Abstract
Inhibition of the HER-2 pathway via the monoclonal antibody trastuzumab has had a major impact in treatment of HER-2 positive breast cancer, but de novo or acquired resistance may reduce its effectiveness. The known interplay between the epidermal growth factor receptor (EGFR) and HER-2 receptors and pathways creates a rationale for combined anti-EGFR and anti-HER-2 therapy in HER-2 positive metastatic breast cancer (MBC), and toxicities associated with the use of multiple chemotherapeutic agents together with biological therapies may also be reduced. We conducted a prospective, single arm, phase I/II trial to determine the efficacy and toxicity of the combination of trastuzumab with the EGFR inhibitor gefitinib and docetaxel, in patients with HER-2 positive MBC. The maximum tolerated dose (MTD) was determined in the phase I portion. The primary end point of the phase II portion was progression-free survival (PFS). Immunohistochemical analysis of biomarker expression of the PKA-related proteins cAMP response element-binding protein (CREB), phospho-CREB and DARPP-32 (dopamine and cAMP-regulated phosphoprotein of 32 kDa) plus t-DARPP (the truncated isoform of DARPP-32); PTEN; p-p70 S6K; and EGFR was conducted on tissue from metastatic sites. Nine patients were treated in the phase I portion of the study and 22 in the phase II portion. The MTD was gefitinib 250 mg on days 2–14, trastuzumab 6 mg/kg, and docetaxel 60 mg/m2 every 21 days. For the 29 patients treated at the MTD, median PFS was 12.7 months, with complete and partial response rates of 18 and 46%, and a stable disease rate of 29%. No statistically significant correlation was found between response and expression of any biomarkers. We conclude that the combination of gefitinib, trastuzumab, and docetaxel is feasible and effective. Expression of the biomarkers examined did not predict outcome in this sample of HER-2 overexpressing metastatic breast cancer.
Keywords: Breast cancer, HER2, Gefitinib, Phosphatase and tensin homolog, Protein kinase A, Trastuzumab
Introduction
Overexpression of HER-2 occurs in 20–25% of breast cancers [1], and is associated with decreased progression-free and overall survival [2]. Inhibition of the HER-2 pathway via the monoclonal antibody trastuzumab has had a profound impact upon the natural history of HER-2 positive breast cancer [3-5]. However, the rate of response of HER-2 positive metastatic breast cancer (MBC) to first line single-agent trastuzumab is only 34% [6], suggesting high levels of inherent resistance. Possible mechanisms of resistance include a variety of molecular changes that converge upon the phosphatidyl-inositol 3-kinase (PI3K)/Akt signaling pathway. Upregulated signaling through alternative receptor tyrosine kinases, such as the insulin-like growth factor-1 receptor [7] and epidermal growth factor receptor (EGFR) [8, 9]; modulation of key regulatory components of the PI3K/Akt pathway, such as PI3K [10, 11] and Akt [12] themselves, phosphatase and tensin homolog (PTEN) [10, 12], and mammalian target of rapamycin (mTOR) [13]; and dysregulation of cross-talk signaling pathways such as protein kinase A (PKA) [14] have all been reported as possible mediators of trastuzumab resistance, all with the net effect of allowing sustained signaling through PI3K/Akt in the presence of trastuzumab.
We and others have reported that trastuzumab-resistant cells have higher levels of phosphorylated EGFR and EGFR/HER-2 heterodimers than their trastuzumab-sensitive counterparts and remain sensitive to EGFR inhibitors [8], particularly in the presence of trastuzumab [9]. This suggests a greater dependence on EGFR in these cells when HER-2 signaling is inhibited by trastuzumab [9]. The efficacy of lapatinib, a dual HER-2/EGFR inhibitor, in combination with trastuzumab in patients whose disease has progressed through prior trastuzumab-containing therapy [15] also suggests that simultaneous HER-2 and EGFR inhibition can overcome trastuzumab resistance in some patients.
In light of the known interplay between EGFR and HER-2, there is a rationale for examining combined anti-EGFR and anti-HER-2 therapy in HER-2 positive MBC. We chose to study trastuzumab in combination with gefitinib, which was the most clinically proven EGFR inhibitor at the time this study was initiated, having shown efficacy in the treatment of lung cancer [16]. Moreover, gefitinib has been shown to induce apoptosis of trastuzumab-resistant cells in culture [8], and studies of single-agent gefitinib in MBC patients not selected for HER-2 status have demonstrated some activity [17, 18].
