Summary
Preventive actions for chronic diseases hold the promise of improving lives and reducing healthcare costs. For several diseases, including breast cancer, multiple risk and protective factors have been identified by epidemiologists. The impact of most of these factors has yet to be fully understood at the organism, tissue, cellular and molecular levels. Importantly, combinations of external and internal risk and protective factors involve cooperativity thus, synergizing or antagonizing disease onset. Models are needed to mechanistically decipher cancer risks under defined cellular and microenvironmental conditions. Here, we briefly review breast cancer risk models based on 3D cell culture and propose to improve risk modeling with lab-on-a-chip approaches. We suggest epithelial tissue polarity, DNA repair and epigenetic profiles as endpoints in risk assessment models and discuss the development of ‘risks-on-chips’ integrating biosensors of these endpoints and of general tissue homeostasis. Risks-on-chips will help identify biomarkers of risk, serve as screening platforms for cancer preventive agents, and provide a better understanding of risk mechanisms, hence resulting in novel developments in disease prevention.
Introduction
The worldwide rise in cancer incidence can only be halted if an organ at risk never becomes a neoplasm. Reducing incidence is the focus of primary prevention, which constitutes a promising and cost-effective approach for healthcare and would complement secondary prevention (i.e., disease detection) and tertiary prevention (i.e., disease control) 1,2,3. However, successful interventions in primary prevention require extensive knowledge of risk and protective factors for any given human population. In contrast to lung and cervical cancers that can each be linked to a main single cause and for which preventive actions are readily identifiable, breast cancer is characterized by a multiplicity of risk factors 4,5 (Figure 1), and large individual variations in susceptibility render the implementation of preventive interventions challenging. Reliable biomarkers are needed to assess risk, screen for protective agents, and monitor the effectiveness of interventions. New in vitro human cell-based models are necessary to achieve these goals.
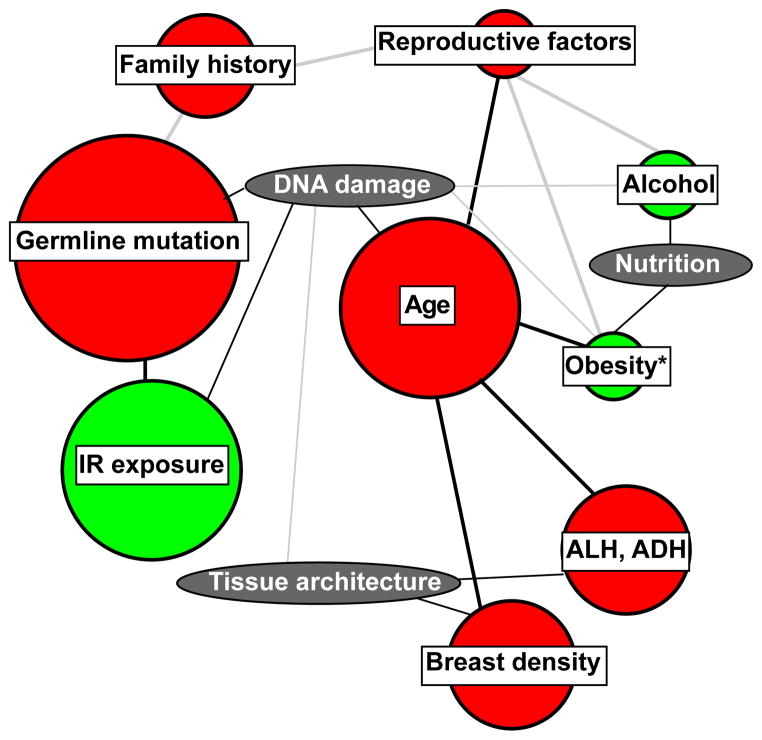
Figure 1.
Flow chart representation of commonly accepted breast cancer risk factors (circles), linked to major tissue and environmental features (ovals) involved in breast cancer onset. The estimated relative risks 5 are represented by circle sizes and interactions between risk factors are tentatively represented with lines. Gray lines indicate limited experimental support. Red and green colors indicate unmodifiable (or not easily modifiable) and modifiable risk factors, respectively. *, Increased risk linked to obesity postmenopause only.
Three major biological aspects have been linked so far to early epithelial changes that control breast cancer development and that can be assessed with human cell culture-based assays typically providing higher throughput compared to animal models. The first aspect that can be used as readout of cancer risk in the breast epithelium is tissue polarity (Figure 2A). The architecture of the mammary epithelium reflects the primary function of the tissue, i.e., lactation. It consists of a layer of luminal cells surrounded by a layer of contractile myoepithelial cells. This epithelium is organized into grape-like milk-secreting gland units (acini) connected to the nipple by branched ductal structures. The main architectural feature of the epithelium is basoapical tissue polarity that not only provides directionality for milk secretion but also controls key processes such as cell quiescence, survival and differentiation 6. Certain tissue models produced using three-dimensional (3D) cell culture protocols 7 recapitulate epithelial polarity. These models permitted the demonstration that disruption of the apical pole of the polarity axis signified by the disruption of cell-cell tight junctions normally located against the lumen of the mammary epithelium is necessary for cell cycle entry 8. Altered apical polarity was also associated with tissues from women who carry a mutation in the breast cancer susceptibility gene BRCA1 9. Moreover, apical polarity was disrupted by a dietary fatty acid linked to increased breast cancer risk 10. Therefore, it is expected that factors altering the polarity axis generate a measurable neoplastic risk for the breast tissue.
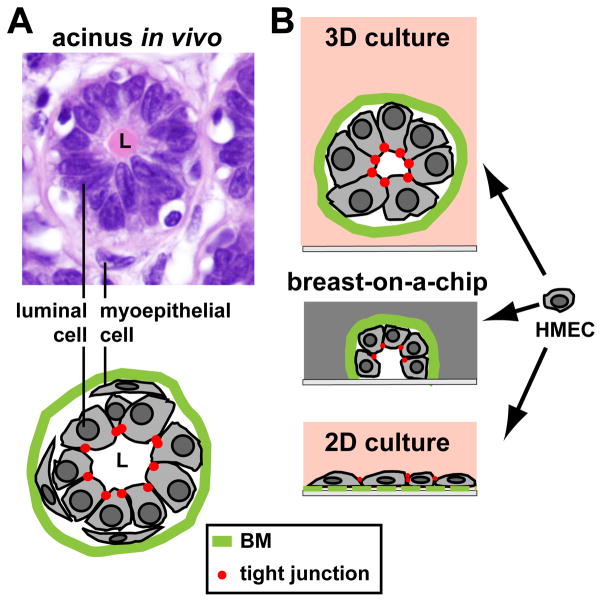
Figure 2.
