that the neuroprotective effects of SN79 are related to its ability to decrease methamphetamine-induced hyperthermia. However, correlation does not always mean causation; temperature modulation may be an important but not obligatory factor in the neuroprotective effects of SN79.
In addition to modulating body temperature at a systems level, σ receptors play an important role in cell death pathways in tumor cell models (van Waarde et al., 2010). In fact, when using an in vitro model of methamphetamine neurotoxicity in which temperature was held constant, AC927, an earlier tested σ receptor putative antagonist that attenuated methamphetamine-induced neurotoxicity and hyperthermia in vivo (Matsumoto et al., 2008), mitigated various effects of methamphetamine including cytotoxicity (Kaushal et al., 2012), demonstrating that neuroprotection can occur independently of changes in temperature. A similar pattern of in vitro results has been shown with SN79 in which SN79 attenuated methamphetamine-induced cytotoxicity and caspases when temperature was held constant (Kaushal et al., 2011c), confirming that the neuroprotection can occur independent of changes in temperature. Moreover, the exacerbated methamphetamine-induced cytotoxicity resulting from elevations in the temperature, were still attenuated by SN79. Therefore, SN79 may provide neuroprotection in vivo by a dual mechanism of decreasing hyperthermia as well as blocking intracellular, neurotoxic cascades.
In addition to acute hyperthermic effects and long lasting neurotoxic effects, methamphetamine at higher doses also causes lethality. The σ receptor agonist DTG exacerbated, whereas SN79 attenuated, the lethal effects of methamphetamine, again indicating an important role for σ receptors in the lethal effects of methamphetamine.
The ability of SN79 to attenuate methamphetamine-induced hyperthermia, lethality and dopaminergic and serotonergic neurotoxicity provides support for it being a novel drug lead against numerous harmful effects of methamphetamine. Since SN79 has good bioavailability, a long half life, and can also attenuate various behavioral and toxic effects of cocaine (Kaushal et al., 2011a), this further strengthens its potential as a drug candidate against many effects of psychostimulants. Further studies were therefore performed to determine the effectiveness of SN79 under more clinically relevant conditions, where subjects have already been exposed to methamphetamine. The post-treatment results obtained using SN79, although not quite statistically significant, indicated a trend towards neuroprotection. This may have important implications in a clinical setting, where a 25% increase in striatal dopamine levels may translate into an asymptomatic condition. However, further studies are needed to study the long term effect of SN79 post-treatment on methamphetamine-induced neurotoxicity and its functional consequences.
Methamphetamine exposure has recently been shown to be an important risk factor for Parkinson’s disease (Callaghan et al., 2010). Studies in the literature have shown σ ligands to provide improvement in animal models of Parkinson’s disease and other neurological disorders associated with neurotoxicity and/or monoaminergic deficiencies (Guitart et al., 2004; Mishina et al., 2005). Therefore, SN79 alone in the long term or in combination with already existing therapies may be effective for treating methamphetamine-induced parkinsonism or other motor and cognitive disorders. Although partial recovery is observed with SN79 post-treatment, the mechanism of neuroprotection is not known and needs to be studied in the future.