The combination of docetaxel and trastuzumab is more effective than docetaxel alone in treating MBC, with an overall response rate of 61%, time to progression (TTP) of 11.7 months, and median overall survival (OS) of 31.2 months [19]. We therefore examined the combination of gefitinib, docetaxel, and trastuzumab in this patient population with a prospective, single arm, phase I/II trial. The goal was to establish the maximum tolerated dose (MTD) during the phase I portion of the study and then determine the progression-free survival (PFS) and response rate in patients treated at the MTD during the phase II portion. Based upon their known association with trastuzumab resistance in pre-clinical and clinical settings, we also sought to determine whether key molecular biomarkers were associated with these outcomes.
Materials and methods
This protocol was approved by the City of Hope Institutional Review Board and followed the ethical standards of that body. Patients ≥18 years of age with histologically confirmed, measurable, or evaluable, stage IV HER-2 overexpressing MBC were eligible. Patients had to have tumors with 3+ HER-2 expression as determined by immunohistochemistry, or HER-2 amplification determined by fluorescence in situ hybridization (FISH). Prior adjuvant chemotherapy and trastuzumab were allowed unless disease had recurred within 6 months of receiving that therapy. Previous treatment with chemotherapy or trastuzumab in the metastatic setting was not permitted.
Other eligibility criteria included absolute granulocyte count ≥1,500/ml; platelet count ≥100,000/ml; creatinine level < 1.6 mg/dl; total bilirubin level < 1.5 × the upper limit of normal; aspartate aminotransferase and alanine aminotransferase levels < 2.5 × the upper limit of normal without demonstrable liver metastases, or <5.0 × the upper limit of normal with liver metastases; ECOG performance status ≤ 2; and left ventricular ejection fraction >55%.
In the phase I portion of the study, subjects received gefitinib 250 mg orally daily, trastuzumab 6 mg/kg intravenously every 3 weeks (after an initial dose of 8 mg/kg with cycle 1), and docetaxel 75 mg/m2 intravenously every 3 weeks. This was to serve as the phase II dose if no dose-limiting toxicities (DLTs) occurred in the first three subjects. If one DLT occurred in the first three subjects, another three subjects were to be enrolled atthis dose, whereas if two DLTs occurred in the first three subjects, the docetaxel dose was to be decreased to 60 mg/m2. The study would then be continued only if no more than one patient had a DLT at this dose. Once the dose of docetaxel was established, all further subjects were to be treated at the phase II MTD dose. The primary endpoint of the phase II portion of the study was PFS.
Reports from the lung cancer study INTACT 2 [20] demonstrated that gefitinib plus concurrent chemotherapy, followed by gefitinib maintenance, was not superior to chemotherapy alone in the treatment of non-small cell lung cancer, suggesting an adverse interaction between gefitinib and chemotherapy. Therefore, the gefitinib schedule was modified to 250 mg daily on days 2–14 of the 21-day cycle after the initiation of the study, and this schedule was used for the entire phase II portion of the study.
Patients were evaluated for response at 9-week intervals, using standard response evaluation criteria in solid tumors. Assessment of complete response (CR) and partial response (PR) was performed at or after completion of the third course of treatment. A patient with a single response assessment of PR and no subsequent confirmatory assessments was considered to have stable disease (SD). Response and SD had to be confirmed on two assessments. Duration of SD or response was by definition at least 9 weeks. Clinical benefit rate (CBR) was defined as the rate of CR plus PR plus SD. PFS was defined as the time from initiation of treatment until objective disease progression or death; patients who discontinued treatment for reasons other than disease progression were included in the PFS analysis. Only subjects with measurable disease were included in the response rate calculation. The National Cancer Institute Common Toxicity Criteria Version 2.0 was used to assess toxicities. Dose-limiting toxicity was defined as any grade 3 or 4 treatment-related toxicity other than hematologic toxicity (which only qualified if grade 4) and, during the phase I portion, any interstitial lung disease that was considered treatment-related.