Cell culture models for the breast epithelium. A Hematoxylin & Eosine staining of normal breast tissue showing the organization of luminal (inner layer) and myoepithelial (outer later) cells in the smallest structural and functional unit (acinus) of the breast glandular epithelium. On the drawing, the basement membrane (BM) and apical tight junctions are represented. B Cultures of human mammary epithelial cells (HMECs): flat cell monolayers (2D culture) do not recreate the organization of the glandular and ductal epithelia. In contrast, three-dimensional (3D) cultures of HMECs in compliant hydrogels yield growth-arrested spheroid structures with basal polarity (cell-BM contacts) and apical polarity (apical tight junctions) mimicking glandular structures or acini. When placed on chip devices resembling a ductal system (breast-on-a-chip), cells divide and spread alongside the walls of the hemichannel, leading to a growth-arrested basoapically polarized monolayer of cells.
The second aspect to take into account in risk assessment in tissue models is DNA repair. Chest exposures to DNA damaging radiations strongly increase the risk of developing breast cancer, as demonstrated in cohort studies of atomic bomb survivors in Japan 11 and for patients treated with chest radiations for pediatric or young adult cancers 12, hence illustrating the critical cancer suppression function of DNA repair in the breast. Germline mutations in breast cancer susceptibility genes are linked to the highest relative risks and concern approximately 5% of patients with breast cancer 13. Most of these genes including BRCA1, BRCA2, TP53, ATM, and RAD51 function in DNA repair pathways 14, highlighting the importance of genome safeguard mechanisms for breast homeostasis. Silencing of the BRCA tumor suppressors has been reported in sporadic cancers 15; therefore even in the absence of mutations, defects in DNA repair linked to the epigenetic regulation of gene transcription may drive cancer progression. Epigenetic regulation encompasses notably DNA methylation and more than two dozen histone modifications (e.g., acetylation, methylation) 16. Other examples of epigenetic modification that modulate the DNA damage response (DDR) are those of histone methylase EZH2, a potential marker of breast cancer risk 17 affecting DNA repair 18 and modifications induced by nightshift, a potential breast cancer risk factor 19.
The epigenome itself represents a third aspect to consider in risk assessment using tissue-based models. It is well accepted that cancer ultimately arises from epigenetic events that modify the expression of a host of genes. Epigenetic modifications are understood as chromatin modifications heritable through mitosis that influence gene expression without changes in DNA sequence. Changes in levels of EZH2, an enzyme that methylates histone 3 on lysine 27, have been monitored in the breast several years before the appearance of cancer 20. The importance of measuring epigenetic modifications in risk models is strengthened by the fact that intrinsic (age, germline mutations) and environmental (nutrition, stress, inactivity, pollutants) breast cancer risk factors have been shown to influence epigenetic mechanisms of gene expression control 21,22,1. Many studies have measured epigenetic changes in the blood under conditions that might favor oncogenesis in the body, but it is essential to determine epigenetic changes that might occur specifically in the breast epithelium in order to decipher mechanisms of breast cancer onset. Importantly, modulators of risk might induce permanent epigenetic changes in the mammary gland, meaning changes that can be propagated through cell division and even to the offspring during specific periods of the life of an individual. For instance obesity significantly increases breast cancer risk upon menopause. But the effect of obesity on risk may depend on the life stage when weight was gained 23. Animal models have revealed that high fat intake in pregnant female rats increases the incidence of mammary tumors in their offspring and changes the DNA methylation pattern of the mammary tissue of the offspring, suggesting that modifications introduced during fetal life have created a sustained risk in the mammary gland 24. The impact of other modulators of breast cancer risk is also dependant on the life stage, it is greater before puberty for nutrients 1 and in middle life for breast density 25.
It is possible to measure different degrees of alteration of tissue polarity corresponding to various impacts on tissue homeostasis 6 as well as the accumulation of DNA and epigenetic alterations. This incremental evaluation of risk will facilitate the study of cooperativity among modulators of risk. The challenge will be to develop risk models that integrate modulators of risk studied under physiologically relevant microenvironments and live evaluation of the molecular readouts of breast cancer risk. There exists few 3D tissue culture models that mimic cancer risk situations for the breast; however, organs-on-chips appear more amenable to the development of high-throughput risk models as they can be modified to integrate biosensors to monitor changes in tissue architecture, genome integrity, and epigenetic marks. Risk modeling is a new frontier for on-a-chip models.
3D cell culture models that reproduce breast tissues at risk
Homeostasis of the breast depends on tightly controlled tissue architecture since cell-extracellular matrix (ECM) and cell-cell adhesion junctions influence gene expression 26. Perturbation of the epithelial polarity axis established through cell adhesion is a necessary step for cancer onset 6,27,8,9. Therefore, loss of polarity has been studied in simple homotypic models with non-neoplastic mammary epithelial cells placed in microenvironments with carefully chosen soluble factors and ECM components (Figure 2A). Several human mammary epithelial cell (HMEC) lines derived from breast cancer-free mammary tissues have been used for the study of mechanisms leading to cancer onset. Possible cell lines encompass MCF-10A 28, MCF-12A 29, 184A1 30, and HMT-3522 S1 31. Note that the popular MCF-10A cells often lack the capability of forming apical tight junction complexes 32,33; therefore, assays focused on phenotypically normal differentiation with this cell line should be interpreted with caution. In classical 3D culture acinar/glandular differentiation is achieved by ligation of cell membrane-bound integrins to basement membrane (BM) components of the ECM in hydrogels leading to the formation of smooth spheroids with diameters similar to those of acini and alveoli in the breast 34,33,7 (Figure 2B). Acini produced with these simple protocols have characteristics of their counterpart in vivo, notably growth arrest, polarization, and lumen formation and hence, approximate well the normal mammary gland. We have been using a model producing fully polarized acini to assess the impact of nutrients considered as either risk or protective factors in the breast. Arachidonic acid, the major polyunsaturated fatty acid in the Western diet increased in obese individuals, disrupts apical polarity as shown using Raman spectromicroscopy. This technique permits the analysis of polarity in live and stain-free acini produced by high-throughput (HTP) 3D culture 33 based on changes in lipid orders in the basal and apical cell membranes 10.