In conclusion, SN79 is a potentially promising drug candidate to mitigate many effects of methamphetamine. Since DTG exacerbated the lethal effects of methamphetamine, but not methamphetamine-induced hyperthermia, this study implicates σ receptors in the direct modulation of some effects of methamphetamine like lethality, while having a modulatory role which can mitigate other methamphetamine-induced effects such as hyperthermia and neurotoxicity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldwin HA, Colado MI, Murray TK, Souza RJ, Grren AR. Striatal dopamine release in vivo following neurotoxic doses of methamphetamine and effect of the neuroprotective drugs, chlormethiazole and dizocilpine. British Journal of Pharmacology. 1993;108:590–596. doi: 10.1111/j.1476-5381.1993.tb12847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer JF, Davis DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies on the role of hyperthermia in methamphetamine neurotoxicity. Journal of Pharmacology and Experimental Therapeutics. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Brunswick DJ, Benmansour S, Tejani-Butt SM, Hauptmann M. Effects of high-dose methamphetamine on monoamine uptake sites in rat brain measured by quantitative autoradiography. Synapse. 1992;11:287–293. doi: 10.1002/syn.890110404. [DOI] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sajeev G, Kish SJ. Incidence of Parkinson’s disease among hospital patients with methamphetamine-use disorders. Movement Disorders. 2010;25:2333–2339. doi: 10.1002/mds.23263. [DOI] [PubMed] [Google Scholar]
- Cappon GD, Pu C, Vorhees CV. Time-course of methamphetamine-induced neurotoxicity in rat caudate-putamen after single-dose treatment. Brain Research. 2000;863:106–111. doi: 10.1016/s0006-8993(00)02107-7. [DOI] [PubMed] [Google Scholar]
- Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Wilkins DG, Gibb JW, Hanson GR. Interaction between hyperthermia and oxygen radical formation in the 5-hydroxytryptaminergic response to a single methamphetamine administration. Journal of Pharmacology and Experimental Therapeutics. 1997;283:281–285. [PubMed] [Google Scholar]
- Fukumura M, Cappon GD, Pu C, Broening HW, Voorhees CV. A single dose model of methamphetamine-induced neurotoxicity in rats: effects on neostriatal monoamines and glial fibrillary acidic protein. Brain Research. 1998;806:1–7. doi: 10.1016/s0006-8993(98)00656-8. [DOI] [PubMed] [Google Scholar]
- Gough B, Imam SZ, Blough D, Slikker W, Jr, Ali SF. Comparative effect of substituted amphetamines (PMA, MDMA, and METH) on monoamines in rat caudate: a microdialysis study. Annals of the New York Academy of Sciences. 2002;965:410–420. doi: 10.1111/j.1749-6632.2002.tb04182.x. [DOI] [PubMed] [Google Scholar]
- Guitart X, Codony X, Monroy X. Sigma receptors: biology and therapeutic potential. Psychopharmacology. 2004;174:301–319. doi: 10.1007/s00213-004-1920-9. [DOI] [PubMed] [Google Scholar]
- Hart CL, Marvin CB, Silver R, Smith EE. Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology. 2012;37:586–608. doi: 10.1038/npp.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Ladenheim B, Rothman RB, Epstein C, Cadet JL. Methamphetamine-induced serotonin neurotoxicity is mediated by superoxide radicals. Brain Research. 1995;677:345–347. doi: 10.1016/0006-8993(95)00218-f. [DOI] [PubMed] [Google Scholar]
- Kaushal N, Robson MJ, Vinnakota H, Narayanan S, Avery BA, McCurdy CR, Matsumoto RR. Synthesis and pharmacological evaluation of 6-acetyl-3-(4-(4-(4-fluorophenyl)piperazin-1-yl)butyl)benzo[d]oxazol-2-(3H)-one (SN79), a cocaine antagonist, in rodents. AAPS Journal. 2011a;13:336–346. doi: 10.1208/s12248-011-9274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Seminerio MJ, Shaikh J, Medina MA, Mesangeau C, Wilson LL, McCurdy CR, Matsumoto RR. CM156, a high affinity sigma ligand, attenuates the stimulant and neurotoxic effects of methamphetamine in mice. Neuropharmacology. 2011b;61:992–1000. doi: 10.1016/j.neuropharm.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Elliott M, Robson MJ, Iyer AK, Rojanasakul Y, Coop A, Matsumoto RR. AC927, a sigma receptor ligands, blocks methamphetamine-induced release of dopamine and generation of reactive oxygen species in NG108-15 cells. Molecular Pharmacology. 2012;81:299–308. doi: 10.1124/mol.111.074120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Matsumoto RR. Role of sigma receptors in methamphetamine-induced neurotoxicity. Current Neuropharmacology. 2011;9:54–57. doi: 10.2174/157015911795016930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, McCurdy CR, Matsumoto RR. SN79 attenuates the neurotoxic effect of methamphetamine: In vivo and in vitro studies. Society for Neuroscience meeting 2011 Program #367.03.2011. [Google Scholar]