Immunohistochemical studies were conducted on tissue samples, when available, using 5-μm thick sections prepared from formalin-fixed, paraffin-embedded tissue. Tissue samples were procured prior to enrollment (required to document first time metastases). While patients were enrolled prospectively, samples were batched, and hence were analyzed in a retrospective fashion. We planned to assess molecular markers involved in EGFR/HER2-driven signal transduction. Due to limitations in available tissue, the biomarkers examined in an exploratory fashion were the PKA-related proteins cAMP response element-binding protein (CREB), phospho-CREB, and DARPP-32 (dopamine and cAMP-regulated phosphoprotein of 32 kDa) plus t-DARPP (the truncated isoform of DARPP-32); PTEN; p-p70 S6K and EGFR. Staining for CREB was performed using the 48H2 rabbit monoclonal antibody (Cell Signaling Technology, Inc., Danvers, MA), 1:300 dilution; for phospho-CREB (Ser133) using the 87G3 rabbit monoclonal antibody (Cell Signaling Technology), 1:50 dilution; for DARPP-32 plus t-DARPP using the H62 rabbit polyclonal antibody against the common C-terminus of these two proteins (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), 1:200 dilution; for PTEN using the 6H2.1 antibody (Dako, Carpinteria, CA), 1:300 dilution; and for p-p70 S6K using a rabbit polyclonal antibody raised against a short amino acid sequence containing phosphorylated Thr421 and Ser424 of human p70S6K (Santa Cruz Biotechnology), 1:300 dilution. For detection of EGFR, the EGFR pharmDx kit system (Dako) was used according to the manufacturer’s instructions. Tissue sections were deparaffinized in xylene, rehydrated in graded alcohol, and then quenched in 3% hydrogen peroxide. For heat-induced epitope retrieval, the CREB, phospho-CREB, DARPP-32 plus t-DARPP, and p-p70S6K sections were steamed with Diva decloaker buffer, pH 6 (Biocare Medical); sections for PTEN were steamed with EDTA; and sections for EGFR were steamed with proteinase K. Slides were blocked for 10 min using Protein Block from DAKO and then incubated with primary antibody overnight at 4°C. The slides were processed in a DAKO Autostainer universal staining system and developed with the EnVision + horseradish peroxidase system for detection of rabbit primary antibodies. Counterstaining was with 50% Mayer’s hematoxylin (DAKO) for 3 min. Stained slides were scored for intensity on a scale of 0–3 and for percent positivity (0–100). The final score used in statistical analysis was the product of the intensity score and the percent positivity score for each antibody.
The primary endpoint of the phase II portion of the study was PFS. The initial sample size goal was 76 patients, established to estimate PFS, presuming that the efficacy of the gefitinib/docetaxel/trastuzumab combination was similar to that of a platinum salt/taxane/trastuzumab combination, which yielded a median PFS of 10.7 months [21]. The trial closed early, however, because availability of lapatinib and use of alternative strategies made accrual more difficult. Secondary endpoints included OS, response rate, CBR, and safety. Duration of PFS and OS was estimated using the Kaplan–Meier method [22]. Data were expressed as medians with 95% confidence intervals (CI) calculated by the Greenwood formula [23]. Determination of significance of association between expression of tissue biomarkers and treatment response was performed using the t-test, with a two-sided alpha level of 0.05, or the Wilcoxon rank sum test when data were skewed. All statistical tests were performed using SAS V9.2 or S-Plus v 7.0, and univariate and multivariate analyses of biomarker data were planned. Given the predictable variability of the biomarkers and the number of samples, we also evaluated the power of this study to detect (with a two-sided P value of 0.05) a 30% difference between the responders and non-responders. The design, immunohistochemical testing, and statistical assessment are in line with Remark criteria [24].
Results
A total of 31 patients were enrolled, 9 in the phase I portion of the study and 22 in the phase II portion. Patient characteristics are summarized in Table 1. One patient in the phase I portion of the study (who had no metastatic disease), and two patients in the phase II portion (one with stage IIIB disease, one with HER-2 negative disease by FISH on retesting) were found to be ineligible. Because these patients received therapy, they are included in the data analysis unless otherwise specified.
Table 1.
Patient demographics (n = 31)
| Characteristic | Years |
|---|---|
| Median age (range) | 52 (34–67) |
| Number (%) | |
| Gender | |
| Female | 31 (100) |
| Race | |
| African American | 3 (10) |
| Asian | 3 (10) |
| Caucasian | 21 (68) |
| Hispanic | 4 (13) |
| Performance status (ECOG) | |
| 0/1 | 19 (61)/11 (36) |
| 2 | 1 (3) |
| Histology | |
| Infiltrating ductal carcinoma | 27 (87) |
| Inflammatory carcinoma | 4 (13) |
| ER/PgR positive disease | 10 (32) |
| Site/s of metastatic disease | |
| Bone/soft tissue/skin | 21 (68) |
| Lung/liver/other | 23 (74) |
| Prior systemic therapy | |
| Adjuvant/neoadjuvant chemotherapya | 16 (52) |
| Antiestrogen therapy for metastatic disease | 3 (10) |
ECOG Eastern Cooperative Oncology Group. NOS not otherwise specified. Numbers may not add up due to overlap among subgroups
Eleven patients received regimens containing an anthracycline but not a taxane. Six patients received regimens containing doxorubicin and paclitaxel (one of these patients also received docetaxel)
The first two patients in the phase I portion of the study at the initial dose level (docetaxel 75 mg/m2) experienced DLTs (detailed below), so the docetaxel dose was decreased to 60 mg/m2 for the remainder of the trial. Seven patients were enrolled at this dose level, including the ineligible patient, who was replaced. This dose was used in the phase II portion of the study. Starting with the last patient in the phase I study, the gefitinib schedule was modified to 250 mg daily on days 2–14 of the 21-day cycle.