The association between increased breast density (i.e., high collagen I and epithelial density) 35 and heightened breast cancer risk has strengthened the importance of producing models in which the mechanical properties of the microenvironment can be manipulated. Stiffening of the ECM is known to affect cytoskeleton dynamics, integrin signaling pathways, and nuclear architecture 36. Cells compensate ECM stiffening by adapting their cytoskeleton (mechanoreciprocity), which profoundly affects gene expression and cell behavior 37. Risk associated with breast density can be compared to desmoplastic tissue, as observed for instance in alterations adjacent to breast tumors and characterized by high content in fibroblasts and ECM molecules as well as reduced adipocyte size and numbers. Engelbreth-Holm-Swarm (EHS) hydrogels 38 commonly used for 3D culture of non-neoplastic cells have an elastic modulus similar to the endogenous BM; this ECM elastic modulus can be fine-tuned by chemical crosslinking 39, thereby reproducing a range of 0.2 – 4 kPa measured in vivo from soft breast tissues to stiff tumors 40. In addition, the choice of materials for cell culture substrates, from soft polydimethylsiloxane (PDMS) to stiff plastics may help further determine mechanical properties of the system. Recreating high matrix density or matrix stiffening in 3D culture is possible by adding, for instance, nonmetabolizable L-ribose to collagen/reconstituted BM gels 41. However, measuring risk indicators such as polarity loss, altered genome maintenance, and epigenetic modifications in the context of stiff or compliant ECM mimicking changes in breast density has not been reported.
While most in vitro studies on breast cancer risk have focused so far on epithelial cells, the complex interplay between epithelial cells and the stroma is increasingly considered to influence breast cancer onset and integrated in studies with risk modulators 42. For instance adipocytes secrete soluble growth factors, notably adipokines and estrogens. They are often in direct contact with the BM and participate in its biosynthesis, which implies that changes in adipocytes linked to obesity may influence epithelial architecture, in turn regulating the DDR 43. Adipocytes also secrete saturated fatty acids that have been shown to down-regulate the DDR 44. Approaches that combine stroma and epithelial cells in 3D culture have been pursued but not applied to the study of risk. Krause and collaborators 45 have combined MCF10A cells, fibroblasts, and adipocytes with different hydrogels in several 3D culture schemes and characterized cell-ECM and cell-cell interactions required for epithelial tubular vs. glandular morphogenesis. Similarly, a coculture system based on collagen/hyaluronic acid hydrogels was shown to support cell differentiation in heterotypic cultures of murine MEC and adipocytes 46. While further developing these coculture systems, it will be of particular interest to reproduce obese and lean stromal microenvironments that modulate breast cancer risk. Possible approaches might include artificially modulating leptin and adiponectin balance, using adipocytes from animals fed regular or high-fat, high-sugar diets and using adipocytes from reduction or, in a limited capacity, augmentation mammoplasty from lean and obese women.
A particularly difficult aspect of studying breast cancer risk is the modeling of breast epithelia corresponding to different maturity stages of the mammary gland. Age is the ultimate risk factor. Only high penetrant BRCA1/2 mutations and strong IR exposures bring higher risk than age (Figure 1). Moreover, cancers that arise in premenopausal women are often of a more aggressive type compared to those occurring in postmenopausal women. A two to three fold increase in breast cancer risk in postmenopausal women has been associated with weight gain in adulthood 23 whereas childhood or teenage obesity may confer protection against breast cancer. It is therefore essential to use cells that correspond to specific stages in life for culture models. To date, a limited number of non-neoplastic HMECs are available with scarce information on the exact menopausal status. This situation is likely to change rapidly, thanks notably to sampling efforts with healthy tissue donors 47. Mimicking immature stages of the mammary gland (e.g., before puberty) that are particularly sensitive to environmental factors is currently impossible since cell lines corresponding to that age period are not available. Nevertheless, culture conditions that restrict cells to an immature stage of differentiation before allowing acinar morphogenesis can be envisioned as crude substitute models to test for a sustained risk created by factors that interfere with the epigenome. We have applied this method to study nutrients associated with the modulation of breast cancer risk, including nutrients known to act as methyl donors, and confirmed their impact on polarity although nutrient exposure was applied during an immature stage (no acini formed yet) of the epithelium (Lelièvre laboratory, unpublished results).
The few 3D cell culture models presented above to study breast cancer risk can be used to measure tissue polarity, DNA repair and epigenetic modifications. The study of epigenetic modifications as readout for breast cancer risk is of increasing interest 48, yet it is still in its infancy. Importantly, relationships between readouts of risk have been identified in 3D culture models, strengthening the importance of such molecular assessment in primary prevention research. The tissue polarity axis has been observed to control DNA repair, as shown with irradiation protocols of acini followed by comet assays adapted for multicellular structures and measurements of staining features of markers of DNA repairs 43. Apical polarity in mammary acini is under epigenetic influence 33, and epigenetic modifiers have been found within tight junctions 49, suggesting that polarity alterations, in turn, can modulate the epigenome. Nevertheless, if assays to evaluate readouts of risk can be applied to 3D cell culture, the production of acini is not ideal for primary prevention research. Instead, breast-on-a-chip models ought to be envisioned for the study of breast cancer risk based on knowledge acquired from 3D cell culture. Indeed, these models better mimic the contextual development of breast cancer, and they are more amenable to the integration of biosensors that will permit live assessment of risk cooperativity.