- Kita T, Matsunari Y, Saraya T, Shimada K, O’Hara K, Kubo K, Wagner GC, Nakashima T. Methamphetamine-induced striatal dopamine release, behavior changes and neurotoxicity in BALB/c mice. International Journal of Developmental Neuroscience. 2000;18:521–530. doi: 10.1016/s0736-5748(00)00022-8. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL, Sharma HS. Brain edema and breakdown of the blood-brain barrier during methamphetamine intoxication: critical role of brain hyperthermia. European Journal of Neuroscience. 2007;26:1242–1253. doi: 10.1111/j.1460-9568.2007.05741.x. [DOI] [PubMed] [Google Scholar]
- Kovachich GB, Aronson CE, Brunswick DJ. Effects of high-dose methamphetamine administration on serotonin uptake sites in rat brain measured using [3H]cyanoimipramine autoradiography. Brain Research. 1989;505:123–129. doi: 10.1016/0006-8993(89)90122-4. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Research Reviews. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Cho AK, Melaga W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. Journal of Neuroscience. 1995;15:1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Friedman MJ, Pouw B, De Costa BR, Bowen WD. Conformationally restricted analogs of BD1008 and an antisense oligodeoxynucleotide targeting σ1 receptor prodice anti-cocaine effects in mice. European Journal of Pharmacology. 2001;419:163–174. doi: 10.1016/s0014-2999(01)00968-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Shaikh J, Wilson LL, Vedam S, Coop A. Attenuation of methampehtamine-induced effects through the antagonism of sigma (σ) receptors: Evidence from in vivo and in vitro studies. European Neuropsychopharmacology. 2008;18:871–881. doi: 10.1016/j.euroneuro.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean S, Weber E. Autoradiographic visualization of haloperidol-sensitive sigma receptors in guinea-pig brain. Neuroscience. 1988;25:259–269. doi: 10.1016/0306-4522(88)90024-3. [DOI] [PubMed] [Google Scholar]
- Mishina M, Ishiwata K, Ishii K, Kitamura S, Kimura Y, Kawamura K, Oda K, Sasaki T, Sakayori O, Hamamoto M, Kobayashi S, Katayama Y. Function of sigma1 receptors in Parkinson’s disease. Acta Neurologica Scandinavia. 2005;112:103–107. doi: 10.1111/j.1600-0404.2005.00432.x. [DOI] [PubMed] [Google Scholar]
- Nguyen EC, McCracken KA, Pouw B, Matsumoto RR. Involvement of sigma receptors in the actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Numachi Y, Ohara A, Yamashita M, Fukushima S, Kobayashi H, Hata H, Watanabe H, Hall FS, Lesch KP, Murphy DL, Uhl GR, Sora I. Methamphetamine-induced hyperthermia and lethal toxicity: role of the dopmaine and serotonin transporters. European Journal of Pharmacology. 2007;572:120–128. doi: 10.1016/j.ejphar.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Research. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Robson MJ, Noorbakhsh B, Seminerio MJ, Matsumoto RR. Sigma-1 receptors: potential targets for the treatment of substance abuse. Current Pharmaceutical Design. 2012;18:902–919. doi: 10.2174/138161212799436601. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychological Reviews. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Commins DL, Vosmer G, Axt K, Marek G. Neurotoxicity in dopamine and 5-hydroxytryptamine terminal fields: a regional analysis in nigrostriatal and mesolimbic projections. Annals of the New York Academy of Sciences. 1988;537:161–172. doi: 10.1111/j.1749-6632.1988.tb42104.x. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Sjoquist PO, Ali SF. Drugs of abuse-induced hyperthermia, blood-brain barrier dysfunction and neurotoxicity: neuroprotective effects of a new antioxidant compound H-290/51. Current Pharmaceutical Design. 2007;13:1903–1923. doi: 10.2174/138161207780858375. [DOI] [PubMed] [Google Scholar]
- van Waarde A, Rybczynska AA, Ramakrishnan N, Ishiwata K, Elsinga PH, Dierckx RA. Sigma receptors in oncology: therapeutic and diagnostic applications of sigma ligands. Current Pharmaceutical Design. 2010;16:3519–3537. doi: 10.2174/138161210793563365. [DOI] [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Walker FO, Matsumoto RR, De Costa BR, Rice KC. Sigma receptors: biology and function. Pharmacological Reviews. 1990;42:355–402. [PubMed] [Google Scholar]
- Xie T, McCann UD, Kim S, Yuan J, Ricaurte GA. Effect of temperature on dopamine transporter function and intracellular accumulation of methamphetamine: implications for methamphetamine-induced dopaminergic neurotoxicity. Journal of Neuroscience. 2000;20:7838–7845. doi: 10.1523/JNEUROSCI.20-20-07838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]