The two patients treated at the initial phase I dose of docetaxel of 75 mg/m2 had stable disease. Of the remaining seven phase I patients, three had a PR and four had SD. Among the phase II patients, there were five CRs, ten PRs, four cases of SD, and two cases of progressive disease. One patient was excluded from the response evaluation because she was found to be ineligible (having HER-2 negative disease) and completed only one course of therapy. The other ineligible phase II patient, who had stage IIIB disease, was included in the response analysis; she completed eight cycles of therapy, with a best response of unconfirmed CR (preceded by a confirmed PR). Twenty-nine patients (7 in the phase I portion, 22 in the phase II portion) were treated at the phase II docetaxel dose; the CR, PR, and SD rates were 18, 46, and 29%, respectively; CBR was 93%.
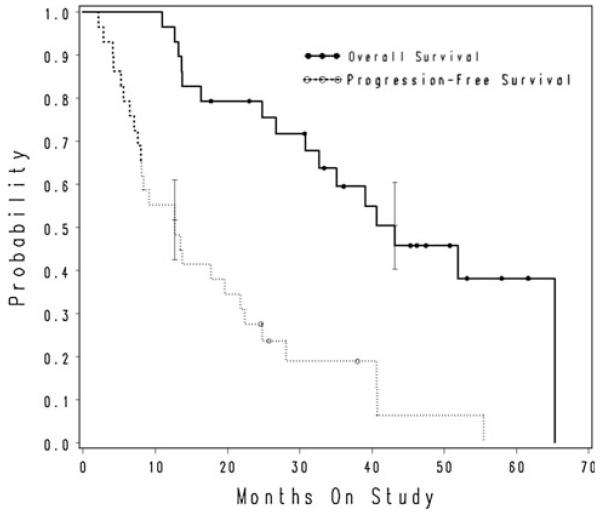
The median PFS for all patients treated at the phase II docetaxel dose was 12.7 months (95% CI 7.6–21.8 months; range 2.1–55.5 months) (see Fig. 1). The median OS was 43.2 months (95% CI 30.8–65.3 months; range 11.0–65.3 months) (see Fig. 1). Reanalysis of the data with exclusion of ineligible patients non-significantly shortened OS to 40.7 months, with no change in PFS (data not shown).
Fig. 1.
Kaplan–Meier plot of time to progression and overall survival for all patients enrolled
Both patients treated at the initial dose level (docetaxel dose 75 mg/m2) experienced the DLT of grade 3 infection. One of these patients also developed grade 4 leukopenia, grade 4 neutropenia, and grade 3 hypophosphatemia. The other patient developed grade 3 fatigue. The overall incidence of grade 3 and 4 toxicities is summarized in Table 2. There were no treatment-related deaths. The incidence of grade 4 leukopenia was 10%, and of grade 4 neutropenia was 26%. Two patients experienced grade 3 pulmonary toxicity (dyspnea) during the phase II portion that was felt to be potentially treatment-related. Other toxicities were largely as expected, including diarrhea and skin toxicity.
Table 2.