Building risk-on-a-chip models
Physiologically relevant tissue models hold promise for the evaluation of cancer risk factors and for the discovery of risk biomarkers. In the previous section the cell culture models were mostly based on the production of mammary acini in 3D cell culture that correspond to the closed ends of terminal ductal lobular units in the breast. However, these acini represent individual units separated from the branched ductal system normally observed in mammary tissues. Producing mammary ducts is especially relevant to breast cancer prevention studies since tumors have been proposed to originate in the terminal ducts leading to acini 50. Mimicking mammary ducts with cell culture is possible. Certain types of HMECs spontaneously form ductal structures in 3D culture, but the mimicry of branched, sizable channels is not achieved under these conditions. Non-neoplastic HMECs have been cultured in microfabricated hemichannels with diameters that approximate the terminal dusts to produce an organon-a-chip model 51 (Figure 2B). Cells are deposited in the channels on dried laminin 111, which triggers the formation of basoapical polarity in the luminal epithelium without inducing the formation of spheroids as EHS would otherwise do. This platform might potentially be used to study soluble factors that modulate breast cancer risk and may also encompass epithelial cells bearing mutations in breast cancer susceptibility genes as these cell lines become available, like those derived from patients with haploinsufficiency in BRCA2 52 or p53 53. However, this system will have to be modified to reproduce risk that includes interaction with the stroma, via adipocytes for instance, or increased stromal density. Although hemichannels are easy to build and practical for cell deposition and treatment, full channels should also be engineered so that multiple factors can be studied separately on-a-chip and appropriate delivery methods of devices/agents for detection and treatment of abnormal cells can be designed.
Coexistence of different cell types for high-throughput studies of cell-ECM interaction with lab-on-chip has been achieved 54,55. In these automated cell culture systems that included fibroblasts and breast cancer cells, differential surface tension on drops deposited at inports and outports drove the flow of medium used to feed and treat cells. The production of tubeless enclosed channels in such microfluidic arrays permits simultaneous studies with different treatments in each of the channels. The cell culture technique chosen here was to produce multiple tumor nodules inside channels containing stromal components. This array may be implemented for the culture of non-neoplastic breast cells on laminin 111 substratum thus, mimicking breast ducts and providing an ideal setting for screening many risk factors separately. However, it is unlikely that the interaction with the stroma could be easily mimicked unless the goal is to produce a multitude of acini within each microchannel intead of a monolayered duct. On-chip microchannels lined with ECM and endothelial cells were also produced by creating lumens through ECM hydrogel and depositing cells afterwards 56. Whether a similar approach may be used to produce mammary ducts with a polarized epithelium remains to be established.
Most organs-on-chips still rely on molding or micromachining synthetic polymer scaffolds supporting tissue growth in a defined 3D environment. A progressive shift towards 3D bioprinting might foster optimization of the models for coculture, in the presence of stroma, by eliminating the need for plastic molds 57,58,59,60,61. Bioprinting, now commercially available, permits not only more complex tissues of different compositions, including branched tubes like in the breast, but also the exact placement of different cell types for the mimicry of complex organs. One of the principles of bioprinting is self-assembly based on differential adhesion and cellular tensile forces, which for instance explains lumen formation by the positioning of certain cell-cell junctions on a restricted part of the cells 62,59. Cell sorting through a mixture of cells is also based on these principles and could apply to dual layered epithelium (myoepithelia cells-luminal cells) engineering for the mammary duct. Bioprinting is also based on the principle of tissue fusion during which two or more cell populations coalesce after making contact 59. Briefly, in bioprinting multicellular building blocks are added in the presence of specifically distributed agarose rods for instance and allowed to fuse, giving rise to tissues with specific arrangements after removal of the agarose. Multiple cell types can be added to make cylindrical tissue structures around a central lumen, and including different cell layers that produce their own ECM. For the breast duct from the inside to outside we would need luminal epithelial cells, myoepithelial cells that contribute to the production of the BM and fibroblasts that make stromal ECM. The composition of the stroma as well as the quantity of epithelial cells could be modified 63 to produce ducts within environments of increasing density, thus recapitulating this particular risk factor.
If all parameters in the risks-on-chip are controlled, one could envision the study of nutritional or breast density factors leading to the epigenetic silencing of the normal BRCA1 allele in cells that are BCRA1 mutant carriers, hence enabling to measure cooperativity of different risk factors. In order to readily decipher the impact of a given factor, instead of a heterotypic model, specific metabolites originated from different cell types (e.g., leptin/adiponectin from adipocytes or inflammatory mediators for inflammatory cells) could be introduced in a breast-on-a-chip only made of the luminal epithelium.
Estimating the risk from factors introduced in organ-on-a-chip models that achieve sizes that properly mimic human features would rely on the three readouts of risk described in the introduction and discussed further in the section on 3D cell culture: tissue polarity, DNA repair and the epigenome. However, these readouts ought to be assessed in live cells to permit the study of risk modulators that might cooperate in a sequential manner and to allow multiplex analysis of the parameters of interest via integration of optical and electrochemical biosensors within the chip (Figure 3). Moreover, biosensing of general aspects of cellular homeostasis and of the actual presence of the risk will also have to be available. Variables linked to cellular homeostasis such as signaling molecules, pH, ions, glucose or cell adhesion, have been assessed by a variety of biosensors 64,65,66,67. Of particular interest are biosensors that measure cell-ECM interaction based on cytoskeletal rearrangement and cell-substratum bonds 68; however, these biosensors will have to be adapted for embedding within organon-a-chip systems. Other variables such as tissue mechanics would be important to measure when modulating the risk based on tissue density. The elastic modulus of tissues is typically measured using a strain rheometer and unconfined compression analysis 41, which has poor spatial resolution and may be difficult to implement for lab-on-chips. Measurements of elastic moduli by atomic force microscopy (AFM) 69 have a much higher resolution but require direct contact or very close proximity between the sample and the cantilever probe. Application of AFM measurements will therefore be limited to ‘open’ organ-on-a-chip configurations such as ductal hemichannels 51. The development of mechanical sensors enabling force measurements in real time, with high resolution, is therefore needed for more complex ‘closed’ (co)culture systems with complete channels.