Treatment-related toxicities
| Toxicity | Grade 3: Number (%) |
Grade 4: Number (%) |
|---|---|---|
| Neutropenia | 12 (39) | 8 (26) |
| Leukopenia | 10 (32) | 3 (10) |
| Diarrhea | 10 (32) | 0 |
| Lymphopenia | 8 (26) | 0 |
| Infection | 6 (19) | 0 |
| Pain | 4 (13) | 0 |
| Fatigue | 4 (13) | 0 |
| Electrolyte abnormalities |
4 (13)a | 0 |
| Pulmonary toxicity | 2 (6)b | 0 |
| Neuropathy | 2 (6) | 0 |
| Skin toxicity | 2 (6) | 0 |
| Nausea/emesis | 2 (6) | 0 |
| Elevated ALT | 2 (6) | 0 |
| Prolonged PTT | 2 (6) | 0 |
| Mucositis | 1 (3) | 0 |
| Cardiac toxicity | 0 | 0 |
| Anemia | 0 | 0 |
| Thrombocytopenia | 0 | 0 |
| Other | 3 (10)c | 0 |
PTT partial thromboplastin time. ALT alanine transaminase
Hyponatremia, hypokalemia, hypophosphatemia
Dyspnea
Anorexia, dehydration
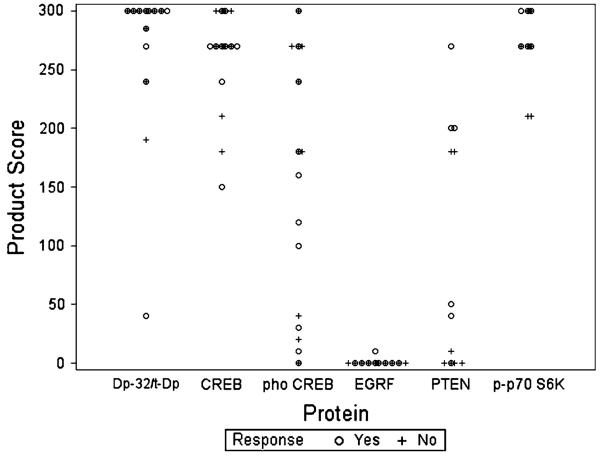
We analyzed expression and phosphorylation levels for several proteins implicated in determining response to receptor tyrosine kinase inhibitors. Twenty-two patient samples were stained for DARPP-32 plus t-DARPP, 20 for CREB and for phospho-CREB, 19 for EGFR, and 13 for PTEN and for p-p70S6K. Sufficient tissue was not available to perform all tests on specimens from each patient. Figure 2 shows the distribution of biomarker expression in responding and non-responding patients. Table 3 summarizes the statistical analysis of expression in these cohorts. The majority of specimens were highly positive for DARPP-32 plus t-DARPP, CREB, and p-p70S6K, whereas only one specimen demonstrated EGFR expression. The range of expression for these biomarkers was too narrow to permit detection of a significant difference between responders to therapy and the small number of non-responders. There was a significant range of expression of phospho-CREB and PTEN, making statistical analysis of these two markers feasible. Neither marker showed a statistically significant correlation with response to therapy. The mean product score for phospho-CREB was 141 ± 108 in responders and 177 ± 115 in non-responders. For PTEN, the mean product score was 127 ± 100 in responders and 53 ± 87 in non-responders (P = 0.09); this was the lowest P value obtained for all markers tested. Due to the non-significant univariate analysis results, multivariate analysis was not initiated. Notably the power to detect a difference between the responders and the non-responders was limited: the power to detect a 30% difference between the two groups (using the pooled estimates for the comparator group and estimates of standard deviation) was only 15 and 7% for Phospho-CREB and PTEN, respectively. The other biomarkers (excluding EGFR due to lack of expression) had power greater than 86% for a 30% change.
Fig. 2.
Expression of biomarkers as measured by the product score (the product of intensity of staining, expressed as a score of 0–3, and the percent positivity of staining). Dp-32/t-Dp: DARPP-32 plus t-DARPP. phoCREB: phospho-CREB
Table 3.
Correlation of response with expression of molecular biomarkers
| Biomarker | Response |
All samples | ||
|---|---|---|---|---|
| No | Yes | |||
| Overall | 10 | 12 | 22 | |
| Phospho-CREB | # of tissue samplesa | 10 | 10 | 20 |
| Mean score (SD)b | 177 (115) | 141 (108) | 159 (110) | |
| CREB | # of tissue samples | 10 | 10 | 20 |
| Mean score (SD) | 267 (41) | 261 (43) | 264 (41) | |
| Dp-32/tDpc | # of tissue samples | 10 | 12 | 22 |
| Mean score (SD) | 282 (37) | 270 (75) | 275 (60) | |
| PTENd | # of tissue samples | 7 | 6 | 13 |
| Mean score (SD) | 53 (87) | 127 (110) | 87 (102) | |
| p-p70 S6K | # of tissue samples | 7 | 6 | 13 |
| Mean score (SD) | 261 (38) | 285 (16.4) | 272 (31) | |
| EGFR | # of tissue samples | 10 | 9 | 19 |
| Mean score (SD) | 0 (0) | 1 (3) | 1 (2) | |
Samples were from the following metastatic sites: liver (5), skin/soft tissue (5), lymph nodes (4), brain (3), bone (2), lung (1), mediastinum (1), pleural fluid (1)
Scores represent the products of the percent positivity score (0–100) and intensity score (0–3) for each antibody
Dp-32/t-Dp DARPP-32 plus t-DARPP
P 0.09, Wilcoxon rank-sum test. All other P-values were greater than 0.09
Discussion
MBC remains incurable, and responses to HER-2-directed therapy in HER-2 positive MBC are relatively brief, with the majority of patients progressing within 1 year [3, 19]. The HER-2-related transmembrane protein EGFR is overexpressed in 18% of breast cancers and is associated with worse survival [25, 26]. Compensatory signaling through EGFR may be one mechanism of trastuzumab resistance [8, 9], suggesting that up-front combination of an EGFR inhibitor with trastuzumab might be more efficacious than trastuzumab alone. Nonetheless, the combination of gefitinib and trastuzumab was not found to be substantially more effective than trastuzumab alone in the treatment of metastatic HER-2 positive MBC, the response rate in previously untreated patients being only 9%, with a TTP of 3 months [27].