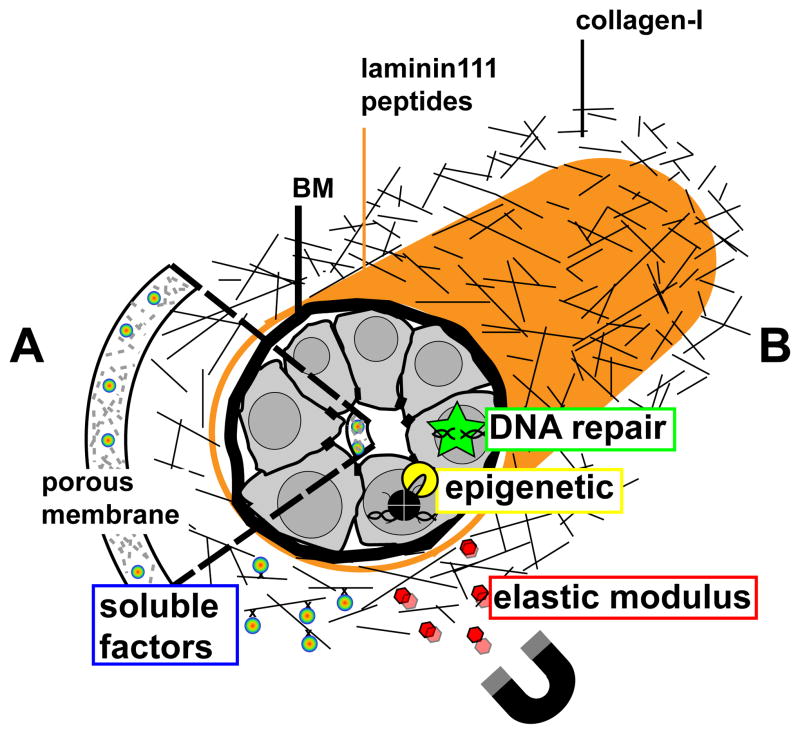
Figure 3.
Risk-on-a-chip for the study of breast cancer risk factors. The drawing represents HMECs that recapitulate a mammary duct in an organ-on-chip context with cells engineered to contain biosensors for DNA repair and epigenetic modifications, and biosensors embedded in the extracellular milieu to measure ECM stiffness (using a magnet to pull the sensors for instance) and the concentration of soluble factors (e.g., glucose, ions, reproductive hormones, pH). Biosensors for soluble factors may be incorporated in mesoporous silica membranes at the basal and apical sides (A) or in a collagen 1-based matrix that provides the stromal ECM microenvironment and might contains different cell types (e.g., adipocytes, fibroblasts- not represented) (B). In both setups, laminin-111 peptides lining the collagen-I ECM stimulate HMEC differentiation.
To specifically assess the readouts of risk different methods will have to be envisioned for integration into organ-on-a-chip settings. For the measurement of apical polarity in live tissues, to the best of our knowledge there is no biosensor. Nevertheless, as described earlier, Raman spectromicroscopy permits label-free distinction between polarized and nonpolarized cells based on lipid orders in cell membranes. As we better understand the different degrees of polarity loss, sensors for local changes in molecules involved in polarity will have to be built. Another key aspect when assessing risk is to precisely measure DNA repair activity in cells. The modified comet assay for measuring DNA damage and repair activity in 3D culture 43 is not compatible for on-chip miniaturization and live cell analysis. Mammalian cells have evolved multiple DNA repair pathways 70 to process different types of DNA damage. BRCA1 and BRCA2 gene products are essential for homologous recombination repair of DNA double-strand breaks. Other DNA repair pathways may also be protective against breast cancer. Specific DNA repair pathways can be assessed by immunostaining using pathway-specific repair factors that accumulate at DNA repair foci. The quantification of these foci in time course experiments reflects pathway usage and repair kinetics. Nevertheless, like for mechanical and cell polarity measurements, more dynamic readouts are desirable for risk-on-a-chip models. Automated quantification of endogenously labeled DDR factors (e.g., fused to fluorescent proteins) is one possibility. An alternative is the implementation of GFP-based biosensors for specific repair pathways, with which the coding sequence of the GFP is restored after cleavage with an endonuclease and subsequent DNA repair 71,72. We have proposed that the ultimate readout to measure cooperativity between multiple risk factors is epigenetic because cancer onset is anchored in the modification of gene expression patterns. Measurements of chromatin organization and modifications are therefore critical to fully understand risk mechanisms and identify new biomarkers of risk. Genome-wide mapping of epigenetic marks (http://www.genome.gov/10005107) is now broadly available but does not have yet single-cell resolution for intact tissues. Fluorescence resonance energy transfer (FRET) has been used to define nucleosome composition and histone modifications on a per gene locus basis in intact but fixed cells 73; translation in live tissue models will require significant technological advances in optical imaging and spectroscopy, nevertheless it is being envisioned. This microscopy-based technology will single out genes of interest and assess the quantity as well as colocalization of different epigenetic marks. This step is particularly important to achieve since permanent epigenetic changes require the presence of several modifications.
Integrative homo- and heterotypic cell culture models in tunable microenvironments offer opportunities to study cancer initiation in physiological conditions. Combining these models into high-throughput ‘risks-on-chips’ that include built-in sensors will provide unprecedented resources to identify optimal biomarkers for use in primary prevention as well as a better understanding of how risk factors impact essential features of tissues, like architecture, DNA integrity and gene transcription control. An important question to address in the future is the source of the cells used in the chips. Cell lines are convenient for repeatability and comparison of high-throughput assays, like those used for drug development. However, immortalized non-neoplastic cells are not completely normal and already present epigenetic alterations observed in breast cancers like, for instance, the methylation of the p16 gene. It is possible to use primary cells from women at average breast cancer risk on a regular basis for these assays possibly from reduction mammoplasty or, if enough material is available, breast augmentation, although these cells will have a limited capacity to divide to cover the channel. But obtaining primary cells from women at high risk for breast cancer would require seeking tissue cores for the experimental purpose. This approach is not sustainable for routine use in risks-on-chips unless there is a direct benefit to the individual like the identification of potential therapies to decrease the breast cancer risk of that particular individual. An alternative to maintain the pool of cells and develop reproducible models will be to derive prestasis cells from tissue core obtained with the help of normal breast tissue banks 47 where donors are from different risk levels. The methods to obtain and maintain these cell lines have been readily developed for mammary epithelial cells over more than two decades by the Stampfer laboratory 74. Ultimately, the key advantage of artificial human tissues on-chips is that a large number of therapeutic approaches can be tested faster and less expensively than would be possible on animal models or human subjects. The lack of risk to humans and animals allows for the design and testing of more innovative preventive interventions that may lead to break-through advances in breast cancer prevention.