We examined the combination of gefitinib, docetaxel, and trastuzumab. Although the initial dose level was too toxic, a docetaxel dose of 60 mg/m2 was well tolerated. The response rate of 64% and PFS of 12.7 months demonstrated in this study are comparable to those achieved with other three-drug combinations that include trastuzumab and a taxane with other chemotherapeutic agents, with potentially lower toxicity through avoidance of a second chemotherapeutic agent [21, 28]. Unfortunately, our study was closed early due to poor accrual, and interpretation of the results is limited by the small sample size.
This trial also examined a number of proteins in an attempt to elucidate markers of response or resistance to therapy. For most of the markers, the range of expression was too narrow to permit meaningful statistical analysis. However, there was a significant range of expression for phospho-CREB and PTEN. We did not see a significant relationship between phospho-CREB and response, in contrast to our previous results which demonstrated higher phospho-CREB levels in residual disease remaining after trastuzumab-containing neoadjuvant therapy [14]. One possible explanation for this is that patients enrolled in the current trial had more advanced disease and PKA signaling (via phospho-CREB) is not associated with therapeutic response in this setting. In the case of PTEN, expression was greater in tumor specimens from patients who responded to therapy; however, this did not reach statistical significance. Previously reported data showed that intact PTEN expression is associated with better response to trastuzumab-containing therapy, suggesting that PTEN loss is a marker of trastuzumab resistance [12]. Although not statistically significant, our data are consistent with these previous findings, and the failure to reach statistical significance could be partly due to the small number of samples available for determination of PTEN expression.
The mTOR effector p-p70S6K may have a role in trastuzumab resistance, as its levels have been correlated with patient outcome during trastuzumab-based therapy when PTEN loss was accounted for [29]. However, we could not demonstrate any association between response to therapy and p-p70S6K levels, since all samples tested were high in p-p70S6K. Further study is needed to determine if this high expression is a uniform feature of MBC.
No relationship between EGFR expression and response to therapy could be demonstrated because 18 of 19 specimens available for testing had little or no detectable EGFR. This is surprising in light of published data demonstrating a correlation between EGFR positivity and HER-2 positivity [25]. The response to therapy in the current study was similar to that reported in studies of trastuzumab-containing regimens without EGFR-directed therapy [21, 28], raising the question of whether a population selected for EGFR expression might have a higher response rate. However, response to anti-EGFR therapy in colon cancer does not correlate with the degree of EGFR expression [30,31], and responses have been shown in colorectal cancer that is EGFR negative by immunohistochemistry (IHC) [32]. Studies in lung cancer suggest that EGFR expression, measured by FISH [33], and EGFR mutations [34] correlate with clinical benefit from EGFR inhibitors. However, neither EGFR positivity by FISH nor EGFR mutations is consistently accompanied by expression of EGFR as measured by IHC [35]. Thus, IHC may not be the optimal way to predict response to EGFR-directed therapy. Further work is needed to determine the relationship between EGFR expression and response to EGFR inhibitors.
Future trials should examine further the potential mechanisms of resistance to HER2 and EGFR-targeting therapy. A wider array of validated markers is needed, assessing the efficacy of multiple pathway-targeting therapies with or without cytotoxic therapies to predict outcome and allow for better treatment selection in this challenging patient population.
Acknowledgments
We wish to thank Kim Robinson for her assistance with data management and Carol Wuenschell, Ph.D., for her editorial assistance. We also thank Sofia Loera and members of the City of Hope Anatomic Pathology Core lab for their help with histology work. This work was supported by the National Cancer Institute at the National Institutes of Health through a National Cancer Institute Comprehensive Cancer Center Grant [CA 33572]; and by AstraZeneca.
Footnotes
This study was presented in part at the 2005 San Antonio Breast Cancer Symposium.