Insight, innovation, integration.
Public health authorities display a growing interest in preventing chronic diseases, which calls for the development of models of disease risk. These models need to integrate human tissues, molecular and environmental risk factors, and biosensors to monitor tissue architecture, genetic/epigenetic statuses and the physicochemical properties of the cellular microenvironment. For diseases like breast cancer for which a direct cause remains unidentified, models will have to take into account cooperativity - notably between environmental risk factors and genetic/epigenetic backgrounds - as well as the degree of maturity of the mammary gland. Integrative models mimicking risk situations in microfabricated chips together with technological innovation in biosensing (‘risk-on-a-chip’) should yield much awaited biomarkers for risk assessment and screening platforms for cancer preventive agents.
Acknowledgments
We thank Dr. Kurt Hodges (Indiana University School of Medicine) for providing microscopic illustrations of normal breast tissue. The authors’ work is supported by the National Institute of Health (R01CA112017 to SAL, 1K99CA163957 to PAV), the Purdue Center for Cancer Research Obesity and Cancer Discovery Group (incentive award to SAL) and a Medical Research Award from the Keck Foundation. SAL and JFL are members of the International Breast Cancer & Nutrition (IBCN) group for the development of primary prevention research.
References
- 1.Teegarden D, Romieu I, Lelievre SA. Redefining the impact of nutrition on breast cancer incidence: is epigenetics involved? Nutr Res Rev. 2012;25:68–95. doi: 10.1017/S0954422411000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Interagency Breast Cancer and Environmental Research Coordinating Committee (IBCERCC) Breast Cancer and the Environment: Prioritizing Prevention. 2013. [Google Scholar]
- 3.Lelièvre SA, Weaver CM. Global Nutrition Research: Nutrition and Breast Cancer Prevention as a Model. Nutrition Rev. doi: 10.1111/nure.12075. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singletary SE. Rating the risk factors for breast cancer. Ann Surg. 2003;237:474–482. doi: 10.1097/01.SLA.0000059969.64262.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz GF, Hughes KS, Lynch HT, Fabian CJ, Fentiman IS, Robson ME, Domchek SM, Hartmann LC, Holland R, Winchester DJ. Proceedings of the international consensus conference on breast cancer risk, genetics, & risk management, April, 2007. Cancer. 2008;113:2627–2637. doi: 10.1002/cncr.23903. [DOI] [PubMed] [Google Scholar]
- 6.Lelièvre SA. Tissue polarity-dependent control of mammary epithelial homeostasis and cancer development: an epigenetic perspective. J Mammary Gland Biol Neoplasia. 2010;15:49–63. doi: 10.1007/s10911-010-9168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidi PA, Bissell MJ, Lelièvre S. Methods in Molecular Biology. 2013;945:193–219. doi: 10.1007/978-1-62703-125-7_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandramouly G, Abad PC, Knowles DW, Lelièvre SA. The control of tissue architecture over nuclear organization is crucial for epithelial cell fate. J Cell Sci. 2007;120:1596–1606. doi: 10.1242/jcs.03439. [DOI] [PubMed] [Google Scholar]
- 9.Maxwell CA, Benitez J, Gomez-Baldo L, et al. Interplay between BRCA1 and RHAMM regulates epithelial apicobasal polarization and may influence risk of breast cancer. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yue S, Cardenas-Mora JM, Chaboub LS, Lelievre SA, Cheng JX. Label-free analysis of breast tissue polarity by Raman imaging of lipid phase. Biophys J. 2012;102:1215–1223. doi: 10.1016/j.bpj.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K. Studies of the mortality of atomic bomb survivors, Report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;177:229–243. doi: 10.1667/rr2629.1. [DOI] [PubMed] [Google Scholar]
- 12.Henderson TO, Amsterdam A, Bhatia S, et al. Systematic review: surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med. 2010;152:444–455. doi: 10.1059/0003-4819-152-7-201004060-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pal T, Vadaparampil ST. Genetic risk assessments in individuals at high risk for inherited breast cancer in the breast oncology care setting. Cancer Control. 2012;19:255–266. doi: 10.1177/107327481201900402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ralhan R, Kaur J, Kreienberg R, Wiesmuller L. Links between DNA double strand break repair and breast cancer: accumulating evidence from both familial and nonfamilial cases. Cancer Lett. 2007;248:1–17. doi: 10.1016/j.canlet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Stefansson OA, Villanueva A, Vidal A, Marti L, Esteller M. BRCA1 epigenetic inactivation predicts sensitivity to platinum-based chemotherapy in breast and ovarian cancer. Epigenetics. 2012;7:1225–1229. doi: 10.4161/epi.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Kunju LP, Cookingham C, Toy KA, Chen W, Sabel MS, Kleer CG. EZH2 and ALDH-1 mark breast epithelium at risk for breast cancer development. Mod Pathol. 2011;24:786–793. doi: 10.1038/modpathol.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeidler M, Varambally S, Cao Q, Chinnaiyan AM, Ferguson DO, Merajver SD, Kleer CG. The Polycomb group protein EZH2 impairs DNA repair in breast epithelial cells. Neoplasia. 2005;7:1011–1019. doi: 10.1593/neo.05472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y, Stevens RG, Hoffman AE, Tjonneland A, Vogel UB, Zheng T, Hansen J. Epigenetic impact of long-term shiftwork: pilot evidence from circadian genes and whole-genome methylation analysis. Chronobiol Int. 2011;28:852–861. doi: 10.3109/07420528.2011.618896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding L, Erdmann C, Chinnaiyan AM, Merajver SD, Kleer CG. Identification of EZH2 as a molecular marker for a precancerous state in morphologically normal breast tissues. Cancer Res. 2006;66:4095–4099. doi: 10.1158/0008-5472.CAN-05-4300. [DOI] [PubMed] [Google Scholar]