Contributor Information
G. Somlo, Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Rd., Duarte, California 91010, USA
C. L. Martel, Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Rd., Duarte, California 91010, USA
S. K. Lau, Anatomic Pathology, City of Hope Comprehensive Cancer Center, Duarte, CA, USA
P. Frankel, Division of Biostatistics, City of Hope Comprehensive Cancer Center, Duarte, CA, USA
C. Ruel, Division of Biostatistics, City of Hope Comprehensive Cancer Center, Duarte, CA, USA
L. Gu, Division of Tumor Cell Biology, City of Hope Comprehensive Cancer Center, Duarte, CA, USA
A. Hurria, Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Rd., Duarte, California 91010, USA
C. Chung, Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Rd., Duarte, California 91010, USA
T. Luu, Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Rd., Duarte, California 91010, USA
R. Morgan, Jr, Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Rd., Duarte, California 91010, USA.
L. Leong, Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Rd., Duarte, California 91010, USA
M. Koczywas, Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Rd., Duarte, California 91010, USA
M. McNamara, Department of Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center, 1500 E. Duarte Rd., Duarte, California 91010, USA
C. A. Russell, Division of Medical Oncology, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
S. E. Kane, Division of Tumor Cell Biology, City of Hope Comprehensive Cancer Center, Duarte, CA, USA
References
- 1.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5(1):63–69. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. doi:10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 4.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. doi:10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 5.Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R, Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N, Wist E, Sanchez Rovira P, Piccart-Gebhart MJ. 2-year followup of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369(9555):29–36. doi: 10.1016/S0140-6736(07)60028-2. doi:10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 6.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93(24):1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 8.Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, Arteaga CL. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13(16):4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. doi:10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 9.Chan CT, Metz MZ, Kane SE. Differential sensitivities of trastuzumab (Herceptin)-resistant human breast cancer cells to phosphoinositide-3 kinase (PI-3 K) and epidermal growth factor receptor (EGFR) kinase inhibitors. Breast Cancer Res Treat. 2005;91(2):187–201. doi: 10.1007/s10549-004-7715-1. doi:10.1007/s10549-004-7715-1. [DOI] [PubMed] [Google Scholar]
- 10.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28(6):1075–1083. doi: 10.1200/JCO.2009.25.3641. doi:10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dave B, Migliaccio I, Gutierrez MC, Wu MF, Chamness GC, Wong H, Narasanna A, Chakrabarty A, Hilsenbeck SG, Huang J, Rimawi M, Schiff R, Arteaga C, Osborne CK, Chang JC. Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J Clin Oncol. 2011;29(2):166–173. doi: 10.1200/JCO.2009.27.7814. doi:10.1200/JCO.2009.27.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3(5):269–280. doi: 10.1038/ncponc0509. doi:10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 13.Miller TW, Forbes JT, Shah C, Wyatt SK, Manning HC, Olivares MG, Sanchez V, Dugger TC, de Matos Granja N, Narasanna A, Cook RS, Kennedy JP, Lindsley CW, Arteaga CL. Inhibition of mammalian target of rapamycin is required for optimal antitumor effect of HER2 inhibitors against HER2-overexpressing cancer cells. Clin Cancer Res. 2009;15(23):7266–7276. doi: 10.1158/1078-0432.CCR-09-1665. doi:10.1158/1078-0432.CCR-09-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu L, Lau SK, Loera S, Somlo G, Kane SE. Protein kinase A activation confers resistance to trastuzumab in human breast cancer cell lines. Clin Cancer Res. 2009;15(23):7196–7206. doi: 10.1158/1078-0432.CCR-09-0585. doi:10.1158/1078-0432.CCR-09-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, Baselga J, O’Shaughnessy J. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124–1130. doi: 10.1200/JCO.2008.21.4437. doi:10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 16.Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, Eek R, Horai T, Noda K, Takata I, Smit E, Averbuch S, Macleod A, Feyereislova A, Dong RP, Baselga J. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003;21(12):2237–2246. doi: 10.1200/JCO.2003.10.038. doi:10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 17.Albain K, Elledge R, Gradishar WJ, Hayes DF, Rowinsky E, Hudis C, Pusztai L, Tripathy D, Modi S, Rubi S. 20: Open-label, phase II, multicenter trial of ZD1839 (‘Iressa’) in patients with advanced breast cancer. Breast Cancer Res Treat. 2002;76(Suppl. 1) doi:10.1023/A:1021560101414. [Google Scholar]