- 21.Gomes MV, Toffoli LV, Arruda DW, Soldera LM, Pelosi GG, Neves-Souza RD, Freitas ER, Castro DT, Marquez AS. Age-related changes in the global DNA methylation profile of leukocytes are linked to nutrition but are not associated with the MTHFR C677T genotype or to functional capacities. PLoS One. 2013;7:e52570. doi: 10.1371/journal.pone.0052570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam LL, Emberly E, Fraser HB, Neumann SM, Chen E, Miller GE, Kobor MS. Factors underlying variable DNA methylation in a human community cohort. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17253–17260. doi: 10.1073/pnas.1121249109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballard-Barbash R, Hunsberger S, Alciati MH, Blair SN, Goodwin PJ, McTiernan A, Wing R, Schatzkin A. Physical activity, weight control, and breast cancer risk and survival: clinical trial rationale and design considerations. J Natl Cancer Inst. 2009;101:630–643. doi: 10.1093/jnci/djp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Assis S, Warri A, Cruz MI, Laja O, Tian Y, Zhang B, Wang Y, Huang TH, Hilakivi-Clarke L. High-fat or ethinyl-oestradiol intake during pregnancy increases mammary cancer risk in several generations of offspring. Nat Commun. 2012;3:1053. doi: 10.1038/ncomms2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra GD, dos Santos Silva I, McNaughton SA, Stephen A, Kuh D. Energy intake and dietary patterns in childhood and throughout adulthood and mammographic density: results from a British prospective cohort. Cancer Causes Control. 2011;22:227–235. doi: 10.1007/s10552-010-9690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 27.Royer C, Lu X. Epithelial cell polarity: a major gatekeeper against cancer? Cell Death Differ. 2011;18:1470–1477. doi: 10.1038/cdd.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- 29.Paine TM, Soule HD, Pauley RJ, Dawson PJ. Characterization of epithelial phenotypes in mortal and immortal human breast cells. Int J Cancer. 1992;50:463–473. doi: 10.1002/ijc.2910500323. [DOI] [PubMed] [Google Scholar]
- 30.Stampfer MR, Bartley JC. Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. Proc Natl Acad Sci U S A. 1985;82:2394–2398. doi: 10.1073/pnas.82.8.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briand P, Petersen OW, Van Deurs B. A new diploid nontumorigenic human breast epithelial cell line isolated and propagated in chemically defined medium. In Vitro Cell Dev Biol. 1987;23:181–188. doi: 10.1007/BF02623578. [DOI] [PubMed] [Google Scholar]
- 32.Underwood JM, Imbalzano KM, Weaver VM, Fischer AH, Imbalzano AN, Nickerson JA. The ultrastructure of MCF-10A acini. J Cell Physiol. 2006;208:141–148. doi: 10.1002/jcp.20639. [DOI] [PubMed] [Google Scholar]
- 33.Plachot C, Chaboub LS, Adissu HA, Wang L, Urazaev A, Sturgis J, Asem EK, Lelièvre SA. Factors necessary to produce basoapical polarity in human glandular epithelium formed in conventional and high-throughput three-dimensional culture: example of the breast epithelium. BMC Biol. 2009;7:77. doi: 10.1186/1741-7007-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 36.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci. 2003;116:1397–1408. doi: 10.1242/jcs.00360. [DOI] [PubMed] [Google Scholar]
- 38.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Tse JR, Engler AJ. Preparation of hydrogel substrates with tunable mechanical properties. Curr Protoc Cell Biol. 2010;Chapter 10(Unit 10):16. doi: 10.1002/0471143030.cb1016s47. [DOI] [PubMed] [Google Scholar]
- 40.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su Y, Shankar K, Rahal O, Simmen RC. Bidirectional signaling of mammary epithelium and stroma: implications for breast cancer--preventive actions of dietary factors. J Nutr Biochem. 2011;22:605–611. doi: 10.1016/j.jnutbio.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Vidi PA, Chandramouly G, Gray M, Wang L, Liu E, Kim JJ, Roukos V, Bissell MJ, Moghe PV, Lelievre SA. Interconnected contribution of tissue morphogenesis and the nuclear protein NuMA to the DNA damage response. J Cell Sci. 2012;125:350–361. doi: 10.1242/jcs.089177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng L, Wu GZ, Goh KJ, Lee YM, Ng CC, You AB, Wang J, Jia D, Hao A, Yu Q, Li B. Saturated fatty acids modulate cell response to DNA damage: implication for their role in tumorigenesis. PLoS One. 2008;3:e2329. doi: 10.1371/journal.pone.0002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krause S, Jondeau-Cabaton A, Dhimolea E, Soto AM, Sonnenschein C, Maffini MV. Dual regulation of breast tubulogenesis using extracellular matrix composition and stromal cells. Tissue Eng Part A. 2012;18:520–532. doi: 10.1089/ten.tea.2011.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell JJ, Davidenko N, Caffarel MM, Cameron RE, Watson CJ. A multifunctional 3D co-culture system for studies of mammary tissue morphogenesis and stem cell biology. PLoS One. 2011;6:e25661. doi: 10.1371/journal.pone.0025661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherman ME, Figueroa JD, Henry JE, Clare SE, Rufenbarger C, Storniolo AM. The Susan G. Komen for the Cure Tissue Bank at the IU Simon Cancer Center: a unique resource for defining the “molecular histology” of the breast. Cancer Prev Res (Phila) 2012;5:528–535. doi: 10.1158/1940-6207.CAPR-11-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dumitrescu RG. Epigenetic markers of early tumor development. Methods Mol Biol. 2012;863:3–14. doi: 10.1007/978-1-61779-612-8_1. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura T, Blechman J, Tada S, Rozovskaia T, Itoyama T, Bullrich F, Mazo A, Croce CM, Geiger B, Canaani E. huASH1 protein, a putative transcription factor encoded by a human homologue of the Drosophila ash1 gene, localizes to both nuclei and cell-cell tight junctions. Proc Natl Acad Sci U S A. 2000;97:7284–7289. doi: 10.1073/pnas.97.13.7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jensen HM. On the origin and progression of human breast cancer. Am J Obstet Gynecol. 1986;154:1280–1284. doi: 10.1016/0002-9378(86)90713-1. [DOI] [PubMed] [Google Scholar]