- 18.Baselga J, Albanell J, Ruiz A, Lluch A, Gascon P, Guillem V, Gonzalez S, Sauleda S, Marimon I, Tabernero JM, Koehler MT, Rojo F. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol. 2005;23(23):5323–5333. doi: 10.1200/JCO.2005.08.326. doi:10.1200/JCO.2005.08.326. [DOI] [PubMed] [Google Scholar]
- 19.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Anton A, Lluch A, Kennedy J, O’Byrne K, Conte P, Green M, Ward C, Mayne K, Extra JM. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23(19):4265–4274. doi: 10.1200/JCO.2005.04.173. doi:10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 20.Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves JA, Wolf MK, Krebs AD, Averbuch SD, Ochs JS, Grous J, Fandi A, Johnson DH. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 2. J Clin Oncol. 2004;22(5):785–794. doi: 10.1200/JCO.2004.07.215. doi:10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 21.Robert N, Leyland-Jones B, Asmar L, Belt R, Ilegbodu D, Loesch D, Raju R, Valentine E, Sayre R, Cobleigh M, Albain K, McCullough C, Fuchs L, Slamon D. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006;24(18):2786–2792. doi: 10.1200/JCO.2005.04.1764. doi:10.1200/JCO.2005.04.1764. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 23.Sun J. Variance estimation of a survival function for interval-censored survival data. Stat Med. 2001;20(8):1249–1257. doi: 10.1002/sim.719. doi:10.1002/sim.719. [DOI] [PubMed] [Google Scholar]
- 24.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MARKer prognostic studies (REMARK) Breast Cancer Res Treat. 2006;100(2):229–235. doi: 10.1007/s10549-006-9242-8. doi:10.1007/s10549-006-9242-8. [DOI] [PubMed] [Google Scholar]
- 25.Rimawi MF, Shetty PB, Weiss HL, Schiff R, Osborne CK, Chamness GC, Elledge RM. Epidermal growth factor receptor expression in breast cancer association with biologic phenotype and clinical outcomes. Cancer. 2010;116(5):1234–1242. doi: 10.1002/cncr.24816. doi:10.1002/cncr.24816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Somlo G, Chu P, Frankel P, Ye W, Groshen S, Doroshow JH, Danenberg K, Danenberg P. Molecular profiling including epidermal growth factor receptor and p21 expression in high-risk breast cancer patients as indicators of outcome. Ann Oncol. 2008;19(11):1853–1859. doi: 10.1093/annonc/mdn402. doi:10.1093/annonc/mdn402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arteaga CL, O’Neill A, Moulder SL, Pins M, Sparano JA, Sledge GW, Davidson NE. A phase I-II study of combined blockade of the ErbB receptor network with trastuzumab and gefitinib in patients with HER2 (ErbB2)-overexpressing metastatic breast cancer. Clin Cancer Res. 2008;14(19):6277–6283. doi: 10.1158/1078-0432.CCR-08-0482. doi:10.1158/1078-0432.CCR-08-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forbes JF, Pienkowski T, Valero V, Eiermann W, Von Minckwitz G, Martin M, Smylie M, Crown JM, Noel N, Pegram M, BCIRG007 investigators BCIRG 007: Randomized phase III trial of trastuzumab plus docetaxel with or without carboplatin first line in HER2 positive metastatic breast cancer (MBC) J Clin Oncol. 2006;24(18S pt.II):LBA516. [Google Scholar]
- 29.Esteva FJ, Guo H, Zhang S, Santa-Maria C, Stone S, Lanchbury JS, Sahin AA, Hortobagyi GN, Yu D. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177(4):1647–1656. doi: 10.2353/ajpath.2010.090885. doi:10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–345. doi: 10.1056/NEJMoa033025. doi:10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 31.Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22(7):1201–1208. doi: 10.1200/JCO.2004.10.182. doi:10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 32.Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L, Klimstra DS, Fridman D, Kelsen DP, Saltz LB. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23(9):1803–1810. doi: 10.1200/JCO.2005.08.037. doi:10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch FR, Varella-Garcia M, Dziadziuszko R, Xiao Y, Gajapathy S, Skokan M, Lin M, O’Neill V, Bunn PA., Jr Fluorescence in situ hybridization subgroup analysis of TRIBUTE, a phase III trial of erlotinib plus carboplatin and paclitaxel in non-small cell lung cancer. Clin Cancer Res. 2008;14(19):6317–6323. doi: 10.1158/1078-0432.CCR-08-0539. doi:10.1158/1078-0432.CCR-08-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. doi:10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 35.Pinter F, Papay J, Almasi A, Sapi Z, Szabo E, Kanya M, Tamasi A, Jori B, Varkondi E, Moldvay J, Szondy K, Keri G, Dominici M, Conte P, Eckhardt S, Kopper L, Schwab R, Petak I. Epidermal growth factor receptor (EGFR) high gene copy number and activating mutations in lung adenocarcinomas are not consistently accompanied by positivity for EGFR protein by standard immunohistochemistry. J Mol Diagn. 2008;10(2):160–168. doi: 10.2353/jmoldx.2008.070125. doi:10.2353/jmoldx.2008.070125. [DOI] [PMC free article] [PubMed] [Google Scholar]