- 51.Grafton MM, Wang L, Vidi PA, Leary J, Lelievre SA. Breast on-a-chip: mimicry of the channeling system of the breast for development of theranostics. Integr Biol (Camb) 2011;3:451–459. doi: 10.1039/c0ib00132e. [DOI] [PubMed] [Google Scholar]
- 52.Lewis CM, Herbert BS, Bu D, Halloway S, Beck A, Shadeo A, Zhang C, Ashfaq R, Shay JW, Euhus DM. Telomerase immortalization of human mammary epithelial cells derived from a BRCA2 mutation carrier. Breast Cancer Res Treat. 2006;99:103–115. doi: 10.1007/s10549-006-9189-9. [DOI] [PubMed] [Google Scholar]
- 53.Hernandez L, Roux KJ, Wong ES, et al. Functional coupling between the extracellular matrix and nuclear lamina by Wnt signaling in progeria. Dev Cell. 2010;19:413–425. doi: 10.1016/j.devcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montanez-Sauri SI, Sung KE, Puccinelli JP, Pehlke C, Beebe DJ. Automation of three-dimensional cell culture in arrayed microfluidic devices. J Lab Autom. 2011;16:171–185. doi: 10.1016/j.jala.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montanez-Sauri SI, Sung KE, Berthier E, Beebe DJ. Enabling screening in 3D microenvironments: probing matrix and stromal effects on the morphology and proliferation of T47D breast carcinoma cells. Integr Biol (Camb) 2013 doi: 10.1039/c3ib20225a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bischel LL, Young EW, Mader BR, Beebe DJ. Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials. 2013;34:1471–1477. doi: 10.1016/j.biomaterials.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang R, Nam J, Sun W. Direct cell writing of 3D microorgan for in vitro pharmacokinetic model. Tissue Eng Part C Methods. 2008;14:157–166. doi: 10.1089/ten.tec.2007.0392. [DOI] [PubMed] [Google Scholar]
- 58.Jakab K, Norotte C, Damon B, Marga F, Neagu A, Besch-Williford CL, Kachurin A, Church KH, Park H, Mironov V, Markwald R, Vunjak-Novakovic G, Forgacs G. Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Eng Part A. 2008;14:413–421. doi: 10.1089/tea.2007.0173. [DOI] [PubMed] [Google Scholar]
- 59.Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, Forgacs G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010;2 doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khalil S, Sun W. Bioprinting endothelial cells with alginate for 3D tissue constructs. J Biomech Eng. 2009;131:111002. doi: 10.1115/1.3128729. [DOI] [PubMed] [Google Scholar]
- 61.Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steinberg MS. Differential adhesion in morphogenesis: a modern view. Curr Opin Genet Dev. 2007;17:281–286. doi: 10.1016/j.gde.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 63.Ker ED, Nain AS, Weiss LE, Wang J, Suhan J, Amon CH, Campbell PG. Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials. 2011;32:8097–8107. doi: 10.1016/j.biomaterials.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 64.Fan X, White IM, Shopova SI, Zhu H, Suter JD, Sun Y. Sensitive optical biosensors for unlabeled targets: a review. Anal Chim Acta. 2008;620:8–26. doi: 10.1016/j.aca.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones IL, Livi P, Lewandowska MK, Fiscella M, Roscic B, Hierlemann A. The potential of microelectrode arrays and microelectronics for biomedical research and diagnostics. Anal Bioanal Chem. 2011;399:2313–2329. doi: 10.1007/s00216-010-3968-1. [DOI] [PubMed] [Google Scholar]
- 66.Prakash S, Pinti M, Bhushan B. Theory, fabrication and applications of microfluidic and nanofluidic biosensors. Philos Transact A Math Phys Eng Sci. 2012;370:2269–2303. doi: 10.1098/rsta.2011.0498. [DOI] [PubMed] [Google Scholar]
- 67.Pemberton RM, Cox T, Tuffin R, et al. Microfabricated glucose biosensor for culture well operation. Biosens Bioelectron. 2013;42:668–677. doi: 10.1016/j.bios.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 68.Saitakis M, Gizeli E. Acoustic sensors as a biophysical tool for probing cell attachment and cell/surface interactions. Cell Mol Life Sci. 2012;69:357–371. doi: 10.1007/s00018-011-0854-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopez JI, Kang I, You WK, McDonald DM, Weaver VM. In situ force mapping of mammary gland transformation. Integr Biol (Camb) 2011;3:910–921. doi: 10.1039/c1ib00043h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pierce AJ, Johnson RD, Thompson LH, Jasin M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 1999;13:2633–2638. doi: 10.1101/gad.13.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akyuz N, Boehden GS, Susse S, Rimek A, Preuss U, Scheidtmann KH, Wiesmuller L. DNA substrate dependence of p53-mediated regulation of double-strand break repair. Mol Cell Biol. 2002;22:6306–6317. doi: 10.1128/MCB.22.17.6306-6317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen J, Miller A, Kirchmaier AL, Irudayaraj JM. Single-molecule tools elucidate H2A.Z nucleosome composition. J Cell Sci. 2012;125:2954–2964. doi: 10.1242/jcs.101592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garbe JC, Bhattacharya S, Merchant B, Bassett E, Swisshelm K, Feiler HS, Wyrobek AJ, Stampfer MR. Molecular distinctions between stasis and telomere attrition senescence barriers shown by long-term culture of normal human mammary epithelial cells. Cancer Res. 2009;69:7557–7568. doi: 10.1158/0008-5472.CAN-09-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]